This cohort study determines whether circulating tumor cell status is associated with radiotherapeutic benefit in terms of overall, local recurrence-free, and disease-free survival in patients with early-stage breast cancer.
Key Points
Question
Does circulating tumor cell status predict local recurrence or survival benefit of adjuvant radiotherapy in early-stage breast cancer?
Findings
In these cohort studies of 1697 patients from a large national database and 1516 patients from a randomized clinical trial with early-stage breast cancer who were evaluated for circulating tumor cell status, patients with at least 1 circulating tumor cell and who received radiotherapy after breast-conserving surgery had significantly longer local recurrence-free survival, disease-free survival, and overall survival compared with those who did not receive radiotherapy. Patients without circulating tumor cells did not experience longer survival outcomes after radiotherapy.
Meaning
Circulating tumor cell status may be an important predictive clinical marker for the benefit of radiotherapy in patients with early-stage breast cancer.
Abstract
Importance
Circulating tumor cells (CTCs) represent the liquid component of solid tumors and are a surrogate marker for residual cancer burden. Although CTC status is prognostic of recurrence and death in breast cancer, its role in guiding clinical management remains unknown.
Objective
To determine whether CTC status is predictive of radiotherapeutic benefit in early-stage breast cancer.
Design, Setting, and Participants
The cohort studies in the present analysis included patients with stages pT1 to pT2 and pN0 to pN1 breast cancer and known CTC status from the National Cancer Database (NCDB) and the multicenter phase 3 SUCCESS clinical trial. Multivariable parametric accelerated failure time models were used to evaluate the association of CTC status and radiotherapy (RT) with survival outcomes. Data were collected from January 1, 2004, through December 31, 2014, from the NCDB cohort. The SUCCESS trial collected data from September 1, 2005, through September 30, 2013. The analyses were completed from November 1, 2016, through December 17, 2017.
Exposure
Adjuvant RT.
Main Outcomes and Measures
Overall survival (OS), local recurrence-free survival (LRFS), and disease-free survival (DFS).
Results
A total of 1697 patients from the NCDB (16 men [0.9%] and 1681 women [99.1%]; median age, 63 years; interquartile range, 53-71 years) and 1516 patients from the SUCCESS clinical trial (median age, 52 years; interquartile range, 45-60 years) were identified. Circulating tumor cells were detected in 399 patients (23.5%) in the NCDB cohort and 294 (19.4%) in the SUCCESS cohort. The association of RT with survival was dependent on CTC status within the NCDB cohort (4-year OS, 94.9% for CTC-positive RT vs 88.0% for CTC-positive non-RT vs 93.9% for CTC-negative RT vs 93.4% for CTC-negative non-RT groups; P < .001) and 5-year DFS within the SUCCESS cohort (88.0% for CTC-positive RT vs 75.2% for CTC-positive non-RT vs 92.3% for CTC-negative RT vs 88.3% for CTC-negative non-RT; P = .04). In the NCDB cohort, RT was associated with longer OS in patients with CTCs (time ratio [TR], 2.04; 95% CI, 1.55-2.67; P < .001), but not in patients without CTCs (TR, 0.80; 95% CI, 0.52-1.25; P = .33). In the SUCCESS cohort, CTC-positive patients treated with RT exhibited longer LRFS (TR, 2.73; 95% CI, 1.62-4.80; P < .001), DFS (TR, 3.03; 95% CI, 2.22-4.13; P < .001), and OS (TR, 1.83; 95% CI, 1.23-2.72; P = .003). Among patients from both cohorts who underwent breast-conserving surgery, RT was associated with longer OS in patients with CTCs (TR, 4.37; 95% CI, 2.71-7.05; P < .001) but not in patients without CTCs (TR, 0.87; 95% CI, 0.47-1.62; P = .77). Radiotherapy was not associated with OS after mastectomy in CTC-positive or CTC-negative patients.
Conclusions and Relevance
Treatment with RT was associated with longer LRFS, DFS, and OS in patients with early-stage breast cancer and detectable CTCs. These results are hypothesis generating; a prospective trial evaluating CTC-based management for RT after breast-conserving surgery in women with early-stage breast cancer is warranted.
Introduction
Of the 255 000 new breast cancer diagnoses made in the United States annually, more than 60% are confined to the breast.1 Despite improving survival rates, 20% to 30% of patients with primary breast cancer will experience locoregional or distant disease recurrence.2 Prospective identification of patients at higher risk of recurrence remains challenging owing to imperfect risk stratification. Although multiple-gene expression panels provide predictive information regarding the benefit of chemotherapy in patients with estrogen receptor (ER)–positive breast cancer, similar advances have not been achieved for the benefit of radiotherapy (RT).3
Circulating tumor cells (CTCs) represent the liquid component of solid tumors and indicate the presence of residual disease. Circulating tumor cells are detected in 10% to 30% of patients with nonmetastatic breast cancer.4,5,6,7 Positive CTC status has been validated as prognostic of recurrence-free survival (RFS), breast cancer–specific survival, and overall survival (OS) in breast cancer.6,7,8,9,10,11,12 The detection of even a single CTC has been shown to be prognostic of shorter RFS and OS.7,10,13,14 Circulating tumor cell status has also been shown to be prognostic of failure of locoregional and adjuvant systemic treatments.9,15,16,17 Furthermore, CTC status has been demonstrated to be prognostic of metastasis and survival in low-risk patients with node-negative disease who did not receive adjuvant therapy.12 No data are yet available regarding CTC status as a predictive factor of the benefit of RT in early-stage breast cancer. To test this concept, survival analyses were performed to test the associations and interactions of CTC status and RT with survival outcomes in patients from the National Cancer Database (NCDB). An external validation was performed using an independent patient cohort from the German SUCCESS (Simultaneous Study of Gemcitabine-Docetaxel Combination Adjuvant Treatment as well as Extended Bisphosphonate and Surveillance) clinical trial.7
Methods
Data Sources
The NCDB is a nationwide, facility-based comprehensive oncology database established by the Commission on Cancer of the American College of Surgeons and the American Cancer Society in 1989.18 The institutional review board of Northwestern University Feinberg School of Medicine determined that local institutional review board approval and informed consent is not required for NCDB analyses. The SUCCESS trial (NCT02181101) was a prospective, multicenter, phase 3 trial of women with nonmetastatic breast cancer and at least 1 high-risk factor randomized to 1 of 2 chemotherapy regimens.7,19 Defining the prognostic significance of CTCs was a study objective.7 The SUCCESS trial was approved by 37 German ethical boards (lead ethical board: Ludwig-Maximilians-University, Munich) and conducted in accordance with the Declaration of Helsinki.20 Deidentified data from the SUCCESS cohort were provided upon request by W.J. for this post hoc secondary analysis.7 Written informed consent was obtained for each patient. Only deidentified data from the SUCCESS cohort were obtained for this secondary analysis, which according to the Common Rule, is not human subjects research and does not require institutional review board approval.7
In accordance with the SUCCESS trial, blood samples were obtained before adjuvant therapy for evaluation of CTCs using CellSearch.22 CellSearch is the only platform for CTC detection that is approved by the US Food and Drug Administration for estimation of prognosis in metastatic disease. To date, no evidence supports a role for CTCs in predicting response to therapy. Further information on the design of the SUCCESS trial design has been described previously7 and is described briefly in the eMethods in the Supplement.
Cohort Selection and Variable Definition
Patients with stages pT1 to pT2 and pN0 to pN1 (microscopic) breast cancer and listed as having positive or negative CTC status in the NCDB from January 1, 2004, to December 31, 2014, and patients with pT1 to pT2 and pN0 to pN1 breast cancer from the SUCCESS trial were included from September 1, 2005, through September 30, 2013 (eFigure 1 in the Supplement). The NCDB began reporting CTC status in 2004 after approval of CellSearch by the US Food and Drug Administration, but most participants (97.7%) were diagnosed after 2010.
Statistical Analyses
Data were analyzed from November 1, 2016, through December 17, 2017. Baseline characteristics between patient groups were compared using the Fisher exact test for categorical data, the Mann-Whitney test for nonnormally distributed numeric or ordinal data, and the 2-tailed t test or analysis of variance (ANOVA) for normally distributed data. Multivariable binomial logistic regression was performed to calculate adjusted odds ratios for factors associated with CTC status within the NCDB cohort. Because median survival was not observed, restricted mean survival times and 4-year (NCDB) or 5-year (SUCCESS) survival proportions were estimated using the Kaplan-Meier method and compared with the log-rank test. Survival curves were plotted as unadjusted Kaplan-Meier estimates.
We performed multivariable analyses of the NCDB and SUCCESS overall cohorts. To have enough patients to perform subset analyses of women grouped by type of surgery, the 2 overall cohorts were pooled for subset analyses. Two-to-one (NCDB) or 3-to-1 (SUCCESS) nearest-neighbor propensity score matching was performed to generate well-balanced, matched cohorts of patients based on CTC status (eTables 1-4 in the Supplement).23
Multivariable parametric accelerated failure time models using the generalized gamma distribution were used to evaluate the association of CTC status and RT with local RFS (LRFS), disease-free survival (DFS), and/or OS. This model was chosen in place of the Cox proportional hazards model owing to violation of the proportional hazards assumption among CTC-positive patients who received vs did not receive RT. When the non–proportional hazards assumption is present, the parametric accelerated failure time model provides better goodness-of-fit of the model to the observed data and therefore provides more robust statistical inference.24,25,26,27 The accelerated failure time model estimates the time ratio (TR), which describes the factor by which the time to event is associated between 2 groups. A TR of greater than 1 therefore describes longer survival.
Covariates used in analyses were selected a priori based on clinical knowledge and availability (eTables 5-10 in the Supplement). Use of chemotherapy was included as a covariate in the multivariable analyses of the NCDB cohort. Type of chemotherapy was included as a covariate in the multivariable analyses of the SUCCESS trial. Because the primary outcomes of the SUCCESS trial have yet to be published, survival estimates associated with type of chemotherapy were not listed. Type of surgery was omitted in the analyses of the individual cohorts owing to its strong association with receipt of RT, but this factor was addressed by performing subset analyses of the pooled cohort by type of surgery. Models including surgery as a covariate were also performed and did not change the findings as reported.
We combined CTC status and RT in a composite variable to calculate survival estimates relative to the reference level (CTC positive without RT). The main effects and pairwise interaction terms of CTC status and RT were tested for all models. Subgroup analyses by CTC status were performed. Propensity score–matched and inverse probability–weighted cohort accelerated failure time multivariable analyses were also performed to reduce treatment assignment biases related to measured covariates.23
All statistical tests were 2-tailed. To test the robustness of the observed results, we explored the sensitivity of TR estimates to a possible unmeasured confounder.28 Statistical analyses were performed using R software (version 3.3.3) with the MatchIt, survival, flexsurv, Hmisc, and rms packages.25,29,30,31,32,33
Results
Characteristics of Cohorts
We included 1697 patients from the NCDB (16 men [0.9%] and 1681 women [99.1%]; median age, 63 years; interquartile range, 53-71 years) and 1516 patients from the SUCCESS clinical trial (median age, 52 years; interquartile range, 45-60 years). We identified CTCs in 399 patients (23.5%) in the NCDB cohort and 294 (19.4%) in the SUCCESS cohort.5,9,34,35 Baseline characteristics of the NCDB and SUCCESS cohorts compared by CTC status are shown in Table 1 and eTable 11 in the Supplement. Inherent to the protocol design, the SUCCESS cohort is a patient population with higher-risk disease than the NCDB cohort.7 Within the NCDB cohort, CTC positivity was associated with younger age, lobular or mixed histologic findings, micrometastatic nodal disease, positive lymphovascular invasion, ER-positive status, ERBB2 (formerly Her2/neu) overexpression, and insurance through the government or no insurance (eTable 12 in the Supplement). In the NCDB cohort, CTC-positive patients without RT were not significantly older than those with RT (median age, 59.5 vs 61.0 years; 1-way ANOVA, P = .81) and did not have greater comorbidity scores (comorbidity scores 1-2, 12.8% vs 13.7%; Kruskal-Wallis, P = .87). Radiotherapy was not more commonly omitted against recommendation in patients with CTCs vs without CTCs (10.3% vs 7.7%; Fisher exact test, P = .36), and higher comorbidity scores were not more common (comorbidity scores 1-2, 13.3% vs 19.3%; Kruskal-Wallis, P = .65). Baseline characteristics of patients within the pooled cohort of the NCDB and SUCCESS trials compared by receipt of RT are presented for all patients and for subsets of patients by type of surgery in eTables 13 and 14 in the Supplement.
Table 1. Characteristics of Patients From the NCDB and SUCCESS Cohorts Grouped by CTC Statusa.
| Variable | NCDB Cohort | SUCCESS Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| CTC Negative (n = 1298) | CTC Positive (n = 399) | P Value | SMD | CTC Negative (n = 1222) | CTC Positive (n = 294) | P Value | SMD | |
| Age, median (IQR), y | 63.0 (53.0-71.0) | 61.0 (52.0-70.0) | .02 | 0.12 | 52.0 (45.0-60.0) | 54.0 (45.0-61.0) | .36 | 0.04 |
| Follow-up, median (IQR), mo | 37.7 (26.4-51.5) | 40.4 (28.7-54.0) | .02 | 0.14 | 64.3 (59.9-69.6) | 64.2 (59.6-72.1) | .91 | 0.02 |
| Tumor stage | ||||||||
| 1 | 1002 (77.2) | 290 (72.7) | .08 | 0.10 | 581 (47.5) | 128 (43.5) | .24 | 0.08 |
| 2 | 296 (22.8) | 109 (27.3) | 641 (52.5) | 166 (56.5) | ||||
| Nodal stage | ||||||||
| 0 | 1244 (95.8) | 366 (91.7) | .002 | 0.17 | 511 (41.8) | 127 (43.2) | .72 | 0.03 |
| Microscopic/1b | 54 (4.2) | 33 (8.3) | 711 (58.2) | 167 (56.8) | ||||
| Grade | ||||||||
| 1 | 343 (26.4) | 124 (31.1) | .14 | 0.11 | 66 (5.4) | 11 (3.7) | .47 | 0.08 |
| 2 | 592 (45.6) | 178 (44.6) | 548 (44.8) | 138 (46.9) | ||||
| 3 | 363 (28.0) | 97 (24.3) | 608 (49.8) | 145 (49.3) | ||||
| Histologic finding | ||||||||
| IDC | 1046 (80.6) | 297 (74.4) | .02 | 0.16 | 1024 (83.8) | 242 (82.3) | .79 | 0.04 |
| ILC/mixed | 182 (14.0) | 78 (19.5) | 111 (9.1) | 29 (10.0) | ||||
| Other | 70 (5.4) | 24 (6.0) | 87 (7.1) | 23 (7.8) | ||||
| ER status | ||||||||
| Positive | 1080 (83.2) | 365 (91.5) | <.001 | 0.25 | 810 (66.3) | 205 (69.7) | .27 | 0.07 |
| Negative | 218 (16.8) | 34 (8.5) | 412 (33.7) | 89 (30.3) | ||||
| PR status | ||||||||
| Positive | 950 (73.2) | 324 (81.2) | .001 | 0.19 | 768 (62.8) | 195 (66.3) | .28 | 0.07 |
| Negative | 348 (26.8) | 75 (18.8) | 454 (37.2) | 99 (33.7) | ||||
| ERBB2 status | ||||||||
| Negative | 1185 (91.3) | 345 (86.5) | .007 | 0.15 | 927 (75.9) | 224 (76.2) | .94 | 0.008 |
| Positive | 113 (8.7) | 54 (13.5) | 295 (24.1) | 70 (23.8) | ||||
| Surgery | ||||||||
| BCS | 777 (59.9) | 241 (60.4) | .89 | 0.01 | 940 (76.9) | 219 (74.5) | .40 | 0.06 |
| Mastectomy | 521 (40.1) | 158 (39.6) | 282 (23.1) | 75 (25.5) | ||||
| Radiotherapy | ||||||||
| No | 563 (43.4) | 172 (43.1) | .95 | 0.005 | 216 (17.7) | 46 (15.6) | .44 | 0.05 |
| Yes | 735 (56.6) | 227 (56.9) | 1006 (82.3) | 248 (84.4) | ||||
| Hormone therapy | ||||||||
| No | 372 (28.7) | 86 (21.5) | .006 | 0.16 | 354 (29.0) | 84 (28.6) | .94 | 0.009 |
| Yes | 926 (71.3) | 313 (78.4) | 868 (71.0) | 210 (71.4) | ||||
| Chemotherapy | ||||||||
| No | 902 (69.5) | 279 (69.9) | .92 | 0.009 | 0 | 0 | >.99 | <0.001 |
| Yes | 396 (30.5) | 120 (30.1) | 1222 (100) | 294 (100) | ||||
| Trastuzumabc | ||||||||
| No | NA | NA | NA | NA | 962 (78.7) | 234 (79.6) | .81 | 0.02 |
| Yes | NA | NA | NA | NA | 260 (21.3) | 60 (20.4) | ||
Abbreviations: BCS, breast-conserving surgery; CTC, circulating tumor cell; ER, estrogen receptor; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; IQR, interquartile range; LVI, lymphovascular invasion; NA, not applicable; NCDB, National Cancer Database; PR, progesterone receptor; SMD, standardized mean difference; SUCCESS, Simultaneous Study of Gemcitabine-Docetaxel Combination Adjuvant Treatment as well as Extended Bisphosphonate and Surveillance.
Significance determined by Fisher exact test or Kruskal-Wallis test. Percentages have been rounded and may not total 100.
Defined as microscopic in the NCDB cohort.
Data were not available for the NCDB cohort.
Kaplan-Meier Estimates
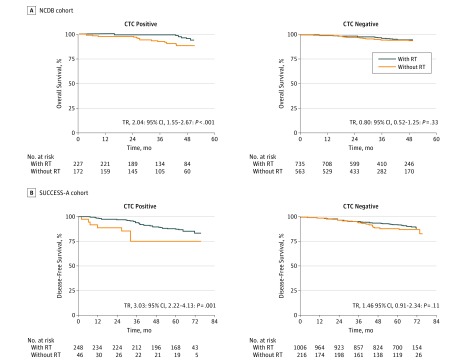
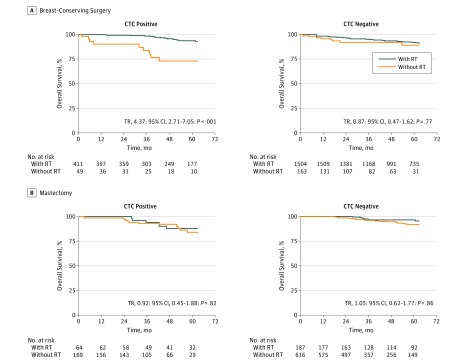
We calculated unadjusted estimates of survival proportions and restricted mean survival times for all analyses (Table 2 and eTables 5-10 in the Supplement). Survival curves were plotted as unadjusted Kaplan-Meier estimates (Figure 1, Figure 2, and eFigure 2 in the Supplement). The CTC-positive patients without RT had significantly shorter 4-year OS compared with CTC-positive patients with RT and CTC-negative patients without and with RT in the NCDB cohort (88.0%; 95% CI, 82.3%-94.2%; P = .005) and shorter 5-year LRFS (85.7%; 95% CI, 73.6%-99.8%; P = .003), DFS (75.2%; 95% CI, 61.5%-91.9%; P = .002), and OS (84.5%; 95% CI, 72.7%-98.2%; P = .008) in the SUCCESS cohort. Similarly, CTC-positive patients without RT from the pooled cohort who underwent breast-conserving surgery (BCS) had significantly shorter 5-year OS (73.3%; 95% CI, 59.4%-90.3%; P < .001) compared with CTC-positive patients with RT and CTC-negative patients without and with RT (Table 2 and eTable 9 in the Supplement). No significant difference was noted in 5-year OS among patients who underwent mastectomy, regardless of CTC status or receipt of RT (84.3%; 95% CI, 76.0%-93.6%; P = .30) (Table 2 and eTable 10 in the Supplement).
Table 2. Kaplan-Meier Estimates and Multivariable Survival Models.
| CTC-RT Group | Kaplan-Meier Estimate, All Patients | AFT Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| All Patients | Matched Cohorts | |||||||
| No. of Patients (No. of Events) | RMS (95% CI), moa | Survival Proportion, % (95% CI)b | Log-Rank P Value | TR (95% CI) | P Valuec | TR (95% CI) | P Valuec | |
| NCDB Cohortd | ||||||||
| CTC-positive, no RT | 172 (18) | 78.6 (74.2-83.0) | 88.0 (82.3-94.2) | .005 | 1 [Reference] | NA | 1 [Reference] | NA |
| CTC-positive, RT | 227 (7) | 85.5 (83.3-87.6) | 94.9 (90.8-99.1) | 2.34 (1.26-4.35) | .006 | 1.65 (1.26-2.17) | <.001 | |
| CTC-negative, no RT | 563 (34) | 82.3 (80.1-84.4) | 93.4 (91.1-95.9) | 1.93 (1.13-3.30) | .02 | 1.90 (1.46-2.48) | <.001 | |
| CTC-negative, RT | 735 (33) | 82.9 (79.5-86.3) | 93.9 (91.7-96.1) | 1.72 (1.07-2.74) | .02 | 1.59 (1.23-2.07) | <.001 | |
| SUCCESS Cohorte | ||||||||
| DFSf | ||||||||
| CTC-positive, no RT | 46 (8) | 73.7 (62.9-84.4) | 75.2 (61.5-91.9) | .002 | 1 [Reference] | NA | 1 [Reference] | NA |
| CTC-positive, RT | 248 (35) | 81.1 (78.0-84.2) | 88.0 (83.9-92.4) | 3.77 (1.77-8.03) | <.001 | 2.83 (2.02-3.98) | <.001 | |
| CTC-negative, no RT | 216 (23) | 82.0 (78.4-85.7) | 88.3 (83.5-93.4) | 3.28 (1.51-7.12) | .002 | 2.16 (1.41-3.33) | <.001 | |
| CTC-negative, RT | 1006 (87) | 84.9 (83.6-86.3) | 92.3 (90.5-94.0) | 5.05 (2.47-10.33) | <.001 | 3.61 (2.58-5.03) | <.001 | |
| LRFSg | ||||||||
| CTC-positive, no RT | 46 (4) | 82.8 (74.1-91.5) | 85.7 (73.6-99.8) | .003 | 1 [Reference] | NA | 1 [Reference] | NA |
| CTC-positive, RT | 248 (7) | 90.3 (88.8-91.8) | 98.2 (96.4-100.0) | 3.59 (1.45-8.94) | .005 | 4.35 (2.60-7.28) | <.001 | |
| CTC-negative, no RT | 216 (7) | 89.8 (88.0-91.6) | 96.2 (93.3-99.2) | 2.65 (1.11-6.36) | .03 | 2.12 (1.21-3.72) | .008 | |
| CTC-negative, RT | 1006 (23) | 90.1 (89.2-91.1) | 98.1 (97.3-99.0) | 4.31 (1.88-9.86) | <.001 | 5.99 (3.36-10.70) | <.001 | |
| OSh | ||||||||
| CTC-positive, no RT | 46 (5) | 81.5 (73.2-89.9) | 84.5 (72.7-98.2) | .008 | 1 [Reference] | NA | 1 [Reference] | NA |
| CTC-positive, RT | 248 (20) | 86.8 (84.8-88.9) | 92.4 (88.9-95.9) | 2.73 (1.16-6.41) | .02 | 2.05 (1.41-2.97) | <.001 | |
| CTC-negative, no RT | 216 (11) | 87.4 (84.9-89.9) | 95.2 (92.0-98.5) | 2.92 (1.22-6.99) | .02 | 1.99 (1.21-3.30) | .007 | |
| CTC-negative, RT | 1006 (41) | 88.9 (88.0-89.8) | 96.7 (95.6-97.9) | 4.04 (1.80-9.08) | <.001 | 2.89 (1.96-4.25) | <.001 | |
| Pooled Cohort | ||||||||
| BCSi | ||||||||
| CTC-positive, no RT | 49 (9) | 73.6 (63.7-83.5) | 73.3 (59.4-90.3) | <.001 | 1 [Reference] | 1 [Reference] | ||
| CTC-positive, RT | 411 (20) | 87.2 (85.6-88.8) | 93.6 (90.8-96.6) | 3.42 (1.47-8.00) | .004 | 3.42 (2.17-5.40) | <.001 | |
| CTC-negative, no RT | 163 (8) | 86.0 (82.7-89.3) | 92.7 (87.3-98.3) | 3.10 (1.33-7.24) | .009 | 2.82 (1.65-4.81) | <.001 | |
| CTC-negative, RT | 1554 (64) | 87.8 (87.0-88.6) | 95.5 (94.3-96.6) | 3.70 (1.69-8.09) | <.001 | 3.41 (2.21-5.28) | <.001 | |
| Mastectomyj | ||||||||
| CTC-positive, no RT | 169 (14) | 82.6 (78.7-86.5) | 84.3 (76.0-93.6) | .30 | 1 [Reference] | NA | 1 [Reference] | NA |
| CTC-positive, RT | 64 (7) | 82.0 (76.1-87.9) | 88.3 (79.9-97.6) | 0.90 (0.51-1.58) | .64 | 0.96 (0.70-1.31) | .78 | |
| CTC-negative, no RT | 616 (37) | 84.0 (81.8-86.2) | 91.0 (87.8-94.2) | 1.34 (0.94-1.92) | .17 | 1.38 (1.14-1.68) | .001 | |
| CTC-negative, RT | 187 (10) | 85.1 (82.1-88.1) | 95.8 (92.6-99.2) | 1.30 (0.80-2.11) | .29 | 1.66 (1.16-2.37) | .006 | |
Abbreviations: AFT, accelerated time failure; BCS, breast-conserving surgery; CTC, circulating tumor cell; DFS, disease-free survival; LRFS, local recurrence-free survival; NCDB, National Cancer Database; NA, not applicable; OS, overall survival; RMS, restricted mean survival; RT, radiotherapy; SUCCESS, Simultaneous Study of Gemcitabine-Docetaxel Combination Adjuvant Treatment as well as Extended Bisphosphonate and Surveillance; TR, time ratio.
Measured as overall survival in the NCDB and pooled cohorts.
Measured as 4-year survival in the NCDB cohort and 5-year survival in the SUCCESS and pooled cohorts.
Determined by Wald test.
For pairwise interaction terms of CTC status and RT in the AFT multivariable analyses, P = .01 for all patients (n = 1697) and P < .001 for matched cohorts (n = 1197).
For AFT multivariable analysis, includes 1516 for all patients and 1176 for matched cohorts.
For pairwise interaction terms of CTC status and RT in the AFT multivariable analysis, P = .04 for all patients and P = .04 for matched cohorts.
For pairwise interaction terms of CTC status and RT in the AFT multivariable analysis, P = .13 for all patients and P = .20 for matched cohorts.
For pairwise interaction terms of CTC status and RT in the AFT multivariable analysis, P = .16 for all patients and P = .23 for matched cohorts.
For pairwise interaction terms of CTC status and RT in the AFT multivariable analysis, P = .04 for all patients (n = 2177) and P < .001 for matched cohorts (n = 1840).
For pairwise interaction terms of CTC status and RT in the AFT multivariable analysis, P = .82 for all patients (n = 1036) and P = .83 for matched cohorts (n = 932).
Figure 1. Unadjusted Survival Curves of the National Cancer Database (NCDB) and SUCCESS Cohorts.
Unadjusted survival curves based on Kaplan-Meier estimates for patients with primary breast cancer who were positive (left) or negative (right) for circulating tumor cells (CTCs) and treated with or without radiotherapy (RT) from the NCDB cohort for overall survival (A) and the SUCCESS clinical trial for disease-free survival (B). TR indicates time ratio.
Figure 2. Unadjusted Survival Curves of the Pooled Cohort of Patients From the National Cancer Database (NCDB) and SUCCESS Cohorts.
Unadjusted survival curves based on Kaplan-Meier estimates for patients with primary breast cancer who were positive (left) or negative (right) for circulating tumor cells (CTCs) and treated with or without radiotherapy (RT) from a pooled cohort of both cohorts for overall survival. Patients were grouped by receipt of breast-conserving surgery (A) or mastectomy (B). TR indicates time ratio.
Multivariable Survival Analyses
In the NCDB cohort, we found a significant interaction of CTC status and use of RT with 4-year OS (94.9% for CTC-positive RT vs 88.0% for CTC-positive non-RT vs 93.9% for CTC-negative RT vs 93.4% for CTC-negative non-RT; P < .001) (Table 2 and eTable 5 in the Supplement). When evaluated independently, CTC-negative patients had longer OS compared with CTC-positive patients (TR, 1.78; 95% CI, 1.34-2.36; P < .001), and patients who received RT had longer OS compared with those who did not receive RT (TR, 2.03; 95% CI, 1.52-2.71; P < .001). In subset analyses, CTC-positive patients with RT had significantly longer OS compared with CTC-positive patients without RT (TR, 2.04; 95% CI, 1.55-2.67; P < .001) (Table 3). Receipt of RT, however, was not associated with longer OS in CTC-negative patients (TR, 0.80; 95% CI, 0.52-1.25; P = .33) (Table 3 and Figure 1). There was no significant association of CTC status and 4-year OS with use of chemotherapy (93.2% for CTC-positive with chemotherapy vs 91.2% for CTC-positive without chemotherapy vs 95.0% for CTC-negative with chemotherapy vs 93.0% for CTC-negative without chemotherapy; P = .46), use of hormone therapy (93.2% for CTC-positive with hormone therapy vs 86.6% for CTC-positive without hormone therapy vs 95.5% for CTC-negative with hormone therapy vs 88.8% for CTC-negative without hormone therapy; P = .70), or tumor subtype (93.8% for CTC-positive with invasive ductal cancer vs 93.4% for CTC-negative with invasive ductal cancer vs 84.9% for CTC-positive with invasive lobular cancer vs 94.9% for CTC-negative with invasive lobular cancer; P = .24).
Table 3. Subset Analyses of Multivariable Survival Models.
| Patient Cohort by CTC and RT Statusa | All Patients | Matched Cohorts | ||
|---|---|---|---|---|
| TR (95% CI) | P Valueb | TR (95% CI) | P Valueb | |
| NCDB Cohortc | ||||
| CTC-positive: RT vs no RT | 2.60 (1.45-4.65) | .001 | 2.04 (1.55-2.67) | <.001 |
| CTC-negative: RT vs no RT | 0.86 (0.60-1.23) | .41 | 0.80 (0.52-1.25) | .33 |
| SUCCESS Cohort | ||||
| DFS | ||||
| CTC-positive: RT vs no RT | 2.38 (1.25-4.55) | .009 | 3.03 (2.22-4.13) | <.001 |
| CTC-negative: RT vs no RT | 1.58 (1.08-2.31) | .02 | 1.46 (0.91-2.34) | .11 |
| LRFS | ||||
| CTC-positive: RT vs no RT | 3.12 (1.10-8.90) | .03 | 2.73 (1.62-4.60) | <.001 |
| CTC-negative: RT vs no RT | 1.63 (0.89-2.97) | .11 | 1.68 (0.82-3.47) | .16 |
| OS | ||||
| CTC-positive: RT vs no RT | 1.98 (0.92-4.27) | .08 | 1.83 (1.23-2.72) | .003 |
| CTC-negative: RT vs no RT | 1.36 (0.93-2.00) | .11 | 1.31 (0.73-2.34) | .36 |
| Pooled Cohortsc | ||||
| Breast-conserving surgery | ||||
| CTC-positive: RT vs no RT | 6.76 (3.56-12.84) | <.001 | 4.37 (2.71-7.05) | <.001 |
| CTC-negative: RT vs no RT | 1.14 (0.59-2.22) | .69 | 0.87 (0.47-1.62) | .77 |
| Mastectomy | ||||
| CTC-positive: RT vs no RT | 0.88 (0.37-2.06) | .77 | 0.92 (0.45-1.88) | .82 |
| CTC-negative: RT vs no RT | 0.99 (0.65-1.49) | .94 | 1.05 (0.62-1.77) | .86 |
Abbreviations: CTC, circulating tumor cell; DFS, disease-free survival; LRFS, local recurrence-free survival; NCDB, National Cancer Database; OS, overall survival; RT, radiotherapy; SUCCESS, Simultaneous Study of Gemcitabine-Docetaxel Combination Adjuvant Treatment as well as Extended Bisphosphonate and Surveillance; TR, time ratio.
For each analysis, the no RT group served as the reference group.
Determined by Wald test.
Measures overall survival only.
In the SUCCESS cohort, a significant interaction of CTC status and use of RT with 5-year DFS was found (88.0% for CTC-positive RT vs 75.2% for CTC-positive non-RT vs 92.3% for CTC-negative RT vs 88.3% for CTC-negative non-RT groups; P = .04 for all patients and matched cohorts) (Table 2 and eTable 6 in the Supplement). No significant interaction of CTC status and receipt of RT with 5-year LRFS (98.2% for CTC-positive RT vs 85.7% for CTC-positive non-RT vs 98.1% for CTC-negative RT vs 96.2% for CTC-negative non-RT groups; P = .20) (Table 2 and eTable 7 in the Supplement) or with 5-year OS (92.4% for CTC-positive RT vs 84.5% for CTC-positive non-RT vs 96.7% for CTC-negative RT vs 95.2% for CTC-negative non-RT groups; P = .23) (Table 2 and eTable 8 in the Supplement) was found within the SUCCESS cohort, although these analyses were likely underpowered for testing the interaction terms. Among all patients within the SUCCESS cohort, CTC-negative status was independently associated with significantly longer DFS (TR, 2.18; 95% CI, 1.42-3.35; P < .001), LRFS (TR, 2.12; 95% CI, 1.21-3.72; P = .008), and OS (TR, 1.97; 95% CI, 1.20-3.23; P = .007) (Table 3). Receipt of RT was also found to be independently associated with significantly longer DFS (TR, 2.89; 95% CI, 2.06-4.04; P < .001), LRFS (TR, 4.35; 95% CI, 2.60-7.28; P < .001), and OS (TR, 1.95; 95% CI, 1.36-2.79; P < .001). In subset analyses, CTC-positive patients with RT had significantly longer DFS (TR, 3.03; 95% CI, 2.22-4.13; P < .001), LRFS (TR, 2.73; 95% CI, 1.62-4.60; P < .001), and OS (TR, 1.83; 95% CI, 1.23-2.72; P = .003) compared with CTC-positive patients without RT (Table 3). CTC-negative patients with RT did not experience longer LRFS (TR, 1.68; 95% CI, 0.82-3.47; P = .16) or OS (TR, 1.31; 95% CI, 0.73-2.34; P = .36) compared with CTC-negative patients without RT but had nonsignificantly longer DFS (TR, 1.46; 95% CI, 0.91-2.34; P = .11) and a small absolute difference in DFS (Table 3).
In a pooled analysis of patients from the NCDB and SUCCESS cohorts who underwent BCS, we found a significant interaction of CTC status and use of RT with OS (93.6% for CTC-positive RT vs 73.3% for CTC-positive non-RT vs 95.5% for CTC-negative RT vs 92.7% for CTC-negative non-RT groups; P < .001) (Table 2 and eTable 9 in the Supplement). Among patients who underwent mastectomy, however, no significant interaction between CTC status and receipt of RT with OS was found (88.3% for CTC-positive RT vs 84.3% for CTC-positive non-RT vs 95.8% for CTC-negative RT vs 91.0% for CTC-negative non-RT groups; P = .83) (Table 2 and eTable 10 in the Supplement). After BCS, CTC-positive patients with RT experienced longer OS compared with CTC-positive patients without RT (TR, 4.37; 95% CI, 2.71-7.05; P < .001) (Table 3). However, CTC-negative patients with RT did not have longer OS compared with CTC-negative patients without RT (TR, 0.87; 95% CI, 0.47-1.62; P = .77) (Table 3 and Figure 2). Of the patients who underwent mastectomy, receipt of RT was not associated with an OS benefit in CTC-positive (TR, 0.92; 95% CI, 0.45-1.88; P = .82) or CTC-negative (TR, 1.05; 95% CI, 0.62-1.77; P = .86) patients (Table 3 and Figure 2). Sensitivity analyses demonstrated that TR estimates for longer survival among patients with CTC-positive status who received RT were robust when tested by the introduction of possible unmeasured confounding (eTable 15 in the Supplement).
Discussion
In this analysis of a large cohort of patients with early-stage breast cancer and known CTC status from the NCDB, CTC status was predictive of a benefit of RT for OS. This finding was validated and extended in an independent cohort of patients from the German phase 3 SUCCESS trial, in which CTC status was predictive of a benefit of RT for LRFS, DFS, and OS in patients treated with surgery followed by systemic therapy. In both cohorts, CTC-positive patients who received RT had longer OS compared with CTC-positive patients who did not receive RT. In the SUCCESS trial, CTC-positive women derived a large benefit in LRFS and DFS from RT, whereas CTC-negative women did not. In a pooled analysis of patients from both cohorts who underwent BCS, receipt of RT was associated with longer OS in CTC-positive patients but not in CTC-negative patients. Among patients who received mastectomy, however, CTC status was not associated with an OS benefit from RT. Determination of CTC status may therefore be useful as a predictive marker for potential benefit from RT in patients with early-stage breast cancer who receive BCS.
Postoperative RT is the standard of care for most patients undergoing BCS.3 The benefit of whole-breast RT on locoregional recurrence has been consistently demonstrated in prospective clinical trials,36,37 whereas a comprehensive meta-analysis of prospective trials revealed an OS advantage associated with RT.2 More recently, investigators have moved toward identifying low-risk patients for whom RT may not provide a meaningful clinical benefit,38,39 with the goal of reducing the burden of treatment-related morbidity while preserving the survival advantage associated with RT for high-risk disease. In the CALGB 9343 trial,38 patients older than 70 years with early-stage, ER-positive breast cancer derived no survival benefit after RT compared with patients for whom RT was omitted. Similar findings have been seen in parallel trials that included younger patient populations.39,40,41,42 Although clinicopathologic factors can help to risk stratify patients to inform potential benefit of RT, no such factor has been shown to predict response to RT.
Prospective clinical trials are currently under way to determine the efficacy of biological markers as predictive of benefit for RT in this context, including multigene assays, such as Oncotype DX43 and Prosigna,44 and the proliferation marker Ki67.45 Retrospective analyses have also evaluated molecular signatures of radiosensitivity for prediction of locoregional recurrence in breast cancer.43,44,45,46,47,48,49,50,51 Despite its known prognostic efficacy for recurrence and death in early-stage breast cancer, evaluation of CTC status is not currently included in clinical practice guidelines, owing to a lack of evidence supporting the predictive value of CTC status in guiding clinical management decisions.3,52 The present study suggests that CTC status may be useful alongside standard clinicopathologic variables to better select patients with early-stage breast cancer for whom RT may be of benefit. This finding may be particularly relevant for patients with lower-risk disease who are considering omission of RT after BCS. Most patients analyzed within the pooled cohort who underwent BCS and did not receive RT were older and had low-grade, ER-positive, ERBB2-nonamplified tumors that were smaller than 2 cm and did not have nodal involvement, reflecting a favorable patient population for whom omission of RT may reasonably be considered.
Although the biological underpinning of the predictive value of CTC status with RT benefit is not immediately clear, CTC positivity may indicate a higher burden of locoregional residual disease or an association with endocrine resistance. In support of this, in the SUCCESS trial, the risk of local recurrence was higher in CTC-positive women who did not receive RT. Although CTC status was associated with benefit of RT among patients who underwent BCS, it was not associated with benefit of RT among patients who underwent mastectomy. This finding suggests that benefit of RT among CTC-positive patients may be limited to those who undergo BCS, perhaps owing to the higher burden of residual local disease in these patients. Additional studies are required to elucidate the potential mechanisms underlying the association of positive CTCs with increased risk of local recurrence.
Limitations
The NCDB is a valuable resource that permits analysis of rare cancers, treatment paradigms, and prognostic variables.18 Although the size of the NCDB cohort used here is large compared with most other studies evaluating CTC status, this analysis remains limited by the small proportion of patients within the NCDB who underwent evaluation for CTCs. Importantly, CTC-positive patients who did not receive RT were not found to be older or to have higher comorbidity scores, nor was RT more commonly omitted against recommendation in CTC-positive patients. The small sample size is a particular limitation when analyzing patients with early-stage breast cancer owing to infrequent recurrences or death, further limiting the statistical power of the analyses. For instance, negative associations in the present analysis may be underpowered for very small differences. Although CTC-positive status appeared predictive of benefit of RT on survival early during follow-up, a small survival advantage associated with use of RT may emerge after longer follow-up in CTC-negative women. In addition, potential biases may exist despite the statistical methods used to reduce bias and adjust for unmeasured confounding. For example, currently used methods for identifying CTCs that rely on the expression of epithelial cell adhesion molecules may underestimate detection of CTCs with nonepithelial phenotypes, such as circulating tumor stem cells or CTCs that have undergone epithelial-to-mesenchymal transition.53,54 Furthermore, CTCs are known to demonstrate remarkable molecular heterogeneity with significant intrapatient and interpatient variability.55,56,57 Specific genotypic and phenotypic features of CTCs may therefore further inform their prognostic and predictive potential. Of importance, however, the observed associations within these analyses were robust to plausible levels of unmeasured confounding.
The particular limitations of the NCDB cohort are less concerning given that we observed consistent results by performing an external validation using data from the SUCCESS trial in which all patients were prospectively evaluated for CTC status. The SUCCESS trial is, to date, the largest clinical trial in which CTC status was evaluated in women with early-stage breast cancer. This trial validated CTC status as independently prognostic of longer survival in early-stage breast cancer.7 Despite the limitations of the NCDB and SUCCESS cohort analyses as well as the modest differences between the inclusion criteria, both cohorts gave remarkably similar results, producing a robust external validation.
Conclusions
These analyses provide initial evidence for the use of CTC status as a predictive marker for benefit of RT in patients with early-stage breast cancer. To date, no prognostic test has been validated in phase 3 trials as a predictive clinical marker of therapeutic benefit of RT. In addition, this study is the first, to our knowledge, to demonstrate that CTC status may predict response to a locoregional treatment in early-stage breast cancer. The results from this study are hypothesis generating and contribute to the growing body of literature supporting prospective clinical trials of CTC status as a predictive marker for treatment. At present, no trials have used CTC status to guide management in early breast cancer. A prospective trial evaluating CTC-based management for RT in early-stage breast cancer merits consideration, with the ultimate goal of refining treatment recommendations to improve clinical outcomes, quality of life, and value of care.
eMethods. Participants, Materials, Outcomes, and Analysis
eFigure 1. CONSORT Diagram of Patient Selection
eFigure 2. Unadjusted Survival Curves for the SUCCESS Cohorts
eTable 1. Characteristics of the Matched Cohorts of All Patients From the NCDB Cohort Grouped by CTC Status
eTable 2. Characteristics of the Matched Cohorts of Patients From the SUCCESS Cohort Grouped by CTC Status
eTable 3. Characteristics of the Matched Cohorts of Patients Who Underwent Breast-Conserving Surgery From the Pooled Cohort
eTable 4. Characteristics of the Matched Cohorts of Patients Who Underwent Mastectomy From the Pooled Cohort
eTable 5. Kaplan-Meier Estimates and Multivariable Survival Models for Overall Survival From the NCDB Cohort
eTable 6. Kaplan-Meier Estimates and Multivariable Survival Models for Disease-Free Survival From the SUCCESS Cohort
eTable 7. Kaplan-Meier Estimates and Multivariable Survival Models for Local Recurrence-Free Survival From the SUCCESS Cohort
eTable 8. Kaplan-Meier Estimates and Multivariable Survival Models for Overall Survival From the SUCCESS Cohort
eTable 9. Kaplan-Meier Estimates and Multivariable Survival Models for Overall Survival of Patients Who Underwent Breast-Conserving Surgery From the Pooled Cohort
eTable 10. Kaplan-Meier Estimates and Multivariable Survival Models for Overall Survival of Patients Who Underwent Mastectomy From the Pooled Cohort
eTable 11. Additional Characteristics of the NCDB Cohort Grouped by CTC Status
eTable 12. Adjusted Odds Ratios for Factors Associated With CTC-Positive Status of Patients From the NCDB Cohort
eTable 13. Characteristics of the Merged Cohorts Grouped by Receipt of Radiation
eTable 14. Characteristics of the Patients Within the Merged Cohorts Who Received Breast-Conserving Surgery or Mastectomy Grouped by Receipt of Radiation
eTable 15. Sensitivity Analyses for Possible Unmeasured Confounding
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):-. [DOI] [PubMed] [Google Scholar]
- 2.Darby S, McGale P, Correa C, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378(9804):1707-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. Breast Cancer, Version 2.2017. 2017. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed September 11, 2017.
- 4.Krishnamurthy S, Cristofanilli M, Singh B, et al. . Detection of minimal residual disease in blood and bone marrow in early stage breast cancer. Cancer. 2010;116(14):3330-3337. [DOI] [PubMed] [Google Scholar]
- 5.Bidard FC, Mathiot C, Delaloge S, et al. . Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol. 2010;21(4):729-733. [DOI] [PubMed] [Google Scholar]
- 6.Janni WJ, Rack B, Terstappen LW, et al. . Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin Cancer Res. 2016;22(10):2583-2593. [DOI] [PubMed] [Google Scholar]
- 7.Rack B, Schindlbeck C, Jückstock J, et al. ; SUCCESS Study Group . Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;106(5):dju066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franken B, de Groot MR, Mastboom WJ, et al. . Circulating tumor cells, disease recurrence and survival in newly diagnosed breast cancer. Breast Cancer Res. 2012;14(5):R133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucci A, Hall CS, Lodhi AK, et al. . Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13(7):688-695. [DOI] [PubMed] [Google Scholar]
- 10.Bidard FC, Belin L, Delaloge S, et al. . Time-dependent prognostic impact of circulating tumor cells detection in non-metastatic breast cancer: 70-month analysis of the REMAGUS02 Study. Int J Breast Cancer. 2013;2013:130470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bidard FC, Hajage D, Bachelot T, et al. . Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: a prospective observational study. Breast Cancer Res. 2012;14(1):R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molloy TJ, Bosma AJ, Baumbusch LO, et al. . The prognostic significance of tumour cell detection in the peripheral blood versus the bone marrow in 733 early-stage breast cancer patients. Breast Cancer Res. 2011;13(3):R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall C, Karhade M, Laubacher B, et al. . Circulating tumor cells after neoadjuvant chemotherapy in stage I-III triple-negative breast cancer. Ann Surg Oncol. 2015;22(suppl 3):S552-S558. [DOI] [PubMed] [Google Scholar]
- 14.Hall CS, Karhade MG, Bowman Bauldry JB, et al. . Prognostic value of circulating tumor cells identified before surgical resection in nonmetastatic breast cancer patients. J Am Coll Surg. 2016;223(1):20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8(5):329-340. [DOI] [PubMed] [Google Scholar]
- 16.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9(4):302-312. [DOI] [PubMed] [Google Scholar]
- 17.Giuliano M, Giordano A, Jackson S, et al. . Circulating tumor cells as early predictors of metastatic spread in breast cancer patients with limited metastatic dissemination. Breast Cancer Res. 2014;16(5):440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ClinicalTrials.gov Simultaneous Study of Gemcitabine-Docetaxel Combination Adjuvant Treatment, as Well as Extended Bisphosphonate and Surveillance-Trial. NCT02181101. https://clinicaltrials.gov/ct2/show/NCT02181101. Accessed October 2017.
- 20.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21.Gradishar WJ, Anderson BO, Balassanian R, et al. . NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J Natl Compr Canc Netw. 2017;15(4):433-451. [DOI] [PubMed] [Google Scholar]
- 22.Riethdorf S, Fritsche H, Müller V, et al. . Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13(3):920-928. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Box GEP, Hunter JS, Hunter WG. Statistics for Experimenters: Design, Innovation and Discovery. 2nd ed Hoboken, NJ: John Wiley & Sons; 2005. [Google Scholar]
- 25.Jackson CH. Flexsurv: a platform for parametric survival modelling in R. J Stat Softw. 2016;70:1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong GPS, Hutton JL. Choice of parametric models in survival analysis: applications to monotherapy for epilepsy and cerebral palsy. J R Stat Soc Appl Stat. 2003;52(pt 2):153-168. [Google Scholar]
- 27.Collett D. Modelling Survival Data in Medical Research. 3rd ed Boca Raton, FL: Chapman and Hall/CRC; 2014. [Google Scholar]
- 28.Mitra N, Heitjan DF. Sensitivity of the hazard ratio to nonignorable treatment assignment in an observational study. Stat Med. 2007;26(6):1398-1414. [DOI] [PubMed] [Google Scholar]
- 29.MatchIt: Nonparametric Preprocessing for Parametric Causal Inference [computer program]. 2011.
- 30.Hmisc: Harrell Miscellaneous. R package version 4.0-2 [computer program]. 2016.
- 31.Therneau TM. A Package for Survival Analysis in S. Version 2.38. 2015. https://cran.r-project.org/web/packages/survival/index.html. Accessed July 2017.
- 32.tableone: Create “Table 1” to Describe Baseline Characteristics. R package version 0.7.3 [computer program]. 2015.
- 33.R Foundation. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 34.Sandri MT, Zorzino L, Cassatella MC, et al. . Changes in circulating tumor cell detection in patients with localized breast cancer before and after surgery. Ann Surg Oncol. 2010;17(6):1539-1545. [DOI] [PubMed] [Google Scholar]
- 35.Riethdorf S, Müller V, Zhang L, et al. . Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16(9):2634-2645. [DOI] [PubMed] [Google Scholar]
- 36.Fisher B, Anderson S, Bryant J, et al. . Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233-1241. [DOI] [PubMed] [Google Scholar]
- 37.Fisher B, Bryant J, Dignam JJ, et al. ; National Surgical Adjuvant Breast and Bowel Project . Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20(20):4141-4149. [DOI] [PubMed] [Google Scholar]
- 38.Hughes KS, Schnaper LA, Bellon JR, et al. . Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM; PRIME II investigators . Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266-273. [DOI] [PubMed] [Google Scholar]
- 40.Fyles AW, McCready DR, Manchul LA, et al. . Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):963-970. [DOI] [PubMed] [Google Scholar]
- 41.Blamey RW, Bates T, Chetty U, et al. . Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer. 2013;49(10):2294-2302. [DOI] [PubMed] [Google Scholar]
- 42.Pötter R, Gnant M, Kwasny W, et al. ; Austrian Breast and Colorectal Cancer Study Group . Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer. Int J Radiat Oncol Biol Phys. 2007;68(2):334-340. [DOI] [PubMed] [Google Scholar]
- 43.ClinicalTrials.gov The IDEA Study (Individualized Decisions for Endocrine Therapy Alone). NCT02400190. https://clinicaltrials.gov/ct2/show/NCT02400190. Accessed October 2017.
- 44.ClinicalTrials.gov. The PRECISION Trial (Profiling Early Breast Cancer for Radiotherapy Omission) : A Phase II Study of Breast-Conserving Surgery Without Adjuvant Radiotherapy for Favorable-Risk Breast Cancer. NCT02653755. https://www.clinicaltrials.gov/ct2/show/NCT02653755. Accessed October 2017.
- 45.ClinicalTrials.gov. A Prospective Cohort Study Evaluating Risk of Local Recurrence Following Breast Conserving Surgery and Endocrine Therapy in Low Risk Luminal A Breast Cancer (LUMINA). NCT01791829. https://clinicaltrials.gov/ct2/show/NCT01791829. Accessed October 2017.
- 46.ClinicalTrials.gov. Endocrine Treatment Alone for Elderly Patients With Estrogen Receptor Positive Operable Breast Cancer and Low Recurrence Score. NCT02476786. https://clinicaltrials.gov/ct2/show/NCT02476786. Accessed October 2017.
- 47.Eschrich SA, Fulp WJ, Pawitan Y, et al. . Validation of a radiosensitivity molecular signature in breast cancer. Clin Cancer Res. 2012;18(18):5134-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Speers C, Zhao S, Liu M, Bartelink H, Pierce LJ, Feng FY. Development and validation of a novel radiosensitivity signature in human breast cancer. Clin Cancer Res. 2015;21(16):3667-3677. [DOI] [PubMed] [Google Scholar]
- 49.Tramm T, Mohammed H, Myhre S, et al. . Development and validation of a gene profile predicting benefit of postmastectomy radiotherapy in patients with high-risk breast cancer: a study of gene expression in the DBCG82bc cohort. Clin Cancer Res. 2014;20(20):5272-5280. [DOI] [PubMed] [Google Scholar]
- 50.Torres-Roca JF, Fulp WJ, Caudell JJ, et al. . Integration of a radiosensitivity molecular signature into the assessment of local recurrence risk in breast cancer. Int J Radiat Oncol Biol Phys. 2015;93(3):631-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weichselbaum RR, Ishwaran H, Yoon T, et al. . An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A. 2008;105(47):18490-18495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris LN, Ismaila N, McShane LM, et al. ; American Society of Clinical Oncology . Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(10):1134-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grover PK, Cummins AG, Price TJ, Roberts-Thomson IC, Hardingham JE. Circulating tumour cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann Oncol. 2014;25(8):1506-1516. [DOI] [PubMed] [Google Scholar]
- 54.Yu M, Bardia A, Wittner BS, et al. . Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gulbahce N, Magbanua MJM, Chin R, et al. . Quantitative whole genome sequencing of circulating tumor cells enables personalized combination therapy of metastatic cancer. Cancer Res. 2017;77(16):4530-4541. [DOI] [PubMed] [Google Scholar]
- 56.Aktas B, Bankfalvi A, Heubner M, Kimmig R, Kasimir-Bauer S. Evaluation and correlation of risk recurrence in early breast cancer assessed by Oncotype DX®, clinicopathological markers and tumor cell dissemination in the blood and bone marrow. Mol Clin Oncol. 2013;1(6):1049-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaw JA, Guttery DS, Hills A, et al. . Mutation analysis of cell-free DNA and single circulating tumor cells in metastatic breast cancer patients with high circulating tumor cell counts. Clin Cancer Res. 2017;23(1):88-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Participants, Materials, Outcomes, and Analysis
eFigure 1. CONSORT Diagram of Patient Selection
eFigure 2. Unadjusted Survival Curves for the SUCCESS Cohorts
eTable 1. Characteristics of the Matched Cohorts of All Patients From the NCDB Cohort Grouped by CTC Status
eTable 2. Characteristics of the Matched Cohorts of Patients From the SUCCESS Cohort Grouped by CTC Status
eTable 3. Characteristics of the Matched Cohorts of Patients Who Underwent Breast-Conserving Surgery From the Pooled Cohort
eTable 4. Characteristics of the Matched Cohorts of Patients Who Underwent Mastectomy From the Pooled Cohort
eTable 5. Kaplan-Meier Estimates and Multivariable Survival Models for Overall Survival From the NCDB Cohort
eTable 6. Kaplan-Meier Estimates and Multivariable Survival Models for Disease-Free Survival From the SUCCESS Cohort
eTable 7. Kaplan-Meier Estimates and Multivariable Survival Models for Local Recurrence-Free Survival From the SUCCESS Cohort
eTable 8. Kaplan-Meier Estimates and Multivariable Survival Models for Overall Survival From the SUCCESS Cohort
eTable 9. Kaplan-Meier Estimates and Multivariable Survival Models for Overall Survival of Patients Who Underwent Breast-Conserving Surgery From the Pooled Cohort
eTable 10. Kaplan-Meier Estimates and Multivariable Survival Models for Overall Survival of Patients Who Underwent Mastectomy From the Pooled Cohort
eTable 11. Additional Characteristics of the NCDB Cohort Grouped by CTC Status
eTable 12. Adjusted Odds Ratios for Factors Associated With CTC-Positive Status of Patients From the NCDB Cohort
eTable 13. Characteristics of the Merged Cohorts Grouped by Receipt of Radiation
eTable 14. Characteristics of the Patients Within the Merged Cohorts Who Received Breast-Conserving Surgery or Mastectomy Grouped by Receipt of Radiation
eTable 15. Sensitivity Analyses for Possible Unmeasured Confounding