Key Points
Question
Are there differences in glioma incidence and survival by race or ethnicity in the United States?
Findings
In this population-based analysis of 244 808 patients with glioma, non-Hispanic whites had the highest incidence and the lowest relative survival rates in most histologies.
Meaning
Differences in incidence and survival by race or ethnicity can inform future discovery of risk factors and reveal unaddressed health disparities.
Abstract
Importance
Glioma is the most commonly occurring malignant brain tumor in the United States, and its incidence varies by age, sex, and race or ethnicity. Survival after brain tumor diagnosis has been shown to vary by these factors.
Objective
To quantify the differences in incidence and survival rates of glioma in adults by race or ethnicity.
Design, Setting, and Participants
This population-based study obtained incidence data from the Central Brain Tumor Registry of the United States and survival data from Surveillance, Epidemiology, and End Results registries, covering the period January 1, 2000, to December 31, 2014. Average annual age-adjusted incidence rates with 95% CIs were generated by glioma histologic groups, race, Hispanic ethnicity, sex, and age groups. One-year and 5-year relative survival rates were generated by glioma histologic groups, race, Hispanic ethnicity, and insurance status. The analysis included 244 808 patients with glioma diagnosed in adults aged 18 years or older. Data were collected from January 1, 2000, to December 31, 2014. Data analysis took place from December 11, 2017, to January 31, 2018.
Results
Overall, 244 808 patients with glioma were analyzed. Of these, 150 631 (61.5%) were glioblastomas, 46 002 (18.8%) were non-glioblastoma astrocytomas, 26 068 (10.7%) were oligodendroglial tumors, 8816 (3.6%) were ependymomas, and 13 291 (5.4%) were other glioma diagnoses in adults. The data set included 137 733 males (56.3%) and 107 075 (43.7%) females. There were 204 580 non-Hispanic whites (83.6%), 17 321 Hispanic whites (7.08%), 14 566 blacks (6.0%), 1070 American Indians or Alaska Natives (0.4%), and 5947 Asians or Pacific Islanders (2.4%). Incidences of glioblastoma, non-glioblastoma astrocytoma, and oligodendroglial tumors were higher among non-Hispanic whites than among Hispanic whites (30% lower overall), blacks (52% lower overall), American Indians or Alaska Natives (58% lower overall), or Asians or Pacific Islanders (52% lower overall). Most tumors were more common in males than in females across all race or ethnicity groups, with the great difference in glioblastoma where the incidence was 60% higher overall in males. Most tumors (193 329 [79.9%]) occurred in those aged 45 years or older, with differences in incidence by race or ethnicity appearing in all age groups. Survival after diagnosis of glioma of different subtypes was generally comparable among Hispanic whites, blacks, and Asians or Pacific Islanders but was lower among non-Hispanic whites for many tumor types, including glioblastoma, irrespective of treatment type.
Conclusions and Relevance
Incidence of glioma and 1-year and 5-year survival rates after diagnosis vary significantly by race or ethnicity, with non-Hispanic whites having higher incidence and lower survival rates compared with individuals of other racial or ethnic groups. These findings can inform future discovery of risk factors and reveal unaddressed health disparities.
Using 14 years of data from the US Central Brain Tumor Registry and the Surveillance, Epidemiology, and End Results registries, this study examines the incidence and survival rates of glioma and its subtypes among 4 racial or ethnic groups.
Introduction
Glioma is the most commonly occurring type of malignant brain tumor in the United States, with an average annual age-adjusted incidence rate (AAAIR) of 6.0 per 100 000 population from 2010 to 2014,1 and causes significant morbidity and mortality. Glioblastoma, the most commonly occurring type of glioma, has a 5-year survival rate of approximately 5%.1 Age and extent of resection (EOR) are 2 of the strongest prognostic factors in glioblastoma.2 Incidence of glioma varies substantially by age, sex, and race or ethnicity; in the United States, incidence is highest among non-Hispanic whites.1 Glioma incidence also varies globally, with the highest rates in the United States, Canada, Australia, and Northern Europe.3 Lifetime risk of developing a malignant brain tumor is more than twice as high among non-Hispanic whites (715/100,000) compared with blacks (347/100,000) and is 25% higher compared with Hispanic whites (559/100,000).4 Survival rates after a diagnosis of malignant brain tumor also vary by race or ethnicity.5,6
The few validated risk factors for glioma include history of allergies or atopic disease (which decreases risk) and exposure to ionizing radiation (which increases risk).7 These risk factors explain a small proportion of glioma incidence and do not fully explain racial or ethnic differences in incidence. The purpose of this study was to use population-based data to quantify differences in glioma incidence and survival by race or ethnicity.
Methods
Data were obtained from the Central Brain Tumor Registry of the United States, through a data-release agreement with the Centers for Disease Control and Prevention National Program of Cancer Registries, which includes incidence data from approximately 99.9% of the US population.1,7 These data are derived from 51 central cancer registries (in 50 states and Washington, DC). The AAAIRs with 95% CIs were generated for adults (aged 18 years or older) using the SEER*Stat software, version 8.3.4 (Surveillance, Epidemiology, and End Results [SEER] Program). These data from January 1, 2000, to December 31, 2014, included glioma histologic groups, race or Hispanic ethnicity8 (non-Hispanic white, Hispanic white, black, Asian or Pacific Islander, and American Indian or Alaska Native), sex, and age groups (18-34, 35-44, 45-54, 55-64, 65-74, and 75 years or older). Race information is abstracted by cancer registrars from medical records, which have been shown to have high sensitivity for blacks and moderate sensitivity for Hispanic whites.9,10 Hispanic ethnicity is further classified using the North American Association of Central Cancer Registries Hispanic Identification algorithm, which uses a combination of data fields (medical record, birthplace, race, and surname) to directly and indirectly classify cases.8 The data set used in this study was deidentified, and as a result the study protocol was ruled exempt from review by the University Hospitals Cleveland Medical Center Institutional Review Board. No patient informed consent was directly obtained. Data were collected from January 1, 2000, to December 31, 2014. Data analysis took place from December 11, 2017, to January 31, 2018.
All rates were standardized to the 2000 US population and reported per 100 000 population to adjust for differences in age distribution among racial or ethnic populations. Incidence rate ratios were generated from these standardized rates. Histologic groups were classified using the International Classification of Diseases for Oncology, Third Edition, codes and were defined according to the histologic grouping scheme of the Central Brain Tumor Registry of the United States (eTable 1 in the Supplement).11,12 Only malignant cases (International Classification of Diseases for Oncology, Third Edition, behavior code of /3) were included. Joinpoint Regression Program, version 4.2.0213 (SEER Program), was used to estimate incidence time trends as well as annual percentage change and 95% CIs. Figures were created in R, version 3.2.314 (R Foundation for Statistical Computing), using ggplot215 and SEER2R statistical packages.16
Most central cancer registries in the United States do not conduct active follow-up for outcomes. These data are publicly available from the National Cancer Institute SEER Program, which represents a subset (approximately 28%) of the cases included in the Central Brain Tumor Registry of the United States data set.13,14,17 Survival data from 2000 to 2014 for adults at the time of diagnosis with histologic confirmation were included in survival analyses (followed until December 31, 2014, regardless of year of diagnosis). A custom data set was obtained containing additional treatment data fields, including chemotherapy and radiation treatments.17 Although the treatment information in the SEER registries has been shown to have high positive predictive value (95% for radiation and 90% for chemotherapy),18 its sensitivity is only moderate. The SEER Program strongly recommends that “no” values are treated as missing data and comparisons are not made between individuals identified as having treatment and those identified as having no treatment. As a result, treatment groups were defined using “yes” values for beam radiation and/or chemotherapy (see eTable 2 in the Supplement for information on treatment data completeness).
One-year and 5-year relative survival (RS) rates were generated using SEER*Stat, version 8.3.4, by glioma histologic groups, race or ethnicity, and insurance status (available only for diagnosis years 2007 to 2014). Insurance status completeness is known to be poor for persons older than 65 years, who may be classified as being uninsured or having private insurance when they are Medicare-eligible. As a result, these data were used to evaluate survival in individuals aged 18 to 64 years only. Additional survival analyses were performed using Cox proportional hazard models adjusted for age and EOR in R, version 3.2.3 (R Foundation for Statistical Computing). We calculated 95% CIs using the method described in Tiwari et al19 and P values for incidence rate ratios were calculated using the method documented in Fay et al.20 All P values were 1-sided, and associations were considered significant at P < .05.
Results
Overall, from 2000 to 2014, there were 244 808 patients with glioma. Of these, 150 631 (61.5%) were glioblastomas, 46 002 (18.8%) were non–glioblastoma astrocytomas, 26 068 (10.7%) were oligodendroglial tumors, 8816 (3.6%) were ependymomas, and 13 291 (5.4%) were other glioma diagnoses in adults (eTable 3 in the Supplement). The data set included 137 733 males (56.3%) and 107 075 (43.7%) females. There were 204 580 non-Hispanic whites (83.6%), 17 321 Hispanic whites (7.08%), 14 566 blacks (6.0%), 1070 American Indians or Alaska Natives (0.4%), and 5947 Asians or Pacific Islanders (2.4%).
Glioblastoma incidence was highest in non-Hispanic whites (AAAIR, 4.71; 95% CI, 4.69-4.74) and lowest in American Indians or Alaska Natives (AAAIR, 1.88; 95% CI, 1.71-2.06), where incidence was approximately 40% that of non-Hispanic whites (Figure 1; eTable 3 in the Supplement). Incidence was highest in males (incidence rate ratio [IRR], 1.59; P < .005). The male to female IRR was highest in Asians or Pacific Islanders (IRR, 1.65) and lowest in Hispanic whites (IRR, 1.49). The median age varied by race or ethnicity, with non-Hispanic whites having the highest median age (64 years) and blacks and American Indians or Alaska Natives having the lowest (59 years). Incidence rates were highest in the age 65 years or older group (eFigure and eTable 4 in the Supplement).
Figure 1. Average Annual Age-Adjusted Incidence Rates and 95% Confidence Intervals for Selected Glioma Histologies by Race or Ethnicity and Incidence Rate Ratios (IRRs) Compared With Non-Hispanic Whites (NHW), 2000-2014.
AIAN indicates American Indian or Alaska Native; API, Asian or Pacific Islander; HW, Hispanic white.
Overall, the 1-year RS after diagnosis was 41.4% (95% CI, 40.8-42.0) and the 5-year RS was 5.4% (95% CI, 5.1-5.7) (Table). The highest 5-year RS was observed in Asians or Pacific Islanders (8.8%; 95% CI, 7.0-10.9) and the lowest in non-Hispanic whites (4.8%; 95% CI, 4.5-5.2). To evaluate the potential period effects between the pre- and post-Stupp treatment (concurrent radiation and temozolomide21) protocol eras (2000-2004 and 2005-2014), RS was evaluated separately within each period. The RS was generally higher in the post-Stupp era (Table). There was no change in the 5-year RS for Asians or Pacific Islanders between the 2 periods, whereas the 1-year RS increased from 39.3% (95% CI, 33.9-44.6) to 54.2% (95% CI, 50.8-57.4). Among those who received radiation, the 1-year RS was highest in Asians or Pacific Islanders (63.1%), whereas the 5-year RS was highest in Hispanic whites (9.6%). For those who received both radiation and chemotherapy, the 1-year and 5-year RS rates were highest in Asians or Pacific Islanders (67.1% and 10.1%) and lowest in non-Hispanic whites (57.2% and 6.6%). For persons aged 18 to 34 years, non-Hispanic whites had the highest 1-year and 5-year survival (78.6% and 27.0%) (eTable 5 in the Supplement). In the 35 to 44 years age cohort, survival was highest among Hispanic whites, with a 1-year RS of 69.6% and a 5-year RS of 19.3%. For all cohorts aged 45 years or older, Asians or Pacific Islanders had the highest survival rate. When survival models were adjusted for EOR and age, being Asian or Pacific Islander was a significant predictor of increased survival (P = 2.4x10−5; Figure 2; eTable 6 in the Supplement). Among only those who received surgery, radiation, and chemotherapy, all racial or ethnic groups had significantly improved survival compared with non-Hispanic whites (see eTable 2 in the Supplement for the completeness of treatment information).
Table. One-Year and 5-Year Relative Survival Rates and 95% CIs for Selected Glioma Histologies in Adults by Race or Ethnicity and Diagnosis Periods, 2000-2014.
| Histologic Group | Race or Ethnicity | Diagnosis Years 2000-2014 | Diagnosis Years 2000-2004 | Diagnosis Years 2005-2014 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | |||||
| 1-Year RS | 5-Year RS | 1-Year RS | 5-Year RS | 1-Year RS | 5-Year RS | |||||
| Glioblastoma | All groups | 30 880 | 41.4 (40.8-42.0) | 5.4 (5.1-5.7) | 9223 | 34.4 (33.5-35.4) | 4.3 (3.9-4.7) | 21 657 | 44.6 (43.9-45.3) | 5.8 (5.4-6.2) |
| Non-Hispanic white | 24 635 | 40.7 (40.1-41.3) | 4.8 (4.5-5.2) | 7581 | 34.1 (33.0-35.2) | 3.9 (3.4-4.3) | 17 054 | 43.8 (43.1-44.6) | 5.2 (4.7-5.6) | |
| Hispanic white | 2972 | 42.9 (41.0-44.8) | 7.8 (6.7-9.0) | 783 | 37.4 (33.9-40.8) | 6.3 (4.7-8.2) | 2189 | 45.1 (42.8-47.3) | 8.4 (7.0-10.0) | |
| Black | 1758 | 42.0 (39.6-44.3) | 6.8 (5.5-8.4) | 487 | 32.7 (28.5-37.0) | 4.6 (2.9-6.8) | 1271 | 45.7 (42.8-48.5) | 7.8 (6.0-9.9) | |
| API | 1331 | 50.2 (47.4-53.0) | 8.8 (7.0-10.9) | 334 | 39.3 (33.9-44.6) | 8.8 (5.9-12.3) | 997 | 54.2 (50.8-57.4) | 8.1 (5.9-10.7) | |
| Non-glioblastoma astrocytomas | All groups | 10 259 | 72.4 (71.5-73.3) | 44.4 (43.3-45.5) | 3443 | 68.8 (67.2-70.3) | 42.0 (40.3-43.7) | 6816 | 74.3 (73.2-75.4) | 45.6 (44.2-47.0) |
| Non-Hispanic white | 7648 | 71.3 (70.2-72.3) | 43.2 (41.9-44.4) | 2630 | 67.5 (65.7-69.3) | 40.7 (38.8-42.7) | 5018 | 73.3 (72.0-74.6) | 44.4 (42.7-46.0) | |
| Hispanic white | 1270 | 76.0 (73.5-78.4) | 50.8 (47.5-53.9) | 411 | 72.7 (68.0-76.8) | 48.6 (43.4-53.5) | 859 | 77.8 (74.6-80.6) | 52.1 (47.9-56.1) | |
| Black | 675 | 72.2 (68.5-75.5) | 45.4 (41.1-49.5) | 236 | 71.3 (64.9-76.7) | 45.8 (39.2-52.2) | 439 | 72.8 (68.2-76.9) | 44.5 (38.9-50.0) | |
| API | 540 | 78.2 (74.3-81.6) | 44.1 (39.1-49.0) | 144 | 75.8 (67.8-82.1) | 38.9 (30.8-47.0) | 396 | 79.2 (74.5-83.0) | 46.6 (40.3-52.7) | |
| Oligodendroglial tumors | All | 6396 | 90.4 (89.6-91.1) | 70.1 (68.8-71.3) | 2238 | 89.4 (88.0-90.6) | 67.6 (65.5-69.5) | 4158 | 91.0 (90.0-91.8) | 71.8 (70.1-73.4) |
| Non-Hispanic white | 4647 | 90.2 (89.3-91.1) | 70.0 (68.5-71.4) | 1694 | 89.1 (87.5-90.5) | 67.9 (65.5-70.1) | 2953 | 90.9 (89.7-91.9) | 71.4 (69.3-73.3) | |
| Hispanic white | 906 | 90.7 (88.5-92.5) | 73.9 (70.3-77.1) | 267 | 89.7 (85.2-92.8) | 72.3 (66.1-77.5) | 639 | 91.2 (88.6-93.3) | 74.9 (70.4-78.8) | |
| Black | 292 | 89.5 (85.1-92.7) | 63.8 (57.1-69.7) | 94 | 85.7 (76.5-91.5) | 56.8 (45.7-66.5) | 198 | 91.4 (86.1-94.7) | 68.0 (59.5-75.1) | |
| API | 454 | 91.5 (88.4-93.8) | 67.5 (62.2-72.2) | 149 | 92.1 (86.4-95.5) | 61.4 (52.9-68.8) | 305 | 91.2 (87.2-94.0) | 72.4 (65.4-78.1) | |
| Ependymoma | All | 2032 | 94.3 (93.1-95.3) | 88.0 (86.2-89.6) | 646 | 93.9 (91.7-95.6) | 86.3 (83.0-89.0) | 1386 | 94.5 (93.0-95.7) | 89.0 (86.7-90.9) |
| Non-Hispanic white | 1395 | 94.2 (92.8-95.4) | 88.2 (86.0-90.1) | 462 | 93.9 (91.2-95.9) | 86.4 (82.4-89.6) | 933 | 94.4 (92.5-95.8) | 89.1 (86.3-91.3) | |
| Hispanic white | 328 | 94.9 (91.6-96.9) | 88.9 (83.7-92.4) | 102 | 98.3 (91.7-99.7) | 89.0 (79.6-94.2) | 226 | 93.2 (88.6-96.0) | 89.1 (82.6-93.3) | |
| Black | 165 | 93.0 (87.4-96.1) | 84.8 (77.0-90.2) | Not presented | Not presented | Not presented | 118 | 95.0 (88.6-97.9) | 85.0 (74.8-91.3) | |
| API | 114 | 93.7 (86.9-97.0) | 84.2 (74.7-90.4) | Not presented | Not presented | Not presented | 81 | 96.2 (87.9-98.8) | 86.4 (73.7-93.3) | |
| Other gliomas | All | 1247 | 65.8 (63.0-68.4) | 41.0 (38.0-44.1) | 437 | 62.1 (57.3-66.5) | 36.8 (32.2-41.5) | 810 | 68.0 (64.4-71.2) | 44.0 (39.9-48.0) |
| Non-Hispanic white | 897 | 65.0 (61.7-68.1) | 40.7 (37.1-44.2) | 328 | 63.8 (58.3-68.8) | 37.8 (32.4-43.2) | 569 | 65.8 (61.5-69.7) | 42.9 (38.1-47.6) | |
| Hispanic white | 159 | 69.2 (60.8-76.1) | 44.0 (35.0-52.6) | Not presented | Not presented | Not presented | 113 | 73.5 (63.7-81.0) | 48.1 (36.7-58.6) | |
| Black | 101 | 65.6 (55.0-74.2) | 44.8 (33.7-55.2) | Not presented | Not presented | Not presented | 62 | 73.0 (59.2-82.8) | 48.0 (32.5-61.9) | |
| API | 71 | 70.0 (57.2-79.7) | 35.6 (22.5-48.9) | Not presented | Not presented | Not presented | Not presented | Not presented | Not presented | |
Abbreviations: API, Asian or Pacific Islander; RS, relative survival.
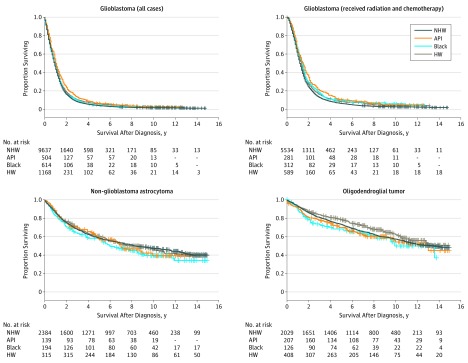
Figure 2. Survival Curves by Histologic Subtype for Individuals Who Received Resection by Race or Ethnicity, Adjusted by Age and Extent of Resection (Subtotal vs Gross Total), 2000-2014.
Glioblastoma survival is significantly improved for people who are not non-Hispanic whites (HNW). No significant differences were observed in survival for non-glioblastoma astrocytoma or oligodendroglial tumors.
API indicates Asian or Pacific Islander; HW, Hispanic white.
Non-glioblastoma astrocytoma incidence was highest in non-Hispanic whites (AAAIR, 1.55; 95% CI, 1.54-1.57) and lowest in blacks (AAAIR, 0.74; 95% CI, 0.72-0.77), where incidence was approximately 48% that of non-Hispanic whites (Figure 1; eTable 3 in the Supplement). Overall incidence was highest in males (IRR, 1.35; P < .005). The male to female IRR was highest in non-Hispanic whites (IRR, 1.55) and Asians or Pacific Islanders (IRR, 1.55) but lowest in American Indians or Alaska Natives, where no significant sex difference was observed (IRR, 1.08; P = .58). The median age varied by race or ethnicity, with non-Hispanic whites having the highest (50 years) and Hispanic whites, Asians or Pacific Islanders, and American Indians or Alaska Natives having the lowest (41 years). Incidence rates were highest in the 65-74 years age cohort in all race or ethnicity groups except Asians or Pacific Islanders, where the highest incidence was in persons 75 years or older (eFigure and eTable 4 in the Supplement).
Overall, the 1-year RS after diagnosis of non-glioblastoma astrocytomas was 72.4% (95% CI, 71.5-73.3) and the 5-year RS was 44.4% (95% CI, 43.3-45.5) (Table). The highest 5-year RS was observed in Hispanic whites (50.8%; 95% CI, 47.5-53.9) and the lowest in non-Hispanic whites (43.2%; 95% CI, 41.9-44.4). For persons aged 18 to 44 years, non-Hispanic whites had the highest 1-year and 5-year RS, whereas in older age groups the highest 5-year RS was in Hispanic whites (eTable 5 in the Supplement). When survival models were adjusted for EOR and age, there was no significant association between survival and any race or ethnicity group (Figure 2; eTable 6 in the Supplement).
Oligodendroglial tumors incidence was highest in non-Hispanic whites (AAAIR, 0.92; 95% CI, 0.91-0.93) and lowest in blacks (AAAIR, 0.33; 95% CI, 0.31-0.35), where incidence was approximately 36% that of non-Hispanic whites (Figure 1; eTable 3 in the Supplement). Overall incidence was highest in males (IRR, 1.33; P < .005). The male to female IRR was highest in non-Hispanic whites (IRR, 1.33) and lowest in Hispanic whites, where there was no significant sex difference (IRR, 1.17). The median age varied by race or ethnicity, with the highest in non-Hispanic whites (44 years) and the lowest in American Indians or Alaska Natives (36 years). Incidence rates were highest in the 35 to 44 years age cohort in non-Hispanic whites, blacks, and Asians or Pacific Islanders; incidence rate in American Indians or Alaska Natives was highest in the 18 to 34 years cohort; and incidence rate in Hispanic whites was highest in the 45 to 54 years age group (eFigure and eTable 4 in the Supplement).
Overall, the 1-year RS after diagnosis of oligodendroglial tumors was 90.4% (95% CI, 89.6-91.1) and the 5-year RS was 70.0% (95% CI, 68.5-71.4) (Table). The highest 5-year RS was observed in Hispanic whites (73.9%; 95% CI, 70.3-77.1) and lowest in blacks (63.8%; 95% CI, 57.1-69.7). The 1-year and 5-year RS increased from 2005 to 2014, but these increases were minimal. For individuals aged 18 to 44 years, non-Hispanic whites had the highest 1-year and 5-year RS, whereas in older age groups, the highest 5-year RS was in Hispanic whites (eTable 5 in the Supplement). When survival models were adjusted for EOR and age, no significant association was found between survival rate and any race or ethnicity group (Figure 2; eTable 6 in the Supplement).
Ependymoma incidence was highest in non-Hispanic whites (AAAIR, 0.29; 95% CI, 0.28-0.29) and lowest in American Indians or Alaska Natives (AAAIR, 0.13; 95% CI, 0.09-0.17), where incidence was approximately 45% that of non-Hispanic whites (Figure 1; eTable 3 in the Supplement). Incidence of ependymoma was slightly higher in males (IRR, 1.33; P < .005). The male to female IRR was highest in non-Hispanic whites (IRR, 1.11), and there was no significant sex difference in incidence in any other group. The median age varied by race or ethnicity, with non-Hispanic whites having the highest (59 years) and Hispanic whites having the lowest (47 years). Incidence of ependymoma was highest in the 45 to 74 years age group in all race or ethnicity groups (eFigure and eTable 4 in the Supplement). Overall, the 1-year RS after diagnosis of ependymoma was 94.3% (95% CI, 93.1-95.3) and the 5-year RS was 88.0% (95% CI, 86.2-89.6) (Table). The highest 5-year RS was observed in Hispanic whites (88.9%; 95% CI, 83.7-92.4) and lowest in Asians or Pacific Islanders (84.2%; 95% CI, 74.7-90.4). No notable changes were observed in the 1-year and 5-year RS rates between 2000 to 2004 and 2005 to 2014.
Discussion
The present study of glioma incidence and survival across a 14-year period demonstrates substantial variation by race or ethnicity, using data from 99.9% of the US population to derive incidence rates and data from approximately 28% of the population to obtain survival rates.22,23 Non-Hispanic whites had the highest incidence of all glioma subtypes, consistent with what has been previously reported.1,18,21 The median age of diagnosis for all histologies was highest also among non-Hispanic whites. There was a male preponderance in incidence of most glioma histologies among all examined racial or ethnic groups, and the scale of the male to female IRR was similar among all race or ethnicity groups. Analyses have consistently found higher glioma incidence in non-Hispanic whites as well as in countries with high proportions of individuals with mostly European ancestry.1,3 The reason for this finding may be, to some extent, the variation in genetic risk susceptibility for glioma, but to date no studies have examined the inherited risk variants associated with glioma risk in blacks or Hispanic whites.
A portion of this incidence difference may be the result of several biases. Incidence estimates are sensitive to ascertainment bias, because of differential access to health care or the variation in diagnostic procedure. Non-Hispanic whites often have improved access to health care, which may lead to earlier or more accurate diagnosis.22,23 Glioma is usually symptomatic (incidental diagnoses represent approximately 2% to 6% of diagnoses in case series studies24,25,26) and often presents with substantial symptoms, including seizures and cognitive impairments. As a result, the chance of ascertainment bias resulting in considerable underdiagnosis is small. Heterogeneous reporting guidelines or the application of histologic classification schemes could result in spuriously different rates among racial or ethnic groups. There is no central pathology review of tumor samples in the data used for this analysis, but it is possible that “true” histologic classification could be confounded with race or ethnicity. Although these factors may affect incidence rates, they fail to explain the incidence difference between non-Hispanic whites and other groups.
Previous analyses have found substantial associations between increased socioeconomic status and glioma, which may contribute to differences in incidence by race or ethnicity.27,28,29 A previous SEER analysis found increased incidence of glioma in areas of higher socioeconomic status after adjusting for sex, race, and age.28 In Sweden, the highest glioma incidence was among those with family incomes in the highest quartile (odds ratio [OR], 1.5; 95% CI, 1.1-2.1), after adjusting for sex, age, and geographic region.29 The underlying risk factors responsible for differential incidence by socioeconomic status are unknown but could be partially the result of confounding with known or currently unknown risk factors.30,31 The population distribution of the SEER registries approximates that these registries contain a greater proportion of persons who are not non-Hispanic white in the SEER data, which may affect race-specific survival estimates.32,33,34 These registries contain greater proportions of foreign-born individuals, who have less than a high school diploma compared with the general US population, which may also affect survival estimates.35
This analysis found that non-Hispanic whites had poorer outcomes after diagnosis of glioblastoma when compared with all other groups. This pattern was present in both the pre- and post-Stupp protocol era period analyses (Table) among those who received treatment beyond resection (eTable 7 in the Supplement) as well as after adjustment for age and EOR (Figure 2). A large US meta-analysis previously reported that blacks have lower 5-year survival rate compared with whites after diagnosis of central nervous system cancers.36 These disparities were reduced after adjustment for disease severity, treatment type, and the underlying mortality rate. Curry et al5 remark that blacks presented with greater disease severity and that there is lower annual cancer-associated craniotomy case volume in the National Inpatient Sample data for patients with Medicaid insurance. Variations in health care access and severity at the time of presentation are likely substantial contributors to the variation in survival patterns.
Furthermore, this analysis found that non-Hispanic whites have a lower survival rate compared with other racial or ethnic groups. People who are not non-Hispanic whites have comparatively low numbers of cases, and some of the observed differences may be the result of these low numbers. These findings are in line with those of some previously reported analyses, such as a SEER study that found increased survival in blacks compared with whites after diagnosis of a central nervous system tumor.37 These differences were consistently observed within sex and across age strata. Both 1-year and 5-year survival rates were lower among non-Hispanic whites, particularly compared with Hispanic whites, regardless of treatment type. This previous analysis also found that differences persisted in glioblastoma among those who received radiation and chemotherapy, suggesting that health care access is not sufficient to explain survival differences.
In older cohorts who have universal access to Medicare, access to health care and treatment type may not adequately explain differential survival rates. For all ages, other factors should be explored in future studies. Survival after diagnosis of cancer of other sites has been shown to correlate with many factors that also vary by race or ethnicity, including body mass index, smoking status, and religious service attendance.38,39,40 Although prediagnostic body mass index has been associated with shorter survival after diagnosis of glioma, these additional factors have not all been sufficiently explored.41 Non-Hispanic whites had the highest RS in younger cohorts in age-stratified analyses (eTable 4 in the Supplement). Compared with persons aged 65 years or older who are eligible for Medicare, young persons diagnosed with cancer may not have access to health insurance. Access to health insurance among young adult oncology patients varies significantly by socioeconomic status, race, and ethnicity.42,43 When survival analyses were performed for glioblastoma stratified by insurance status within the 18 to 64 years age cohorts, non-Hispanic whites had the poorest survival within each cohort (eTable 8 in the Supplement). This finding suggests that the observed survival benefit among non-Hispanic whites in younger cohorts may be, to some extent, due to differences in health care access.
Limitations
This study has limitations. The data used for this analysis are limited in the variables available for each individual. Many of these factors may be associated with differences in survival, including comorbidities, tumor volume and location, EOR, Karnofsky performance status, and treatment pattern.2,21 Cancer registry data represent the most complete data set for characterizing patterns of cancer incidence and survival, but several limitations are inherent in this type of data, including known problems with completeness of treatment information.18 The 2016 World Health Organization Classification of Tumors of the Central Nervous System summary contains considerable revisions to glioma histologic classification.44 The largest of these revisions is the incorporation of isocitrate dehydrogenase 1/2 mutation status and 1p/19q codeletion status into the diagnostic criteria for astrocytoma and oligodendroglioma. This revision also resulted in the elimination of oligoastrocytoma as a valid glioma histologic subtype.45 To date, data on isocitrate dehydrogenase 1/2 mutation status have not been collected in both the National Program of Cancer Registries or SEER systems, although collection will begin in 2018. Since 2011, 1p/19q codeletion status has been required by SEER registries, but completeness varies significantly by histologic subtype.46 As a result, these molecular classifications were not incorporated into this analysis, and it is not possible to assess whether these may be driving the observed survival differences. An analysis of time trends by race or ethnicity and glioma histologic subtype (eTable 9 in the Supplement) shows substantial decreases in incidence of oligodendroglial tumors in all groups over the period examined in this study, which may be the result of changing classification schemes.
Conclusions
To our knowledge, this study represents the most complete and up-to-date reporting of patterns of glioma incidence and survival by race or ethnicity in the United States. These estimates of incidence are population-based and include approximately 99.9% of the US population, while SEER data cover approximately 28% of the population.22 Incidence and survival patterns of most glioma histologies varied significantly by race or ethnicity, with non-Hispanic whites having higher incidence and lower survival compared with individuals of other race or ethnicity. Further examination is necessary to determine the factors that contribute to these differences. Expanding investigations of germline genetic associations in blacks and Hispanic whites may identify genetic contributions to incidence differences. Somatic characterization of tumors in multiethnic cohorts is also necessary to determine that the observed survival differences may not be due to differences in proportions of molecular subtypes within histologic subtypes among racial or ethnic groups. Examination of treatment patterns in databases with more extensive clinical information is necessary to determine the extent to which health care access may affect differences in outcomes.
eTable 1. Definitions of Glioma Histology Groups
eTable 2. Availability of Treatment Information for SEER Cases, by Histology Group and Race
eTable 3. Median Age at Diagnosis, Total Cases, Average Annual Age-Adjusted Incidence Rates (AAAIR), 95% Confidence Intervals (95% CI), and Incidence Rate Ratios (Males:Females) for Selected Glioma Histologies in Adults (ages 18+) by Race, Hispanic Ethnicity, and Sex, 2000-2014
eTable 4. Total Cases, Average Annual Age-Adjusted Incidence Rates (AAAIR), 95% Confidence Intervals (95% CI) for Selected Glioma Histologies in Adults (Ages 18+) by Race, Hispanic Ethnicity, and Age Groups, CBTRUS 2000-2014
eTable 5. One- and Five-Year Relative Survival Rates and 95% Confidence Intervals (95% CI) for Selected Glioma Histologies in Adults (Ages 18+) by Race, Ethnicity, and Age at Diagnosis, SEER 18 Registries, 2000-2014
eTable 6. Hazard Ratios, P Values, Median Survival and 95% Confidence Intervals for Individuals That Received Resection by Race and Ethnicity, Adjusted by Age at Diagnosis and Extent of Resection (Subtotal vs Gross Total), 2000-2014
eTable 7. One- and Five-Year Relative Survival Rates and 95% Confidence Intervals (95% CI) for Selected Glioma Histologies in Adults (Ages 18+) by Race, Ethnicity, and Treatment Groups, SEER 18 Registries, 2005-2014
eTable 8. One- and Five-Year Relative Survival Rates and 95% Confidence Intervals (95% CI) for Selected Glioma Histologies in Adults (Ages 18-64) by Race, Ethnicity, and Insurance Status, SEER 18 Registries, 2007-2014
eTable 9. Annual Percent Change (APC) and 95% Confidence Intervals by Glioma Histology and Race/Ethnicity, CBTRUS 2000-2014
eFigure. Average Annual Age-Adjusted Incidence Rates and 95% Confidence Intervals for Selected Glioma Histologies by Race, Ethnicity, and Age at Diagnosis, 2000-2014
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl_5):v1-v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190-198. [DOI] [PubMed] [Google Scholar]
- 3.Leece R, Xu J, Ostrom QT, Chen Y, Kruchko C, Barnholtz-Sloan JS. Global incidence of malignant brain and other central nervous system tumors by histology, 2003-2007. Neuro Oncol. 2017;19(11):1553-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute. DevCan—Probability of developing or dying of cancer. https://surveillance.cancer.gov/devcan/. Accessed January 31, 2018.
- 5.Curry WT Jr, Carter BS, Barker FG II. Racial, ethnic, and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988-2004. Neurosurgery. 2010;66(3):427-437. [DOI] [PubMed] [Google Scholar]
- 6.Barnholtz-Sloan JS, Maldonado JL, Williams VL, et al. Racial/ethnic differences in survival among elderly patients with a primary glioblastoma. J Neurooncol. 2007;85(2):171-180. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. United States Cancer Statistics: 1999-2014 cancer incidence and mortality data. https://nccd.cdc.gov/uscs/. Accessed January 31, 2018.
- 8.NAACCR Race and Ethnicity Work Group. NAACCR guideline for enhancing Hispanic/Latino identification: revised NAACCR Hispanic/Latino identification algorithm [NHIA v2.2.1]. https://www.naaccr.org/wp-content/uploads/2016/11/NHIA-v2.2.1.pdf. Revised September 12, 2011. Accessed January 31, 2018.
- 9.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States). Cancer Causes Control. 2006;17(6):771-781. [DOI] [PubMed] [Google Scholar]
- 10.West CN, Geiger AM, Greene SM, et al. Race and ethnicity: comparing medical records to self-reports. J Natl Cancer Inst Monogr. 2005;(35):72-74. [DOI] [PubMed] [Google Scholar]
- 11.Fritz APC, Jack A, Shanmugaratnam K, Sobin L, Perkin DM, Whelan S, eds. International Classification of Diseases for Oncology. 3rd ed Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 12.Melin BS, Barnholtz-Sloan JS, Wrensch MR, et al. ; GliomaScan Consortium . Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat Genet. 2017;49(5):789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute Surveillance Epidemiology and End Results Program. Overview of the SEER program. http://seer.cancer.gov/about/overview.html. Accessed January 31, 2018.
- 14.National Cancer Institute Surveillance Epidemiology and End Results Program. Number of persons by race and Hispanic ethnicity for SEER participants (2010 Census data). http://seer.cancer.gov/registries/data.html. Accessed January 31, 2018.
- 15.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer Science+Business Media, LLC; 2009.
- 16.Luo J. Reading and writing SEER*STAT data files. Package ‘SEER2R.’ https://cran.r-project.org/web/packages/SEER2R/index.html. Published February 19, 2015. Accessed January 31, 2018.
- 17.National Cancer Institute Surveillance Epidemiology and End Results Program. SEER*Stat database: November 2016 submission. https://seer.cancer.gov/data-software/documentation/seerstat/nov2016/. Accessed January 31, 2018.
- 18.Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with Medicare claims. Med Care. 2016;54(9):e55-e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547-569. [DOI] [PubMed] [Google Scholar]
- 20.Fay MP, Tiwari RC, Feuer EJ, Zou Z. Estimating average annual percent change for disease rates without assuming constant change. Biometrics. 2006;62(3):847-854. [DOI] [PubMed] [Google Scholar]
- 21.Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 22.Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017;109(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curry WT Jr, Barker FG II. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009;93(1):25-39. [DOI] [PubMed] [Google Scholar]
- 24.Opoku-Darko M, Lang ST, Artindale J, Cairncross JG, Sevick RJ, Kelly JJP. Surgical management of incidentally discovered diffusely infiltrating low-grade glioma. J Neurosurg. 2017;6:1-8. [DOI] [PubMed] [Google Scholar]
- 25.Morris Z, Whiteley WN, Longstreth WT Jr, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stark AM, van de Bergh J, Hedderich J, Mehdorn HM, Nabavi A. Glioblastoma: clinical characteristics, prognostic factors and survival in 492 patients. Clin Neurol Neurosurg. 2012;114(7):840-845. [DOI] [PubMed] [Google Scholar]
- 27.Porter AB, Lachance DH, Johnson DR. Socioeconomic status and glioblastoma risk: a population-based analysis. Cancer Causes Control. 2015;26(2):179-185. [DOI] [PubMed] [Google Scholar]
- 28.Plascak JJ, Fisher JL. Area-based socioeconomic position and adult glioma: a hierarchical analysis of surveillance epidemiology and end results data. PLoS One. 2013;8(4):e60910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wigertz A, Lönn S, Hall P, Feychting M. Non-participant characteristics and the association between socioeconomic factors and brain tumour risk. J Epidemiol Community Health. 2010;64(8):736-743. [DOI] [PubMed] [Google Scholar]
- 30.Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99(20):1544-1550. [DOI] [PubMed] [Google Scholar]
- 31.Braganza MZ, Kitahara CM, Berrington de González A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 2012;14(11):1316-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariotto A, Capocaccia R, Verdecchia A, et al. Projecting SEER cancer survival rates to the US: an ecological regression approach. Cancer Causes Control. 2002;13(2):101-111. [DOI] [PubMed] [Google Scholar]
- 33.Kuo TM, Mobley LR. How generalizable are the SEER registries to the cancer populations of the USA? Cancer Causes Control. 2016;27(9):1117-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 suppl):IV-3-IV-18. [DOI] [PubMed] [Google Scholar]
- 35.National Cancer Institute Surveillance Epidemiology and End Results Program Population characteristics. https://seer.cancer.gov/registries/characteristics.html. Accessed January 29, 2017.
- 36.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287(16):2106-2113. [DOI] [PubMed] [Google Scholar]
- 37.Ries LAG, Eisner MP, Kosary CL, et al. SEER Cancer Statistics Review, 1975-2002. http://seer.cancer.gov/csr/1975_2002/. Accessed January 31, 2018.
- 38.Wang N, Khankari NK, Cai H, et al. Prediagnosis body mass index and waist-hip circumference ratio in association with colorectal cancer survival. Int J Cancer. 2017;140(2):292-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan C, Morales-Oyarvide V, Babic A, et al. Cigarette smoking and pancreatic cancer survival. J Clin Oncol. 2017;35(16):1822-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S, Stampfer MJ, Williams DR, VanderWeele TJ. Association of religious service attendance with mortality among women. JAMA Intern Med. 2016;176(6):777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegel EM, Nabors LB, Thompson RC, et al. Prediagnostic body weight and survival in high grade glioma. J Neurooncol. 2013;114(1):79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alvarez EM, Keegan TH, Johnston EE, et al. The Patient Protection and Affordable Care Act dependent coverage expansion: disparities in impact among young adult oncology patients. Cancer. 2018;124(1):110-117. [DOI] [PubMed] [Google Scholar]
- 43.Okoro CA, Zhao G, Fox JB, Eke PI, Greenlund KJ, Town M. Surveillance for health care access and health services use, adults aged 18-64 years—Behavioral Risk Factor Surveillance System, United States, 2014. MMWR Surveill Summ. 2017;66(7):1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803-820. [DOI] [PubMed] [Google Scholar]
- 45.Sahm F, Reuss D, Koelsche C, et al. Farewell to oligoastrocytoma: in situ molecular genetics favor classification as either oligodendroglioma or astrocytoma. Acta Neuropathol. 2014;128(4):551-559. [DOI] [PubMed] [Google Scholar]
- 46.Ostrom QT, Gittleman H, Kruchko C, et al. Completeness of required site-specific factors for brain and CNS tumors in the Surveillance, Epidemiology and End Results (SEER) 18 database (2004-2012, varying). J Neurooncol. 2016;130(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definitions of Glioma Histology Groups
eTable 2. Availability of Treatment Information for SEER Cases, by Histology Group and Race
eTable 3. Median Age at Diagnosis, Total Cases, Average Annual Age-Adjusted Incidence Rates (AAAIR), 95% Confidence Intervals (95% CI), and Incidence Rate Ratios (Males:Females) for Selected Glioma Histologies in Adults (ages 18+) by Race, Hispanic Ethnicity, and Sex, 2000-2014
eTable 4. Total Cases, Average Annual Age-Adjusted Incidence Rates (AAAIR), 95% Confidence Intervals (95% CI) for Selected Glioma Histologies in Adults (Ages 18+) by Race, Hispanic Ethnicity, and Age Groups, CBTRUS 2000-2014
eTable 5. One- and Five-Year Relative Survival Rates and 95% Confidence Intervals (95% CI) for Selected Glioma Histologies in Adults (Ages 18+) by Race, Ethnicity, and Age at Diagnosis, SEER 18 Registries, 2000-2014
eTable 6. Hazard Ratios, P Values, Median Survival and 95% Confidence Intervals for Individuals That Received Resection by Race and Ethnicity, Adjusted by Age at Diagnosis and Extent of Resection (Subtotal vs Gross Total), 2000-2014
eTable 7. One- and Five-Year Relative Survival Rates and 95% Confidence Intervals (95% CI) for Selected Glioma Histologies in Adults (Ages 18+) by Race, Ethnicity, and Treatment Groups, SEER 18 Registries, 2005-2014
eTable 8. One- and Five-Year Relative Survival Rates and 95% Confidence Intervals (95% CI) for Selected Glioma Histologies in Adults (Ages 18-64) by Race, Ethnicity, and Insurance Status, SEER 18 Registries, 2007-2014
eTable 9. Annual Percent Change (APC) and 95% Confidence Intervals by Glioma Histology and Race/Ethnicity, CBTRUS 2000-2014
eFigure. Average Annual Age-Adjusted Incidence Rates and 95% Confidence Intervals for Selected Glioma Histologies by Race, Ethnicity, and Age at Diagnosis, 2000-2014