Key Points
Question
Does administration of trastuzumab concomitantly with a taxane for a brief period (9 weeks) lead to similar survival outcomes as chemotherapy and 1 year of trastuzumab in women with early human epidermal growth factor receptor 2–positive breast cancer?
Findings
In this randomized clinical trial including 2174 women, both treatments were associated with high 5-year distant disease–free survival and overall survival rates, but the 9-week trastuzumab regimen resulted in a shorter disease-free survival than the 1-year regimen. The shorter treatment was associated with fewer cardiac adverse effects and better maintained cardiac function.
Meaning
Trastuzumab continued beyond chemotherapy increases efficacy but may lead to more cardiac adverse effects.
This randomized clinical trial evaluates the efficacy and safety of adjuvant trastuzumab continued beyond chemotherapy for a duration of 9 weeks vs 1 year in women with HER2-positive early breast cancer treated with up-front chemotherapy containing a taxane and trastuzumab.
Abstract
Importance
Trastuzumab plus chemotherapy is the standard adjuvant treatment for patients with human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. While the standard duration of trastuzumab treatment is 12 months, the benefits and harms of trastuzumab continued beyond the chemotherapy are unclear.
Objective
To evaluate the efficacy and safety of adjuvant trastuzumab continued beyond chemotherapy in women treated with up-front chemotherapy containing a taxane and trastuzumab.
Design, Setting, and Participants
Open-label, randomized (1:1) clinical trial including women with HER2-positive breast cancer. Chemotherapy was identical in the 2 groups, consisting of 3 cycles of 3-weekly docetaxel (either 80 or 100 mg/m2) plus trastuzumab for 9 weeks, followed by 3 cycles of fluorouracil, epirubicin, and cyclophosphamide. Thereafter, no trastuzumab was administered in the 9-week group, whereas controls received trastuzumab to complete 1 year of administration. Disease-free survival (DFS) was compared between the groups using a Cox model and the noninferiority approach. The estimated sample size was 2168 patients (1-sided testing, with a relative noninferiority margin of 1.3). From January 3, 2008, to December 16, 2014, 2176 patients were accrued from 7 countries.
Intervention
Docetaxel plus trastuzumab for 9 weeks, followed by 3 cycles of fluorouracil, epirubicin, and cyclophosphamide in both groups. Controls continued trastuzumab to 1 year.
Main Outcomes and Measures
The primary objective was DFS; secondary objectives included distant disease–free survival, overall survival, cardiac DFS, and safety.
Results
In the 2174 women analyzed, median age was 56 (interquartile range [IQR], 48-64) years. The median follow-up was 5.2 (IQR, 3.8-6.7) years. Noninferiority of the 9-week treatment could not be demonstrated for DFS (hazard ratio, 1.39; 2-sided 90% CI, 1.12-1.72). Distant disease–free survival and overall survival did not differ substantially between the groups. Thirty-six (3%) and 21 (2%) patients in the 1-year and the 9-week groups, respectively, had cardiac failure; the left ventricle ejection fraction was better maintained in the 9-week group. An interaction was detected between the docetaxel dose and DFS; patients in the 9-week group treated with 80 mg/m2 had inferior and those treated with 100 mg/m2 had similar DFS as patients in the 1-year group.
Conclusions and Relevance
Nine weeks of trastuzumab was not noninferior to 1 year of trastuzumab when given with similar chemotherapy. Cardiac safety was better in the 9-week group. The docetaxel dosing with trastuzumab requires further study.
Trial Registration
ClinicalTrials.gov Identifier: NCT00593697
Introduction
Patients with breast cancer treated with adjuvant trastuzumab, an antibody targeting human epidermal growth factor receptor 2 (HER2), and chemotherapy after surgery for localized cancer have fewer recurrences and live longer compared with patients treated with chemotherapy alone.1,2 The most notable adverse effect of trastuzumab therapy is congestive heart failure (CHF), which occurs in 1% to 3% of the patients treated in clinical trials,1,3 but the proportion is higher in elderly populations with risk factors for CHF.4 Trastuzumab-related decrease in the cardiac left ventricular ejection fraction (LVEF) usually resolves after drug discontinuation and initiation of therapy for CHF.5,6,7
Adjuvant trastuzumab is recommended to be administered for 1 year and in part concomitantly with chemotherapy.8,9 The choice of this duration was arbitrary10 in the trials that established the current standard 12-month duration.11,12,13 While the optimal duration remains unknown, 6 months of adjuvant trastuzumab did not lead to noninferior survival outcomes compared with 1-year administration in randomized trials, although the results tended to favor the longer duration,14,15 and 2-year administration was not superior to 1 year of administration.16
Observations from in vitro studies,17,18 randomized trials in the treatment of advanced breast cancer,19,20 and data from 1 adjuvant trial21 suggest that concomitant administration of a taxane with trastuzumab improves efficacy, and may be synergistic. In 2 randomized trials22,23 the experimental group patients were treated with trastuzumab and a concomitant taxane and received no trastuzumab after chemotherapy, whereas the standard group patients were treated with trastuzumab both during chemotherapy and after chemotherapy for a total duration of 1 year. Neither trial found the standard group to have superior disease-free survival (DFS) or overall survival (OS), but these trials had relatively limited power. We studied in the present Synergism or Long Duration (SOLD) trial the benefits and harms of adjuvant single-agent trastuzumab continued beyond chemotherapy in a patient population treated with upfront chemotherapy containing docetaxel and trastuzumab. The study hypothesis was that a brief course of trastuzumab, administered concomitantly with potentially synergistic chemotherapy, leads to similar efficacy as chemotherapy plus trastuzumab administered for 1 year, and to fewer cardiac toxic effects.
Methods
Patients
Patients who had histologically confirmed HER2-positive breast cancer with either regional node-positive or node-negative disease and with cancer size 5 mm or greater were eligible (when size was 6-10 mm, histological grade was required to be 2 or 3). Cancer HER2 positivity was verified by demonstrating either the presence of ERBB2/HER2 amplification using in situ hybridization or strong HER2 protein expression by immunohistochemical analysis (a score of 3+ on a scale from negative to 3+) according to the institutional guidelines. Other eligibility criteria included age 18 years or older; World Health Organization performance score 0 or 1 (on a scale of 0-5); and adequate renal, hepatic, and bone marrow function. An LVEF of at least 50% was required (or within the institutional reference range). Patients with clinically significant cardiac disease were excluded. Other exclusion criteria included presence of distant metastases, neoadjuvant systemic therapy, and history of another malignant neoplasm within the past 5 years. Independent ethics committees of the participating hospitals and the relevant medical authorities of the participating countries approved the study. All patients provided written informed consent prior to study inclusion.
Study Procedures
The primary objective in this open-label phase 3 trial was DFS, defined as the interval between the date of randomization and date of diagnosis of invasive cancer recurrence (distant recurrence, locoregional recurrence, contralateral breast cancer, or any invasive second cancer) or death if the patient died prior to recurrence. The secondary objectives included distant disease–free survival (DDFS, the time from randomization to the date of first diagnosis of distant recurrence of breast cancer, or to death), cardiac DFS (the period from randomization to the date of a cardiac event, cancer recurrence, or death; a cardiac event was defined as CHF necessitating medication or medical intervention, myocardial infarction, cardiac or coronary artery surgery, or stenting), OS (the period from randomization to the date of death), and treatment safety.
Patients were randomized centrally with blinded computer-assisted allocation to the study groups. Randomization (1:1) was performed using dynamic minimization, with a 90% chance of allocation to the group with the lowest count. Patients were stratified at randomization between the groups by axillary nodal status, HER2 analysis method (in situ hybridization or immunohistochemical analysis), cancer estrogen receptor expression (positive vs negative), and study center.
Chemotherapy was identical in the groups. It consisted of 3 cycles of 3-weekly docetaxel given concomitantly with trastuzumab, followed by 3 cycles of 3-weekly fluorouracil, epirubicin hydrochloride, and cyclophosphamide (FEC). The only difference in the treatment between the groups was that in the experimental group (the 9-week group) no further trastuzumab was given after completion of chemotherapy, whereas in the standard treatment group (1-year group) trastuzumab was given to complete 1 year of administration (for a total of 51 weeks).
The docetaxel dose was prespecified for each center, either 80 or 100 mg/m2 intravenously, based on center preference. Use of the starting dose of 100 mg/m2 in patients younger than 60 years and 80 mg/m2 among those 60 years or older was allowed, provided that this practice was followed throughout the study. FEC consisted of intravenous fluorouracil (600 mg/m2), epirubicin hydrochloride (75 mg/m2), and cyclophosphamide (600 mg/m2), each given on day 1 of the 3-week cycle. Intravenous trastuzumab was administered with docetaxel either weekly or 3-weekly, or subcutaneously 3-weekly (weekly: first dose 4 mg/kg, subsequently 2 mg/kg; 3-weekly: first dose 8 mg/kg, subsequently 6 mg/kg; 3-weekly subcutaneously: each dose 600 mg regardless of body weight). When trastuzumab was administered after chemotherapy (standard group only), it was given 3-weekly 14 times (either intravenously or subcutaneously; intravenously: first dose 8 mg/kg, subsequently 6 mg/kg; subcutaneously: 600 mg regardless of body weight).
Hematopoietic growth factor support was permitted at the discretion of the investigator. Patients with estrogen receptor–positive and/or progesterone receptor–positive cancer received adjuvant endocrine therapy for a minimum of 5 years on completion of chemotherapy. The choice of endocrine therapy was at the treating physician’s discretion. The institutional criteria for hormone receptor–positive cancer were followed when the decision about adjuvant endocrine treatment was made, but tumors with at least 10% positive cancer cells by immunohistochemical analysis were considered receptor positive for the stratification. Locoregional radiotherapy was given according to institutional practice. In the 1-year group, single-agent trastuzumab was started 3 weeks (±1 week) after the last FEC cycle.
Adverse effects were graded according to the Common Terminology Criteria for Adverse Events, version 3.0. Docetaxel and FEC doses were reduced 20% in case of febrile neutropenia, neutropenic infection, grade 4 neutropenia lasting longer than 7 days, any grade 3 or 4 nonhematological toxic effect, or any treatment-related toxic effect that resulted in hospitalization. Trastuzumab doses were not reduced despite docetaxel dose reduction. When docetaxel administration was deferred, trastuzumab administration was also deferred. In the 1-year group no trastuzumab dose reductions were performed, but trastuzumab was discontinued when grade 3 or 4 nonhematological toxic effects considered trastuzumab related occurred, or when a grade 3 or 4 cardiac event, symptomatic cardiac failure, or cardiac failure necessitating medical management took place, or when the LVEF decreased more than 10 percentage points from the baseline value to a value less than 50%, or to less than 45% from any baseline value.
Staging examinations were done according to the institutional practice. However, computed tomography or radiography of the chest; isotope bone scan or skeletal radiography; and computed tomography, magnetic resonance imaging, or ultrasound of the liver were mandatory at screening for patients with at least 4 positive axillary nodes, metastases in the internal mammary, infraclavicular, or supraclavicular nodes, or when the primary tumor was larger than 5 cm, or classified as pT4. The LVEF was measured at baseline, at study weeks 18, 31, 43, and 61, and 36 months after study entry with either echocardiography (1295 patients [60%]) or isotope cardiography (876 patients [40%]), using the same method throughout the study. The patients were scheduled for a minimum of 8 years of follow-up after randomization.
Statistical Analysis
The study was designed as a superiority trial with an estimated recruitment period of 4 years. The sample calculation was carried out considering a 4% difference in DFS between the groups, 80% vs 84% after 5 years of follow-up, a power of 0.80, a 2-sided significance level of .05, a hazard ratio (HR) of 0.781, and 3% of the patients estimated to be lost to follow-up or to discontinue the study. With these assumptions, 516 events and a sample size of 3000 patients (1500 per group) were estimated to be needed.
These power calculations were revised and the study protocol (Supplement 1) was amended on February 21, 2014, because new data2,24 suggested that the assumptions made for DFS were too low, and it seemed unreasonable to assume that DFS in the experimental group could be superior to the standard arm because trastuzumab-related cardiac toxic effects were reported relatively rarely. Therefore, a noninferiority design seemed a more reasonable approach. Coincidentally, patient accrual was slower than anticipated, and the longer-than-expected accrual period affected the power calculations. A 5-year DFS of 85.0% was estimated in the 1-year group,2 and absolute 5-year DFS differences of less than 4% were not considered clinically significant, leading to a relative noninferiority margin of 1.3. The noninferiority margin corresponding to the true (observed) 5-year DFS rate of 88.7% is 1.385 (Statistical Analysis Plan in Supplement 1). Based on an estimated accrual duration of 7.5 years, 1-sided testing,25,26 a relative noninferiority margin of 1.3, and a 3% dropout rate, the final sample size was 2168 patients (1084 patients per group). The primary analysis was planned for when approximately 366 DFS events were reached or when the last patient entered was followed up for 2.0 years after randomization, whichever occurred first. The sample size was calculated using nQuery Advisor, version 6.0 (Statistical Solutions Ltd).
Efficacy analyses were based on the intention-to-treat principle. Exploratory subgroup analyses were defined in the Statistical Analysis Plan approved prior to the study analysis on February 28, 2017, but not in the original study protocol. The safety population included patients who received at least 1 dose of the study drugs. Frequency tables were analyzed using the χ2 test. The LVEF between the groups with time was compared with repeated-measures analysis of variance. Survival between groups was compared using the Kaplan-Meier life-table method and with a Cox proportional hazards model adjusted with the stratification factors. The subgroup analyses were done by including the treatment group, the subgroup variable, and their interaction in the Cox model. Survival analysis results are provided with a 90% 2-sided confidence interval (corresponding to the 1-sided 95% upper limit used in the evaluation of noninferiority). The P values are 2 sided and not adjusted for multiple testing. Statistical analyses were performed with SAS, version 9.3 for Windows (SAS Institute, Inc).
Results
Between January 3, 2008, and December 16, 2014, 2176 patients were accrued from 65 centers (9-week group, 1087; 1-year group, 1089). On December 16, 2016, when the last patient accrued had been followed up for 2 years after randomization, fewer than the anticipated number of DFS events were reported, and therefore the study was analyzed as per the protocol based on the landmark follow-up time. December 31, 2016, was set as the data collection cutoff date.
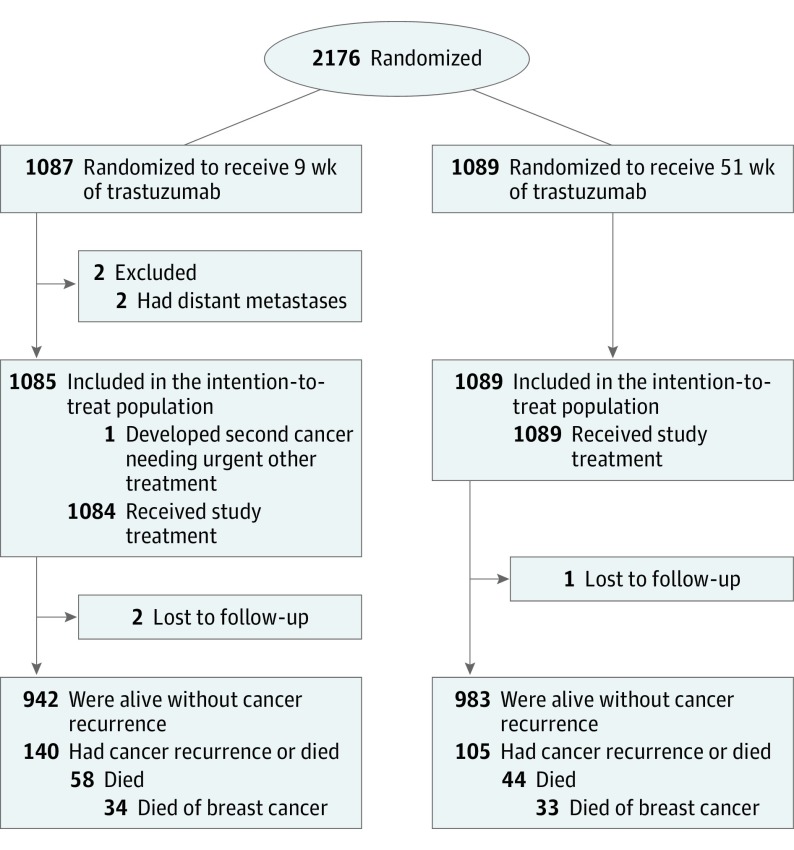
Two patients with overt metastases at the time of study entry were excluded from the intention-to-treat analysis (Figure 1). Two patients in the 9-week group and 1 in the 1-year group were lost to follow-up. The patient and breast cancer characteristics were similar in the groups; most had axillary node–negative cancer (Table 1). Adjuvant endocrine therapy was administered to 714 (66%) and 727 (67%) participants after chemotherapy in the 9-week and 1-year groups, respectively, and locoregional radiotherapy to 824 (76%) and 811 (74%).
Figure 1. CONSORT Diagram.
The numbers of individuals screened for eligibility and the reasons for exclusion were not captured.
Table 1. Baseline Demographic and Clinical Characteristics.
| Characteristic | No. (%)a | |
|---|---|---|
| Trastuzumab for 9 wk (n = 1085) |
Trastuzumab for 51 wk (n = 1089) |
|
| Age, median (IQR), y | 56 (49-64) | 56 (48-63) |
| Weight, median (IQR), kg | 70 (62-80) | 70 (61-79) |
| Race | ||
| White | 1061 (98) | 1066 (98) |
| Other | 24 (2) | 23 (2) |
| Menopausal status | ||
| Premenopausal | 353 (33) | 364 (33) |
| Postmenopausal | 731 (67) | 724 (66) |
| Not available | 1 (<1) | 1 (<1) |
| World Health Organization performance status | ||
| 0 | 975 (90) | 963 (88) |
| 1 | 102 (9) | 112 (10) |
| Not available | 8 (1) | 14 (1) |
| Breast tumor diameter, mm | ||
| ≤10 | 129 (12) | 155 (14) |
| 11-20 | 473 (44) | 453 (42) |
| 21-50 | 447 (41) | 452 (42) |
| >50 | 36 (3) | 29 (3) |
| No. of axillary lymph nodes with cancer | ||
| 0 | 647 (60) | 649 (60) |
| 1-3 | 322 (30) | 320 (29) |
| >3 | 116 (11) | 120 (11) |
| Stage | ||
| I | 427 (39) | 430 (39) |
| II | 529 (49) | 528 (48) |
| III | 129 (12) | 131 (12) |
| Histological grade | ||
| 1 | 26 (2) | 27 (2) |
| 2 | 340 (31) | 327 (30) |
| 3 | 714 (66) | 731 (67) |
| Not available | 5 (<1) | 4 (<1) |
| Histological type | ||
| Ductal | 1000 (92) | 1000 (92) |
| Lobular | 44 (4) | 49 (4) |
| Other | 39 (4) | 39 (4) |
| Not available | 2 (<1) | 1 (<1) |
| Estrogen receptor status | ||
| Positive | 711 (66) | 723 (66) |
| Negative | 374 (34) | 366 (34) |
| Progesterone receptor status | ||
| Positive | 504 (46) | 517 (47) |
| Negative | 576 (53) | 565 (52) |
| Not available | 5 (<1) | 7 (1) |
| HER2 status | ||
| Positive | 1078 (99) | 1084 (100) |
| Negative | 0 | 1 (<1) |
| Unconfirmed, IHC++ | 7 (1) | 4 (1) |
Abbreviations: HER2, human epidermal growth factor-2; IHC, immunohistochemistry; IQR, interquartile range.
Percentages may not total 100 because of rounding.
Survival
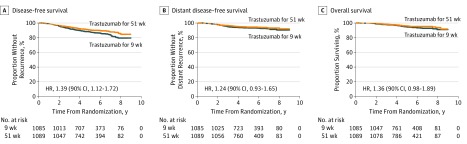
During a median follow-up time of 5.2 years (interquartile range [IQR], 3.8-6.7 years), 140 and 105 DFS events were recorded in the 9-week and 1-year groups, respectively (Table 2). Noninferiority of the 9-week treatment as compared with the 1-year treatment was not demonstrated (5-year DFS, 88.0% vs 90.5%, respectively; HR, 1.39; 2-sided 90% CI, 1.12-1.72) (Figure 2). When the analysis was adjusted for axillary nodal status and cancer estrogen receptor content using a Cox model, the HR was 1.42 (90% CI, 1.15-1.76). The DDFS and OS did not differ substantially between groups (5-year DDFS, 93.2% vs 94.2%; HR, 1.24; 90% CI, 0.93-1.65; 5-year OS, 94.7% vs 95.9%; HR, 1.36; 90% CI, 0.98-1.89). Five-year cardiac DFS was 86.6% and 87.5%, respectively (HR, 1.16; 90% CI, 0.96-1.40) (eFigure 1 in Supplement 2).
Table 2. Cancer Recurrence and Survival.
| Variable | No. (%) | Hazard Ratioa (90% CI) | |
|---|---|---|---|
| Trastuzumab for 9 wk (n = 1085) |
Trastuzumab for 51 wk (n = 1089) |
||
| Any recurrence or deathb | 140 (13) | 105 (10) | 1.39 (1.12-1.72) |
| Distant recurrence | 73 (7) | 61 (6) | 1.24 (0.93-1.65) |
| Locoregional recurrence | 17 (2) | 13 (1) | 1.35 (0.74-2.48) |
| Contralateral breast cancer | 15 (1) | 7 (1) | 2.24 (1.05-4.75) |
| Second cancer | 27 (3) | 24 (2) | 1.16 (0.73-1.84) |
| Death without cancer | 14 (1)c | 5 (0) | 2.88 (1.22-6.78) |
| Death from any cause | 58 (5) | 44 (4) | 1.36 (0.98-1.89) |
| Death from breast cancer | 34 (3) | 33 (3) | 1.06 (0.71-1.59) |
| Death from other caused | 24 (2) | 11 (1) | 2.24 (1.23-4.08) |
Calculated with an unadjusted Cox proportional hazards model.
Six patients in the 9-week group and 5 patients in the 1-y group had 2 disease-free survival events.
The cause of death was unknown in 3 cases.
Eleven patients died of second cancer, 5 of infection, 2 of a cardiac cause, 3 of other causes, and 3 of an unknown cause in the 9-week group; in the 1-year group, 6 died of second cancer, 2 of infection, 1 of a cardiac cause, and 2 of other causes.
Figure 2. Kaplan-Meier Estimates of Survival Outcomes.
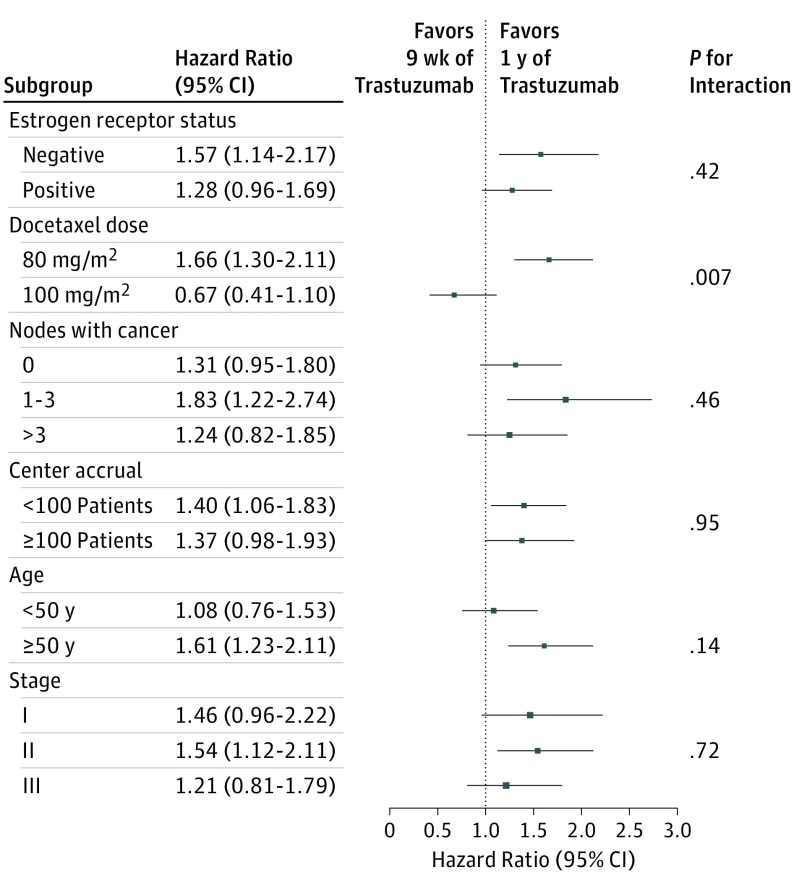
The subgroup analyses on DFS generally favored the 1-year treatment duration (Figure 3). The subgroups were formed with a stratification factor (cancer estrogen receptor content) or factors predefined in the study Statistical Analysis Plan (docetaxel dosing, the number of axillary nodes with cancer, center accrual, patient age at study entry, and cancer stage) (Supplement 1). A significant interaction (P = .007) was found between docetaxel dosing and DFS; the 1678 patients who received docetaxel, 80 mg/m2, had inferior and the 480 who received 100 mg/m2 had similar DFS to patients in the 1-year group treated with the same docetaxel dosing (eFigure 2 in Supplement 2).
Figure 3. Disease-Free Survival in Prespecified Subgroups.
Treatment Tolerance
Twenty-two (2%) of the 1085 patients in the 9-week group and 42 (4%) of the 1089 patients in the 1-year group had a protocol-defined cardiac adverse event (P = .01). Most were CHF (21 and 36, respectively). The LVEF was better maintained in the 9-week group, with less decrease in LVEF during follow-up (mean LVEF was 63% in the 9-week group and 61% in the 1-year group 61 weeks after study entry; P < .001) (eFigure 3 in Supplement 2). As expected, chemotherapy-related adverse effects were similar in both groups (eTables 1 and 2 in Supplement 2). A total of 185 (17%) and 203 (19%) patients in the 9-week and 1-year groups had the docetaxel dose reduced on 1 or more occasion, respectively, and 128 (12%) and 120 (11%) had FEC administered at a reduced dose.
Ninety-six (9%) patients in the 9-week group and 217 (20%) in the 1-year group discontinued scheduled trastuzumab administration, usually due to adverse effects. Most patients completed all 6 chemotherapy cycles (9-week group, 1041; 1-year group, 1038).
Discussion
Women with HER2-positive breast cancer treated with adjuvant chemotherapy and trastuzumab for 1 year had higher DFS compared with patients who received trastuzumab during chemotherapy only. Because the only difference between the regimens was administration of trastuzumab after chemotherapy in the 1-year group, postchemotherapy trastuzumab improved DFS despite prior trastuzumab plus docetaxel. The HR for OS was in agreement with that for DFS, although this analysis was based on a relatively small number of events. The numbers of deaths considered to have resulted from breast cancer were similar in the groups. The 9-week regimen was associated with fewer cardiac adverse effects and a smaller decline in the LVEF.
The numerical difference in the DFS events recorded between the groups was 35 (140 [13%] vs 105 [10%]). The number of distant recurrences was numerically higher in the 9-week group (73 [7%] vs 61 [6%] events). The numbers of death from a cause other than breast cancer (24 [2%] vs 11 [1%]) and the numbers of contralateral breast cancers (15 [1%] vs 7 [1%]) were also higher in the 9-week group, which may have been due to chance because there are no biological explanations for a higher incidence of these events with less trastuzumab. There was only approximately 1% absolute difference between the groups in the 5-year DDFS and OS rates, which are clinically relevant survival end points. Considering this, patients who are unable to complete the longer duration of trastuzumab therapy due to treatment toxic effects or other reasons usually still have a favorable outcome.
We found an interaction between docetaxel dosing and DFS. A subgroup of the patients were treated in centers where the 100 mg/m2 docetaxel dose was preferred, and in this patient population the 1-year duration of trastuzumab did not yield better DFS than the 9-week duration. This observation needs to be viewed with caution because this is a subgroup analysis and site-related confounding factors cannot be excluded. Other trials have suggested that docetaxel and trastuzumab have additive or synergistic effects for breast cancer.17,18,19,20,21 In the Short-HER trial, which compared a regimen of trastuzumab given for 9 weeks concomitantly with 100 mg/m2 of docetaxel27 vs a modified standard chemotherapy regimen including 1 year of trastuzumab administration,2 patients assigned to 9 weeks of trastuzumab did not have inferior DFS or OS compared with those treated with the 1-year trastuzumab regimen despite larger cumulative doses of epirubicin and docetaxel administered in the 1-year trastuzumab group.23 Similarly, in the small randomized E2198 trial, in which the patients first received 12 weeks of a full dose of 3-weekly paclitaxel (175 mg/m2) and concomitant trastuzumab, followed by 4 cycles of doxorubicin and cyclophosphamide, or the aforementioned treatment followed by 1 year of trastuzumab, trastuzumab continued beyond chemotherapy did not improve DFS or OS.22
Collectively, these results from SOLD, E2198, and Short-HER suggest that a relatively high dose of the coadministered taxane should be given with trastuzumab when a brief course of trastuzumab will be evaluated in further clinical trials. The biological explanation for the interaction between taxane dosing and trastuzumab remains speculative. Trastuzumab action likely involves the host immune system and activation of antibody-dependent cellular cytotoxicity.28,29 Hypothetically, a large enough dose of a taxane might reduce the numbers of immunosuppressive lymphocytes and other cells in the cancer microenvironment to a level that allows immune system activation for a period long enough to enhance trastuzumab efficacy.
Limitations
The study has some limitations. Many patients had node-negative cancer. Some investigators may have preferred not to enroll patients with a high risk of cancer recurrence, and some study centers participated in a concurrent trial.30 The likely too low estimation for DFS and the longer-than-planned accrual time led to a revision of the study design and the power calculations during the study. The protocol-defined median follow-up time, rather than the number of events, triggered the analysis because it seemed not possible to reach the planned number of events within a reasonable time frame, leading to a lower-than-planned study power. Yet, the present analysis seems to be adequately powered for the primary objective of evaluating noninferiority.
Conclusions
Noninferiority of 9-week administration of adjuvant trastuzumab, when given with docetaxel, could not be demonstrated compared with chemotherapy and 1-year duration of trastuzumab therapy. Although the shorter regimen was associated with fewer cardiac toxic effects and there was little absolute difference in clinically important survival outcomes, DDFS and OS, in the overall assessment chemotherapy plus 1 year of anti-HER2 therapy should remain the standard treatment. Adequate dosing of the partner chemotherapy agents is likely important and requires further evaluation.
Trial Protocol and Statistical Analysis Plan
eFigure 1. Cardiac Disease-Free Survival
eFigure 2. Kaplan-Meier Estimates of Disease-Free Survival Stratified by the Scheduled Docetaxel Dose
eFigure 3. Mean Cardiac Ejection Fractions Stratified by the Treatment Group
eTable 1. Most Frequent Adverse Events in the Safety Population During Docetaxel Plus Trastuzumab Administration (Cycles 1 to 3)
eTable 2. Most Frequent Adverse Events in the Safety Population During Fluorouracil, Epirubicin, and Cyclophosphamide (FEC) Administration (Cycles 4 to 6)
References
- 1.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;(4):CD006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long HD, Lin YE, Zhang JJ, Zhong WZ, Zheng RN. Risk of congestive heart failure in early breast cancer patients undergoing adjuvant treatment with trastuzumab: a meta-analysis. Oncologist. 2016;21(5):547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavez-MacGregor M, Zhang N, Buchholz TA, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31(33):4222-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldhar HA, Yan AT, Ko DT, et al. The temporal risk of heart failure associated with adjuvant trastuzumab in breast cancer patients: a population study. J Natl Cancer Inst. 2015;108(1):djv301. [DOI] [PubMed] [Google Scholar]
- 6.Advani PP, Ballman KV, Dockter TJ, Colon-Otero G, Perez EA. Long-term cardiac safety analysis of NCCTG N9831 (Alliance) adjuvant trastuzumab trial. J Clin Oncol. 2016;34(6):581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Azambuja E, Procter MJ, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01). J Clin Oncol. 2014;32(20):2159-2165. [DOI] [PubMed] [Google Scholar]
- 8.Coates AS, Winer EP, Goldhirsch A, et al. ; Panel Members . Tailoring therapies—improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26(8):1533-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology (NCCN Guidelines). Breast cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed November 19, 2017.
- 10.Pinto AC, Ades F, de Azambuja E, Piccart-Gebhart M. Trastuzumab for patients with HER2 positive breast cancer: delivery, duration and combination therapies. Breast. 2013;22(suppl 2):S152-S155. [DOI] [PubMed] [Google Scholar]
- 11.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673-1684. [DOI] [PubMed] [Google Scholar]
- 12.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. ; Herceptin Adjuvant (HERA) Trial Study Team . Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659-1672. [DOI] [PubMed] [Google Scholar]
- 13.Slamon D, Eiermann W, Robert N, et al. ; Breast Cancer International Research Group . Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pivot X, Romieu G, Debled M, et al. ; PHARE Trial Investigators . 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14(8):741-748. [DOI] [PubMed] [Google Scholar]
- 15.Mavroudis D, Saloustros E, Malamos N, et al. ; Breast Cancer Investigators of Hellenic Oncology Research Group (HORG), Athens, Greece . Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol. 2015;26(7):1333-1340. [DOI] [PubMed] [Google Scholar]
- 16.Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. ; Herceptin Adjuvant (HERA) Trial Study Team . 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the Herceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pegram MD, Lopez A, Konecny G, Slamon DJ. Trastuzumab and chemotherapeutics: drug interactions and synergies. Semin Oncol. 2000;27(6)(suppl 11):21-25. [PubMed] [Google Scholar]
- 18.Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96(10):739-749. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Nakagami K, Mizutani M, et al. Randomized phase III trial of trastuzumab monotherapy followed by trastuzumab plus docetaxel versus trastuzumab plus docetaxel as first-line therapy in patients with HER2-positive metastatic breast cancer: the JO17360 Trial Group. Breast Cancer Res Treat. 2010;119(1):127-136. [DOI] [PubMed] [Google Scholar]
- 20.Hamberg P, Bos MM, Braun HJ, et al. ; Dutch Breast Cancer Trialists’ Group (BOOG) . Randomized phase II study comparing efficacy and safety of combination-therapy trastuzumab and docetaxel vs sequential therapy of trastuzumab followed by docetaxel alone at progression as first-line chemotherapy in patients with HER2+ metastatic breast cancer: HERTAX trial. Clin Breast Cancer. 2011;11(2):103-113. [DOI] [PubMed] [Google Scholar]
- 21.Perez EA, Suman VJ, Davidson NE, et al. Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol. 2011;29(34):4491-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider BP, O’Neill A, Shen F, et al. Pilot trial of paclitaxel-trastuzumab adjuvant therapy for early stage breast cancer: a trial of the ECOG-ACRIN cancer research group (E2198). Br J Cancer. 2015;113(12):1651-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conte PF, Bisagni G, Frassoldati A, et al. 9 weeks vs 1 year adjuvant trastuzumab in combination with chemotherapy: results of the phase III multicentric Italian Short-HER study. J Clin Oncol. 2017;35(suppl): abstr 501. [Google Scholar]
- 24.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothmann M, Li N, Chen G, Chi GY, Temple R, Tsou HH. Design and analysis of non-inferiority mortality trials in oncology. Stat Med. 2003;22(2):239-264. [DOI] [PubMed] [Google Scholar]
- 26.Saad ED, Buyse M. Non-inferiority trials in breast and non-small cell lung cancer: choice of non-inferiority margins and other statistical aspects. Acta Oncol. 2012;51(7):890-896. [DOI] [PubMed] [Google Scholar]
- 27.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. ; FinHer Study Investigators . Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809-820. [DOI] [PubMed] [Google Scholar]
- 28.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27(34):5838-5847. [DOI] [PubMed] [Google Scholar]
- 29.Collins DM, Gately K, Hughes C, et al. Tyrosine kinase inhibitors as modulators of trastuzumab-mediated antibody-dependent cell-mediated cytotoxicity in breast cancer cell lines. Cell Immunol. 2017;319:35-42. [DOI] [PubMed] [Google Scholar]
- 30.von Minckwitz G, Procter M, de Azambuja E, et al. ; APHINITY Steering Committee and Investigators . Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eFigure 1. Cardiac Disease-Free Survival
eFigure 2. Kaplan-Meier Estimates of Disease-Free Survival Stratified by the Scheduled Docetaxel Dose
eFigure 3. Mean Cardiac Ejection Fractions Stratified by the Treatment Group
eTable 1. Most Frequent Adverse Events in the Safety Population During Docetaxel Plus Trastuzumab Administration (Cycles 1 to 3)
eTable 2. Most Frequent Adverse Events in the Safety Population During Fluorouracil, Epirubicin, and Cyclophosphamide (FEC) Administration (Cycles 4 to 6)