Key Points
Question
Does cancer increase the risk of diabetes?
Findings
In a Korean general population cohort of 524 089 men and women observed for up to 10 years, participants who developed cancer had a clear increase in the subsequent risk of diabetes, even after taking into account precancer risk factors.
Meaning
Physicians should remember that patients with cancer develop other clinical problems, such as diabetes, with higher frequency than individuals without cancer, and should consider routine diabetes screening in these patients.
This Korean national cohort study investigates whether the development of cancer is associated with increasing risk of subsequent diabetes.
Abstract
Importance
Diabetes is an established risk factor for developing cancer. A limited body of evidence also suggests that cancer can increase the risk of developing new cases of diabetes, but the evidence is inconclusive.
Objective
To evaluate whether the development of cancer is associated with increasing risk of subsequent diabetes.
Design, Setting, and Participants
This cohort study included a nationally representative sample of the Korean general population observed for up to 10 years (January 1, 2003, to December 31, 2013). A total of 524 089 men and women 20 to 70 years of age without diabetes and with no history of cancer at baseline were included.
Exposures
Incident cancer (time-varying exposure).
Main Outcomes and Measures
Incident type 2 diabetes using insurance claim codes.
Results
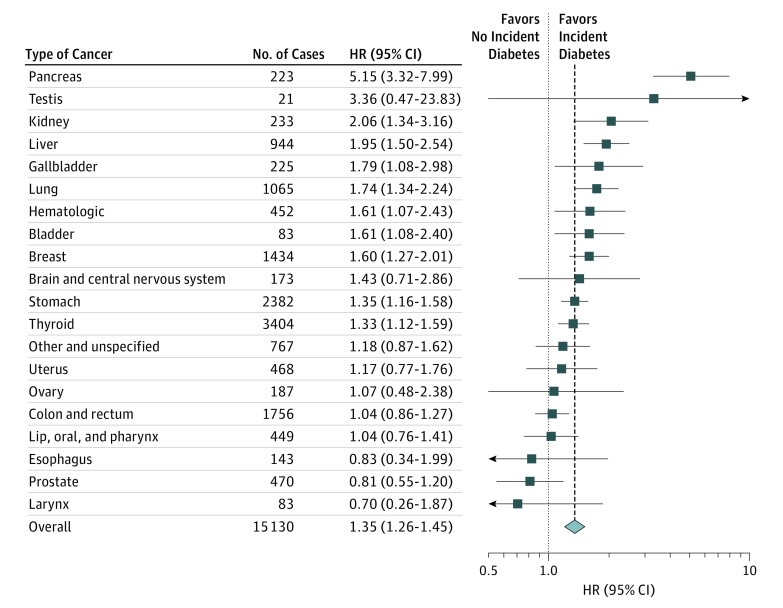
During 3 492 935.6 person-years of follow-up (median follow-up, 7.0 years) in 494 189 individuals (50.0% female; mean [SD] age, 41.8 [12.5] years), 15 130 participants developed cancer and 26 610 participants developed diabetes. After adjustment for age, sex, precancer diabetes risk factors, metabolic factors, and comorbidities, the hazard ratio (HR) for diabetes associated with cancer development was 1.35 (95% CI, 1.26-1.45; P < .001). The excess risk for diabetes was highest in the first 2 years after cancer diagnosis, but it remained elevated throughout follow-up. By cancer type, development of pancreatic (HR, 5.15; 95% CI, 3.32-7.99), kidney (HR, 2.06; 95% CI, 1.34-3.16), liver (HR, 1.95; 95% CI, 1.50-2.54), gallbladder (HR, 1.79; 95% CI, 1.08-2.98), lung (HR, 1.74; 95% CI, 1.34-2.24), blood (HR, 1.61; 95% CI, 1.07-2.43), breast (HR, 1.60; 95% CI, 1.27-2.01), stomach (HR, 1.35; 95% CI, 1.16-1.58), and thyroid cancer (HR, 1.33; 95% CI, 1.12-1.59) was associated with a significantly increased risk of diabetes.
Conclusions and Relevance
In this large Korean cohort, cancer development increased the risk of subsequent diabetes. These data provide evidence that cancer is associated with an increased risk of diabetes in cancer survivors independent of traditional diabetes risk factors. Physicians should remember that patients with cancer develop other clinical problems, such as diabetes, with higher frequency than individuals without cancer, and should consider routine diabetes screening in these patients.
Introduction
Cancer is a global burden for clinical and public health systems, with 14 million newly diagnosed cases in 2012.1 Early detection and improved treatment have increased survival in many types of cancer,2,3 making long-term quality of life a consideration in patient management. Chronic comorbidities, including diabetes, are key determinants of quality of life in cancer.4 Diabetes, a major risk factor of cardiovascular disease,5 is the main cause of noncancer mortality among cancer survivors6 and is associated with increased mortality in patients with cancer.7,8,9
While multiple studies have established that diabetes is a risk factor for the development of several types of cancer10,11,12,13 and a prognostic factor for cancer-related mortality,14 a limited body of evidence suggests that cancer can increase the risk of developing new cases of diabetes, especially after pancreatic, colorectal, and breast cancer.15,16,17 Current studies, however, are limited by small sample sizes or by restriction to specific cancer types. Furthermore, obesity, physical inactivity, and smoking are common causes of diabetes and cancer, but few studies have information on diabetes risk factors prior to the development of cancer and are unable to separate the specific contribution of cancer or cancer-related therapies to the development of diabetes.15,18
We thus studied a large cohort of 524 089 men and women 20 to 70 years of age without diabetes and with no history of cancer at baseline. We followed study participants for up to 10 years and determined whether the risk of developing diabetes increased after the development of cancer, adjusting for precancer diabetes risk factors.
Methods
Study Population and Design
The National Health Insurance Service–National Sample Cohort (NHIS-NSC) is a population-based retrospective cohort based on a 2.2% representative sample of Korean citizens, covering all regions in Korea.19 Korea has a single-payer national health system, and the NHIS maintains national records of all covered inpatient and outpatient visits, procedures, and prescriptions. Sampling consisted of a systematic stratified random sample with proportional allocation within each stratum. The sampling procedures and representativeness of the cohort are described in elsewhere.19 We used person-level longitudinal NHIS-NSC registration and claims data between January 1, 2002, and December 31, 2013.19
In Korea, the NHIS provides, free of charge, annual or biennial health screening examinations that include assessment of cardiovascular and diabetes risk factors to all insured individuals. Approximately 72% of eligible beneficiaries receive the screening examinations.20 Our study population thus included all men and women 20 to 70 years of age in the NHIS-NSC cohort who underwent at least 1 health screening examination between January 1, 2003, and December 31, 2013 (N = 524 089).
Participants were included in the study on the date of their first health screening examination (baseline examination). We then excluded participants who had claims for cancer (n = 4266) or diabetes (n = 8987) between January 1, 2002, and the baseline screening examination, as well as participants with fasting serum glucose level of 126 mg/dL or greater (n = 15 609; to convert to millimoles per liter, multiply by 0.0555) or a self-reported history of diabetes (n = 1281) at the baseline screening examination. The final sample size was 494 189 (247 008 men and 247 181 women) (eFigure 1 in the Supplement). The Institutional Review Board of the National Cancer Center approved this study and waived the requirement for informed consent because we used only deidentified data.
Data Collection
The NHIS-NSC cohort comprises 4 databases on insurance eligibility, medical treatments, medical care institutions, and general health examinations. The insurance eligibility database contains information on age, sex, residential area, type of health insurance, income level, and disability. The medical treatment database contains information from treatment bills, including details of diseases and prescriptions. The general health examination database includes results from the health screening examinations conducted by the NHIS.19
Baseline information was collected at health screening examinations. Smoking habits, history of diabetes, and medication use were collected by self-administered questionnaires. Height, weight, and blood pressure were measured, and body mass index was calculated as weight in kilograms divided by height in meters squared. Glucose and total cholesterol levels were measured in fasting samples.
The NHIS claims for inpatient and outpatient visits, procedures, and prescriptions were coded using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), which was adopted in Korea in 1995, and the Korean Drug and Anatomical Therapeutic Chemical Codes.21,22 The NHIS routinely audits the claims, and the data are considered reliable and have been used in numerous peer-reviewed publications.19,23 Type 2 diabetes was defined as the presence of identical E11-E14 codes (ICD-10) at least 2 times, or a diabetes drug code (including biguanides, sulfonylurea, meglitinides, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, α-glucosidase inhibitors, sodium-glucose co-transporter 2 inhibitors, insulin, or glucagon-like peptide 1 agonists) plus an E11-E14 code.
Cancer was defined as the presence of the same C code more than 3 times within a year or an inpatient hospitalization with a C code. C codes are carefully reviewed by the NHIS because they have implications for additional benefits for patients. In Korea, once a person receives a cancer diagnosis, he or she is registered to the National Cancer Registry with a specific code (called C code) that indicates to the system that the person has received a diagnosis of cancer and that provides special insurance benefits. Once a person has a C code, it is carried forward in medical records and claims created for that patient. Therefore, cancer diagnoses based on claims are considered reliable and a 1-year look-back window to exclude patients with a prior diagnosis of cancer (C code) effectively excludes patients with a prior diagnosis at any time (had history of cancer), and not only patients who received a diagnosis of cancer in the prior year.
Comorbidities during the year prior to the screening examination were defined using ICD-10 codes21 and were summarized using the Charlson index.24,25 In addition, baseline hypertension was defined as the presence of at least 1 I10-I13 or I15 code during the year prior to the screening examination or a systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg at the baseline screening examination; and baseline hyperlipidemia was defined as the presence of at least 1 E78 code or a total cholesterol level greater than 240 mg/dL (to convert to millimoles per liter, multiply by 0.0259) at the baseline screening examination.
Statistical Analysis
The study end point was the development of diabetes. Participants were included in the study at the baseline screening examination and were observed until the development of diabetes, death, or the end of the study period (December 31, 2013). The study exposure was cancer development, considered as a time-varying variable (participants who developed cancer contributed unexposed person-time prior to the development of cancer). Cancer cases that occurred after development of diabetes were not included in the study because follow-up ended when a participant developed diabetes. In addition, to reduce the potential impact of surveillance bias, we excluded from the analysis cases of diabetes that occurred in the first 31 days after cancer diagnosis (n = 72) by censoring observation time for these individuals at the time of cancer development.
We calculated hazard ratios (HRs) with 95% confidence intervals for developing diabetes using a proportional hazards regression model. We used 3 models with increasing degrees of adjustment to account for potential confounding factors at baseline. Model 1 was adjusted for age (20-29, 30-39, 40-49, 50-59, and 60-69 years) and sex. Model 2 was further adjusted for body mass index (continuous), smoking (never, former, current, and missing), and frequency of alcohol intake (<1 time per month, 1-2 times per week, 3-4 times per week, almost every day, and missing). Model 3 was further adjusted for hypertension (yes or no), hyperlipidemia (yes or no), Charlson comorbidity index (0, 1, 2, ≥3), systolic blood pressure (continuous), fasting glucose (continuous), and total cholesterol (continuous). To account for competing risks due to mortality, we fitted a proportional subdistribution hazards regression model26 with death as the competing event, with similar findings. We examined the proportional hazards assumption using plots of the log(−log) survival function and Schoenfeld residuals. All analyses were performed using STATA, version 14 (StataCorp LP). All P values reported are 2 sided. A P < .05 was considered statistically significant.
Results
During 3 492 935.6 person-years of follow-up (median follow-up, 7.0 years [interquartile range, 4.0-10.0 years]), 15 130 participants developed cancer (eTable 1 in the Supplement). Compared with participants who did not develop cancer, those who did were older, more likely to be female, to drink alcohol every day, to have a higher body mass index, and to have more comorbidities.
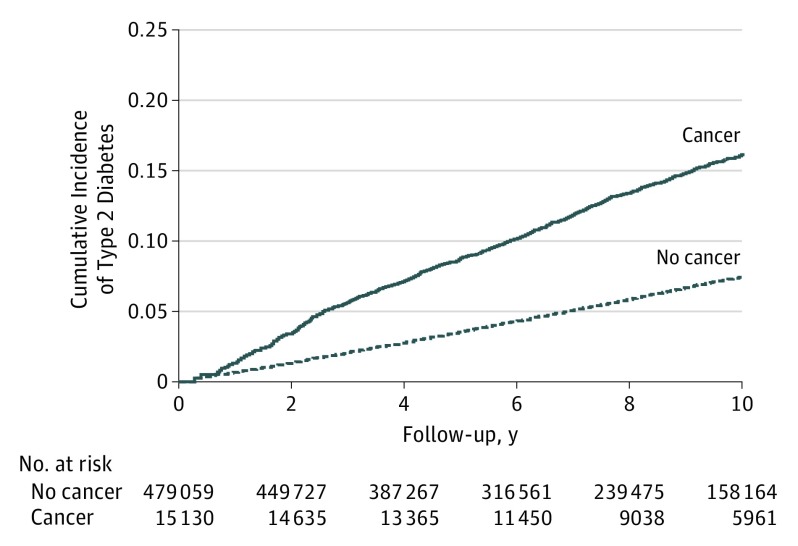
The number of incident cases of diabetes observed during follow-up was 26 610. Of these, 834 cases occurred after the development of cancer (incidence rate, 17.4 per 1000 person-years), and 25 776 cases occurred prior to the development of cancer or in participants who did not develop cancer (incidence rate, 7.5 per 1000 person-years) (Table and Figure 1). The age- and sex-adjusted HR for diabetes associated with the development of cancer was 1.36 (95% CI, 1.27-1.46). The association did not materially change after adjustment for precancer diabetes risk factors, comorbidities, or metabolic factors (fully adjusted HR, 1.35; 95% CI, 1.26-1.45), and it persisted after taking into account competing risks due to mortality (eTable 2 in the Supplement).
Table. Hazard Ratios (HRs) for Incident Diabetes Associated With Cancer Development in a Cohort of 494 189 Patients.
| Parameter | Person-Years | Cases, No. | Diabetes Incidence Rate, per 1000 Person-Years | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||||
| Cancer | |||||||||
| None | 3 445 098.6 | 25 776 | 7.5 | 1 [Reference] | <.001 | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| Incident cancer | 47 837.0 | 834 | 17.4 | 1.36 (1.27-1.46) | 1.38 (1.28-1.48) | 1.35 (1.26-1.45) | |||
| Time after cancer diagnosis, y | |||||||||
| 0 | 3 445 098.6 | 25 776 | 7.5 | 1 [Reference] | <.001 | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| <3 | 30 487.5 | 560 | 18.4 | 1.47 (1.35-1.60) | 1.48 (1.36-1.61) | 1.47 (1.35-1.60) | |||
| ≥3 to <5 | 9994.8 | 152 | 15.2 | 1.15 (0.98-1.35) | 1.16 (0.99-1.36) | 1.14 (0.97-1.33) | |||
| ≥5 | 7354.6 | 122 | 16.6 | 1.23 (1.02-1.47) | 1.25 (1.05-1.47) | 1.19 (1.00-1.43) | |||
Model 1: adjusted for age (20-29, 30-39, 40-49, 50-59, and 60-69 y) and sex.
Model 2: further adjusted for body mass index (continuous), smoking (never, former, current, and missing), and frequency of alcohol intake (<1 time per month, 1-2 times per week, 3-4 per week, almost every day, and missing).
Model 3: further adjusted for hypertension (yes or no), hyperlipidemia (yes or no), Charlson comorbidity index (0, 1, 2, ≥3), systolic blood pressure (continuous), fasting glucose (continuous), and total cholesterol (continuous).
Figure 1. Cumulative Incidence of Diabetes by Cancer Status.
Cumulative incidence was calculated using Kaplan-Meier curves. Participants who developed cancer contributed person-time to the exposed group from the time of cancer development. Unexposed person-time was contributed by participants who did not develop cancer and by participants who developed cancer prior to diabetes development. To reduce the potential impact of surveillance bias, we excluded from the analysis cases of diabetes that occurred in the first 31 days after cancer diagnosis (n = 72).
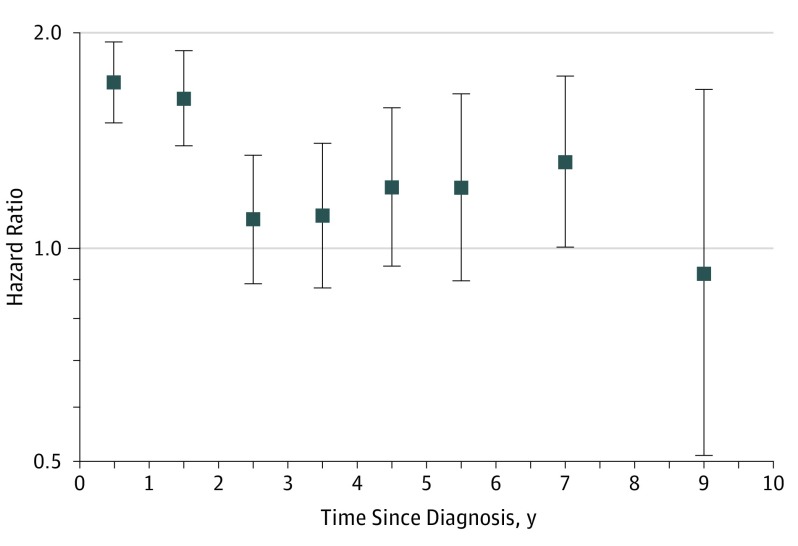
The excess risk for diabetes after cancer development was higher in the first 2 years after cancer diagnosis, although it persisted throughout the duration of follow-up (Table and Figure 2). The fully adjusted HRs for diabetes in years 1 to 2, 3 to 5, or 6 to 10 after a cancer diagnosis were 1.47 (95% CI, 1.35-1.60), 1.14 (95% CI, 0.97-1.33), and 1.19 (95% CI, 1.00-1.43), respectively.
Figure 2. Hazard Ratios for Incident Diabetes Associated With Cancer Development by Time Since Cancer Diagnosis.
Adjusted for age (20-29, 30-39, 40-49, 50-59, and 60-69 years) and sex. Model 2 was further adjusted for body mass index (continuous), smoking (never, former, current, and missing), and frequency of alcohol intake (<1 time per month, 1-2 times per week, 3-4 per week, almost every day, and missing). Model 3 was further adjusted for hypertension (yes or no), hyperlipidemia (yes or no), Charlson comorbidity index (0, 1, 2, ≥3), systolic blood pressure (continuous), fasting glucose (continuous), and total cholesterol (continuous). Error bars indicate 95% confidence intervals.
When diabetes risk was evaluated by type of cancer (Figure 3), the association was particularly strong for pancreatic (fully adjusted HR, 5.15; 95% CI, 3.32-7.99), kidney (HR, 2.06; 95% CI, 1.34-3.16), and liver cancer (HR, 1.95; 95% CI, 1.50-2.54). Development of gallbladder, lung, blood, breast, stomach, and thyroid cancers was also associated with a significantly increased risk of diabetes. Development of testicular and brain cancer was associated with increased risk of diabetes, but the number of cases was small and these associations were not statistically significant. Development of uterine, ovarian, colorectal, head and neck, esophageal, or prostate cancers was not associated with increased risk of diabetes.
Figure 3. Hazard Ratios (HRs) for Incident Diabetes Associated With Cancer Development by Type of Cancer.
Adjusted for age (20-29, 30-39, 40-49, 50-59, and 60-69 years) and sex. Model 2 was further adjusted for body mass index (continuous), smoking (never, former, current, and missing), and frequency of alcohol intake (<1 time per month, 1-2 times per week, 3-4 per week, almost every day, and missing). Model 3 was further adjusted for hypertension (yes or no), hyperlipidemia (yes or no), Charlson comorbidity index (0, 1, 2, ≥3), systolic blood pressure (continuous), fasting glucose (continuous), and total cholesterol (continuous). Error bars indicate 95% confidence intervals.
In participants who developed cancer, there was no clear association between the HR for diabetes and the annual number of inpatient or outpatient visits (eFigure 2 in the Supplement). In sensitivity analyses, we repeated the analysis after censoring participants if they developed thyroid cancer or gastric cancer, 2 types of cancer with very high incidence in Korea. The results were essentially unchanged (eTable 3 in the Supplement). Finally, plots of the log(−log) survival function and Schoenfeld residuals did not show major departures from the proportional hazards assumption (eFigure 3 in the Supplement).
Discussion
In this large national cohort, cancer development increased the risk of subsequent development of diabetes. The increased risk of diabetes was evident shortly after cancer development, was strongest in the first 2 years after cancer diagnosis, and remained elevated throughout the rest of follow-up. The risk of diabetes varied by type of cancer: development of pancreatic, kidney, liver, gallbladder, lung, blood, breast, stomach, and thyroid cancers was associated with a significantly increased risk of diabetes.
The increased risk of diabetes after cancer may be related to cancer-management interventions. Corticosteroids are widely used for a variety of purposes in patients with cancer, including the prevention of chemotherapy-induced emesis and hypersensitivity,27 the management of brain metastasis and metastatic spinal cord compression,27 the control of hematologic cancers,28 and the reduction of cancer-related fatigue, and improvement of quality of life.29 Corticosteroid use is associated with hyperglycemia and with diabetes development, a process mediated primarily by reduced insulin sensitivity.30 Several small studies have also reported that corticosteroid-containing treatment regimens induced hyperglycemia or diabetes in patients with several types of cancer.31,32,33
Treatment with chemotherapy agents may also cause hyperglycemia and increase diabetes risk.34,35,36,37 L-asparaginase, which directly inhibits insulin release, is an established cause of hyperglycemia.38 Total body irradiation39 and immunosuppressive agents, such as calcineurin inhibitors, may also increase the risk of diabetes.40 Tamoxifen, an anti-estrogen, was also associated with an increased risk of diabetes in breast cancer.41 Furthermore, the combined use of chemotherapy agents and corticosteroids may have a synergistic effect on diabetes risk. Indeed, the increased risk of diabetes in postmenopausal women with breast cancer observed in a previous study was highest early during follow-up in patients receiving corticosteroid-containing chemotherapy.15 The metabolic consequences of many chemotherapy agents, however, have not been fully characterized, and further research is needed to elucidate the impact of chemotherapy on diabetes risk.36 In our study, the increased risk of diabetes seemed to be highest in the period immediately after cancer development, further suggesting a role for toxicity of cancer treatments in diabetes development.
Diabetes risk may also be increased by direct cancer effects. Many patients with advanced cancer experience substantial weight loss, muscle wasting, and loss of appetite, a syndrome described as cancer cachexia.42 Cancer cachexia is often associated with increased insulin resistance, impaired glucose tolerance, and diabetes.43 Various cytokines and cachectic factors, such as tumor necrosis factor and interleukin 6, are associated with skeletal muscle wasting and development of insulin resistance.44,45 Patients with cancer are more likely to experience acute illnesses, hospitalizations, and stressful events such as surgery, infection, or bleeding. These events may result in stress-hyperglycemia,46 induced by a complex interplay of regulatory hormones, cytokines, and insulin resistance.46 Hyperglycemia during acute illness is a risk factor for subsequent development of diabetes,47 and its cumulative effects may be substantial in patients with cancer.
In our study, diabetes risk varied by cancer type. Pancreatic cancer was associated with the highest risk of diabetes. In addition to direct destruction of pancreatic tissue by the cancer, surgery and radiation therapy can result in loss of pancreatic islet cell mass, inducing hyperglycemia and diabetes.48,49,50 Patients with liver and kidney cancer also had a high risk of subsequent diabetes. Liver cancer commonly originates in patients with chronic liver disease and cirrhosis, 2 conditions frequently associated with diabetes.51 Several mechanisms have been suggested for glucose intolerance in cirrhosis, including decreased hepatic insulin clearance, increased advanced glycation end products, and hypoxia.52 For kidney cancer, the mechanism underlying an increased risk of diabetes is unclear, although nephrectomy, the primary treatment strategy in kidney cancer, is a risk factor for chronic kidney disease, which has been linked to insulin resistance.53,54 Further research is needed to elucidate the precise mechanisms that lead to diabetes in different types of cancer.
An increased risk of diabetes in patients with cancer could also be mediated by common risk factors for cancer and diabetes, such as obesity, physical inactivity, unbalanced diet, smoking, and excess alcohol consumption.18 Hyperinsulinemia, hyperglycemia, or chronic inflammation are possible mediating mechanisms,18 particularly in obesity-related cancers, such as colorectal, endometrial, breast, and kidney cancer.55 A strength of our analysis was that we could adjust for precancer risk factors for diabetes. In addition, the increased risk in diabetes did not affect all obesity-related cancers because colorectal and endometrial cancer were not associated with an increased risk of subsequent diabetes. Our data thus provide strong evidence that some types of cancer are associated with an increased risk of diabetes in cancer survivors independent of traditional risk factors for diabetes.
Limitations
Several limitations need to be considered in the interpretation of our findings. We did not have information on cancer stage, and we had only limited information on cancer treatments and disease management based on claims. As a consequence, we could not establish the precise mechanisms responsible for increased diabetes risk in each type of cancer. Also, patients who develop cancer have more frequent contacts with the health care system, which may induce surveillance bias. However, we excluded all participants with diabetes after a baseline screening, as well as diabetes cases that were identified very early after cancer development. Furthermore, the lack of a clear association between the average number of inpatient or outpatient visits for each type of cancer and its HR for diabetes and the fact that several types of cancer did not show an increased risk of diabetes argue against a major role of surveillance bias in the findings.
In addition, data on clinical outcomes were based on claims data. Use of claims data may have caused the risk of developing diabetes to be underestimated in our study. With respect to cancer, identification of cancer cases based on claims is considered reliable in Korea because cancer codes are reviewed by NHIS and have implications for additional benefits for patients. While these data are subject to errors, claims data for the clinical outcomes evaluated are highly specific and have been widely used in population studies.
Finally, the overall association of cancer with diabetes will depend on the mix of cancer types, which may be different in different populations. Compared with Western countries, Korea has a higher frequency of thyroid and gastric cancer, and a lower frequency of prostate cancer.1 Although the overall association persisted after excluding thyroid and gastric cancers from the analyses, our findings may not be generalizable to other settings with different incidence rates for different types of cancer.
Clinical studies in cancer traditionally focus on cancer progression, cancer-related mortality, and treatment-related complications but often neglect long-term consequences of cancer and its treatment. Increased survival due to advances in cancer diagnosis and treatment,56 however, is driving the emphasis toward chronic diseases and long-term outcomes. Diabetes is a common complication in patients with cancer that increases their mortality and their risk of cardiovascular disease.7 Furthermore, because disease-specific and treatment-related factors may contribute to diabetes development in patients with cancer, it is possible that the clinical course and response to therapy in diabetes in patients with cancer may be different than in patients with type 2 diabetes who do not have cancer. Further research is needed to establish the specific causes, natural history, and optimal management strategy for diabetes that occurs in patients with cancer.
Conclusions
Cancer development was associated with an increased risk of diabetes. This increase was evident after development of pancreatic, kidney, liver, gallbladder, lung, blood, breast, stomach, and thyroid cancers. The increased hazard of diabetes was greatest in the 2 years following the development of cancer but remained elevated over almost a decade of follow-up. Physicians should remember that patients with cancer develop other clinical problems, such as diabetes, with higher frequency than individuals without cancer, and should consider routine diabetes screening in these patients.
eTable 1. Baseline characteristics of study participants (N = 494,189)
eTable 2. Fully adjusted subhazard ratios (95% confidence intervals) for incident diabetes associated with cancer development in models with all-cause mortality as a competing risk
eTable 3. Hazard ratios (95% confidence intervals) for incident diabetes associated with cancer development, excluding thyroid and stomach cancers
eFigure 1. Flowchart of study participants
eFigure 2. Hazard ratios for diabetes vs. average annual number of total, inpatient, and outpatient visits by type of cancer
eFigure 3. Graphical evaluation of the proportional hazards assumption using a log(-log) transformation of the survival probabilities (top) and scaled Schoenfeld residuals (bottom) in univariate Cox proportional hazards models for the association of cancer development with incident diabetes
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. [DOI] [PubMed] [Google Scholar]
- 3.Oh CM, Won YJ, Jung KW, et al. ; Community of Population-Based Regional Cancer Registries . Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016;48(2):436-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes HM, Nguyen HT, Nayak P, Oh JH, Escalante CP, Elting LS. Chronic conditions and health status in older cancer survivors. Eur J Intern Med. 2014;25(4):374-378. [DOI] [PubMed] [Google Scholar]
- 5.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747-1757. [DOI] [PubMed] [Google Scholar]
- 6.Shin DW, Ahn E, Kim H, Park S, Kim YA, Yun YH. Non-cancer mortality among long-term survivors of adult cancer in Korea: national cancer registry study. Cancer Causes Control. 2010;21(6):919-929. [DOI] [PubMed] [Google Scholar]
- 7.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300(23):2754-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16(4):1103-1123. [DOI] [PubMed] [Google Scholar]
- 9.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. [DOI] [PubMed] [Google Scholar]
- 10.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer: a meta-analysis. JAMA. 1995;273(20):1605-1609. [PubMed] [Google Scholar]
- 11.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(22):1679-1687. [DOI] [PubMed] [Google Scholar]
- 12.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121(4):856-862. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130(7):1639-1648. [DOI] [PubMed] [Google Scholar]
- 14.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35(2):299-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipscombe LL, Chan WW, Yun L, Austin PC, Anderson GM, Rochon PA. Incidence of diabetes among postmenopausal breast cancer survivors. Diabetologia. 2013;56(3):476-483. [DOI] [PubMed] [Google Scholar]
- 16.De Bruijn KM, van Eijck CH. New-onset diabetes after distal pancreatectomy: a systematic review. Ann Surg. 2015;261(5):854-861. [DOI] [PubMed] [Google Scholar]
- 17.Singh S, Earle CC, Bae SJ, et al. Incidence of diabetes in colorectal cancer survivors. J Natl Cancer Inst. 2016;108(6):djv402. [DOI] [PubMed] [Google Scholar]
- 18.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017. 46(2):e15. [DOI] [PubMed] [Google Scholar]
- 20.National Health Insurance Service (NHIS) National Health Examination Statistical Yearbook. Seoul, Korea: NHIS; 2014. [Google Scholar]
- 21.Chun C-B, Kim S-Y, Lee J-Y, Lee S-Y. Republic of Korea: health system review. Health Syst Transit. 2009;11(7). [Google Scholar]
- 22.Korea Pharmaceutical Information Service. Korea Pharmaceutical Information. http://biz.kpis.or.kr. Accessed June 14, 2016.
- 23.Shin DW, Cho B, Guallar E. Korean National Health Insurance database. JAMA Intern Med. 2016;176(1):138. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 25.Kim KH. Comparative study on three algorithms of the ICD-10 Charlson comorbidity index with myocardial infarction patients [in Korean]. J Prev Med Public Health. 2010;43(1):42-49. [DOI] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 27.Wooldridge JE, Anderson CM, Perry MC. Corticosteroids in advanced cancer. Oncology (Williston Park). 2001;15(2):225-234. [PubMed] [Google Scholar]
- 28.Greenstein S, Ghias K, Krett NL, Rosen ST. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin Cancer Res. 2002;8(6):1681-1694. [PubMed] [Google Scholar]
- 29.Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31(25):3076-3082. [DOI] [PubMed] [Google Scholar]
- 30.Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15(5):469-474. [DOI] [PubMed] [Google Scholar]
- 31.Lee SY, Kurita N, Yokoyama Y, et al. Glucocorticoid-induced diabetes mellitus in patients with lymphoma treated with CHOP chemotherapy. Support Care Cancer. 2014;22(5):1385-1390. [DOI] [PubMed] [Google Scholar]
- 32.Harris D, Barts A, Connors J, et al. Glucocorticoid-induced hyperglycemia is prevalent and unpredictable for patients undergoing cancer therapy: an observational cohort study. Curr Oncol. 2013;20(6):e532-e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowas SR, Marks D, Malempati S. Prevalence of transient hyperglycemia during induction chemotherapy for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;52(7):814-818. [DOI] [PubMed] [Google Scholar]
- 34.Feng JP, Yuan XL, Li M, et al. Secondary diabetes associated with 5-fluorouracil-based chemotherapy regimens in non-diabetic patients with colorectal cancer: results from a single-centre cohort study. Colorectal Dis. 2013;15(1):27-33. [DOI] [PubMed] [Google Scholar]
- 35.Mohn A, Di Marzio A, Capanna R, Fioritoni G, Chiarelli F. Persistence of impaired pancreatic beta-cell function in children treated for acute lymphoblastic leukaemia. Lancet. 2004;363(9403):127-128. [DOI] [PubMed] [Google Scholar]
- 36.Ariaans G, de Jong S, Gietema JA, Lefrandt JD, de Vries EG, Jalving M. Cancer-drug induced insulin resistance: innocent bystander or unusual suspect. Cancer Treat Rev. 2015;41(4):376-384. [DOI] [PubMed] [Google Scholar]
- 37.Hwangbo Y, Lee EK. Acute hyperglycemia associated with anti-cancer medication. Endocrinol Metab (Seoul). 2017;32(1):23-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida H, Imamura T, Saito AM, et al. ; Japan Association of Childhood Leukemia Study . Protracted administration of L-asparaginase in maintenance phase is the risk factor for hyperglycemia in older patients with pediatric acute lymphoblastic leukemia. PLoS One. 2015;10(8):e0136428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer. increased risk associated with radiation therapy: a report for the Childhood Cancer Survivor study. Arch Intern Med. 2009;169(15):1381-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davidson J, Wilkinson A, Dantal J, et al. ; International Expert Panel . New-onset diabetes after transplantation: 2003 international consensus guidelines. proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003;75(10)(suppl):SS3-SS24. [DOI] [PubMed] [Google Scholar]
- 41.Lipscombe LL, Fischer HD, Yun L, et al. Association between tamoxifen treatment and diabetes: a population-based study. Cancer. 2012;118(10):2615-2622. [DOI] [PubMed] [Google Scholar]
- 42.Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14(11):754-762. [DOI] [PubMed] [Google Scholar]
- 43.Honors MA, Kinzig KP. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J Cachexia Sarcopenia Muscle. 2012;3(1):5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153-166. [DOI] [PubMed] [Google Scholar]
- 45.Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gornik I, Vujaklija-Brajkovic A, Renar IP, Gasparovic V. A prospective observational study of the relationship of critical illness associated hyperglycaemia in medical ICU patients and subsequent development of type 2 diabetes. Crit Care. 2010;14(4):R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shirakawa S, Matsumoto I, Toyama H, et al. Pancreatic volumetric assessment as a predictor of new-onset diabetes following distal pancreatectomy. J Gastrointest Surg. 2012;16(12):2212-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Vathaire F, El-Fayech C, Ben Ayed FF, et al. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: a retrospective cohort study. Lancet Oncol. 2012;13(10):1002-1010. [DOI] [PubMed] [Google Scholar]
- 50.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10(1):88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15(3):280-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elkrief L, Rautou PE, Sarin S, Valla D, Paradis V, Moreau R. Diabetes mellitus in patients with cirrhosis: clinical implications and management. Liver Int. 2016;36(7):936-948. [DOI] [PubMed] [Google Scholar]
- 53.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7(9):735-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shehab-Eldin W, Shoeb S, Khamis S, Salah Y, Shoker A. Susceptibility to insulin resistance after kidney donation: a pilot observational study. Am J Nephrol. 2009;30(4):371-376. [DOI] [PubMed] [Google Scholar]
- 55.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17(8):328-336. [DOI] [PubMed] [Google Scholar]
- 56.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20(10):1996-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline characteristics of study participants (N = 494,189)
eTable 2. Fully adjusted subhazard ratios (95% confidence intervals) for incident diabetes associated with cancer development in models with all-cause mortality as a competing risk
eTable 3. Hazard ratios (95% confidence intervals) for incident diabetes associated with cancer development, excluding thyroid and stomach cancers
eFigure 1. Flowchart of study participants
eFigure 2. Hazard ratios for diabetes vs. average annual number of total, inpatient, and outpatient visits by type of cancer
eFigure 3. Graphical evaluation of the proportional hazards assumption using a log(-log) transformation of the survival probabilities (top) and scaled Schoenfeld residuals (bottom) in univariate Cox proportional hazards models for the association of cancer development with incident diabetes