Key Points
Question
What is the association of atopic dermatitis severity and disease control with the patient-reported disease burden in clinical practice?
Findings
Among 1519 adults in this cross-sectional study, patients reported a multidimensional burden that is higher with greater disease severity and inadequate disease control.
Meaning
For patients with moderate to severe dermatitis, there is a need for more effective therapies and for greater incorporation of the patient’s perspective into clinical assessment.
This cross-sectional study examines the association of atopic dermatitis severity and disease control with the patient-reported disease burden in clinical practice.
Abstract
Importance
Real-world data are limited on the patient-reported burden of adult atopic dermatitis (AD).
Objective
To characterize the patient-reported burden of AD with regard to impact of disease severity and inadequate control in adults from clinical settings.
Design, Setting, and Participants
In this cross-sectional study using data from 6 academic medical centers in the United States collected by a self-administered internet-based questionnaire, 1519 adult patients with AD were stratified by AD severity as mild or moderate/severe using the Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD). Patients with moderate/severe disease using systemic immunomodulators/phototherapy were further stratified as having adequate or inadequate disease control. Strata were compared for all outcomes.
Main Outcomes and Measures
Outcomes included validated measures and stand-alone questions assessing itch (pruritus numerical rating scale; PO-SCORAD itch visual analog scale), pain (numerical rating scale), sleep (PO-SCORAD sleep visual analog scale; sleep interference with function), anxiety and depression (Hospital Anxiety and Depression Scale), and health-related quality of life (Dermatology Life Quality Index).
Results
Among the 1519 adult patients with AD, relative to mild AD (n = 689, 64% women; mean [SD] age, 46.5 [18.0] years), patients with moderate/severe AD (n = 830, 66.8% women; mean [SD] age, 45.1 [16.9] years) reported more severe itching and pain, greater adverse effects on sleep, higher prevalence of anxiety and depression (417 [50.2%] vs 188 [27.3%]), and greater health-related quality-of-life impairment. The 103 patients with moderate/severe AD with inadequate disease control despite treatment with systemic immunomodulators or phototherapy (55.7%) reported higher burdens of itch and sleeping symptoms vs patients with controlled disease including more days per week with itchy skin (5.7 vs 2.7) and higher proportions with itch duration greater than half a day (190 [22.8%] vs 20 [2.9%]). Sleep symptoms included trouble sleeping (3.9 vs 1.1 on the PO-SCORAD VAS), longer sleep latency (38.8 vs 21.6 minutes), more frequent sleep disturbances (2.6 vs 0.4 nights in past week), and greater need for over-the-counter sleep medications (324 [39%] vs 145 [21%]).
Conclusions and Relevance
Inadequate disease control was common among patients with moderate/severe AD, and was associated with a higher patient-reported burden than patients with controlled disease. Regardless of disease control, the burden of moderate/severe AD was higher than mild AD, suggesting a need for more effective therapies for moderate/severe disease.
Introduction
Onset of atopic dermatitis (AD) most often begins in early childhood with a variable disease course and symptoms persisting into adulthood for many patients.1,2 There remain substantial gaps in our understanding of the impact of AD on the lives of adults with this disease,3 especially those with moderate-to-severe disease. Moderate AD has been reported in 20% to 37% of adult patients and severe AD in 10% to 34%.4,5 A recent clinical trial conducted in adults with moderate-to-severe AD reported the presence of a multidimensional baseline disease burden that included substantial disease activity, symptoms, and a variety of comorbid conditions with a significant impact on health-related quality-of-life (HRQoL).6 A similarly broad burden was observed based on self-report among adults with AD in the general population participating in the National Health and Wellness Survey.7,8 Although these results reflect a population-based sample, they may not be generalizable to patients encountered in clinical practice.
The Adults With Atopic dermatitis Reporting on their Experience (AD-AWARE) study, a cross-sectional burden-of-illness study in adult patients with AD from clinical practices in the United States, presented the opportunity to assess the patient burden across multiple domains in a large, well-characterized sample in a real-world setting. The objectives of this study were to characterize the association of patient-reported burden of AD with regard to disease severity and patient assessment of AD control.
Methods
Study Design and Patient Population
The AD-AWARE study was conducted in clinical practices at 6 US academic medical centers via a self-administered internet-based questionnaire among adult patients with AD who agreed to participate: University of California, San Diego; Brown University, Rhode Island; Wake Forest Baptist Health, North Carolina; Icahn School of Medicine at Mount Sinai, New York; University of Pennsylvania, Pennsylvania; and the Oregon Health and Science University, Oregon. The protocol received institutional review board approval from each study center. Participants provided written informed consent and data collection was performed in accordance with the Health Insurance Portability and Accountability Act of 1996.
Adult patients (≥18 years) with 1 or more visits to any clinic in the facility associated with AD (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 691.8) between January 1, 2013, and December 31, 2014, were identified for inclusion and initially contacted by postal letters, which included the URL and sign-in information to access the online consent form and questionnaire. Initial contact letters included a preincentive ($5 bill), and eligible patients who completed the questionnaire received a post-incentive ($35 mailed check or Visa gift card) as recommended by the Tailored Design Method for surveys.9 Reminders were sent via postal letters if the quota of 200 patients per site had not been met. Inclusion criteria were amended if necessary to meet site quotas by allowing participation of patients diagnosed with International Classification of Diseases, Ninth Revision (ICD-9) 692.9 (ie, contact dermatitis and other eczema, unspecified cause) if they also had 1 or more prescriptions for a systemic agent. The AD was confirmed by patients self-reporting that they had received a diagnosis by a health care provider in response to a screening question at the beginning of the online questionnaire. Paper versions of the screening questions, consent form, and full questionnaire were mailed to patients upon request.
Patients were stratified by AD severity using the Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD) that was included in the survey instrument,10 with scores less than 25 and 25 or more considered mild and moderate/severe disease, respectively.11 Treatment patterns were evaluated based on patient-reported use of medications within the past 7 days, including topical agents (corticosteroids and calcineurin inhibitors), systemic steroids, immunomodulatory therapy (Im-tx), and phototherapy. Patients with moderate/severe AD receiving Im-tx (azathioprine, cyclophosphamide, cyclosporine, methotrexate, mycophenolate, tacrolimus) or phototherapy within the past 7 days (with/without topical agents) were further categorized as having controlled or inadequately controlled AD. Inadequately controlled AD was defined as patients who somewhat or completely disagreed with the statement “I feel my current treatments are effective in controlling my atopic dermatitis”; all other responses were considered controlled AD.
Outcomes
The pruritus numerical rating scale (NRS; 0 = no itch to 10 = worst imaginable itch) assessed worst itch within the past 24 hours,12 and the PO-SCORAD itch visual analog scale (VAS; 0 = no itching, 10 = unbearable itching) assessed average itch over the past 3 days.10 Standalone questions evaluated days in the past week and hours per day with itchy skin. Worst pain within the past 24 hours was assessed using an NRS (0 = no pain, 10 = worst imaginable pain).
Sleep outcomes included trouble sleeping over the past 3 days using the PO-SCORAD sleep VAS (0 = no insomnia, 10 = total insomnia),10 and standalone questions on hours of sleep per night, nights with sleep disturbance during the past week, and sleep interference with function during the past week using a Likert scale (1 = not at all to 5 = very much).
The presence of anxiety and depression symptoms was evaluated using the Hospital Anxiety and Depression Scale (HADS), which consists of anxiety (HADS-A) and depression (HADS-D) subscales. Scores range from 0 to 21 on each subscale, with scores of 8 or more indicative of anxiety or depression symptoms.13 Participant HRQoL was evaluated using the Dermatology Life Quality Index (DLQI),14 which evaluates the impact of skin conditions on HRQoL over the past week (score range, 0-30; higher scores indicate greater impact).
Statistical Analyses
Outcomes were compared for patients with moderate/severe vs mild AD, and patients who self-reported having controlled disease vs inadequately controlled on their current therapy (among patients with moderate/severe AD who were treated with Im-tx or phototherapy in the past 7 days). χ2 tests were used for categorical variables, and for continuous variables, equality of variances was tested first and, based on the results, pooled variances or Satterthwaite variances were used in the student t test; P < .05 was considered significant. Analyses were performed using SAS statistical software (version 9.3, SAS Institute).
Results
Response Rate and Population Characteristics
Of 6737 patients who were contacted, 1519 agreed to participate and completed the questionnaire (22.5% response rate); 160 of these, at a single site (University of California San Diego), were identified using the amended criteria. A total of 997 (65.7%) of respondents were women, 1028 (67.7%) were white non-Hispanic, with a mean (SD) age of 45.7 (17.4) years; 830 (54.6%) had moderate/severe AD (Table 1). Significant differences between patients with mild AD and moderate/severe AD were observed for age distribution, race/ethnicity, education, body mass index (calculated as weight in kilograms divided by height in meters squared), education, and family income (Table 1).
Table 1. Demographic and Disease Onset Characteristics of the Populations.
| Variable | All Patients (n = 1519) | By Severity | Moderate/Severe Patients Treated With Im-tx or Phototherapy in Past 7 Days | ||||
|---|---|---|---|---|---|---|---|
| Mild (n = 689) | Moderate/Severe (n = 830) | P Value | Controlled (n = 82) | Inadequately Controlled (n = 103) | P Value | ||
| Age (n = 1517), mean (SD), y | 45.7 (17.4) | 46.5 (18.0) | 45.1 (16.9) | .10 | 49.8 (17.4) | 44.6 (16.6) | .04 |
| Sex (n = 1517), No. (%) | .32 | .67 | |||||
| Male | 520 (34.3) | 245 (35.6) | 275 (33.2) | 44 (53.7) | 52 (50.5) | ||
| Female | 997 (65.7) | 443 (64.4) | 554 (66.8) | 38 (46.3) | 51 (49.5) | ||
| BMI (n = 1519), mean (SD) | 27.2 (6.5) | 26.0 (5.4) | 28.1 (7.1) | <.001 | 27.5 (5.8) | 27.4 (6.8) | .92 |
| Race/ethnicity (n = 1519), No. (%) | |||||||
| White, non-Hispanic | 1028 (67.7) | 498 (72.3) | 530 (63.9) | .01 | 55 (67.1) | 69 (67.0) | .53 |
| White, Hispanic | 68 (4.5) | 28 (4.1) | 40 (4.8) | 3 (3.7) | 8 (7.8) | ||
| African-American, non-Hispanic | 201 (13.2) | 82 (11.9) | 119 (14.3) | 10 (12.2) | 7 (6.8) | ||
| African-American, Hispanic | 28 (1.8) | 8 (1.2) | 20 (2.4) | 4 (4.9) | 3 (2.9) | ||
| Asian | 132 (8.7) | 54 (7.8) | 78 (9.4) | 8 (9.8) | 10 (9.7) | ||
| Pacific Islander | 8 (0.5) | 2 (0.3) | 6 (0.7) | 1 (1.2) | 1 (1.0) | ||
| Other | 54 (3.6) | 17 (2.5) | 37 (4.5) | 1 (1.2) | 5 (4.9) | ||
| Education (n = 1519), No. (%) | |||||||
| High school, did not graduate | 34 (2.2) | 9 (1.3) | 25 (3.0) | .002 | 5 (6.1) | 5 (4.9) | .49 |
| High school graduate | 170 (11.2) | 74 (10.7) | 96 (11.6) | 10 (12.2) | 10 (9.7) | ||
| GED or alternative | 22 (1.4) | 10 (1.5) | 12 (1.4) | 1 (1.2) | 1 (1.0) | ||
| Some college, or Associate’s degree | 379 (25.0) | 147 (21.3) | 232 (28.0) | 17 (20.7) | 30 (29.1) | ||
| Bachelor’s degree | 448 (29.5) | 210 (30.5) | 238 (28.7) | 30 (36.6) | 26 (25.2) | ||
| Graduate degree | 466 (30.7) | 239 (34.7) | 227 (27.3) | 19 (23.2) | 31 (30.1) | ||
| Marital status (n = 1519), No. (%) | .39 | .12 | |||||
| Single | 518 (34.1) | 221 (32.1) | 297 (35.8) | 20 (24.4) | 42 (40.8) | ||
| Married, or remarried | 779 (51.3) | 371 (53.8) | 408 (49.2) | 42 (51.2) | 44 (42.7) | ||
| Married, but separated | 25 (1.6) | 12 (1.7) | 13 (1.6) | 1 (1.2) | 3 (2.9) | ||
| Divorced | 155 (10.2) | 69 (10.0) | 86 (10.4) | 13 (15.9) | 9 (8.7) | ||
| Widowed | 42 (2.8) | 16 (2.3) | 26 (3.1) | 6 (7.3) | 9 (4.9) | ||
| Family income (n = 1519), No. (%), $ | |||||||
| <50 000 | 472 (31.1) | 167 (24.3) | 305 (36.7) | <.001 | 28 (34.1) | 44 (42.7) | .53 |
| 50 000-99 999 | 385 (25.3) | 192 (27.9) | 193 (23.3) | 21 (25.6) | 19 (18.5) | ||
| ≥100 000 | 425 (28.0) | 204 (29.6) | 221 (26.6) | 20 (24.4) | 31 (30.1) | ||
| Prefer not to answer | 237 (15.6) | 126 (18.3) | 111 (13.4) | 13 (15.9) | 9 (8.7) | ||
| Age when first diagnosed (n = 1512), mean (SD), y | 27.6 (22.1) | 31.1 (22.1) | 24.7 (21.7) | <.001 | 25.8 4.7 | 22.4 (19.5) | .30 |
| Age when first diagnosed (n = 1512), mean (SD), y | |||||||
| <18 | 617 (40.8) | 232 (34.0) | 385 (46.4) | <.001 | 38 (46.3) | 51 (49.5) | .67 |
| ≥18 | 895 (59.2) | 450 (66.0) | 445 (53.6) | 44 (53.7) | 52 (50.5) | ||
| Time since disease onset (n = 1498), mean (SD), y | 18.3 (17.6) | 15.7 (17.1) | 20.5 (17.7) | <.001 | 24.0 (18.9) | 22.2 (16.7) | .49 |
| Atopic dermatitis therapies in past 7 d, No. (%) | |||||||
| ≥1 Topical therapya | 1038 (68.3) | 454 (65.9) | 584 (70.4) | .06 | 70 (85.4) | 88 (85.4) | .99 |
| ≥1 Systemic therapyb | 366 (24.1) | 97 (14.1) | 269 (32.4) | <.001 | 60 (73.2) | 68 (66.0) | .30 |
| ≥1 Systemic therapy/phototherapyc | 450 (29.6) | 124 (18.0) | 326 (39.3) | <.001 | 81 (100) | 103 (100) | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); Im-tx, immunomodulatory therapy; NA, not applicable.
Topical agents include: baths with additives, bleach in bath, bag balm, cold tar, cold compress, cocoa butter, moisturizers, pimecrolimus cream, steroid cream, soaking and smearing, tacrolimus ointment, and vitamin E oil.
Systemic AD therapy includes: azathioprine, cyclosporine prescriptions, cyclophosphamide, methotrexate, mycophenolate mofetil, tacrolimus, and oral steroid prescriptions.
Systemic AD therapy or phototherapy includes systemic immunosuppressant, oral steroid, and phototherapy.
Although most patients in both severity groups were diagnosed with AD as adults, moderate/severe patients were characterized by a higher proportion of patients diagnosed during childhood or adolescence (385 [46.4%] vs 232 [34.0%]; P < .001), and had been living with AD for a longer time since diagnosis than patients with mild disease (20.5 vs 15.7 years; P < .001) (Table 1). The proportion of patients with at least 1 systemic therapy or systemic/phototherapy within the past 7 days was higher among those with moderate/severe AD, but use of topical agents was similar between groups (Table 1).
A total of 185 (22.3%) patients with moderate/severe disease received Im-tx or phototherapy, of whom 103 (55.7%) assessed themselves as inadequately controlled (Table 1). Patients with inadequately controlled AD were younger than those with controlled AD (44.6 vs 49.8 years; P = .04). There were no differences between patients with controlled and inadequately controlled disease for age of onset, disease duration, or use of topical therapies and any systemic therapies (Table 1).
Itch, Pain, and Sleep
All measures of itch showed greater severity and duration among patients with moderate/severe disease relative to mild (all P < .001) (Table 2), including more days per week with itchy skin (5.7 vs 2.7) and higher proportions with itch duration greater than half a day (190 [22.8%] vs 20 [2.9%]). Itch intensity and frequency were higher among patients with inadequately controlled disease relative to controlled (Table 2). Pain severity was higher among moderate/severe vs mild, and inadequately controlled vs controlled disease (Table 2).
Table 2. Itch and Sleep Symptoms.
| Variable | By Severity | Moderate/Severe Patients Treated With Im-tx or Phototherapy in Past 7 Days | ||||
|---|---|---|---|---|---|---|
| Mild (n = 689) | Moderate/Severe (n = 830) | P Value | Controlled (n = 82) | Inadequately Controlled (n = 103) | P Value | |
| PO-SCORAD pruritus VAS, mean (SD) | 2.0 (2.0) | 5.9 (2.4) | <.001 | 5.4 (2.5) | 6.9 (2.4) | <.001 |
| Pruritus NRS, mean (SD) | 2.1 (2.1) | 5.8 (2.5) | <.001 | 5.7 (2.2) | 6.8 (2.4) | .001 |
| Itch frequency, mean (SD), days/week | 2.7 (2.5) | 5.7 (1.9) | <.001 | 5.7 (1.8) | 6.3 (1.5) | .01 |
| Itch duration, No. (%), hours per day | ||||||
| <6 | 627 (91.0) | 419 (50.5) | <.001 | 34 (41.5) | 34 (33.0) | .66 |
| 6-12 | 42 (6.1) | 221 (26.6) | 22 (26.8) | 31 (30.1) | ||
| 12-23 | 10 (1.4) | 110 (13.2) | 15 (18.3) | 24 (23.3) | ||
| All day | 10 (1.5) | 80 (9.6) | 11 (13.4) | 14 (13.6) | ||
| Pain NRS, mean (SD) | 0.9 (1.6) | 3.3 (2.8) | <.001 | 3.2 (2.7) | 4.7 (3.0) | <.001 |
| PO-SCORAD sleep VAS, mean (SD) | 1.1 (1.9) | 3.9 (3.2) | <.001 | 3.9 (3.1) | 4.9 (3.2) | .04 |
| Hours of sleep at night over the past week, mean (SD) | 6.9 (1.2 | 6.2 (1.4) | <.001 | 6.1 (1.5) | 5.9 (1.7) | .46 |
| Nights with sleep disturbed over the past week, mean (SD) | 0.4 (1.1) | 2.6 (2.5) | <.001 | 3.0 (1.0) | 3.7 (2.7) | .07 |
| Minutes until falling asleep over the past week, mean (SD) | 21.6 (20.1) | 38.8 (36.3) | <.001 | 37.7 (28.2) | 55.7 (50.1) | .002 |
| Sleep problems interfere with daily function during the past week, No. (%) | ||||||
| Not at all | 342 (49.6) | 158 (19.0) | <.001 | 16 (19.5) | 14 (13.6) | .15 |
| A little/somewhat | 300 (43.5) | 470 (56.6) | 47 (57.3) | 52 (50.5) | ||
| Much/very much | 47 (6.8) | 202 (24.3) | 19 (23.2) | 37 (35.9) | ||
| Need for OTC sleep medication during the last week, No. (%) | ||||||
| Not during the past week | 544 (79.0) | 506 (61.0) | <.001 | 36 (43.9) | 49 (47.6) | .85 |
| Once or twice per week | 66 (9.6) | 107 (12.9) | 14 (17.1) | 15 (14.6) | ||
| Three or more times per week | 79 (11.5) | 217 (26.1) | 32 (39.0) | 39 (37.9) | ||
Abbreviations: Im-tx, immunomodulatory therapy; NRS, numerical rating scale; OTC, over-the-counter; PO-SCORAD, Patient-Oriented Scoring of Atopic Dermatitis scale; VAS, visual analog scale.
Patients with moderate/severe AD reported more sleep problems vs those with mild AD (all P < .001) (Table 2), including trouble sleeping (3.9 vs 1.1 on the PO-SCORAD VAS), longer sleep latency (38.8 vs 21.6 minutes), more frequent sleep disturbances (2.6 vs 0.4 nights in past week), and greater need for over-the-counter sleep medications (324 [39%] vs 145 [21%]) (Table 2). These sleep problems “much” or “very much” interfered with daily function in a higher proportion of patients with moderate/severe AD (202 [24.3%] vs 47 [6.8%]; P < .001).
Greater sleep problems were generally observed among the patients with inadequately controlled relative to those with controlled disease. However, only for the sleep VAS and sleep latency were the differences significant (Table 2).
Mental Health
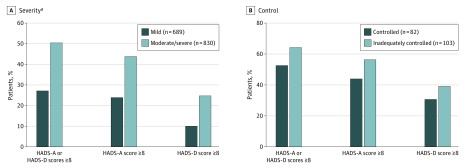
Symptoms of anxiety or depression were reported by 417 (50.2%) patients with moderate/severe AD vs 188 (27.3%) with mild (P < .001) (Figure 1A). The proportion of patients with scores of 8 or higher (indicating symptoms) on the individual anxiety and depression subscales was higher among patients with moderate/severe AD relative to those with mild disease (all P < .001), with anxiety more frequent than depression, regardless of AD severity.
Figure 1. Frequency of Depression and Anxiety Assessed Using the Hospital Anxiety and Depression Scale (HADS).
Symptoms of anxiety or depression were reported more frequently by patients with moderate/severe AD relative to those with mild AD (A), and by patients with inadequate disease control relative to those with controlled AD (B). AD indicates atopic dermatitis.
aP < .001 vs mild.
Anxiety and depression symptoms trended consistently higher among patients with inadequately controlled AD relative to those with controlled disease (Figure 1B).
Health-Related Quality of Life
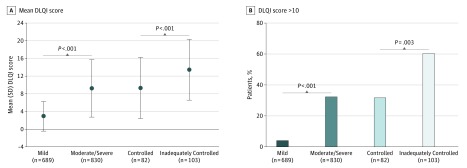
Mean DLQI scores were higher among patients with moderate/severe vs those with mild disease (9.2 vs 2.9; P < .001), and among patients with inadequately controlled vs controlled disease (13.4 vs 9.3; P < .001) (Figure 2A). Higher proportions of patients with moderate/severe and those with inadequately controlled disease reported that AD had a very large effect on their HRQoL (DLQI >10) relative to their respective comparator categories (Figure 2B). Higher proportions of patients with moderate/severe disease also reported that AD had substantial impacts on all individual DLQI items (all P < .001); patients with inadequately controlled disease reported a greater impact across items except for “influence clothes worn” and “sexual difficulties” (Table 3). In particular, item 7 on the DLQI, which elicits information on prevention of work or study, was reported by 116 (14.0%) and 18 (2.6%) patients with moderate/severe and mild disease, respectively (P < .001), and by 28 (27.2%) and 15 (18.3%) patients with inadequately controlled and controlled disease, respectively (P < .001).
Figure 2. Impact of Atopic Dermatitis on Health-Related Quality of Life Assessed Using the Dermatology Life Quality Index (DLQI).
Patients with moderate/severe AD and those with inadequately controlled disease had poorer quality of life relative to patients with mild AD and controlled disease, respectively, as indicated by the total DLQI score (A) and percentage of patients who reported a DLQI total score higher than 10, which is indicative of a very large effect (B). AD indicates atopic dermatitis.
Table 3. Individual Items on the Dermatology Life Quality Index (DLQI).
| DLQI Item | No. (%) | |||||
|---|---|---|---|---|---|---|
| By Severity | Moderate/Severe Patients Treated With Im-tx or Phototherapy in Past 7 Days | |||||
| Mild (n = 689) | Moderate/Severe (n = 830) | P Value | Controlled (n = 82) | Inadequately Controlled (n = 103) | P Value | |
| Itchy, sore, painful, stinging skin | ||||||
| Not at all | 214 (31.1) | 15 (1.8) | <.001 | 0 | 1 (1.0) | <.001 |
| A little | 428 (62.1) | 395 (47.6) | 43 (52.4) | 20 (19.4) | ||
| A lot or very much | 47 (6.8) | 420 (50.6) | 39 (47.6) | 82 (79.6) | ||
| Embarrassed/self-conscious | ||||||
| Not at all | 417 (60.5) | 145 (17.5) | <.001 | 14 (17.1) | 4 (3.9) | <.001 |
| A little | 212 (30.8) | 344 (41.4) | 39 (47.6) | 32 (31.1) | ||
| A lot or very much | 60 (8.7) | 341 (41.1) | 29 (35.4) | 67 (65.0) | ||
| Interfered with shopping, home, garden activities | ||||||
| Not at all | 595 (86.4) | 419 (50.5) | <.001 | 40 (48.8) | 27 (26.2) | .003 |
| A little | 63 (9.1) | 224 (27.0) | 27 (32.9) | 34 (33.0) | ||
| A lot or very much | 14 (2.0) | 157 (18.9) | 13 (15.9) | 35 (34.0) | ||
| Does not apply to me | 17 (2.5) | 30 (3.6) | 2 (2.4) | 7 (6.8) | ||
| Influenced clothes worn | ||||||
| Not at all | 451 (65.5) | 224 (27.0) | <.001 | 22 (26.8) | 16 (15.5) | .13 |
| A little | 157 (22.8) | 249 (30.0) | 26 (31.7) | 28 (27.2) | ||
| A lot or very much | 57 (8.3) | 343 (41.3) | 33 (40.2) | 56 (54.4) | ||
| Does not apply to me | 24 (3.5) | 14 (1.7) | 1 (1.2) | 3 (2.9) | ||
| Affected social/leisure activities | ||||||
| Not at all | 545 (79.1) | 373 (44.9) | <.001 | 39 (47.6) | 23 (22.3) | <.001 |
| A little | 101 (14.7) | 260 (31.3) | 24 (29.3) | 33 (32.0) | ||
| A lot or very much | 19 (2.8) | 171 (20.6) | 16 (19.5) | 45 (43.7) | ||
| Does not apply to me | 24 (3.5) | 26 (3.1) | 3 (3.7) | 2 (1.9) | ||
| Affected sports participation | ||||||
| Not at all | 520 (75.5) | 335 (40.4) | <.001 | 26 (31.7) | 22 (21.4) | .05 |
| A little | 45 (6.5) | 154 (18.6) | 15 (30.5) | 23 (22.3) | ||
| A lot or very much | 17 (2.5) | 129 (15.5) | 12 (14.6) | 31 (30.1) | ||
| Does not apply to me | 107 (15.5) | 212 (25.5) | 19 (23.2) | 27 (26.2) | ||
| Prevented work/studying | ||||||
| Yes | 18 (2.6) | 116 (14.0) | <.001 | 15 (18.3) | 28 (27.2) | <.001 |
| No | 553 (80.3) | 552 (66.5) | 53 (64.6) | 55 (53.4) | ||
| Does not apply to me | 118 (17.1) | 162 (19.5) | 14 (17.1) | 20 (19.4) | ||
| Affected work/studyinga | ||||||
| Not at all | 459 (66.6) | 244 (29.4) | NA | 26 (31.7) | 14 (13.6) | NA |
| A little | 89 (12.9) | 262 (31.6) | 27 (32.9) | 26 (25.2) | ||
| A lot | 5 (0.7) | 46 (5.5) | 0 | 15 (14.6) | ||
| Affected relationships | ||||||
| Not at all | 589 (85.5) | 422 (50.8) | <.001 | 42 (51.2) | 30 (29.10 | .009 |
| A little | 55 (8.0) | 238 (28.7) | 23 (28.0) | 48 (46.6) | ||
| A lot or very much | 8 (1.2) | 99 (11.9) | 9 (11.0) | 18 (17.5) | ||
| Does not apply to me | 37 (5.4) | 71 (8.6) | 8 (9.8) | 7 (6.8) | ||
| Sexual difficulties | <.001 | .06 | ||||
| Not at all | 536 (77.8) | 437 (52.7) | 46 (56.1) | 38 (36.9) | ||
| A little | 44 (6.4) | 146 (17.6) | 17 (20.7) | 25 (24.3) | ||
| A lot or very much | 11 (1.6) | 73 (8.8) | 8 (.8) | 16 (15.5) | ||
| Does not apply to me | 98 (14.2) | 174 (21.0) | 11 (13.4) | 24 (23.3) | ||
| Impact of treatment | ||||||
| Not at all | 476 (69.1) | 285 (34.3) | <.001 | 22 (26.8) | 22 (21.4) | .03 |
| A little | 141 (20.5) | 347 (41.8) | 43 (52.4) | 39 (37.9) | ||
| A lot or very much | 17 (2.5) | 156 (18.8) | 16 (19.5) | 37 (35.9) | ||
| Does not apply to me | 55 (8.0) | 42 (5.1) | 1 (1.2) | 5 (4.9) | ||
Abbreviations: Im-tx, immunomodulatory therapy; NA, not applicable.
Participants who responded “No” to “Prevented work/studying”; statistical comparisons between groups not done.
Discussion
This study, which reflects real-world clinical settings and included a large sample size, describes the impact of moderate-to-severe AD on patients’ daily lives. In addition, the high proportion of patients with inadequate control among those with moderate/severe AD on Im-tx or phototherapy highlights the needs and challenges associated with treating AD, especially given that therapy had been escalated in these patients to include systemic agents not currently indicated for AD treatment.
In contrast to studies that have reported few differences in the burden between mild and moderate/severe AD,7,8 the current study reported a significantly greater burden of moderate/severe AD across all outcomes compared with mildly affected patients. This difference between studies may be owing to sample size limitations of the other studies, and may also reflect differences in severity assessment; whereas the current study used an objective, validated, patient-reported measure to assess disease activity based on specific signs and symptoms, other studies asked patients to self-rate their AD severity. Lack of a standardized measure of AD severity increases the challenge of severity assessment in clinical settings,15,16 as exemplified by previously reported discordance, where one-third of physician- and patient-rated AD severity ratings were not in agreement.17,18
A novel aspect of this study was the exploration of patients’ perceptions of disease control among patients with moderate/severe disease for whom therapy has been escalated beyond topicals to include Im-tx or phototherapy. This group likely represents the more severe end of the spectrum given the escalation of therapy to include off-label use of systemic Im-tx medications, which lack a strong evidence base for long-term use.19,20,21 Despite use of these guideline-recommended therapies, the results suggest many of these patients do not experience good disease control. Not surprisingly, patients with inadequately controlled disease reported higher burdens relative to controlled patients, although not all of the differences were statistically significant, suggesting some burdens are similar to those of patients with uncontrolled disease. For example, patients with “controlled” AD still reported a high frequency of itch, sleep problems that interfere with daily activities, psychosocial issues, and impaired HRQoL. Thus, even so-called “controlled” disease still profoundly affects the lives of patients.
Atopic dermatitis is often considered a disease of childhood, yet 59.2% of patients reported diagnosis during adulthood, similar to a previous study that found that approximately 40% of patients were diagnosed as adults.6 However, age at diagnosis does not necessarily reflect age at onset, and some adults may not recall having AD as a child.22 Regardless of onset, patients were living with AD for more than half of their lives, supporting an associated long-term burden.23
Patients reported frequent, almost daily, itching. Itch drives the burden of AD because it is the primary complaint of patients,24 and affects sleep (the second most common complaint),24 as well as daily activities as indicated by the DLQI total score and its subscales.
In addition to itch, pain was also reported by patients. Although mean scores for pain were lower than for itch, the large standard deviations suggest wide variability in presence or perceptions of pain. Because use of pain medications was not specifically queried, the possibility of concomitant medications to manage pain cannot be excluded. Pain has rarely been evaluated in patients with AD, and this lack has been identified as an important gap in characterizing the burden of the disease.3 However, recent reports, including this study, highlight pain as an important dimension in moderate-to-severe AD,6,25 and support pain as a relevant outcome in determining AD-related treatment response.26 Future studies should assess use of pain medications in patients with AD, characterize different dimensions of AD-related pain, and explore associations of pain with other signs and symptoms of AD.
As previously reported in clinical trials and cross-sectional population-based studies,6,27,28 AD had profound effects on sleep, including sleep disturbances, longer sleep latency, and interference with function. These effects were greater in patients with moderate/severe AD and among those with inadequately controlled disease, and are consistent with the described relationship between itch and sleep.29,30 These results also support the association of sleep problems with fatigue and daily activities,28 with implications for greater work absenteeism and more clinician visits in addition to poorer overall health.27
The mental health burden was significantly higher among those with moderate/severe AD but only numerically higher in inadequately controlled relative to controlled AD, suggesting a similarly high psychosocial burden regardless of disease control. This burden appeared to be driven by the presence of anxiety, which was greater than depression, consistent with previous reports.6,8 These results emphasize the need for mental health assessment and support because suicidal ideation is also a feature associated with AD, especially among those with more severe disease.31,32,33
Results from the DLQI indicated greater HRQoL impairment at higher AD severity and when inadequately controlled. The DLQI subscales provide further indication of the substantial effects on patients’ daily lives, especially with regard to participatory activities, including preventing or interfering with school/work. In addition, high proportions of patients with moderate/severe and inadequately controlled AD reported being embarrassed/self-conscious, which may potentially exacerbate psychological distress through stigmatization.24,34
Limitations
In addition to potential recall bias because the data are based on patient self-report, this study may be subject to several different types of selection biases: patients who agreed to participate may have characteristics different from those who did not agree; a survey conducted through the internet may reflect a population and perspective different from a survey conducted by other methods (eg, phone); and the patients may not necessarily reflect community practice since they were recruited from academic medical centers. Although it is also possible that some patients did not have AD, studies have suggested that report of a physician diagnosis of AD through use of a targeted question designed to elicit such information (as used in this study) may accurately identify patients with the disease.35,36 The definition that was used to characterize disease control may also be considered a limitation because other definitions may provide different results. Finally, this study is cross-sectional and causal inferences cannot be made.
Conclusions
This study provides real-world evidence of the multidimensional patient burden present in adults with AD. Patients with moderate/severe AD reported a significantly greater burden than mild patients on all outcomes. Inadequate disease control among patients with moderate/severe AD despite treatment with Im-tx or phototherapy is high. These results highlight the need for more effective therapies to better control AD, and support the importance of incorporating the patient perspective into assessment of AD beyond using measures of disease activity. It should be noted that this study characterized the patient-reported burden before the introduction of the first biologic agent approved for the treatment of patients with moderate-to-severe AD.37
References
- 1.Ellis CN, Mancini AJ, Paller AS, Simpson EL, Eichenfield LF. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31(3)(suppl):S18-S22. doi: 10.1016/j.sder.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 2.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150(6):593-600. doi: 10.1001/jamadermatol.2013.10271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drucker AM, Wang AR, Qureshi AA. Research gaps in quality of life and economic burden of atopic dermatitis: the National Eczema Association Burden of Disease Audit. JAMA Dermatol. 2016;152(8):873-874. doi: 10.1001/jamadermatol.2016.1978 [DOI] [PubMed] [Google Scholar]
- 4.Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): A systematic review and meta-analysis. J Am Acad Dermatol. 2016;75(4):681-687.e11. doi: 10.1016/j.jaad.2016.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The World Allergy Organization (WAO) The World Allergy Organization (WAO) White Book on Allergy 2013 Update. 2013; http://www.worldallergy.org/UserFiles/file/WhiteBook2-2013-v8.pdf. Accessed June 1, 2018.
- 6.Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): Insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491-498. doi: 10.1016/j.jaad.2015.10.043 [DOI] [PubMed] [Google Scholar]
- 7.Eckert L, Gupta S, Amand C, Gadkari A, Mahajan S, Gelfand JM. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: An analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77(2):247-279. [DOI] [PubMed] [Google Scholar]
- 8.Whiteley J, Emir B, Seitzman R, Makinson G. The Burden of atopic dermatitis in U.S. adults: results from the 2013 National Health and Wellness Survey. Curr Med Res Opin. 2016;32(10):1645-1651. doi: 10.1080/03007995.2016.1195733 [DOI] [PubMed] [Google Scholar]
- 9.Dillman DA, Smyth JD, Christian LM. Internet, Phone, Mail, and Mixed-Mode Surveys: The Tailored Design Method. Hoboken, New Jersey. 4th ed Hoboken, NJ: John Wiley & Sons, Inc; 2014. [Google Scholar]
- 10.Stalder JF, Barbarot S, Wollenberg A, et al. ; PO-SCORAD Investigators Group . Patient-Oriented SCORAD (PO-SCORAD): a new self-assessment scale in atopic dermatitis validated in Europe. Allergy. 2011;66(8):1114-1121. doi: 10.1111/j.1398-9995.2011.02577.x [DOI] [PubMed] [Google Scholar]
- 11.Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007;157(4):645-648. doi: 10.1111/j.1365-2133.2007.08112.x [DOI] [PubMed] [Google Scholar]
- 12.Phan NQ, Blome C, Fritz F, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92(5):502-507. doi: 10.2340/00015555-1246 [DOI] [PubMed] [Google Scholar]
- 13.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 14.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210-216. doi: 10.1111/j.1365-2230.1994.tb01167.x [DOI] [PubMed] [Google Scholar]
- 15.Rehal B, Armstrong AW. Health outcome measures in atopic dermatitis: a systematic review of trends in disease severity and quality-of-life instruments 1985-2010. PLoS One. 2011;6(4):e17520. doi: 10.1371/journal.pone.0017520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Futamura M, Leshem YA, Thomas KS, Nankervis H, Williams HC, Simpson EL. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: Many options, no standards. J Am Acad Dermatol. 2016;74(2):288-294. doi: 10.1016/j.jaad.2015.09.062 [DOI] [PubMed] [Google Scholar]
- 17.Wei W, Anderson P, Gadkari A, et al. Discordance between physician- and patient-reported disease severity in adults with atopic dermatitis: A US cross-sectional survey. Am J Clin Dermatol. 2017;18(6):825-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fivenson D, Arnold RJ, Kaniecki DJ, Cohen JL, Frech F, Finlay AY. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8(5):333-342. [DOI] [PubMed] [Google Scholar]
- 19.Ring J, Alomar A, Bieber T, et al. ; European Dermatology Forum; European Academy of Dermatology and Venereology; European Task Force on Atopic Dermatitis; European Federation of Allergy; European Society of Pediatric Dermatology; Global Allergy and Asthma European Network . Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol. 2012;26(9):1176-1193. doi: 10.1111/j.1468-3083.2012.04636.x [DOI] [PubMed] [Google Scholar]
- 20.Sidbury R, Davis DM, Cohen DE, et al. ; American Academy of Dermatology . Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327-349. doi: 10.1016/j.jaad.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133(2):429-438. doi: 10.1016/j.jaci.2013.07.049 [DOI] [PubMed] [Google Scholar]
- 22.Hanifin JM. Adult-Onset Atopic Dermatitis: Fact or Fancy? Dermatol Clin. 2017;35(3):299-302. doi: 10.1016/j.det.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 23.Simpson E, Guttman-Yassky E, Margolis DJ, et al. Chronicity, Comorbidity and Life Course Impairment in Atopic Dermatitis: Insights from a Cross-Sectional Study in US Adults. Poster presented at the 25th European Academy of Dermatology and Venereology, September 28–October 2, 2016, Vienna, Austria. 2016. [Google Scholar]
- 24.Wittkowski A, Richards HL, Griffiths CE, Main CJ. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. J Psychosom Res. 2004;57(2):195-200. doi: 10.1016/S0022-3999(03)00572-5 [DOI] [PubMed] [Google Scholar]
- 25.Simpson EL. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther (Heidelb). 2017;7(2):243-248. doi: 10.1007/s13555-017-0181-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Kobyletzki LB, Thomas KS, Schmitt J, et al. What Factors are important to patients when assessing treatment response: an international cross-sectional survey. Acta Derm Venereol. 2017;97(1):86-90. doi: 10.2340/00015555-2480 [DOI] [PubMed] [Google Scholar]
- 27.Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135(1):56-66. doi: 10.1038/jid.2014.325 [DOI] [PubMed] [Google Scholar]
- 28.Yu SH, Attarian H, Zee P, Silverberg JI. Burden of Sleep and Fatigue in US Adults With Atopic Dermatitis. Dermatitis. 2016;27(2):50-58. doi: 10.1097/DER.0000000000000161 [DOI] [PubMed] [Google Scholar]
- 29.Bender BG, Ballard R, Canono B, Murphy JR, Leung DY. Disease severity, scratching, and sleep quality in patients with atopic dermatitis. J Am Acad Dermatol. 2008;58(3):415-420. doi: 10.1016/j.jaad.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 30.Bender BG, Leung SB, Leung DY. Actigraphy assessment of sleep disturbance in patients with atopic dermatitis: an objective life quality measure. J Allergy Clin Immunol. 2003;111(3):598-602. doi: 10.1067/mai.2003.174 [DOI] [PubMed] [Google Scholar]
- 31.Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135(4):984-991. doi: 10.1038/jid.2014.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieris-Hirche J, Gieler U, Kupfer JP, Milch WE. [Suicidal ideation, anxiety and depression in adult patients with atopic dermatitis]. Hautarzt. 2009;60(8):641-646. doi: 10.1007/s00105-009-1744-y [DOI] [PubMed] [Google Scholar]
- 33.Kimata H. Prevalence of suicidal ideation in patients with atopic dermatitis. Suicide Life Threat Behav. 2006;36(1):120-124. doi: 10.1521/suli.2006.36.1.120 [DOI] [PubMed] [Google Scholar]
- 34.Zuberbier T, Orlow SJ, Paller AS, et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol. 2006;118(1):226-232. doi: 10.1016/j.jaci.2006.02.031 [DOI] [PubMed] [Google Scholar]
- 35.Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43(4):649-655. doi: 10.1067/mjd.2000.107773 [DOI] [PubMed] [Google Scholar]
- 36.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131(1):67-73. doi: 10.1038/jid.2010.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dupixent [dupilumab injection for subcutaneous use] prescribing information. Regeneron Pharmaceuticals Inc., Tarrytown, NY; sanofi-aventis U.S. LLC, Bridgewater, NJ. 2017. https://www.regeneron.com/sites/default/files/Dupixent_FPI.pdf. Accessed April 26, 2018.