Key Points
Question
Is human cutaneous leishmaniasis endemic in North America?
Findings
This cross-sectional observational study identified 41 novel cases of endemic cutaneous leishmaniasis occurring in humans since 2007, mostly in Texas. Endemic cases represented 59% of all cases identified.
Meaning
Human cutaneous leishmaniasis appears to be endemic in the United States and is acquired endemically more frequently than it is via travel, which argues in favor of making it a federally reportable disease.
This observational study reviews cases of endemic human leishmaniasis occurring in the United States, mostly in Texas, over a 10-year period.
Abstract
Importance
Leishmaniasis is recognized as an endemic human disease in Africa, the Middle East, Asia, and South America. Yet despite case reports of endemic human leishmaniasis in the United States, and well-documented occurrences of disease in various animal vectors and reservoirs, the endemicity of leishmaniasis in North America has not yet been established. Moreover, leishmaniasis is not a federally reportable disease in the United States. Clinical awareness of endemic disease therefore remains low, with North American physicians considering leishmaniasis a tropical disease.
Objective
To assess the endemicity of human leishmaniasis in the United States.
Design, Setting, and Participants
This cross-sectional multicenter observational study reviewed cases of human leishmaniasis occurring in the United States from 2007 through 2017. Previously diagnosed, deidentified cases of leishmaniasis were reported by the institutions of the authors and acknowledged contributors, as well as the Texas Department of State Health Services. Cases of leishmaniasis were identified by searching by disease name (leishmaniasis) or International Classification of Diseases, 9th and 10th Revisions diagnosis codes in the respective laboratory information systems.
Exposures
Via examination of deidentified demographics, cases of leishmaniasis were classified as one of the following: (1) documentation of no history of travel outside of the United States within 10 years; (2) positive history of travel outside of the United States within 10 years; or (3) unknown or no documentation of travel history.
Main Outcomes and Measures
Cases of leishmaniasis were considered endemic if identified in patients with documentation of no travel history outside of the United States within 10 years.
Results
Of the 69 novel cases of human cutaneous leishmaniasis identified in this study, 41 (59%) were endemic; the median age at diagnosis was 61 years (range, 3-89 years), and 28 (68%) of the endemic cases occurred in female patients. Twenty-two (32%) cases had documentation of Leishmania speciation performed by polymerase chain reaction, and in 100% of these cases the infectious organism was identified as Leishmania mexicana.
Conclusions and Relevance
Human cutaneous leishmaniasis is endemic in the United States, and, at least regionally, is acquired endemically more frequently than it is via travel. Our data argue in favor of making leishmaniasis a federally reportable disease and may have substantial implications on North American public health initiatives, with climate models predicting the number of citizens exposed to leishmaniasis will double by 2080.
Introduction
Leishmaniasis is a chronic disease caused by obligate intracellular protozoan parasites. There are more than 20 pathogenic species of the Leishmania parasite worldwide. The organisms are transmitted by approximately 70 species of hematophagous sand flies in the Phlebotomus and Lutzomyia genera.1 In the New World (Western hemisphere), Lutzomyia sand flies feed on and transmit Leishmania between various mammalian reservoirs, including wood rats, cotton rats, other rodents, opossums, and armadillos.2,3,4,5,6,7,8 In the United States, leishmaniasis has also been documented in domesticated cats and dogs (Table 1).2,3,4,5,6,7,8,9,10,11,12
Table 1. Leishmania Disease Vectors and Reservoirs in the United States2,3,4,5,6,7,8,9,10,11,12.
| Species | Vectors | Reservoirs |
|---|---|---|
| Leishmania mexicana |
|
|
| Leishmania infantum | NA | C lupus familiaris (foxhound) |
Leishmania species are classified as Old World or New World depending on the geographic region in which they primarily occur. Leishmania speciation helps to define the clinical subtype of disease (eTable in the Supplement)1,11,13 and, therefore, expected severity, prognosis, and required treatment. In humans, leishmaniasis comprises a spectrum of disease with 3 major clinical subtypes: cutaneous, mucocutaneous, and visceral. Cutaneous leishmaniasis is the most common form of the disease worldwide. Mucocutaneous leishmaniasis is less common, occurring almost exclusively in the New World (Central America and South America).13 Visceral disease, also known as kala-azar, is the most severe and persistent type of leishmaniasis and occurs primarily in the Old World.14
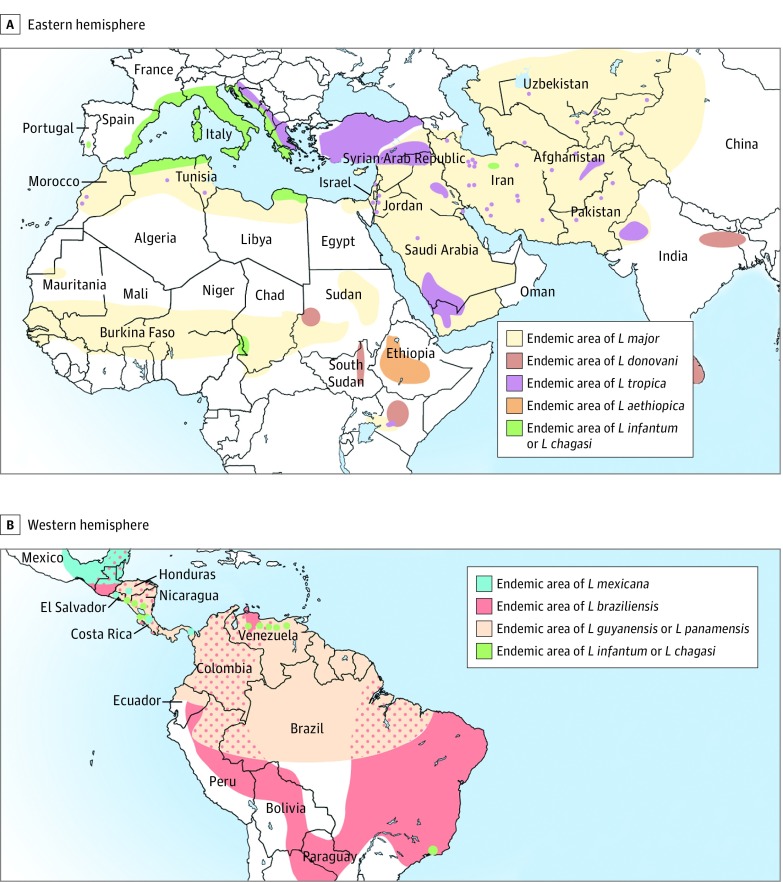
In the United States, leishmaniasis is widely considered a disease of travelers. Numerous American health authorities echo similar statements: “The cases of leishmaniasis evaluated in the United States reflect travel and immigration patterns.… Almost all of the cases of leishmaniasis diagnosed in the United States are in people who became infected while traveling or living in other countries.”15 Medical literature and the most current editions of infectious disease, tropical medicine, dermatology, and other medical textbooks present leishmaniasis as a uniquely tropical disease.1,13,14,16,17,18,19,20,21,22,23,24 Published maps highlighting endemic regions of cutaneous leishmaniasis conspicuously exclude the United States (Figure 1).13,14,18,19,25
Figure 1. Published Maps of the Geographic Distribution of Cutaneous Leishmaniasis.
Maps published in 2016 and 2017 show the geographic distribution of endemic cutaneous leishmaniasis as defined by the Infectious Diseases Society of America and the American Society of Tropical Medicine and Hygiene. Note that the United States is not included on these maps.14,18,19 L indicates Leishmania genus. Reproduced with permission from the American Journal of Tropical Medicine and Hygiene.19
The current American medical view of cutaneous leishmaniasis fails to take into account veterinary and environmental literature that has repeatedly demonstrated American endemicity of leishmaniasis,10,26 with climate change spurring habitat expansion of both disease vectors and reservoirs.2,27,28,29,30 In fact, leishmaniasis has been so well documented in various American animal species that zoologists and parasitologists accept North American endemicity of leishmaniasis as a universal reality (email communication, Nicole Evert, MS, Texas Department of State Health Services [DSHS], 2015). However, this view of leishmaniasis endemicity is not reflected in human medical literature, nor is it espoused by American medical authorities. Despite the World Health Organization classifying the United States as a leishmaniasis-endemic country in 2015, leishmaniasis still is not a federally reportable disease.31
It is therefore not surprising that US physicians often consider a diagnosis of leishmaniasis only in returning vacationers, military service members, or immigrants from endemic regions. Yet over the past century, the incidence of autochthonous cases of cutaneous leishmaniasis in the United States has grown alongside vector and reservoir habitats, with more than 40 human cases reported in the literature since 1903.2,27,32 In Texas, leishmaniasis has become so prevalent that it is now reportable to the Texas DSHS. In the absence of nationally reported data, however, assessment of leishmaniasis incidence and prevalence across the rest of the United States is speculative at best.
The aim of this study is to evaluate the incidence of leishmaniasis in the United States over a 10-year period, with a focus on the relative frequency of autochthonous vs travel-acquired cases.
Methods
Institutional review board exemption for this review of previously collected, deidentified demographic data was obtained from the University of North Texas Health Science Center in December 2014 and the Texas DSHS in June 2016. The Texas DSHS provided the deidentified demographics of all cases of leishmaniasis formally reported to it from 2007—when reporting became mandatory in Texas—through 2017. Cases of leishmaniasis reported by the laboratories of the authors and acknowledged contributors were diagnosed histopathologically by board-certified dermatopathologists from January 2007 through September 2017. Laboratory cases were identified within each laboratory by searching disease name (leishmaniasis) or International Classification of Diseases, 9th and 10th Revisions diagnostic codes in the respective laboratory information systems. The source laboratories accept specimens from varied medical subspecialties, including dermatology, primary care, surgery, podiatry, and dentistry.
Deidentified demographics of all leishmaniasis cases were reviewed. The demographics collected for each case included the following, if available: patient age at diagnosis, sex, race, county of residence, date of leishmaniasis diagnosis, anatomic location of sampled lesion, relevant exposures, treatments undertaken, Leishmania speciation, whether disease was contracted within or outside of the country, and travel history outside of Texas and/or the United States. Recorded travel history for each case was classified as either documentation of no history of travel outside of the United States within the past 10 years, positive history of travel outside of the United States within the past 10 years, or unknown or no documentation of travel history.
Results
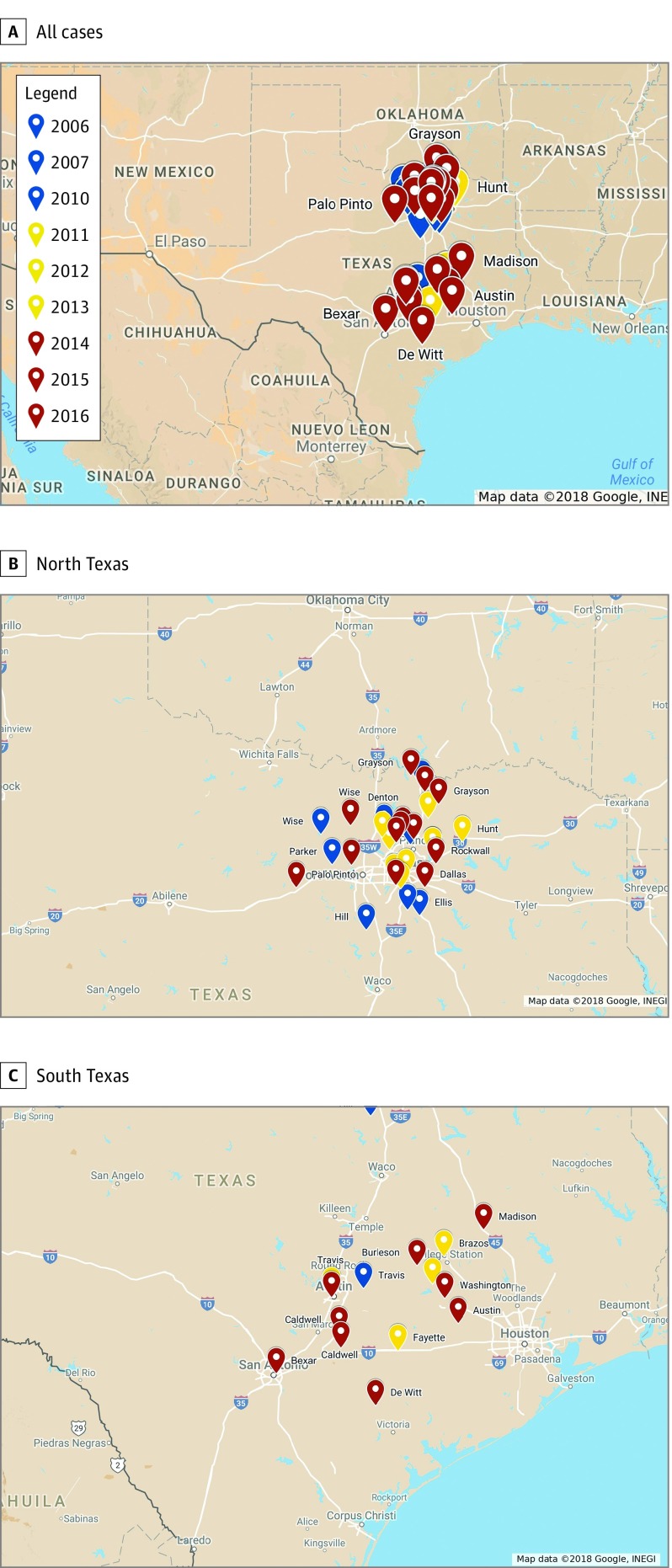
Collection of histopathologically confirmed cases from defined data sources resulted in the identification of 75 total cases of leishmaniasis. Of these, 6 cases matched the demographics of those previously reported in the medical literature and were excluded from further analysis.27,33 Of the remaining 69 novel cases of leishmaniasis identified, 41 (59%) were autochthonous, occurring in patients with documentation of no history of travel outside of the United States (Figure 2). The median age at diagnosis was 61 years (range, 3-89 years), and 28 (68%) of the endemic cases occurred in female patients. Cases of endemic leishmaniasis showed a predilection for areas of skin that are commonly exposed, with 20 (49%) cases diagnosed on the face, head, or neck, and 19 (46%) diagnosed on the upper extremities (Table 2). All identified cases of endemic leishmaniasis were from Texas. Of these cases, only 14 (20%) were reported to the Texas DSHS as required by law. Twenty-two (32%) cases had documentation of Leishmania speciation performed by polymerase chain reaction, and in 100% of these cases the infectious organism was Leishmania mexicana (Figure 3).
Figure 2. Previously Unreported Cases of Endemic Cutaneous Leishmaniasis Diagnosed in Humans From 2006 Through 2017.
Cases of endemic human cutaneous leishmaniasis are coded by year of diagnosis and labeled with Texas county of diagnosis. Copyright: Google, Instituto Nacional de Estadística y Geografía.
Table 2. Autochthonous Cases of Leishmaniasis Identified, 2006-2017.
| Case No./Sex | Ethnicity | Patient County in Texas | Year of Diagnosis | Anatomic Location | Exposures | Leishmania (L) Speciation |
|---|---|---|---|---|---|---|
| 1/M | NR | Wise | 2006 | R abdomen | NR | NR |
| 2/Fa | White | Collin | 2006 | Nose | NR | NR |
| 3/F | White | Hill | 2007 | Upper arm | NR | L mexicana |
| 4/F | NR | Travis | 2007 | Chin, neck | Rabbits, insect bites | NR |
| 5/Fa | White | Denton | 2007 | Face | None, maybe bites | L mexicana |
| 6/Ma | White | Ellis | 2007 | L back | NR | NR |
| 7/Fa | NR | Ellis | 2007 | R upper chest | NR | L mexicana |
| 8/F | White | Grayson | 2007 | Forearm, wrist | NR | L mexicana |
| 9/M | NR | Parker | 2010 | L wrist | NR | NR |
| 10/F | White | Dallas | 2011 | Face, L elbow, buttock | NR | L mexicana |
| 11/F | White | Rockwall | 2011 | Upper arm | Insect bite | NR |
| 12/M | White | Rockwall | 2012 | Face | NR | L mexicana |
| 13/F | White | Hunt | 2013 | R wrist | Lives on farm, works in garden often | L mexicana |
| 14/F | White | Denton | 2013 | Upper arm | NR | NR |
| 15/F | NR | Dallas | 2013 | R forehead | NR | L mexicana |
| 16/F | NR | Brazos | 2013 | Forehead | NR | NR |
| 17/M | White | Denton | 2013 | Forearm | NR | NR |
| 18/F | White | Travis | 2013 | Upper arm | US travel: Louisiana, Washington, Alaska | L mexicana |
| 19/F | White | Dallas | 2013 | Shoulder | NR | NR |
| 20/M | NR | Collin | 2013 | L arm | NR | NR |
| 21/F | NR | Grayson | 2013 | R lower eyelid | NR | NR |
| 22/F | White | Fayette | 2013 | L eyelid | NR | NR |
| 23/F | White | Burleson | 2013 | Upper shoulder | NR | L mexicana |
| 24/M | NR | Bexar | 2014 | Upper eyelid | Hunter, frequent camping | L mexicana |
| 25/F | White | Caldwell | 2014 | Face | Several gnat bites | L mexicana |
| 26/F | White | Madison | 2014 | L temple | NR | L mexicana |
| 27/M | White | Wise | 2014 | Forearm, large portion of arm | NR | L mexicana |
| 28/F | White | Grayson | 2014 | Face, eyelid | NR | NR |
| 29/F | NR | Grayson | 2014 | R forehead | NR | NR |
| 30/F | NR | Caldwell | 2014 | L cheek | NR | L mexicana |
| 31/M | White | Tarrant | 2014 | R ear | Lives in rural area, outside often | L mexicana |
| 32/F | NR | Grayson | 2014 | L upper arm | NR | NR |
| 33/F | NR | Rockwall | 2015 | L upper arm | NR | NR |
| 34/M | White | DeWitt | 2015 | Face, cheek | NR | L mexicana |
| 35/F | NR | Washington | 2015 | R dorsal hand | NR | NR |
| 36/F | White | Denton | 2015 | Forearm | No history of insect bite | L mexicana |
| 37/F | NR | Burleson | 2015 | L earlobe | NR | NR |
| 38/M | White | Travis | 2015 | Elbows | NR | Leishmania species |
| 39/M | White | Dallas | 2015 | L forearm | NR | NR |
| 40/F | White | Collin | 2015 | Face | NR | Leishmania species |
| 41/M | White | Collin | 2015 | Ear, back | Sells firewood for a living | L mexicana |
| 42/F | NR | Austin | 2016 | R anterior upper neck | NR | NR |
| 43/M | NR | Denton | 2016 | R upper arm | NR | L mexicana |
| 44/F | NR | Palo Pinto | 2016 | L forehead | Insect bites | L mexicana |
| 45/F | NR | Dallas | 2016 | R face | Insect bite | NR |
Abbreviations: L, left; NR, not reported; R, right.
Four cases were also reported in previous publications.
Figure 3. Clinical and Histopathologic Presentation of Endemic Cutaneous Leishmaniasis.
Cutaneous leishmaniasis was diagnosed in a patient who had never left their home county in central Texas. The sample from the patient was sent to the Centers for Disease Control and Prevention for speciation via polymerase chain reaction and confirmed to be Leishmania mexicana. A, The patient’s lesion showed granulomatous inflammation without overlying ulceration. B-D, Hematoxylin-eosin stained lesional specimens. B, Dense, granulomatous inflammatory infiltrate is present. The infiltrate comprises lymphocytes, parasitized macrophages, occasional giant cells, plasma cells, and several eosinophils. The overlying epidermis is unaffected. C, At higher power, Leishmania organisms are readily visible rimming parasitized histiocytes. D, Under oil immersion, kinetoplasts are also visible.
Discussion
Our data demonstrate 4 major points: (1) cutaneous leishmaniasis appears to be endemic in humans in the southern United States; (2) in the southern United States, cutaneous leishmaniasis is acquired endemically more frequently than it is via travel; (3) endemic cases of leishmaniasis in the United States are caused by L mexicana; and (4) low awareness of the disease and associated reporting requirements complicate accurate assessment of disease prevalence.
Our data directly contradict current expert medical statements regarding human cutaneous leishmaniasis in the United States. These statements assert that “almost all [cases] of [leishmaniasis] evaluated in North America occur among immigrants, international travelers, expatriates, and military personnel.”18 Our data also contradict American medical authorities who state that “…almost all of the cases of leishmaniasis diagnosed in the United States are in people who became infected while traveling or living in other countries.”15
According to Kerr et al,7(p379) “The factor determining whether zoonotic transmission of Leishmania is possible [is] what species of Lutzomyia occur in [a specific] area.” In areas of the United States where autochthonous leishmaniasis has been diagnosed, there exist both sufficient vector (Lutzomyia species) and reservoir (eg, Neotoma species) populations to support the persistence and spread of disease. In Texas, various species of Lutzomyia sand flies have been captured and identified, including Lutzomyia diabolica, Lutzomyia anthophora, Lutzomyia californica, Lutzomyia oppidana, Lutzomyia texana, and Lutzomyia vexator.34 At least 3 species of wood rat known to harbor leishmaniasis—Neotoma micropus, Neotoma floridana, and Neotoma albigula—inhabit Texas.2,35 Considering the additional cases of human leishmaniasis reported over the past century, leishmaniasis should be considered, both by definition and by data, an endemic disease in the southern United States.
In a 2010 modeling study, González et al demonstrated that climate change will continue to drive northward expansion of vector and reservoir habitats, thus increasing the incidence of autochthonous leishmaniasis in the United States.2 They used computer-generated ecological niche models (ENMs) to predict future habitats of numerous leishmaniasis vector and reservoir species based on climate-change scenarios (Third Intergovernmental Panel on Climate Change Assessment Report).2 These EMNs suggest that by 2050, the range for human leishmaniasis in North America may extend as far north as southeastern Canada. Even under the most restrictive of ENMs, it is predicted that climate-driven expansion of vector and reservoir habitats will expose more than 27 million North Americans to autochthonous leishmaniasis by 2080. This number is more than twice the current estimate of 12 million individuals at risk.2
Indeed, in 2013 Clarke et al27 captured and documented new county and state records for various Lutzomyia species, including L anthophora in Tucson, Arizona (greater than 528 miles west of its previously documented habitat), L anthophora in Collin County, Texas (290 miles north of its nearest previous collection near San Antonio, Texas), and L anthophora in McCurtain County, Oklahoma (a new county and state record for the species). In 2001, McHugh et al35 reported the finding of an eastern woodrat (N floridana) with extensive cutaneous L mexicana infection in Grimes County, Texas. As N floridana had not previously been considered a reservoir for leishmaniasis, this represented not only a new host record but also one occurring in a previously undescribed ecologic region—much further east of any previously reported leishmania-infected Neotoma species. At that time, McHugh et al surmised that this finding indicated the potential for zoonotic transmission of leishmaniasis in an area far beyond the suspected range of natural disease. The subsequent additional diagnoses of autochthonous human leishmaniasis in further eastern and northern Texas suggest this suspicion may be correct.26,33 Because the habitat of N floridana extends from California to North Carolina, its relatively new status as a reservoir host extends the potential range for zoonotic transmission of L mexicana across nearly the entire southern United States, assuming presence of appropriate vectors.35
These findings suggest a burgeoning public health concern, particularly in the setting of low clinical suspicion.2 The American medical profession’s unfamiliarity with endemic leishmaniasis greatly decreases the likelihood of suspecting and diagnosing the disease. All North American physicians, not just those in Texas or the Southwest, must be clinically aware of endemic human leishmaniasis. Relegating discussion of cutaneous leishmaniasis to the tropical disease sections of medical literature limits sufficient training of American physicians in the widely varied clinical presentations of the disease. The photographs of Old World, mucocutaneous, and visceral leishmaniasis frequently presented in medical textbooks are often quite dramatic, with ulceration, deformation of tissue, and loss of chondrocutaneous landmarks. In contrast, American cases of leishmaniasis caused by L mexicana often present more subtly and may take myriad clinical forms, including that of a papular, nodular, or ulcerative dermatosis. Unsurprisingly, clinical recognition of endemic leishmaniasis remains low among American physicians: in this study, no confirmed cases of leishmaniasis were initially suspected as such by the clinician. North American dermatologists, family physicians, and other clinicians should keep leishmaniasis in mind when constructing clinical differential diagnoses for cutaneous lesions. When taking a biopsy of such lesions, including cutaneous leishmaniasis in the clinical differential diagnosis provided with the sample can be instructive to the evaluating dermatopathologist.
Dermatopathologists and general pathologists must likewise be aware of the rising prevalence of endemic leishmaniasis in the United States. In our experience with consultation material of unrecognized cases of leishmaniasis, the original pathologic differential diagnosis often included acneiform eruptions, pseudolymphoma, juvenile xanthogranuloma, and squamous cell carcinoma, among others.
Teaching American physicians that cutaneous leishmaniasis is a tropical disease also limits their training in appropriate treatment of the disease. The majority of patients with cutaneous leishmaniasis caused by L mexicana—as all endemic American cases thus far have been—do not require systemic therapy. These cases may be treated with minor, office-based procedures, such as cryotherapy or brachytherapy, and monitored clinically because there is no risk of progression to visceral leishmaniasis.36,37 American physicians who are taught that leishmaniasis is a life-threatening tropical disease requiring toxic systemic therapy (eg, pentavalent antimonials) could, after receiving a histopathologic diagnosis of leishmaniasis in their domestic patient, subject that patient to unnecessary and harmful systemic medications.
Despite a lack of national reporting requirements, leishmaniasis has been a reportable disease in Texas since 2007. Texas state law requires the reporting of leishmaniasis cases to the Texas DSHS within 1 week of diagnosis. The Texas state law is broad in its requirements for reporting infectious diseases: clinicians, hospitals, laboratories, schools, parents, first responders, or anyone else who may become aware of a notifiable condition is compelled by law to report it to the DSHS.38 Having numerous designated reporters theoretically reduces the risk of failing to identify a case; however, of the leishmaniasis cases identified in the current study, only 20% were reported to the DSHS as required by state law.
Low compliance with mandatory reporting illustrates the medical profession’s lack of awareness regarding human leishmaniasis, even in Texas, where the disease is common enough to be reportable. Throughout the rest of the United States, awareness and diagnosis of human leishmaniasis is likely even lower. Climate models suggest that the habitats of leishmaniasis vectors and reservoirs will continue to spread across the United States in response to climate change, exposing more Americans each year to endemic leishmaniasis. However, lack of a federal reporting requirement makes it impossible to know and track the true prevalence of cutaneous leishmaniasis in the United States.39,40
Limitations
Limitations of this study include the geographic recruitment area of the source laboratories. Although all laboratories used in this study accept specimens from across the United States, the physical laboratories are located within Texas and receive a majority of their specimens locally. It would be beneficial to look at leishmaniasis cases reported by laboratories in other geographic regions of the United States. Furthermore, because leishmaniasis is not a federally reportable disease, reported data on disease cases are not available for states outside of Texas. Requiring federal reporting of cases of human leishmaniasis would allow discernment of true disease incidence across the United States. Well-publicized federal reporting requirements would also increase clinical awareness of and suspicion for endemic disease among American physicians.
Conclusions
Cutaneous human leishmaniasis appears to be endemic in the United States. These data show that, at least in Texas, endemic human leishmaniasis is currently diagnosed more commonly in the United States than is travel-acquired disease. Including this study, there have now been more than 80 published cases of endemic human leishmaniasis in the United States. But with medical literature continuing to discuss leishmaniasis as an exotic illness, physician awareness of endemic disease remains low, as does adherence to state reporting requirements. The prevalence of endemic human leishmaniasis in North America is therefore likely even higher than reported here, but it can only be accurately tracked if leishmaniasis is made federally reportable. Ecologic modeling suggests that the habitats of leishmaniasis vectors and reservoirs may extend as far north as the Canadian border by 2050. We thus propose institution of requisite federal reporting for any human cases of cutaneous leishmaniasis. In such cases, North American physicians have 2 main categories of responsibility: (1) to recognize, diagnose, and treat the disease appropriately; and (2) to report the condition to the applicable local, state, or federal authorities. Labeling leishmaniasis as an endemic North American disease will greatly increase awareness of the disease in the medical community, encouraging clinical suspicion and timely diagnosis, proper reporting, and safe and appropriate treatment. This, coupled with mandated federal reporting, will equip the United States to better identify human cutaneous leishmaniasis and track disease prevalence over time.
eTable. Worldwide Distribution of Leishmania Species Pathogenic in Humans.
References
- 1.Boelaert M, Sundar S. Leishmaniasis In: Farrar J, ed. Manson’s Tropical Infectious Diseases. 23rd ed Philadelphia, PA: Elsevier Inc; 2014:631-651. doi: 10.1016/B978-0-7020-5101-2.00048-0 [DOI] [Google Scholar]
- 2.González C, Wang O, Strutz SE, González-Salazar C, Sánchez-Cordero V, Sarkar S. Climate change and risk of leishmaniasis in north america: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4(1):e585. doi: 10.1371/journal.pntd.0000585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerr SF, McHugh CP, Dronen NO Jr. Leishmaniasis in Texas: prevalence and seasonal transmission of Leishmania mexicana in Neotoma micropus. Am J Trop Med Hyg. 1995;53(1):73-77. doi: 10.4269/ajtmh.1995.53.73 [DOI] [PubMed] [Google Scholar]
- 4.McHugh CP. Leishmaniasis in Washington County, Texas. J Am Acad Dermatol. 2003;49(6):1203. doi: 10.1016/S0190-9622(03)02489-7 [DOI] [PubMed] [Google Scholar]
- 5.Grogl M, Kreutzer RD, McHugh CP, Martin RK. Characterization of a Leishmania isolate from the rodent host Neotoma micropus collected in Texas and comparison with human isolates. Am J Trop Med Hyg. 1991;45(6):714-722. doi: 10.4269/ajtmh.1991.45.714 [DOI] [PubMed] [Google Scholar]
- 6.McHugh CP, Grogl M, Kerr SF. Isolation of Leishmania mexicana from Neotoma micropus collected in Texas. J Parasitol. 1990;76(5):741-742. doi: 10.2307/3282995 [DOI] [PubMed] [Google Scholar]
- 7.Kerr SF, McHugh CP, Merkelz R. Short report: a focus of Leishmania mexicana near Tucson, Arizona. Am J Trop Med Hyg. 1999;61(3):378-379. doi: 10.4269/ajtmh.1999.61.378 [DOI] [PubMed] [Google Scholar]
- 8.Raymond RW, McHugh CP, Witt LR, Kerr SF. Temporal and spatial distribution of Leishmania mexicana infections in a population of Neotoma micropus. Mem Inst Oswaldo Cruz. 2003;98(2):171-180. doi: 10.1590/S0074-02762003000200002 [DOI] [PubMed] [Google Scholar]
- 9.Petersen CA. Leishmaniasis, an emerging disease found in companion animals in the United States. Top Companion Anim Med. 2009;24(4):182-188. doi: 10.1053/j.tcam.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trainor KE, Porter BF, Logan KS, Hoffman RJ, Snowden KF. Eight cases of feline cutaneous leishmaniasis in Texas. Vet Pathol. 2010;47(6):1076-1081. doi: 10.1177/0300985810382094 [DOI] [PubMed] [Google Scholar]
- 11.Alvar J, Vélez ID, Bern C, et al. ; WHO Leishmaniasis Control Team . Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kipp EJ, Mariscal J, Armijos RX, Weigel M, Waldrup K. Genetic evidence of enzootic leishmaniasis in a stray canine and Texas mouse from sites in west and central Texas. Mem Inst Oswaldo Cruz. 2016;111(10):652-654. doi: 10.1590/0074-02760160225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolognia JLJJ, Schaffer JV. Dermatology. 3rd ed Philadelphia, PA: W.B. Saunders; 2012. [Google Scholar]
- 14.Magill A. Leishmania Species: Visceral (Kala-Azar), Cutaneous, and Mucosal. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed Philadelphia, PA: Elsevier; 2015:3091-3107. [Google Scholar]
- 15.US Centers for Disease Control and Prevention Leishmaniasis FAQs. 2013; https://www.cdc.gov/parasites/leishmaniasis/gen_info/faqs.html. Accessed September 20, 2017.
- 16.Dockrell D, Sundar S, Angus B, Hobson R. Infectious disease In: Walker B, ed. Davidson’s Principles and Practice of Medicine. 22nd ed London, England: Churchill Livingstone; 2014:362-367. [Google Scholar]
- 17.Kumar V. Leishmaniasis. Robbins and Cotran Pathologic Basis of Disease. 9th ed Philadelphia, PA: Elsevier; 2014. [Google Scholar]
- 18.Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63(12):e202-e264. doi: 10.1093/cid/ciw670 [DOI] [PubMed] [Google Scholar]
- 19.Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg. 2017;96(1):24-45. doi: 10.4269/ajtmh.16-84256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habif TP, Campbell JL, Chapman MS, Dinulos JGH, Zug KA. Leishmaniasis Skin Disease. 3rd ed Philadelphia, PA: Elsevier; 2011:632-637. doi: 10.1016/B978-0-323-07700-2.00024-X [DOI] [Google Scholar]
- 21.Patterson J. Protozoal Infections. Weedon’s Skin Pathology. 4th ed London, England: Elsevier Ltd; 2016. [Google Scholar]
- 22.Croft S, Buffet P. Leishmaniasis In: Goldman LSA, ed. Goldman-Cecil Medicine. 25th ed Philadelphia, PA: W.B. Saunders; 2015. [Google Scholar]
- 23.Fort G. Leishmaniasis In: Ferri F, ed. Ferri’s Clinical Advisor. Philadelphia, PA: Elsevier; 2017:712-713. [Google Scholar]
- 24.Melby P. Leishmaniasis (Leishmania) In: Kliegman RM, Stanton BS, Geme JS, et al. , eds. Nelson Textbook of Pediatrics. 20th ed Philadelphia, PA: Elsevier Health Sciences; 2015. [Google Scholar]
- 25.US Centers for Disease Control and Prevention National Notifiable Diseases Surveillance System (NNDSS): Current and historical disease list. 2017; https://wwwn.cdc.gov/nndss/conditions/. Accessed August 12, 2017.
- 26.Maloney DM, Maloney JE, Dotson D, Popov VL, Sanchez RL. Cutaneous leishmaniasis: Texas case diagnosed by electron microscopy. J Am Acad Dermatol. 2002;47(4):614-616. doi: 10.1067/mjd.2002.124606 [DOI] [PubMed] [Google Scholar]
- 27.Clarke CF, Bradley KK, Wright JH, Glowicz J. Case report: Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88(1):157-161. doi: 10.4269/ajtmh.2012.11-0717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mead DG, Cupp EW. Occurrence of Lutzomyia anthophora (Diptera: Psychodidae) in Arizona. J Med Entomol. 1995;32(5):747-748. doi: 10.1093/jmedent/32.5.747 [DOI] [PubMed] [Google Scholar]
- 29.Pavlovsky EN, Plous FK, Levine ND. Natural nidality of transmissible diseases. Am J Med Sci. 1966;252(5):161. doi: 10.1097/00000441-196611000-00067 [DOI] [Google Scholar]
- 30.Shaw J. The leishmaniases—survival and expansion in a changing world: a mini-review. Mem Inst Oswaldo Cruz. 2007;102(5):541-547. doi: 10.1590/S0074-02762007000500001 [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization Leishmaniasis: status of endemicity of cutaneous leishmaniasis. 2015; http://www.who.int/leishmaniasis/burden/en/. Accessed May 24, 2018.
- 32.McHugh CP, Melby PC, LaFon SG. Leishmaniasis in Texas: epidemiology and clinical aspects of human cases. Am J Trop Med Hyg. 1996;55(5):547-555. doi: 10.4269/ajtmh.1996.55.547 [DOI] [PubMed] [Google Scholar]
- 33.Wright NA, Davis LE, Aftergut KS, Parrish CA, Cockerell CJ. Cutaneous leishmaniasis in Texas: a northern spread of endemic areas. J Am Acad Dermatol. 2008;58(4):650-652. doi: 10.1016/j.jaad.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 34.Gustafson TL, Reed CM, McGreevy PB, Pappas MG, Fox JC, Lawyer PG. Human cutaneous leishmaniasis acquired in Texas. Am J Trop Med Hyg. 1985;34(1):58-63. doi: 10.4269/ajtmh.1985.34.58 [DOI] [PubMed] [Google Scholar]
- 35.McHugh CP, Thies ML, Melby PC, et al. Short report: a disseminated infection of Leishmania mexicana in an eastern woodrat, Neotoma floridana, collected in Texas. Am J Trop Med Hyg. 2003;69(5):470-472. [PubMed] [Google Scholar]
- 36.Blum J, Desjeux P, Schwartz E, Beck B, Hatz C. Treatment of cutaneous leishmaniasis among travellers. J Antimicrob Chemother. 2004;53(2):158-166. doi: 10.1093/jac/dkh058 [DOI] [PubMed] [Google Scholar]
- 37.Andrade-Narváez FJ, Vargas-González A, Canto-Lara SB, Damián-Centeno AG. Clinical picture of cutaneous leishmaniases due to Leishmania (Leishmania) mexicana in the Yucatan peninsula, Mexico. Mem Inst Oswaldo Cruz. 2001;96(2):163-167. doi: 10.1590/S0074-02762001000200005 [DOI] [PubMed] [Google Scholar]
- 38.Texas Legislature Texas Health and Safety Code: Texas Communicable Disease Prevention and Control Act. 1989.
- 39.de Vries HJ, Reedijk SH, Schallig HD. Cutaneous leishmaniasis: recent developments in diagnosis and management. Am J Clin Dermatol. 2015;16(2):99-109. doi: 10.1007/s40257-015-0114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGwire BS, Satoskar AR. Leishmaniasis: clinical syndromes and treatment. QJM. 2014;107(1):7-14. doi: 10.1093/qjmed/hct116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Worldwide Distribution of Leishmania Species Pathogenic in Humans.