Key Points
Question
How does a climate of expanding targeted and immune therapies but declining funding from the National Institutes of Health affect the conduct of radiotherapy trials compared with other oncological trials?
Findings
In a cross-sectional analysis of 25 907 interventional oncological trials registered in ClinicalTrials.gov from 2007 to 2017, only 1378 (5.3%) were radiotherapy trials. Sponsorship or funding for radiotherapy trials was significantly less than that for other oncological studies, and the proportion of radiotherapy trials with a sample size of more than 100 patients has decreased in the past decade.
Meaning
The decline of radiotherapy trials warrants discussion and collaboration among oncologists, funding agencies, industry leaders, and other concerned parties across multiple geographic regions.
Abstract
Importance
Modern precision radiotherapy is an innovative and effective treatment of cancer, yet it is unclear how radiotherapy trials are affected by expanding targeted and immune therapies and declining National Institutes of Health funding.
Objective
To analyze and compare the characteristics of radiotherapy trials with other oncological trials registered on ClinicalTrials.gov.
Design, Setting, and Participants
This is a cross-sectional analysis of trials registered on ClinicalTrials.gov between June 1, 2007, and May 8, 2017. Records of all 243 758 clinical studies registered by May 8, 2017, were downloaded, but only 25 907 interventional oncological trials registered between June 1, 2007, and May 8, 2017, and whose primary purpose was “treatment” were included in the final analysis. Trials were categorized according to cancer type and other registration information.
Main Outcomes and Measures
Characteristics of radiotherapy trials were compared with characteristics of other oncological trials. Chronological shifts in radiotherapy trials were also analyzed.
Results
Of the 25 907 trials selected, 1378 (5.3%) were radiotherapy trials and 24 529 (94.7%) were other oncological studies. The number of radiotherapy trials increased gradually from 94 (June 1, 2007, through May 31, 2008) to 192 (June 1, 2015, through May 31, 2016). Radiotherapy trials were less likely than other oncological studies to be registered before participant enrollment (763 of 1370 [55.7%] vs 16 105 of 24 434 [65.9%]; P < .001), to be blinded (45 of 1378 [3.3%] vs 2784 of 24 529 [11.3%]; P < .001), or to involve multiple geographic regions (2.4% vs 9.5%; P < .001), but they were more likely to be phase 2 to 3 (773 of 1124 [68.8%] vs 12 910 of 22 300 [57.9%]; P < .001) and to have a data-monitoring committee (839 of 1264 [66.4%] vs 11 728 of 21 060 [55.7%]; P < .001). Only a minority of radiotherapy trials were industry sponsored, which was significantly lower than for other oncological trials (80 of 1378 [5.8%] vs 10 651 of 24 529 [43.4%]; P < .001; adjusted odds ratio, 0.08; 95% CI, 0.06-0.10). The number of National Institutes of Health–sponsored radiotherapy trials decreased from 80 of 544 trials (14.7%) from 2007 to 2012 to 72 of 834 trials (8.6%) from 2012 to 2017 (P < .001). Radiotherapy trials with a sample size of more than 100 patients decreased from 155 of 543 trials (28.5%) from 2007 to 2012 to 157 of 833 trials (18.8%) from 2012 to 2017 (P < .001).
Conclusions and Relevance
The limited number of and the scarcity of funding for radiotherapy trials is concerning given the integral role of radiotherapy in the clinical management of patients with cancer worldwide. A multidisciplinary collaboration to promote and fund more radiotherapy research is warranted.
This analysis examines the characteristics of radiotherapy interventional trials compared with other oncological interventional trials reported in ClinicalTrials.gov from 2007 through 2017.
Introduction
Radiotherapy is one of the cornerstone treatment modalities for cancer. It can be used alone or in combination with chemotherapy, surgery, or both.1 As many as 50% of patients with cancer receive radiotherapy.2 Many important advances in radiotherapy have emerged in recent years, including intensity-modulated radiotherapy, stereotactic ablative radiotherapy,3,4,5 tomotherapy, and particle beam radiotherapy.6,7 However, aside from individual reports on the results of clinical trials involving radiotherapy, little is known about contemporary trials evaluating radiotherapy and its ability to advance clinical care.
In 2004, the International Committee of Medical Journal Editors announced a policy as a prerequisite for publication that requires the registration of clinical trials before the enrollment of participants.8 ClinicalTrials.gov is the most robust of the international registries for clinical trials and represents a unique resource for exploring clinical trials that have been registered worldwide. Presently, ClinicalTrials.gov contains detailed information on more than 240 000 clinical research studies conducted in more than 200 countries.9
For this study, we examined all of the interventional oncology studies registered on ClinicalTrials.gov as of May 8, 2017. We compared the fundamental characteristics of trials focusing on radiotherapy with the characteristics of other nonradiotherapy oncological trials; then, we evaluated the changes over time.
Methods
Data Source and Study Sample
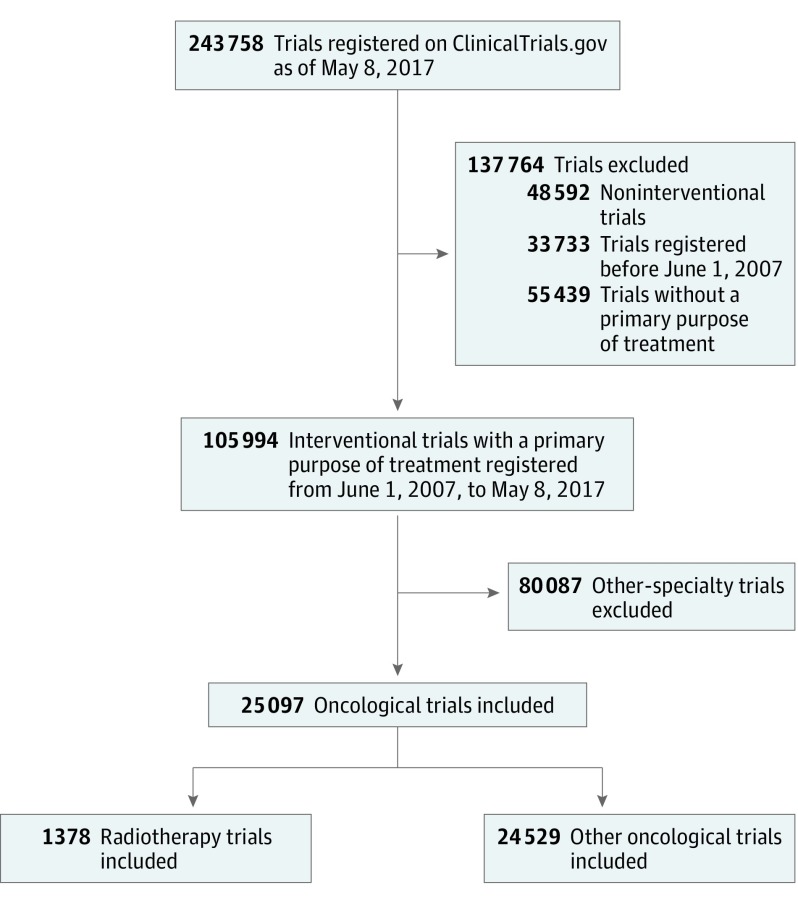
We downloaded the records of all 243 758 clinical studies registered on ClinicalTrials.gov as of May 8, 2017, using the database of the Aggregate Analysis of ClinicalTrials.gov (to see this database as well as data definitions and data dictionaries, go to the Clinical Trials Transformation Initiative website at https://www.ctti-clinicaltrials.org/aact-database).9 We restricted our selection to interventional trials registered between June 1, 2007, and May 8, 2017, whose “primary purpose” was “treatment” (N = 105 994; Figure 1). This study was submitted to the institutional review board of Sun Yat-sen University Cancer Center and was found not to constitute human subjects research.
Figure 1. Flowchart Identifying Trials Registered on ClinicalTrials.gov From 2007 to 2017.
Two oncologists (X.L. and Y.Z.) independently identified the oncological trials, and a third oncologist (L.-L.T.) adjudicated any disagreements. Trials were then categorized according to cancer type, identified by the term condition, brief title, or official title. If the disease type was not clear, other registration information (eg, eligibility and detailed description) was reviewed. Trials that included 2 or more cancer types were grouped into a “multiple neoplasm” category. Similar methods were used to classify these studies into radiotherapy trials or other oncological trials.
We defined radiotherapy trials as studies that (1) added radiotherapy to the standard of care; (2) compared any treatment regimens with or without radiotherapy; (3) investigated novel radiotherapy regimes, such as radiotherapy techniques, volumes, doses, or fractionations; (4) compared different radiotherapy regimens; and (5) assessed interventions for radiotherapy-associated complications. We did not deem trials comparing radiotherapy with or without other treatment modalities or imaging as radiotherapy trials because such trials do not address the optimization of radiotherapy use in the clinic.
Study Variables
We examined these 9 dimensions for each trial: (1) registration before participant enrollment, as required by the International Committee of Medical Journal Editors; (2) presence or absence of a data monitoring committee (DMC); (3) phase of the trial; (4) number of treatment arms; (5) blinding and (6) allocation methods—randomized or nonrandomized; (7) sample size; (8) funding source; and (9) details of the study site.
We used methods described in previous studies10,11,12 of the Aggregate Analysis of ClinicalTrials.gov database. If a trial reported only 1 treatment arm, the allocation methods (if missing) were assigned as nonrandomized and the blinding category (if missing) was assigned as open label.12 Funding sources were classified, on the basis of recorded lead sponsor and/or collaborator for each clinical trial, as the National Institutes of Health (NIH), industry, and other government or academic institutions. If industry was listed as the lead sponsor or a collaborator without NIH as the lead sponsor or collaborator, the trial was classified as an industry-funded trial. If the NIH was the lead sponsor or a collaborator with a nonindustry lead sponsor, the trial was considered to be an NIH-funded trial. All other trials were considered as other-funded trials.11
Statistical Analysis
Descriptive statistics were used primarily to summarize the characteristics of the clinical trial. Categorical variables are reported as frequencies and percentages. Unless stated otherwise, missing values were excluded from the analyses. The Pearson χ2 test was used to compare trial characteristics, and the Fisher exact test was used if indicated. Logistic regression analysis was performed to assess the association between radiotherapy trials and industry or NIH funding, controlling for the cancer types studied and the trial phases. All statistical tests were 2-sided with statistical significance at the α = .05 level. Analyses were undertaken using SPSS, version 22.0 (IBM Corp).
Results
Characteristics of Radiotherapy Trials and Other Oncological Trials
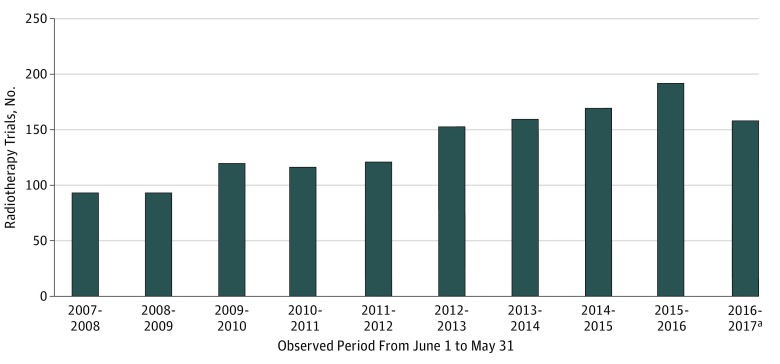
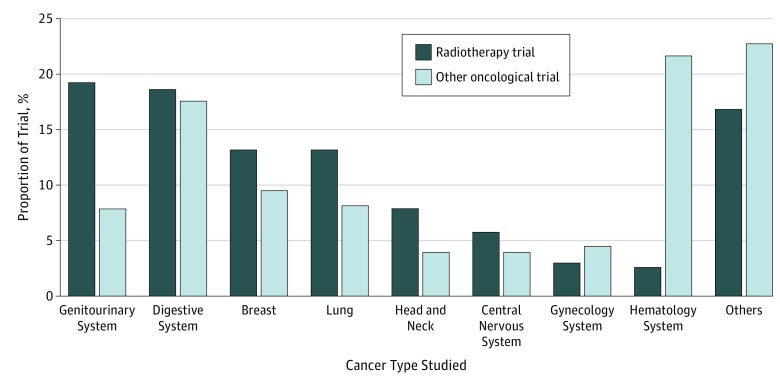
Of the total 105 994 interventional trials registered in ClinicalTrials.gov between June 1, 2007, and May 8, 2017, we identified 25 907 oncological trials (24.4%), of which 1378 (5.3%) were radiotherapy trials and 24 529 (94.7%) were other oncological trials (Figure 1). The number of radiotherapy trials increased gradually from 94 (June 1, 2007, to May 31, 2008) to a peak of 192 (June 1, 2015, to May 31, 2016) but declined to 159 (June 1, 2016, to May 8, 2017) (Figure 2). A higher proportion of radiotherapy trials than other oncological trials were conducted for patients with genitourinary cancer (19.3% vs 7.8%; P < .001), lung cancer (13.1% vs 8.2%; P < .001), and head and neck cancer (7.8% vs 3.9%; P < .001), whereas a lower proportion of radiotherapy trials than other oncological trials were conducted for patients with hematological malignant neoplasm (2.5% vs 21.6%; P < .001) (Figure 3). These observations are consistent with the clinical oncology practice in tumor types for which radiotherapy is the primary treatment modality.
Figure 2. Number of Radiotherapy-Focused Trials Registered on ClinicalTrials.gov per Year Between 2007 and 2017.
aObserved period was June 1, 2016, to May 8, 2017.
Figure 3. Comparison of Cancer Types Studied in Radiotherapy Trials and Other Oncological Trials.
Table 1 presents the characteristics of radiotherapy and other oncological trials. Radiotherapy trials were less likely than other oncological trials to be registered before participant enrollment (763 of 1370 [55.7%] vs 16 105 of 24 434 [65.9%]; P < .001) and to be blind (45 of 1378 [3.3%] vs 2784 of 24 529 [11.3%]; P < .001). The proportion of trials with a DMC was higher in radiotherapy trials than in other oncological trials (839 of 1264 [66.4%] vs 11 728 of 21 060 [55.7%]; P < .001). With regard to phases, radiotherapy trials were more likely than other oncological trials to be phase 2 to 3 trials (773 of 1124 [68.8%] vs 12 910 of 22 300 [57.9%]; P < .001) and less likely to be phase 1 trials (205 of 1124 [18.2%] vs 5711 of 22 300 [25.6%]; P < .001). The use of randomization was comparable between radiotherapy trials and other oncological trials.
Table 1. Comparison of Characteristics of Radiotherapy Trials and Other Oncological Trials Registered on ClinicalTrials.gov From June 1, 2007, to May 8, 2017.
| Characteristic | No./Total No. (%) | P Valueb | |
|---|---|---|---|
| Radiotherapy Trials (n = 1378)a | Other Oncological Trials (n = 24 529)a | ||
| Registration before participant enrollment | 763/1370 (55.7) | 16 105/24 434 (65.9) | <.001 |
| With a DMC | 839/1264 (66.4) | 11 728/21 060 (55.7) | <.001 |
| Phase | |||
| 0 | 12/1124 (1.1) | 135/22 300 (0.6) | <.001 |
| 1 | 205/1124 (18.2) | 5711/22 300 (25.6) | |
| 1-2 | 117/1124 (10.4) | 2825/22 300 (12.7) | |
| 2 | 545/1124 (48.5) | 9747/22 300 (43.7) | |
| 2-3 | 32/1124 (2.8) | 434/22 300 (1.9) | |
| 3 | 196/1124 (17.4) | 2729/22 300 (12.2) | |
| 4 | 17/1124 (1.5) | 719/22 300 (3.2) | |
| Enrollment, No. of patients | |||
| <50 | 630/1376 (45.8) | 12 244/24 472 (50.0) | .009 |
| 50-100 | 434/1376 (31.5) | 7103/24 472 (29.0) | |
| >100 | 312/1376 (22.7) | 5125/24 472 (21.0) | |
| No. of study arms | |||
| 1 | 770/1356 (56.8) | 13 270/24 206 (54.8) | <.001 |
| 2 | 500/1356 (36.9) | 8252/24 206 (34.1) | |
| ≥3 | 86/1356 (6.3) | 2684/24 206 (11.1) | |
| Blinding | |||
| Open label | 1333/1378 (96.7) | 21 745/24 529 (88.7) | <.001 |
| Blind | 45/1378 (3.3) | 2784/24 529 (11.3) | |
| Allocation | |||
| Randomized | 460/1350 (34.1) | 8412/24 240 (34.7) | .02 |
| Nonrandomized | 890/1350 (65.9) | 15 828/24 240 (65.3) | |
| Excludes children (aged <18 y) | 1269/1315 (96.5) | 22 164/23 441 (94.6) | .002 |
| Excludes elderly (aged >65 y) | 77/382 (20.2) | 2095/6864 (30.5) | <.001 |
| Funding source | |||
| Industry | 80/1378 (5.8) | 10 651/24 529 (43.4) | <.001 |
| NIH | 152/1378 (11.0) | 2784/24 529 (11.3) | |
| Other | 1146/1378 (83.2) | 11 094/24 529 (45.2) | |
| No. of geographic regions | |||
| 1 | 1340/1373 (97.6) | 20 405/22 552 (90.5) | <.001 |
| 2 | 22/1373 (1.6) | 1067/22 552 (4.7) | |
| ≥3 | 11/1373 (0.8) | 1080/22 552 (4.8) | |
| Regionc | |||
| United States or Canada | 771/1373 (56.2) | 12 870/22 552 (57.1) | <.001 |
| Europe | 355/1373 (25.9) | 6592/22 552 (29.2) | <.001 |
| Asia | 243/1373 (17.7) | 5361/22 552 (23.8) | <.001 |
| Other | 46/1373 (3.4) | 1453/22 552 (6.4) | <.001 |
| Recruitment status | |||
| Ongoingd | 926/1378 (67.2) | 11 947/24 529 (48.7) | <.001 |
| Stopped earlye | 139/1378 (10.1) | 3107/24 529 (12.7) | .005 |
| Completed | 190/1378 (13.8) | 7634/24 529 (31.1) | <.001 |
| Unknown or others | 123/1378 (8.9) | 1841/24 529 (7.5) | .05 |
Abbreviations: DMC, Data monitoring committee; NIH, National Institutes of Health.
Different denominators were the number of trials with available data for different variables.
Calculated using the χ2 test or the Fisher exact test if indicated.
The sum of the percentages may exceed 100% because categories are not mutually exclusive.
This status includes trials that were “not yet recruiting,” “recruiting,” “enrolling by invitation,” “active, not recruiting,” or “suspended” in the database.
This status includes trials that were “terminated” or “withdrawn” in the database.
With respect to age selection, radiotherapy trials were less likely to exclude elderly patients (aged >65 years) compared with other oncological trials (77 of 382 [20.2%] vs 2095 of 6864 [30.5%]; P < .001). Differences in the funding source were also apparent; only 80 of 1378 trials (5.8%) evaluating radiotherapy were sponsored by industry, which was significantly lower than that for other oncological trials (10 651 of 24 529 trials [43.4%]; P < .001). Multivariable regression analysis confirmed this result (adjusted odds ratio, 0.08; 95% CI, 0.06-0.10). Involvement of multiple (≥2) geographic regions was less common in radiotherapy than other oncological trials (2.4% vs 9.5%; P < .001). Most of the radiotherapy trials involved a study site in the United States or Canada (771 of 1373 [56.2%]), followed by Europe (355 of 1373 [25.9%]). This situation was similar in other oncological studies.
As for recruitment status, radiotherapy trials were more likely to be ongoing compared with other oncological trials (926 of 1378 [67.2%] vs 11 947 of 24 529 [48.7%]; P < .001). The difference between radiotherapy trials and other oncological trials in proportions of trials that stopped early was statistically significant, despite the small magnitude of difference (139 of 1378 [10.1%] vs 3107 of 24 529 [12.7%]; P = .005). However, the proportion of “completed” trials was significantly lower for radiotherapy trials than for other oncological trials (190 of 1378 [13.8%] vs 7634 of 24 529 [31.1%]; P < .001).
Chronological Shifts in Radiotherapy Trial Characteristics
Table 2 lists the characteristics of radiotherapy trials of the 2 periods between June 1, 2007, to May 31, 2012 (n = 544), and June 1, 2012, to May 8, 2017 (n = 834). Compared with trials from 2007 to 2012, a higher proportion of trials from 2012 to 2017 were registered before the first participants were enrolled (298 of 538 [55.4%] vs 523 of 832 [62.9%]; P < .001) and randomized (158 of 519 [30.4%] vs 302 of 834 [36.2%]; P < .001). However, a lower proportion of trials from 2012 to 2017 than from 2007 to 2012 had a DMC (494 of 778 [63.5%] vs 345 of 486 [71.0%]; P = .006). Moreover, the proportion of trials with a sample size of more than 100 patients decreased from 155 of 543 trials (28.5%) from 2007 to 2012 to 157 of 833 trials (18.8%) from 2012 to 2017 (P < .001).
Table 2. Trend Changes in Characteristics of Radiotherapy Trials Registered on ClinicalTrials.gov Between June 1, 2007, to May 31, 2012, and June 1, 2012, to May 8, 2017.
| Characteristic | No./Total No. (%) | P Valueb | |
|---|---|---|---|
| 2007-2012 (n = 544)a | 2012-2017 (n = 834)a | ||
| Registration before participant enrollment | 298/538 (55.4) | 523/832 (62.9) | <.001 |
| With a DMC | 345/486 (71.0) | 494/778 (63.5) | .006 |
| Phase | |||
| 0 | 5/480 (1.0) | 7/644 (1.1) | .06 |
| 1 | 94/480 (19.6) | 111/644 (17.2) | |
| 1-2 | 52/480 (10.8) | 65/644 (10.1) | |
| 2 | 217/480 (45.2) | 328/644 (50.9) | |
| 2-3 | 9/480 (1.9) | 23/644 (3.6) | |
| 3 | 91/480 (19.0) | 105/644 (16.3) | |
| 4 | 12/480 (2.5) | 5/644 (0.8) | |
| Enrollment, No. of patients | |||
| <50 | 249/543 (45.9) | 381/833 (47.4) | <.001 |
| 50-100 | 139/543 (25.6) | 295/833 (33.9) | |
| >100 | 155/543 (28.5) | 157/833 (18.8) | |
| No. of study arms | |||
| 1 | 312/522 (59.8) | 458/834 (54.9) | .21 |
| 2 | 178/522 (34.1) | 322/834 (38.6) | |
| ≥3 | 32/522 (6.1) | 54/834 (6.5) | |
| Blinding | |||
| Open label | 535/544 (98.3) | 798/834 (95.7) | .007 |
| Blind | 9/544 (1.7) | 36/834 (4.3) | |
| Allocation | |||
| Randomized | 158/519 (30.4) | 302/834 (36.2) | <.001 |
| Nonrandomized | 361/519 (69.6) | 529/834 (63.4) | |
| Funding source | |||
| Industry | 38/544 (7.0) | 42/834 (5.0) | <.001 |
| NIH | 80/544 (14.7) | 72/834 (8.6) | |
| Other | 426/544 (78.3) | 720/834 (86.4) | |
| No. of geographic regions | |||
| 1 | 522/544 (96.0) | 818/829 (98.7) | .006 |
| 2 | 15/544 (2.7) | 7/829 (0.8) | |
| ≥3 | 7/544 (1.3) | 4/829 (0.5) | |
| Regionc | |||
| United States or Canada | 368/544 (67.6) | 403/829 (48.6) | <.001 |
| Europe | 126/544 (23.2) | 229/829 (27.5) | .04 |
| Asia | 62/544 (11.4) | 181/829 (21.8) | <.001 |
| Unknown or other | 17/554 (3.1) | 29/829 (3.5) | .71 |
Abbreviations: DMC, Data monitoring committee; NIH, National Institutes of Health.
Different denominators were the number of trials with available data for different variables.
Calculated using the χ2 test or the Fisher exact test if indicated.
The sum of the percentages may exceed 100% because categories are not mutually exclusive.
The proportion of NIH-funded trials decreased from 80 of 544 trials (14.7%) from 2007 to 2012 to 72 of 834 trials (8.6%) from 2012 to 2017 (P < .001). The proportion of trials involving at least 1 study site in the United States decreased from 368 of 544 trials (67.6%) from 2007 to 2012 to 403 of 829 trials (48.6%) from 2012 to 2017 (P < .001), whereas an increase was observed in the proportion of trials involving sites in Europe (126 of 544 trials [23.2%] from 2007 to 2012 vs 229 of 829 trials [27.5%] from 2012 to 2017; P = .04) or Asia (62 of 544 trials [11.4% ] from 2007 to 2012 vs 181 of 829 trials [21.8%] from 2012 to 2017; P < .001). Other characteristics did not change substantially between the 2 periods.
Discussion
To our knowledge, this study is the first comprehensive study to assess the critical characteristics of registered radiotherapy trials. Our study helps us to better understand the current state of radiotherapy trials and provides insights for future development.
Radiotherapy is an indispensable treatment modality for many cancers, but radiotherapy trials account for only a minority (1378 of 25 907 [5.3%]) of all oncological trials registered on ClinicalTrials.gov. In recent years, multiple technological and biological advances have changed the landscape of radiation oncology.1 The clinical gain of improved radiation dosimetry from innovative radiotherapy techniques ought to be tested and proven in clinical trials. However, a systematic analysis of 454 phase 3 randomized clinical trials involving radiotherapy from 1993 to 2016 found that intensity-modulated radiotherapy was used in only 1.3% of trial arms, whereas 3-dimensional conformal radiotherapy was the most commonly used technique (32.4%).13 Another study of 228 phase 1 radiochemotherapy trials observed comparable results.14 These 2 studies highlight a significant discrepancy between radiotherapy techniques that are routinely applied in clinical practice and those reported in the literature. Therefore, more well-designed trials are crucial to generate robust clinical evidence that better defines the clinical implementation of novel radiation techniques in the future.
Only 763 of 1370 radiotherapy trials (55.7%) were registered before the first participants were enrolled, and this number was significantly lower than that for other oncological trials (16 105 of 24 434 [65.9%]). This delayed registration may have hindered the publication of many radiotherapy trials because they did not comply with registration regulations before enrolling participants, a practice that is adopted widely by many medical journals.8,15 To maximize the effect of research, efforts are needed to ensure the timely registration of clinical trials before enrollment. Notably, the number of radiotherapy trials registered before enrollment of the first participants has increased from 298 of 538 trials (55.4%) from 2007 to 2012 to 523 of 832 trials (62.9%) from 2012 to 2017.
Although radiotherapy trials were similar to other oncological trials in using randomization (460 of 1350 [34.1%] vs 8412 of 24 240 [34.7%]; P = .02), they were more likely to be open-label trials (1333 of 1378 [96.7%] vs 21 745 of 24 529 [88.7%]; P < .001). This finding is not surprising because blinding is usually not feasible in trials evaluating radiotherapy. Conversely, more radiotherapy trials had a DMC than did other oncological trials (839 of 1264 [66.4%] vs 11 728 of 21 060 [55.7%]). However, the proportion of radiotherapy trials that had a DMC has decreased in recent years. A DMC is particularly useful for monitoring safety and accrual in clinical trials. However, the appropriate criteria to determine when a DMC is useful or required remain controversial. Consensus is needed to guide researchers when designing trials.10
The proportion of NIH sponsorship was equal for radiotherapy and other oncological trials (152 of 1378 [11.0%] vs 2784 of 24 529 [11.3%]; P = .72) across the entire study period. However, the proportion of radiotherapy trials sponsored by NIH decreased significantly from 80 of 544 trials (14.7%) from 2007 to 2012 to 72 of 834 trials (8.6%) from 2012 to 2017. In addition, only a minority of radiotherapy trials (80 of 1378 [5.8%]) were sponsored by industry, which was significantly lower than for other oncological trials (10 651 of 24 529 trials [43.4%]). This funding discrepancy highlights the critical need to foster closer collaborations among oncologists, industry leaders, funding agencies, and other concerned parties.
Most radiotherapy trials (1340 of 1373 [97.6%]) were conducted in 1 geographic region, which was significantly more common than with other oncological studies (20 405 of 22 552 [90.5%]). Although this finding may be partly attributable to the logistical demands of executing and ensuring robust radiotherapy quality assurance in the setting of a clinical trial, this is nonetheless an important finding because establishing a comprehensive network of global clinical trials would facilitate the gathering of data to enable the most thorough examination of evidence to support medical decision making.10 Global networks have been or are being established for certain cancer types such as gynecological or head and neck cancers.16,17 These efforts will help to accelerate the pace of progress in the treatment of these cancers overall and in radiotherapy in particular.
More important, our analysis of trial recruitment status highlighted 2 key issues. First, early trial stoppages may seem infrequent (approximately 10%) and may seem to occur similarly for both radiotherapy and other oncological trials. However, marginally fewer (but nonetheless statistically significant) radiotherapy trials than other oncological studies were stopped prematurely (139 of 1378 [10.1%] vs 3107 of 24 529 [12.7%]; P = .005). Second, the observation that the proportion of completed radiotherapy trials is significantly lower than the proportion of completed other oncological trials (190 of 1378 [13.8%] vs 7634 of 24 529 [31.1%]; P < .001) points to the difficulty of patient recruitment for radiotherapy trials; consequently, radiotherapy trials tend to take longer to complete than other trials. An important caveat associated with this analysis is that researchers might not update the recruitment status information in a timely manner. Nonetheless, both issues hint at the demands on resources and the difficulty of initiating, conducting, and completing radiotherapy trials.
Compared with the trials registered from 2007 to 2012, a lower proportion of trials that were registered from 2012 to 2017 had a sample size of more than 100 patients (155 of 543 [28.5%] vs 157 of 833 [18.8%]; P < .001). Small trials may be appropriate in certain cases (eg, investigations of early-phase dose evaluations), but these trials are unlikely to establish new standards of care.18,19 Thus, in the future, oncologists, academia, industry leaders, funding agencies, and governments need to find ways to promote collaboration across different regions to conduct larger and more representative trials.
Limitations
Our study had 2 main limitations. First, the ClinicalTrials.gov registry does not include all clinical trials; investigators and sponsors could use other worldwide registries to fulfill the registration-before-enrollment requirement by the International Committee of Medical Journal Editors. Nevertheless, ClinicalTrials.gov contains more than 70% of all clinical trials in the International Clinical Trials Registry of the World Health Organization. Second, the US National Library of Medicine, which runs ClinicalTrials.gov, cannot verify the validity of all registered information; missing data and free-text input may have complicated this study’s conclusions.
Conclusions
This study is a comprehensive overview of radiotherapy trials registered on ClinicalTrials.gov from 2007 to 2017. It highlights the limited number of and the scarcity of funding for radiotherapy trials, which is a concern because of the integral role radiotherapy plays in the clinical management of patients with cancer worldwide. Our findings form the basis for facilitating discussions and collaborations among oncologists, industry leaders, funding agencies, and other concerned parties to promote the conduct of radiotherapy trials that can improve the care of patients with cancer.
References
- 1.Citrin DE. Recent developments in radiotherapy. N Engl J Med. 2017;377(11):1065-1075. [DOI] [PubMed] [Google Scholar]
- 2.DeVita VT Jr, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology. 10th ed Philadelphia: Lippincott Williams & Wilkins, 2014. [Google Scholar]
- 3.Nyman J, Hallqvist A, Lund JA, et al. SPACE—A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121(1):1-8. [DOI] [PubMed] [Google Scholar]
- 4.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88(2):254-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shirvani SM, Chang JY. Proton therapy for non-small cell lung cancer: current evidence and future directions. Thorac Cancer. 2012;3(2):99-108. [DOI] [PubMed] [Google Scholar]
- 7.Holliday EB, Frank SJ. Proton radiation therapy for head and neck cancer: a review of the clinical experience to date. Int J Radiat Oncol Biol Phys. 2014;89(2):292-302. [DOI] [PubMed] [Google Scholar]
- 8.De Angelis C, Drazen JM, Frizelle FA, et al. ; International Committee of Medical Journal Editors . Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004;351(12):1250-1251. [DOI] [PubMed] [Google Scholar]
- 9.US National Institutes of Health About ClinicalTrials.gov. https://clinicaltrials.gov. Accessed October 24, 2017.
- 10.Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007-2010. JAMA. 2012;307(17):1838-1847. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch BR, Califf RM, Cheng SK, et al. Characteristics of oncology clinical trials: insights from a systematic analysis of ClinicalTrials.gov. JAMA Intern Med. 2013;173(11):972-979. [DOI] [PubMed] [Google Scholar]
- 12.Chen YP, Lv JW, Liu X, et al. The landscape of clinical trials evaluating the theranostic role of PET imaging in oncology: insights from an analysis of ClinicalTrials.gov database. Theranostics. 2017;7(2):390-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trone JC, Espenel S, Rehailia-Blanchard A, et al. Navigating the highlights of phase III trials: a watchful eye on evidence-based radiotherapy. Ann Oncol. 2017;28(11):2691-2697. [DOI] [PubMed] [Google Scholar]
- 14.Rivoirard R, Vallard A, Langrand-Escure J, et al. Thirty years of phase I radiochemotherapy trials: latest development. Eur J Cancer. 2016;58:1-7. [DOI] [PubMed] [Google Scholar]
- 15.Laine C, Horton R, DeAngelis CD, et al. Clinical trial registration: looking back and moving ahead. Lancet. 2007;369(9577):1909-1911. [DOI] [PubMed] [Google Scholar]
- 16.Trimble EL, Birrer MJ, Hoskins WJ, et al. ; Gynecologic Cancer Intergroup/National Cancer Institute Writing Committee . Current academic clinical trials in ovarian cancer: Gynecologic Cancer Intergroup and US National Cancer Institute Clinical Trials Planning Meeting, May 2009. Int J Gynecol Cancer. 2010;20(7):1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le QT, Welch JJ, Vermorken JB, Rischin D, Mehanna H; All HNCIG investigators . Formation of an international intergroup to coordinate clinical trials in head and neck cancers: HNCIG. Oral Oncol. 2017;71:180-183. [DOI] [PubMed] [Google Scholar]
- 18.Peto R, Collins R, Gray R. Large-scale randomized evidence: large, simple trials and overviews of trials. J Clin Epidemiol. 1995;48(1):23-40. [DOI] [PubMed] [Google Scholar]
- 19.Geddes JR. Clinical trial design: horses for courses. World Psychiatry. 2009;8(1):28-29. [DOI] [PMC free article] [PubMed] [Google Scholar]