Key Points
Question
What is the rate of recurrence of lentigo maligna (LM) in patients treated with neoadjuvant imiquimod, 5%, cream prior to conservatively staged excisions?
Findings
In this medical record review, 334 patients with 345 LM tumors were treated with off-label imiquimod for a mean of 2.5 months prior to conservatively staged excisions. The recurrence rate was 3.9% with a mean time to recurrence of 4.3 years and a mean follow-up of 5.5 years.
Meaning
Eighty-one percent of patients treated with imiquimod prior to conservatively staged excisions had clearance of LM after 1 stage with recurrence rates comparable with other excisional techniques.
Abstract
Importance
Staged excision of lentigo maligna (LM) often requires multiple stages and can result in significant cosmetic morbidity. Imiquimod cream has been used off-label as monotherapy in the treatment of LM and may be used in the neoadjuvant setting prior to staged excision as a strategy to reduce the size of the surgical margins required to confirm negative histologic margins.
Objective
To examine the rate of recurrence of LM in patients treated with neoadjuvant topical imiquimod, 5%, cream prior to conservatively staged excisions.
Design, Setting, and Participants
This was a retrospective medical record review of 334 patients with 345 biopsy-confirmed LM tumors from June 2004 to January 2012 who were treated with imiquimod prior to undergoing staged excisions at the University of Utah Medical Center and Huntsman Cancer Institute, large academic hospitals in Salt Lake City.
Interventions
Patients were treated with off-label imiquimod, 5%, cream 5 nights per week for 2 to 3 months. Those deemed to have an inadequate inflammatory response were also treated with tazarotene, 0.1%, gel twice weekly. Conservatively staged excisions, beginning with 2-mm margins, were then performed.
Main Outcomes and Measures
The rate of recurrence of LM after long-term follow-up.
Results
Patients included 235 men (70%) and 99 women (30%) with a mean (SD) age of 67 (13) years. Patients were treated with imiquimod cream for a mean of 2.5 months prior to undergoing conservatively staged excisions. There were 12 local recurrences (a rate of 3.9%) with a mean time to recurrence of 4.3 years and a mean length of follow-up of 5.5 years.
Conclusions and Relevance
Neoadjuvant topical imiquimod, 5%, cream prior to conservatively staged excisions for LM allowed for negative histologic margins with a median final margin of 2 mm and a rate of recurrence similar to reported recurrence rates with standard staged excisions by either Mohs surgery or en face permanent sections.
This medical record review examines the rate of recurrence of lentigo maligna in patients treated with neoadjuvant topical imiquimod, 5%, cream prior to conservatively staged excisions.
Introduction
Lentigo maligna (LM) is a subtype of melanoma in situ commonly found on the head and neck in patients who are relatively older compared with those with other melanoma subtypes. Although LM is characterized by a prolonged radial growth phase, there is a risk of progression to lentigo maligna melanoma (LMM). There is a wide range of estimates regarding the risk of progression of LM to LMM, ranging from 5% to 50%.1,2,3 In a previous study at our institution, 16% of LM lesions without clinical signs to suggest invasion were found to harbor a nidus of invasion when excised and were consequently upstaged to LMM.4 When adjusted for Breslow thickness, LMM is as likely to metastasize as other melanoma subtypes; therefore, the treatment of LM must take into account the potential risk of progression to LMM.
In the United States, there is a predilection to treat LM surgically, whereas radiotherapy is more commonly used as primary therapy for LM in Europe, Australia, and New Zealand. With regard to surgery, it is not uncommon to encounter residual tumor cells with the standard 5-mm margins recommended by a 1992 consensus statement from the National Institutes of Health,5,6 and published rates of recurrence of LM following wide local excision (WLE) range from 8% to 20%.7 Most academic institutions in the United States approach LM with a staged surgical technique, such as Mohs micrographic surgery with intraoperative horizontal frozen sections, “square” or “polygonal” excision with permanent sections cut en face, or radial sections with either frozen or permanent sections.8 Many staged techniques using frozen sections are augmented with immunohistochemical stains, such as Mart-1/Melan-A or SOX10, since freezing leads to bloating and other artifactual changes in the melanocytes that make the histologic interpretation of cellular atypia extremely difficult. While the staged surgical approaches have reportedly lower rates of local recurrence (0%-9.7%),7 multiple stages are often required, with an average requirement of 7.1-mm margins to confirm negative histologic results.9 These large surgical margins are associated with substantial cosmetic penalty given that most tumors occur on the head and neck.
Imiquimod is a nucleoside analogue of the imidazoquinoline family with antitumoral activity, the mechanism of which has been proposed to involve 3 pathways.10 The predominant pathway is thought to be agonistic activity for Toll-like receptors 7 and 8, resulting in activation of nuclear factor–κB and the production of proinflammatory cytokines. Imiquimod may also interfere with adenosine receptor pathways, increasing adenylyl cyclase activity. Finally, it may have proapoptotic activity against tumor cells. Imiquimod, 5%, cream has been used in the off-label treatment of LM, both as a primary agent11,12 as well as adjuvant therapy after WLE with positive or narrow (<1 mm) peripheral margins.12 When used as a primary agent, imiquimod has been reported to leave residual tumor cells in 25%13 to 53% of cases12; therefore, caution must be taken when using imiquimod as monotherapy in the treatment of LM.
It is our practice to use imiquimod in the neoadjuvant setting prior to staged excision in an attempt to reduce the size of the required surgical margins to confirm negative histologic findings. We previously described a cohort of 40 patients who were treated with topical imiquimod followed by conservatively staged excisions beginning with 2-mm margins.13 In that study, there were no recurrences after a mean follow-up of 1.5 years. We have also published the results of a randomized prospective trial of neoadjuvant topical imiquimod with or without tazarotene prior to conservatively staged excisions. In that study, there were no recurrences after a mean follow-up of 3.5 years.14 Herein, we present a retrospective review of the rate of recurrence in a larger cohort of patients pretreated with neoadjuvant topical imiquimod prior to conservatively staged excisions.
Methods
After approval by the University of Utah’s institutional review board, a retrospective medical record review was conducted of patients treated with neoadjuvant imiquimod prior to undergoing conservatively staged excisions of LM from June 2004 to January 2012.
As mentioned herein, a previous study at our institution showed that 16% of LM cases referred to us for treatment were actually LMM.4 This same percentage was reported by Hazan et al.9 Because of these cases of unsuspected invasion, excisional biopsies were performed on any LM lesions with clinical signs of residual tumor, when feasible, to verify that the tumors were truly melanoma in situ. If an invasive component was observed, the patient was not included in this study protocol and was instead treated with a staged excision starting with a 1-cm margin to the depth of the fascia as recommended by guidelines published by the National Comprehensive Cancer Network (NCCN).15 A Wood’s lamp was used to identify preoperative tumor margins as accurately as possible, and the tumor outline was traced on a piece of transparent plastic to make a template of the original tumor perimeter. A permanent tattoo was placed in the center of the biopsy site to aid the patient in applying the topical cream to the right location and to enable centering of the template at the time of surgery. Intention to treat with imiquimod, 5%, cream was 5 nights a week for 2 to 3 months. Patients were instructed to treat an area extending at least 2 cm beyond the outlined tumor margins. Patients were monitored monthly to assess the inflammatory response as well as to rule out any signs of recurrent tumor. The frequency of application was decreased if grade 3 inflammation was observed (grade 0, no inflammation; grade 1, pink; grade 2, red with no surface erosion; and grade 3, red with surface erosion). If the inflammatory response was deemed inadequate (grade 0) on follow-up examination, twice-weekly tazarotene, 0.1%, gel was added to the regimen in an effort to improve drug penetration.
After completing the topical therapy, a recuperation period of at least 2 months followed to allow for resolution of the inflammation. At the time of surgery, the original tumor outline was retraced on the skin using the template, which was centered on the tattoo. A 2-mm margin was then inscribed around the tumor outline to completely circumscribe any remnant from the biopsy scar. The tissue was processed by using frozen sections, which were cut radially16 and stained with routine hematoxylin-eosin augmented with a MART-1 immunostain, which tags the cytoplasm of the melanocytes. We prefer radial sections because if there is any residual LM in the center of the specimen, the clinician can appreciate the diminution of melanocyte density from the center radiating out toward the perimeter surgical margin.16 In some patients, a 10-mm–long fusiform sample of clinically uninvolved sun-damaged skin was obtained as a negative control, which allows for comparative melanocyte density counts (ie, if the melanocyte density of the LM treatment site is less than or equal to the melanocyte density of the negative control, we consider the surgical margin to be negative).17 For tumors that were not cleared on the first stage, subsequent stages were taken with 4-mm margins with the specimen processed en face. Follow-up times were calculated from the date of surgery to the date of recurrence or the most recent clinical visit with a dermatologist, mid-level dermatology professional, or surgical oncologist specifically seeing the patient for their history of LM. Visits to physicians for reasons other than the specific LM history were not considered legitimate follow-up to rule out LM recurrence. Recurrence was determined by biopsy.
Recurrence rates and pointwise confidence bands were estimated using the Kaplan-Meier product limit approach. To analyze any difference between patients who did and did not receive tazarotene, we excluded 74 patients who had taken part in a previously published study14 because they had been randomized to tazarotene treatment and thus may have received tazarotene regardless of their inflammatory response, and vice versa. Comparison of recurrence rates between the tazarotene and nontazaratene groups was done using the log-rank test. Logistic regression was used to examine potential predictors (sex, age, and number of stages) of nonresponse to imiquimod. All statistical analyses were performed using Stata software (version 14; StataCorp).
Results
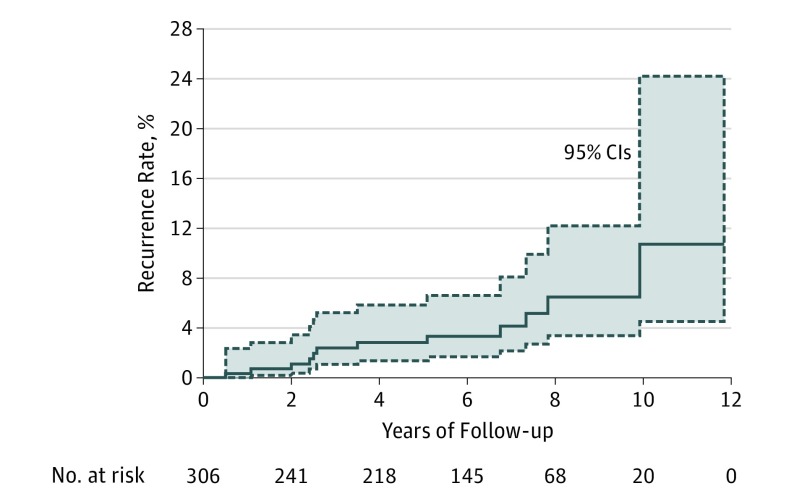
Three hundred thirty-four patients had 345 LM tumors treated with neoadjuvant topical imiquimod for a mean of 2.5 months prior to undergoing conservatively staged excisions with 2-mm margins (Table). Patients included 235 men (70%) and 99 women (30%) with a mean (SD) age of 67 (13) years. A mean (SD) of 1.2 (0.5) stages were required to clear the tumor with a mean (SD) margin of 3.5 (2.9) mm (median, 2.0 mm). A negative control biopsy specimen was taken in 34% of cases (119). Residual LM was present in 18% of specimens (51) cleared with 1 stage; 67% of whom (32 patients) had been noted to have a complete response to imiquimod clinically prior to staged excision. Thirty-seven patients (39 tumors) were lost to follow-up, and 7 of them died. The mean (SD) length of follow-up for the remaining 297 patients (306 tumors) was 5.5 (3.1) years (median, 5.7 years). There was a recurrence rate of 3.9% (Figure 1) with a mean time to recurrence of 4.3 (2.9) years (median, 3.1 years). Figure 1 shows the rate of recurrence over time. Two of the recurrences were invasive melanoma (mean thickness, 0.83 mm). One patient was in his 70s with an LM on the ear treated with imiquimod for 2 months prior to staged excision requiring 2 stages for tumor clearance. He had a recurrence of a 0.35-mm-thick LMM 3 years after the initial surgery. The other patient was a patient in his 70s with an LM on the scalp treated with imiquimod for 3 months and tazarotene for 1 month prior to staged excision required 1 stage for tumor clearance. He had a recurrence of a 1.30-mm-thick LMM 10 years after the initial surgery.
Table. Patient Demographics, Tumor and Treatment Variables, and Local Recurrence.
| Variable | Total No. Patients (n = 334 and 345 Tumors) |
Lost to Follow-up (n = 37 Patients and 39 Tumors) | Recurrence | |
|---|---|---|---|---|
| None (n = 285 Patients and 294 Tumors) | Local (n = 12 Patients and Tumors) | |||
| Demographics | ||||
| Age, mean (SD), y | 67 (13) | 68 (11) | 66 (13) | 70 (10) |
| Male, No. (%) | 235 (70.4) | 22 (59.5) | 204 (71.6) | 9 (75.0) |
| White, No. (%)a | 255 (99) | 24 (100) | 223 (99) | 8 (100) |
| Tumor and Treatment Variables | ||||
| Classification | ||||
| Primary | 340 | 38 | 291 | 11 |
| Recurrent | 5 | 1 | 3 | 1 |
| Tumor site | ||||
| Ear | 39 | 6 | 31 | 2 |
| Lip | 9 | NA | 9 | NA |
| Nose | 52 | 3 | 49 | NA |
| Periocular | 5 | 1 | 4 | NA |
| Other face | 206 | 26 | 173 | 7 |
| Scalp | 16 | 1 | 14 | 1 |
| Neck, trunk, hand/upper extremity | 18 | 2 | 14 | 2 |
| Length of imiquimod therapy, mean (SD), mo | 2.5 (0.5) | 2.5 (0.5) | 2.5 (0.5) | 2.6 (0.5) |
| Length of tazarotene therapy, mean (SD), mo | 2.0 (0.9) | 1.9 (0.9) | 2.0 (0.9) | 2.1 (0.9) |
| Final margin, mean (SD), mm | 3.5 (2.9) | 3.5 (2.9) | 3.4 (2.9) | 4.5 (4.0) |
| Median | 2.0 | 3.0 | 2.0 | 2.5 |
| Stages, mean (SD) | 1.2 (0.5) | 1.1 (0.3) | 1.2 (0.5) | 1.6 (0.6) |
Abbreviation: NA, not applicable.
Ethnicity not known for all patients. There were 2 Hispanic patients in the cohort.
Figure 1. Rate of Local Recurrence After Neoadjuvant Imiquimod Prior to Staged Excision.
Solid line indicates the cumulative incidence; dashed lines, 95% CIs.
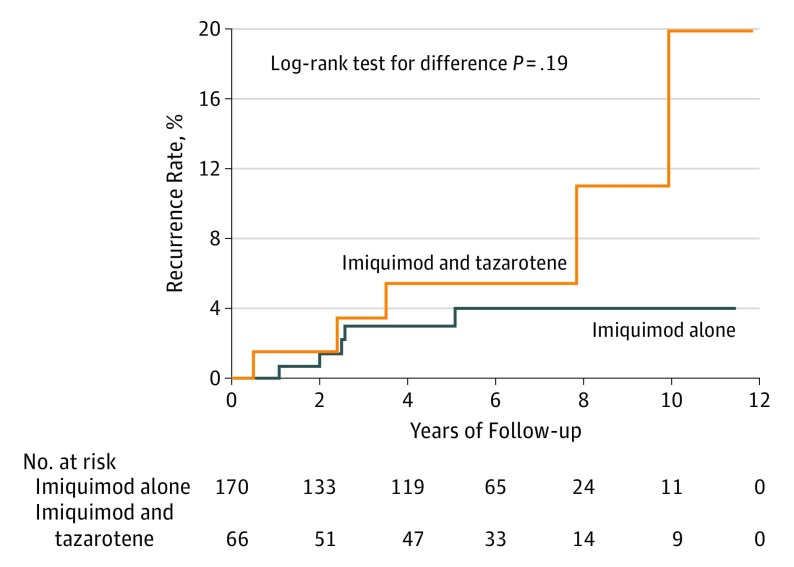
After removing patients who were included in the previously published study,14 74 patients (with 75 tumors) in the cohort used tazarotene in combination with imiquimod, while 188 patients (with 196 tumors) used imiquimod alone. There was not a significant difference in the age, number of stages, or final margins between the 2 groups. Nine patients (9 tumors) in the combination group and 24 patients (26 tumors) in the imiquimod-alone group were lost to follow-up. The mean (SD) length of follow-up for the remaining patients was 5.6 (3.4) years (median, 6.0 years) in the combination group and 5.1 (2.9) years (median, 5.4 years) in the imiquimod-alone group. In the combination group, the rate of recurrence was 7.6% compared with 2.9% in the imiquimod-alone group. The mean time to recurrence in the combination group was 4.8 (3.9) years (median, 3.5 years), and 2.7 (1.5) years (median, 2.5 years) in the single-agent group. The difference between the rate of recurrence between these 2 groups is shown was not significant (P = .19) (Figure 2).
Figure 2. Comparison of Rate of Local Recurrence Between Patients Who Did and Did Not Use Tazarotene in Addition to Imiquimod Prior to Staged Excision.
Discussion
Several studies have been published regarding recommended surgical margins for the treatment of LM with WLE.9,18 The latest version of the NCCN guidelines states that a margin greater than 5 mm may be necessary to achieve tumor clearance.15 Hazan et al9 reported that the mean surgical margin required for excision of LM was 7.1 mm. This corroborates findings from another study in which a 5-mm margin successfully cleared LM in only 65% of tumors; a 15-mm margin was required to clear 97% of tumors.19 These findings may help to explain the 8% to 20% recurrence rate of LM after standard WLE.7 Fortunately, the rate of recurrence after staged excisions is lower (0%-9.7%), but these surgical procedures are often complicated by comparatively large surgical defects in cosmetically sensitive areas.7
In our cohort of patients, the use of neoadjuvant topical imiquimod, 5%, cream prior to conservatively staged excisions for LM was associated with mean and median surgical margin requirements of 3.5 mm and 2.0 mm, respectively, and a recurrence rate of 3.9% with a mean time to recurrence of 4.3 years, and a mean follow-up period of 5.5 years. In 1 patient, a margin of 31.0 mm was required to confirm negative histologic margins, but this was 10 times the standard deviation for the cohort. The recurrence rate in our cohort was similar to that of Connolly et al,20 who reported a recurrence rate of 4% with a mean follow-up of 5 years. Those authors performed staged excisions with initial 5-mm margins and used permanent sections with radial sectioning.9 In their cohort, the mean surgical margin requirements were 7.1 mm for LM and a mean time to recurrence of 5.9 years.20 In a more recent study by Moyer et al,21 a local recurrence rate of 1.8% was reported for melanoma in situ with a median follow-up of 9.3 years. Those authors performed staged excisions with initial 5-mm margins and a square technique with en face permanent sections. In their cohort, a mean margin of 9.3 mm was required for tumor clearance, and predicted margins required for clearance increased with larger primary lesions size (ie, 7-mm margins for a 50-mm2 lesion compared with 8-mm margins for a 100-mm2 lesions).21
Eighty-one percent of patients in our cohort had clear margins after 1 stage. In 18% of these patients, there was residual tumor with an adequate surrounding margin. There are 2 possible explanations for the 82% of cases in which there was no residual LM. First, excisional biopsies were performed on LM lesions with clinical signs of residual tumor, which may have been effective in clearing the tumor. Second, imiquimod may have been effective in tumor clearance, as past reports have stated that 47% to 75% of tumors clear when imiquimod is used as monotherapy.12,13 While the absence of tumor in 67% of patients may be interpreted as a large proportion of patients undergoing unnecessary surgery, 67% of patients who had experienced residual tumor clearance on the first stage did not have clinically evident tumor on physical examination the day of excision. Thus, it may not be possible to make the decision of tumor clearance without tissue sampling.
Overall, these results support our opinion that neoadjuvant topical imiquimod allows for the confirmation of a negative histologic margin for LM, with a smaller mean surgical margin requirement with consequential lessened cosmetic penalty associated with the surgery. The local recurrence rate with our conservatively surgical margins is similar to that in patients treated with staged excisions without neoadjuvant topical imiquimod, indicating that our approach does not put patients at increased risk of recurrence compared with standard practice. An interesting observation within the data set is the higher rate of recurrence seen in the group who had an inadequate inflammatory response to imiquimod alone and thus were given tazarotene. While this difference was not statistically significant, one might speculate that the induction of inflammation by itself (ie, induced by the addition of tazarotene gel) is insufficient to treat LM; rather the quality of the inflammatory response induced by imiquimod likely holds the key to therapeutic efficacy.
Limitations
This study was conducted at a single institution with 2 Mohs surgeons performing the procedures and reading the pathological results (M.L.H. and G.M.B). Continued follow-up is necessary given that some recurrences occur after the mean follow-up of 5.5 years in this study.
Conclusions
The off-label use of neoadjuvant topical imiquimod, 5%, cream prior to conservatively staged excision allows for confirmation of negative histologic surgical margins with a median margin requirement of 2 mm with a local rate of recurrence comparable with those published from patient cohorts treated with varying techniques of staged excisions.
References
- 1.Tsao H, Sober AJ. Cutaneous Melanoma. 4th ed St Louis, MO: Quality Medical Publishing, Inc; 2003. [Google Scholar]
- 2.Wayte DM, Helwig EB. Melanotic freckle of Hutchinson. Cancer. 1968;21(5):893-911. [DOI] [PubMed] [Google Scholar]
- 3.Bax MJ, Johnson TM, Harms PW, et al. . Detection of occult invasion in melanoma in situ. JAMA Dermatol. 2016;152(11):1201-1208. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47(5):743-748. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health Consensus Development Conference Statement on Diagnosis and Treatment of Early Melanoma, January 27-29, 1992. Am J Dermatopathol. 1993;15(1):34-43. [DOI] [PubMed] [Google Scholar]
- 6.Felton S, Taylor RS, Srivastava D. Excision margins for melanoma in situ on the head and neck. Dermatol Surg. 2016;42(3):327-334. [DOI] [PubMed] [Google Scholar]
- 7.McLeod M, Choudhary S, Giannakakis G, Nouri K. Surgical treatments for lentigo maligna: a review. Dermatol Surg. 2011;37(9):1210-1228. [DOI] [PubMed] [Google Scholar]
- 8.Bowen GM, Bowen AR, Florell SR. Lentigo maligna: one size does not fit all. Arch Dermatol. 2011;147(10):1211-1213. [DOI] [PubMed] [Google Scholar]
- 9.Hazan C, Dusza SW, Delgado R, Busam KJ, Halpern AC, Nehal KS. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58(1):142-148. [DOI] [PubMed] [Google Scholar]
- 10.Schon MP, Schon M. Imiquimod: mode of action. Br J Dermatol. 2007;157(suppl 2):8-13. doi: 10.1111/j.1365-2133.2007.08265.x [DOI] [PubMed] [Google Scholar]
- 11.Kai AC, Richards T, Coleman A, Mallipeddi R, Barlow R, Craythorne EE. Five-year recurrence rate of lentigo maligna after treatment with imiquimod. Br J Dermatol. 2016;174(1):165-168. [DOI] [PubMed] [Google Scholar]
- 12.Swetter SM, Chen FW, Kim DD, Egbert BM. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72(6):1047-1053. [DOI] [PubMed] [Google Scholar]
- 13.Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34(2):147-151. [DOI] [PubMed] [Google Scholar]
- 14.Hyde MA, Hadley ML, Tristani-Firouzi P, Goldgar D, Bowen GM. A randomized trial of the off-label use of imiquimod, 5%, cream with vs without tazarotene, 0.1%, gel for the treatment of lentigo maligna, followed by conservative staged excisions. Arch Dermatol. 2012;148(5):592-596. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Melanoma. Version 2.2018. February 21, 2018. https://www.nccn.org/professionals/physician_gls/pdf/melanoma_blocks.pdf. Accessed March 3, 2017.
- 16.Bub JL, Berg D, Slee A, Odland PB. Management of lentigo maligna and lentigo maligna melanoma with staged excision: a 5-year follow-up. Arch Dermatol. 2004;140(5):552-558. [DOI] [PubMed] [Google Scholar]
- 17.Bowen AR, Thacker BN, Goldgar DE, Bowen GM. Immunohistochemical staining with melan-A of uninvolved sun-damaged skin shows features characteristic of lentigo maligna. Dermatol Surg. 2011;37(5):657-663. [DOI] [PubMed] [Google Scholar]
- 18.Kunishige JH, Brodland DG, Zitelli JA. Surgical margins for melanoma in situ: when 5-mm margins are really 9 mm. J Am Acad Dermatol. 2015;72(4):745. [DOI] [PubMed] [Google Scholar]
- 19.McKenna JK, Florell SR, Goldman GD, Bowen GM. Lentigo maligna/lentigo maligna melanoma: current state of diagnosis and treatment. Dermatol Surg. 2006;32(4):493-504. [DOI] [PubMed] [Google Scholar]
- 20.Connolly KL, Nijhawan RI, Dusza SW, Busam KJ, Nehal KS. Time to local recurrence of lentigo maligna: Implications for future studies. J Am Acad Dermatol. 2016;74(6):1247-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moyer JS, Rudy S, Boonstra PS, et al. . Efficacy of staged excision with permanent section margin control for cutaneous head and neck melanoma. JAMA Dermatol. 2017;153(3):282-288. [DOI] [PubMed] [Google Scholar]