Key Points
Question
Can dermoscopy aid in the differentiation of subungual melanoma in situ from benign longitudinal melanonychia?
Findings
In this cohort study of 45 patients with pigmented nails, asymmetry, border fading, multicolor pigmentation, width of the pigmentation of at least 3 mm, and presence of the Hutchinson sign were features associated with subungual melanoma in situ. A predictive scoring model incorporating these features was reliable for the detection of subungual melanoma in situ, with a sensitivity of 89% and a specificity of 62%.
Meaning
A predictive scoring model for the detection of subungual melanoma in situ may assist clinicians in the examination of patients with adult-onset longitudinal melanonychia affecting a single digit.
Abstract
Importance
Subungual melanoma in situ (SMIS) is a malignant neoplasm that requires early diagnosis and complete surgical excision; however, little is known about the usefulness of the detailed dermoscopic features of longitudinal melanonychia (LM) to predict the diagnosis of SMIS.
Objectives
To investigate the characteristic dermoscopic findings of SMIS and to establish a predictive scoring model for the diagnosis of SMIS in patients with adult-onset LM affecting a single digit.
Design, Setting, and Participants
A cohort study of 19 patients with biopsy-proven SMIS and 26 patients with benign LM diagnosed in a tertiary referral hospital in Seoul, South Korea, from September 1, 2013, to July 31, 2017.
Main Outcomes and Measures
Patient demographics, frequency of specific dermoscopic findings, and a predictive scoring model.
Results
Of the total 45 patients with pigmented nails, the 19 patients with SMIS included 14 women and had a mean (SD) age of 52.0 (14.4) years, and the 26 patients with benign LM included 18 women and had a mean (SD) age of 48.1 (13.2) years. Asymmetry (odds ratio [OR], 34.00; 95% CI, 3.88-297.70), border fading (OR, 9.33; 95% CI, 2.37-36.70), multicolor (OR, 11.59; 95% CI, 2.21-60.89), width of the pigmentation of at least 3 mm (OR, 5.31; 95% CI, 1.01-28.07), and presence of the Hutchinson sign (OR, 18.18; 95% CI, 2.02-163.52) were features of LM that were significantly associated with SMIS. A predictive scoring model incorporating these dermoscopic features of SMIS was assessed. The model, ranging from 0 to 8 points, showed a reliable diagnostic value (the receiver operating characteristic curve had an area under the curve [C statistic] of 0.91) in differentiating SMIS from benign LM at a cutoff value of 3, with a sensitivity of 89% and a specificity of 62%.
Conclusions and Relevance
This study suggests characteristic dermoscopic features for SMIS. A predictive scoring model based on these morphologic features may help differentiate SMIS from benign LM.
This cohort study identifies the dermoscopic features of subungual melanoma in situ in a Korean population and assesses a predictive scoring model that distinguishes the diagnosis of subungual melanoma in situ from longitudinal melanonychia in patients with pigmented nails.
Introduction
Patients with longitudinal melanonychia (LM) require clinical attention because of the potential for subungual melanoma (SUM) to appear as LM.1 Histopathologic analysis of the nail matrix is the criterion for establishing a cause for LM; however, biopsy of the nail matrix must be performed cautiously because of the potential sequelae of severe pain and onychodystrophy. Dermoscopy, a noninvasive imaging modality, improves the diagnostic accuracy for various causes of LM, including SUM, lentigines, nail matrix nevi, and subungual hemorrhage.2,3,4,5,6,7,8,9,10
The clinical ABCDEF mnemonic was developed by Levit et al11 to describe the clinical features and risk factors for the development of subungual melanoma. The ABCDEF mnemonic incorporates the following criteria: A, for age and Asian, African American, or Native American race/ethnicity; B, brown-black pigment, breadth of at least 3 mm, or blurred border; C, change in the nail band or lack of change subsequent to adequate treatment; D, digit affected or involving the dominant hand; E, extension of pigment onto the proximal or lateral nail folds; and F, family or personal history of melanoma or dysplastic nevus. The criteria are designed to assist clinicians in predicting the potential for malignant neoplasm while examining patients with LM. Limitations of the mnemonic include lack of validation in a clinical setting, the inclusion of clinical and demographic variables that may not be practical for the diagnosis of SUM, and the limited use among dermatologists in the United States (range of dermatologists, 12%-32%).12
The optimal management of SUM includes early diagnosis and complete surgical excision.13 Although dermoscopy is not a replacement for histopathologic diagnosis,14,15 the detailed examination of magnified morphologic patterns of the nail unit may aid physicians in the detection of SUM.16,17,18,19 However, the detailed dermoscopic features of subungual melanoma in situ (SMIS) have not been sufficiently elucidated.18 In this study, we investigated the characteristic dermoscopic findings of SMIS in comparison with those of benign LM. Furthermore, we propose a predictive scoring model for the diagnosis of SMIS based on the characteristic dermoscopic features of SMIS.
Methods
Cases of LM were retrospectively collected from Seoul National University Hospital in Seoul, South Korea, from September 1, 2013, to July 31, 2017. The cases of LM included in the study were determined as follows: (1) adult-onset of the disease, (2) involvement of a single digit, (3) diagnosis of SMIS or benign LM, and (4) availability of clinical and dermoscopic photographs (DermLite DL3; 3Gen Inc). The diagnosis of all 19 cases of SMIS was confirmed by histopathologic examination. We included 26 cases of benign LM as a control group for comparative analysis to identify the characteristics of SMIS. Cases of benign LM were diagnosed by nail experts (K.H.C. or J.-H.M.) and based on (1) histopathologic examination with clinical information or (2) typical clinical and dermoscopic features without change in at least 1 year of longitudinal follow-up. We included both biopsy-proven and clinically diagnosed cases of benign LM to avoid selection bias because biopsies of the nail matrix are generally performed in cases of LM with a suspicion for malignant neoplasm. This study was approved by the institutional review board at Seoul National University Hospital, Seoul, South Korea. The review board approved a waiver of written informed consent for retrospective deidentified patient data.
Analysis of Dermoscopic Findings
The dermoscopic features of SMIS were analyzed and compared with those of benign LM. Widths of pigmentation of at least 3 mm and at least 6 mm were used as cutoffs suggestive of a malignant origin of LM as was previously reported in the literature.11,20 Colors were classified as gray, light brown, dark brown, and black. The investigated dermoscopic patterns were defined as follows: (1) multicolor pigmentation (LM is composed of ≥2 colors); (2) asymmetry (drawing a midline along the longitudinal band results in colors and patterns that differ between the halves); (3) border fading (the borders of pigmentation are ill defined and the band shows gradual fading at the periphery); (4) triangular sign (wider pigmentation at the proximal aspect of the band compared with the distal aspect); (5) dots and globules (small, round to oval pigmentation); (6) the Hutchinson sign (periungual pigmentation of the nail fold or hyponychium); and (7) nail plate dystrophy.21 All dermoscopic findings were independently evaluated by 2 of us (J.O. and J.-H.M.), and any disagreement was resolved by consensus.
Statistical Analysis
The sensitivity, specificity, and accuracy rates were calculated for each dermoscopic variable. Sensitivity was defined as the number of SMIS cases showing the dermoscopic finding divided by the total number of SMIS cases; specificity was defined as the number of benign LM cases without the dermoscopic finding divided by the total number of benign LM cases; and accuracy was defined as the number of SMIS cases with the dermoscopic finding plus the number of benign LM cases without the dermoscopic finding, with the sum divided by the total number of SMIS and benign LM cases.
The Pearson χ2 test or Fisher exact test (for <5 cells expected) and the unpaired, 2-tailed independent t test were used to compare dermoscopic variables in cases of SMIS and benign LM. Two-sided P < .05 was considered statistically significant. Univariate logistic regression analysis was performed to calculate the odds ratio (OR) of statistically significant dermoscopic features predictive of SMIS compared with those of benign LM. A predictive scoring model for SMIS was established from the β coefficients derived from the logistic regression. The sensitivity and specificity of different cutoff values for the SMIS predictive scoring model were calculated and a receiver operating characteristic curve was generated. Interobserver agreement was represented by the Cohen’s κ coefficient. All statistical analyses were performed using SPSS, version 22.0 (IBM Corporation).
Results
Of the total 45 patients with pigmented nails, 19 patients with SMIS included 14 women who had a mean (SD) age of 52.0 (14.4) years, and 26 patients with benign melanonychia included 18 women and had a mean (SD) age of 48.1 (13.2) years. Histopathologic analysis was performed in all cases of SMIS. Histopathologic examination was also conducted in 17 of the 26 cases of benign LM and revealed 1 case of nail matrix nevus and 16 cases of melanocytic activation of the nail matrix. The mean follow-up duration of the 9 clinically diagnosed cases of benign LM was 24.04 months (range, 15.14-41.50 months).
Patient Demographics
There were no statistically significant differences in sex, age, duration of melanonychia, and affected digit between the SMIS and benign LM groups. A history of trauma was recalled in 3 of 19 cases of SMIS (16%) and 1 of 26 cases of benign LM (4%) (Table 1).
Table 1. Demographic Characteristics of 45 South Korean Patients With Benign LM or SMIS.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| SMIS (n = 19) | Benign LM (n = 26) | ||
| Sex | |||
| Male | 5 (26) | 8 (31) | .75 |
| Female | 14 (74) | 18 (69) | |
| Age, mean (SD), y | 52.0 (14.4) | 48.1 (13.2) | .34 |
| Duration, mean (SD), mo | 75.11 (71.73) | 41.96 (62.23) | .11 |
| Location | |||
| Right hand or foot | 12 (63) | 13 (50) | .38 |
| Left hand or foot | 7 (37) | 13 (50) | |
| Finger | 15 (79) | 18 (69) | .47 |
| Toe | 4 (21) | 8 (31) | |
| Affected digit | |||
| First digit, total | 15 (79) | 12 (46) | .15 |
| Thumb | 11 | 7 | |
| Great toe | 4 | 5 | |
| Second digit, total | 2 (10) | 4 (15) | |
| Finger | 2 | 4 | |
| Toe | 0 | 0 | |
| Third digit, total | 0 | 5 (19) | |
| Finger | 0 | 4 | |
| Toe | 0 | 1 | |
| Fourth digit, total | 2 (10) | 4 (15) | |
| Finger | 2 | 2 | |
| Toe | 0 | 2 | |
| Fifth digit, total | 0 | 1 (4) | |
| Finger | 0 | 1 | |
| Toe | 0 | 0 | |
| Recall of trauma history | 3 (16) | 1 (4) | .30 |
Abbreviations: LM, longitudinal melanonychia; SMIS, subungual melanoma in situ.
Comparative Analysis of Dermoscopic Findings
Mean (SD) pigmentation width was significantly broader in the SMIS cases than in the benign melanonychia cases (9.03 [3.96] vs 3.94 [3.07] mm; P = .001). Widths of the pigmentation of at least 3 mm were seen in 17 of 19 cases (89%) of SMIS vs 16 of 26 cases (62%) of benign LM (OR, 5.31; 95% CI, 1.01-28.07; P = .05). Widths of at least 6 mm were demonstrated in 16 cases (84%) of SMIS and 4 (15%) of benign LM (OR, 29.33; 95% CI, 5.75-149.65; P < .001) (Table 2).
Table 2. Univariate Analysis of Dermoscopic Variables Associated With SMIS vs Benign LM in 45 South Korean Patients.
| Dermoscopic Variable | No. (%) | Sensitivity for SMIS | Specificity for SMIS | Overall Accuracy | OR (95% CI) | P Value | |
|---|---|---|---|---|---|---|---|
| SMIS (n = 19) | Benign LM (n = 26) | ||||||
| Width of pigmentation, mean (SD), mm | 9.03 (3.96) | 3.94 (3.07) | .001 | ||||
| ≥3 | 17 (89) | 16 (62) | 0.90 | 0.38 | 0.60 | 5.31 (1.01-28.07)a | .05 |
| ≥6 | 16 (84) | 4 (15) | 0.84 | 0.85 | 0.84 | 29.33 (5.75-149.65)a | <.001 |
| Pigmentation | |||||||
| Multicolor | 17 (89) | 11 (42) | 0.90 | 0.58 | 0.71 | 11.59 (2.21-60.89)a | .004 |
| Unicolor | 2 (11) | 15 (58) | 0.11 | 0.42 | 0.29 | 0.09 (0.02-0.45)a | |
| Pattern | |||||||
| Asymmetry | 18 (95) | 9 (35) | 0.95 | 0.65 | 0.78 | 34.00 (3.88-297.70)a | .001 |
| Border fading | 14 (74) | 6 (23) | 0.74 | 0.77 | 0.76 | 9.33 (2.37-36.70)a | .001 |
| Triangular pattern | 4 (21) | 1 (4) | 0.21 | 0.96 | 0.64 | 6.67 (0.68-65.37) | .10 |
| Dots or globules | 2 (11) | 1 (4) | 0.11 | 0.96 | 0.60 | 2.94 (0.25-35.06) | .39 |
| Hutchinson signb | 8 (42) | 1 (4) | 0.42 | 0.96 | 0.73 | 18.18 (2.02-163.52)a | .01 |
| Nail plate dystrophy | 4 (21) | 2 (8) | 0.21 | 0.92 | 0.62 | 3.20 (0.52-19.67) | .21 |
Abbreviations: LM, longitudinal melanonychia; OR, odds ratio; SMIS, subungual melanoma in situ.
Indicates ORs that are significantly different.
Defined as periungual pigmentation of the nail fold or hyponychium.
The most commonly observed color in 19 lesions of SMIS was dark brown (16 of 19 lesions [84%]) followed by gray (13 [68%]), light brown (12 [63%]), and black (9 [47%]). In 26 cases of benign LM, the most common color was gray (15 of 26 lesions [58%]) followed by dark brown (13 [50%]), light brown (9 [35%]), and black (4 [15%]). A multicolor pigmentation was seen in 17 of 19 cases (89%) in the SMIS group (OR, 11.59; 95% CI, 2.21-60.89; P = .004); however, in the benign LM group, the presence of a single color was more common and was observed in 15 of 26 cases (58%).
Among the analyzed dermoscopic patterns, statistical significance was shown for asymmetry, border fading, and the Hutchinson sign (Figure 1 and Figure 2). Asymmetry was the most commonly identified dermoscopic feature and was observed in 18 of 19 cases (95%) of SMIS and 9 of 26 cases (35%) of benign LM (OR, 34.00; 95% CI, 3.88-297.70; P = .001). Border fading was more commonly observed in the SMIS group than in the benign LM group (14 [74%] vs 6 [23%]; OR, 9.33; 95% CI, 2.37-36.70; P = .001). The Hutchinson sign was observed in 8 of 19 cases (42%) of SMIS and 1 of 26 cases (4%) of benign LM (OR, 18.18; 95% CI, 2.02-163.52; P = .01). The range of Cohen κ coefficients spanned from 0.34 to 0.72, showing fair to substantial interobserver agreement for all variables.
Figure 1. Dermoscopic and Clinical Manifestations of Subungual Melanoma In Situ.
A, Longitudinal melanonychia showing multicolored pigmentation with asymmetry based on the longitudinal midline (dashed line) and border fading (blue arrowhead). B, Subungual melanoma in situ with the Hutchinson sign (red arrowhead). Inset, Clinical appearance of the involved nail. Scale units are in millimeters.
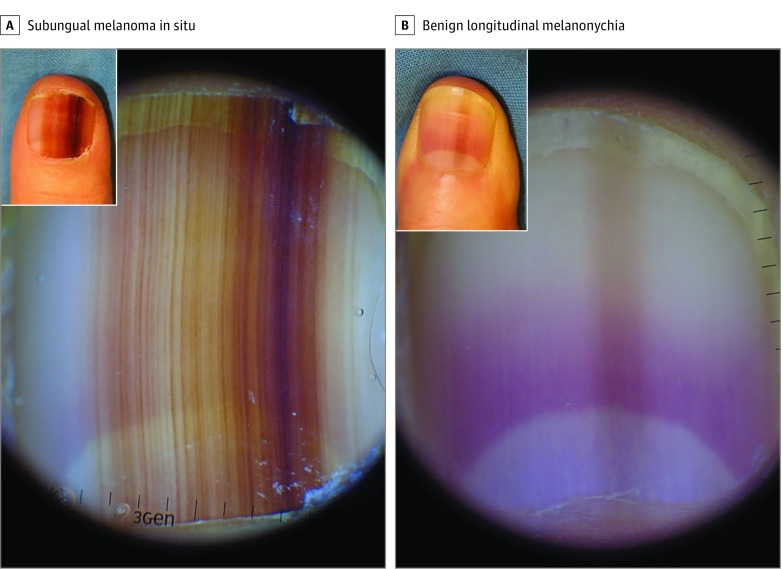
Figure 2. Dermoscopic and Clinical Manifestations of Subungual Melanoma In Situ and Benign Longitudinal Melanonychia.
A, Subungual melanoma in situ showing a 10-mm band with asymmetry, border fading, and multicolor pigmentation (dark and light brown). Inset, Clinical appearance of the involved nail. B, Benign longitudinal melanonychia showing a light brown band with a width of less than 2 mm on dermoscopic examination. Inset, Clinical appearance of the involved nail. Scale units are in millimeters.
Scoring Model for the Diagnosis of SMIS
Based on the logistic regression analysis, the coefficients of each factor were used to develop a scoring model for predicting SMIS by incorporating the following 6 variables: width of pigmentation of at least 3 mm, width of pigmentation of at least 6 mm, multicolor pigmentation, asymmetry, border fading, and the Hutchinson sign (Table 3). The total scores range from 0 to 8, with points assigned as follows: 2 points each for asymmetry, the Hutchinson sign, and a width of pigmentation of at least 6 mm; 1 point each for border fading, multicolor pigmentation, and a width of pigmentation of at least 3 mm. The sensitivity and specificity rates for each cutoff score of the model are shown in the eTable in the Supplement. The cutoff value of 3 points coincided with a sensitivity of 89% and a specificity of 62%. The receiver operating characteristic curve for the model had an area under the curve (C statistic) of 0.91 (eFigure 1 in the Supplement).
Table 3. Predictive Scoring Model for the Diagnosis of Subungual Melanoma In Situ.
| Dermoscopic Variable | β Coefficient (95% CI) | Scorea |
|---|---|---|
| Width of pigmentation, mm | ||
| ≥3b | 1.67 (0.00-3.33) | 1 |
| ≥6 | 3.38 (1.75-5.01) | 2 |
| Multicolor pigmentation | 2.45 (0.79-4.11) | 1 |
| Pattern | ||
| Asymmetry | 3.53 (1.36-5.70) | 2 |
| Border fading | 2.23 (0.86-3.60) | 1 |
| Hutchinson signc | 2.90 (0.70-5.10) | 2 |
Scores were rounded to the nearest integer.
Used as reference regression unit for score standardization.
Defined as periungual pigmentation of the nail fold or hyponychium.
Discussion
We found the clinical and dermoscopic features (width of pigmentation ≥3 mm, width of pigmentation ≥6 mm, multicolor pigmentation, asymmetry, border fading, and the Hutchinson sign) to be predictive of SMIS in adult-onset monodactyl melanonychia. Incorporating these findings, we developed a predictive scoring model for the diagnosis of SMIS with a C statistic of 0.91.
Prior studies have described demographic and morphologic features (clinical and dermoscopic) associated with SUM.7,11,22 Levit et al11 reported age in the fifth to seventh decades of life and thumbnail pigmentation to be risk factors for SUM.11 Consistent with that previous report, the mean (SD) age of patients with SMIS in this study was the sixth decade of life (52.0 [14.4] years), and the thumb was the most commonly affected digit. Patients in the benign LM group were of similar age (mean [SD] age, 48.1 [13.2] years), and the thumb was also the most commonly affected digit in this group. Therefore, age and affected digit may not aid in the differentiation between SMIS and benign causes of LM.
Family history of melanoma has been reported as a risk factor for SUM11; however, large-scale studies on the strength of this association are lacking because of the rare nature of SUM. None of the 19 patients with SMIS in our study reported a family history of melanoma. Therefore, additional studies on the value of family history in the setting of SUM, including SMIS, are needed. Both SUM and benign LM may be associated with trauma.7,22 There was no significant difference in the frequency of patient-reported trauma between the SMIS and benign LM groups in our data set, with 3 cases (16%) of SMIS and 1 case (4%) of benign LM associated with a history of trauma. Sex and duration of symptoms along with age, affected digit, family history, and history of trauma did not differ between the SMIS and benign LM groups and were excluded from our predictive scoring model.
Width of pigmentation is an important risk factor for SMIS. Previous studies suggested that either a width of pigmentation of at least 3 mm or at least 6 mm be used to distinguish malignant from benign causes of LM.11,20 However, the significance of these measures has not been fully established. In this study, the mean (SD) pigmentation width was larger in the SMIS group than in the benign LM group (9.03 [3.96] mm vs 3.94 [3.07] mm, P = .001). While the minimum 3-mm width was useful in detecting SMIS (OR, 5.31; 95% CI, 1.01-28.07), the minimum 6-mm width was more strongly associated with SMIS (OR, 29.33; 95% CI, 5.75-149.65).
Brown-black pigmentation is incorporated into the existing clinical ABCDEF mnemonic of SUM reported by Levit et al.11 In addition, gray pigmentation was suggested to be associated with nail apparatus melanomas.16 In this study of cases of SMIS, dark brown was the most common color (84%) followed by gray (68%). However, in benign cases of LM, gray (58%) was the most common color. The significant distinction between SMIS and benign LM was the presence of multicolor pigmentation (89%) for most SMIS lesions. Univariate analysis revealed multicolor pigmentation to be useful in differentiating SMIS from benign LM (OR, 11.59; 95% CI, 2.21-60.89).
Asymmetry is used for the diagnosis of cutaneous melanomas23; however, it has not been formally described in the evaluation of LM to establish the diagnosis of SMIS or SUM. In this study, we evaluated asymmetry by drawing a longitudinal midline to bisect the band of the nail plate and then we compared the patterns and colors of the 2 segments (Figure 1). Asymmetry was associated with a higher OR for the diagnosis of SMIS than the diagnosis of benign LM (OR, 34.00; 95% CI, 3.88-297.70). The discrepant proliferation of atypical melanocytes in the different regions of the affected matrix could result in clinical or dermoscopic asymmetry of the nail plate pigmentation. In previous studies, an irregular pigmentation pattern was proposed as an important feature of SUM.9,19 However, an irregular pigmentation pattern may be difficult to appreciate because of its subjective attributes, resulting in low interobserver or intraobserver agreement.15,19 Evaluation for asymmetry by drawing a hypothetical longitudinal midline and by comparing the pattern and pigmentation of the bisected halves may be more straightforward than evaluation of an irregular pigmentation pattern. Therefore, we propose asymmetry as a valuable measure in determining the malignant potential of LM.
We found border fading to be a useful characteristic in the diagnosis of SMIS. Border irregularity is already used in the clinical evaluation of pigmented lesions.23 As the atypical melanocytes in SMIS infiltrate the adjacent nail unit, this radial growth may result in the clinical or dermoscopic appearance of a faded border. Border fading was associated with a higher OR for the diagnosis of SMIS compared with the diagnosis of benign LM (OR, 9.33; 95% CI, 2.37-36.70).
The Hutchinson sign is an important clue to the diagnosis of SUM,24 and this factor was reiterated in our data set. The presence of the Hutchinson sign was observed in 42% of the SMIS cases compared with 4% of the benign LM cases and was a useful sign in differentiating SMIS from benign LM (OR, 18.18; 95% CI, 2.02-163.52). In the early stage of SMIS, the Hutchinson sign may be subtle and difficult to appreciate by examination using the naked eye. Dermoscopy aids in the evaluation of a clinically subtle Hutchinson sign (the micro-Hutchinson sign) (eFigure 2 in the Supplement).
Although biopsy of the nail matrix is the criterion standard diagnostic approach for LM, physician and patient concerns regarding its invasiveness and potential for complications may limit its use. A screening model to assess the potential for SMIS could be used to initiate risk-benefit discussions on whether to proceed with a biopsy of the nail matrix. In this study, we propose an 8-point scoring model for distinguishing SMIS from benign LM by assessing the likelihood of a diagnosis of SMIS based on the presence of specific dermoscopic findings, including width of the pigmentation, asymmetry, the Hutchinson sign, multicolor pigmentation, and border fading. Cutoff scores of 0 to 2 demonstrated a 100% sensitivity for diagnosis of SMIS, with specificities ranging from 0 to 50% (eTable 1 in the Supplement). A cutoff point of 3 yielded a sensitivity of 89% and a specificity of 62%.
Based on the analysis of dermoscopic variables, we propose that the following dermoscopic features be checked in the evaluation of adult-onset LM affecting a single digit: (1) asymmetry: the bisected halves of the pigmentation band are asymmetric based on a hypothetical midline; (2) border fading: the borders of pigmentation are faded or ill defined; (3) pigmentation: the lesion displays multicolor pigmentation; (4) width: the width of the pigmented lesion is greater than or equal to 3 mm or 6 mm; and (5) the Hutchinson sign: the pigment extends to the periungual skin. This 5-item checklist, consisting entirely of morphologic features, may be practical for clinician use during nail examinations.
Strengths and Limitations
Our findings must be interpreted in the context of the study design and population. This study included only patients with adult-onset LM affecting a single digit. Thus, the scoring model is not valid for LM diagnosed in the pediatric population or LM involving multiple digits, such as drug-induced melanonychia or melanonychia associated with systemic disease. We included clinically diagnosed cases of benign LM in addition to histopathologically confirmed cases to minimize selection bias. In our institution, biopsies of the nail matrix are typically performed in patients with LM when there is clinical suspicion for malignancy. To avoid misclassification, we included only cases with patients clinically diagnosed with benign LM by our nail experts. The proposed model is based on morphologic features only. Nonmorphologic risk factors (such as a change in a pigmented lesion over time) also could be considered in a study. The number of SMIS cases was limited because of the relatively rare nature of this disease. Further validation of our scoring model is necessary using a large sample size.
Conclusions
Our results showed distinctive dermoscopic patterns in SMIS that aided in the differentiation between benign LM and SMIS. These dermoscopic findings were incorporated into a predictive scoring model for the detection of SMIS. We suggest that clinicians use this predictive scoring model during physical examination to assist in screening for SMIS. By facilitating the early diagnosis of SUM and by assisting patients and physicians with decisions to pursue a biopsy of the nail matrix, this scoring model has the potential to improve the prognosis of SUM and to promote informed decision making regarding the management of LM.
eTable. Sensitivity and Specificity of the Different Cutoff Score for the Subungual Melanoma In Situ
eFigure 1. Receiver Operating Characteristic Curve for a Predictive Scoring Model in Detecting Subungual Melanoma In Situ
eFigure 2. Clinical, Dermoscopic, and Histopathologic Features of Subungual Melanoma In Situ
References
- 1.Decker A, Connolly KL, Lee EH, Busam KJ, Nehal KS. Frequency of subungual melanoma in longitudinal melanonychia: a single-center experience. Dermatol Surg. 2017;43(6):798-804. [DOI] [PubMed] [Google Scholar]
- 2.Boespflug A, Debarbieux S, Depaepe L, et al. Association of subungual melanoma and subungual squamous cell carcinoma: a case series. J Am Acad Dermatol. 2018;78(4):760-768. [DOI] [PubMed] [Google Scholar]
- 3.Tosti A, Schneider SL, Ramirez-Quizon MN, Zaiac M, Miteva M. Clinical, dermoscopic, and pathologic features of onychopapilloma: a review of 47 cases. J Am Acad Dermatol. 2016;74(3):521-526. [DOI] [PubMed] [Google Scholar]
- 4.Ohn J, Choe YS, Mun JH. Dermoscopic features of nail matrix nevus (NMN) in adults and children: a comparative analysis. J Am Acad Dermatol. 2016;75(3):535-540. [DOI] [PubMed] [Google Scholar]
- 5.Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74(6):1121-1127. [DOI] [PubMed] [Google Scholar]
- 6.Thomas L, Dalle S. Dermoscopy provides useful information for the management of melanonychia striata. Dermatol Ther. 2007;20(1):3-10. [DOI] [PubMed] [Google Scholar]
- 7.Braun RP, Baran R, Le Gal FA, et al. Diagnosis and management of nail pigmentations. J Am Acad Dermatol. 2007;56(5):835-847. [DOI] [PubMed] [Google Scholar]
- 8.Hirata SH, Yamada S, Almeida FA, et al. Dermoscopy of the nail bed and matrix to assess melanonychia striata. J Am Acad Dermatol. 2005;53(5):884-886. [DOI] [PubMed] [Google Scholar]
- 9.Ronger S, Touzet S, Ligeron C, et al. Dermoscopic examination of nail pigmentation. Arch Dermatol. 2002;138(10):1327-1333. [DOI] [PubMed] [Google Scholar]
- 10.Mun JH, Kim GW, Jwa SW, et al. Dermoscopy of subungual haemorrhage: its usefulness in differential diagnosis from nail-unit melanoma. Br J Dermatol. 2013;168(6):1224-1229. [DOI] [PubMed] [Google Scholar]
- 11.Levit EK, Kagen MH, Scher RK, Grossman M, Altman E. The ABC rule for clinical detection of subungual melanoma. J Am Acad Dermatol. 2000;42(2, pt 1):269-274. [DOI] [PubMed] [Google Scholar]
- 12.Halteh P, Scher R, Artis A, Lipner SR. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76(5):994-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banfield CC, Redburn JC, Dawber RP. The incidence and prognosis of nail apparatus melanoma: a retrospective study of 105 patients in four English regions. Br J Dermatol. 1998;139(2):276-279. [DOI] [PubMed] [Google Scholar]
- 14.Knackstedt T, Jellinek NJ. Limitations and challenges of nail unit dermoscopy in longitudinal melanonychia. J Am Acad Dermatol. 2017;76(2):e71-e72. [DOI] [PubMed] [Google Scholar]
- 15.Di Chiacchio N, Hirata SH, Enokihara MY, Michalany NS, Fabbrocini G, Tosti A. Dermatologists’ accuracy in early diagnosis of melanoma of the nail matrix. Arch Dermatol. 2010;146(4):382-387. [DOI] [PubMed] [Google Scholar]
- 16.Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31(4):732-736. [DOI] [PubMed] [Google Scholar]
- 17.Ohn J, Mun JH. Reply to: “Limitations and challenges of nail unit dermoscopy in longitudinal melanonychia”. J Am Acad Dermatol. 2017;76(2):e73-e74. [DOI] [PubMed] [Google Scholar]
- 18.Duarte AF, Correia O, Barros AM, Ventura F, Haneke E. Nail melanoma in situ: clinical, dermoscopic, pathologic clues, and steps for minimally invasive treatment. Dermatol Surg. 2015;41(1):59-68. [DOI] [PubMed] [Google Scholar]
- 19.Koga H, Saida T, Uhara H. Key point in dermoscopic differentiation between early nail apparatus melanoma and benign longitudinal melanonychia. J Dermatol. 2011;38(1):45-52. [DOI] [PubMed] [Google Scholar]
- 20.Saida T, Ohshima Y. Clinical and histopathologic characteristics of early lesions of subungual malignant melanoma. Cancer. 1989;63(3):556-560. [DOI] [PubMed] [Google Scholar]
- 21.Ohn J, Choe YS, Park J, Mun JH. Dermoscopic patterns of fungal melanonychia: a comparative study with other causes of melanonychia. J Am Acad Dermatol. 2017;76(3):488-493.e2. [DOI] [PubMed] [Google Scholar]
- 22.Möhrle M, Häfner HM. Is subungual melanoma related to trauma? Dermatology. 2002;204(4):259-261. [DOI] [PubMed] [Google Scholar]
- 23.Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA. 2004;292(22):2771-2776. [DOI] [PubMed] [Google Scholar]
- 24.Baran R, Kechijian P. Hutchinson’s sign: a reappraisal. J Am Acad Dermatol. 1996;34(1):87-90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Sensitivity and Specificity of the Different Cutoff Score for the Subungual Melanoma In Situ
eFigure 1. Receiver Operating Characteristic Curve for a Predictive Scoring Model in Detecting Subungual Melanoma In Situ
eFigure 2. Clinical, Dermoscopic, and Histopathologic Features of Subungual Melanoma In Situ