This epidemiologic assessment and cost analysis determines the public health burden in terms of resources and cost of a 2013 measles outbreak in New York City.
Key Points
Question
What is the burden of a measles outbreak on public health?
Findings
This epidemiologic assessment and cost analysis of a 2013 measles outbreak in New York City, New York, found 58 cases of measles infection and 3351 exposed contacts, with 78% of the age-eligible cases unvaccinated owing to refusal or intentional delay. Total direct costs to the Department of Health and Mental Hygiene were $394 448, and 10 054 personnel hours were consumed responding to and controlling the outbreak.
Meaning
Measles vaccine refusals or delays can lead to large outbreaks following measles importations, with costly and resource intensive response and containment.
Abstract
Importance
Internationally imported cases of measles into the United States can lead to outbreaks requiring extensive and rapid control measures. Importation of measles from an unvaccinated adolescent in 2013 led to what has been the largest outbreak of measles in New York City, New York, since 1992.
Objective
To describe the epidemiology and public health burden in terms of resources and cost of the 2013 measles outbreak in New York City.
Design, Setting, and Participants
This epidemiologic assessment and cost analysis conducted between August 15, 2013, and August 5, 2014, examined all outbreak-associated cases of measles among persons residing in New York City in 2013.
Exposures
Measles virus.
Main Outcomes and Measures
Numbers of measles cases and contacts. Total personnel time and total direct cost to the New York City Department of Health and Mental Hygiene (DOHMH), calculated as the sum of inputs (supplies and materials, equipment, and logistics) and personnel time (salary and fringe benefits).
Results
Between March 13, 2013, and June 9, 2013, 58 persons in New York City with a median age of 3 years (range, 0-32 years) were identified as having measles. Among these individuals, 45 (78%) were at least 12 months old and were unvaccinated owing to parental refusal or intentional delay. Only 28 individuals (48%) visited a medical health care professional who suspected measles and reported the case to the DOHMH at the initial clinical suspicion. Many case patients were not immediately placed into airborne isolation, resulting in exposures in 11 health care facilities. In total, 3351 exposed contacts were identified. Total direct costs to the New York City DOHMH were $394 448, and a total of 10 054 hours were consumed responding to and controlling the outbreak.
Conclusions and Relevance
Vaccine refusals and delays appeared to have propagated a large outbreak following importation of measles into the United States. Prompt recognition of measles along with rapid implementation of airborne isolation of individuals suspected of measles infection in health care facilities and timely reporting to public health agencies may avoid large numbers of exposures. The response and containment of measles outbreaks are resource intensive.
Introduction
Measles is a viral infection characterized by a generalized maculopapular rash with fever. Complications may include diarrhea, otitis media, pneumonia, encephalitis, and death. Measles virus is transmitted both by airborne and respiratory droplets. The virus is highly contagious, with up to 90% of susceptible exposed persons becoming infected. The infectious period begins as early as 4 days prior to rash onset and continues through 4 days after rash onset, further contributing to opportunities for transmission.1
Because of the airborne transmission and the highly contagious nature of measles virus, even a single case of measles can lead to a large number of exposures and secondary cases. Postexposure prophylaxis with measles-mumps-rubella (MMR) vaccine or immune globulin may prevent infection or limit the severity of infection if people become ill. However, the window of opportunity is narrow, with recommended administration of the vaccine within 3 days of exposure and of immune globulin within 6 days of exposure.1 Measles exposures require extensive and rapid control measures by public health agencies and medical facilities.
Measles was declared eliminated in the United States in 2000 owing to a prolonged absence of local transmission following a 2-dose MMR vaccine recommendation and sustained, high vaccine coverage nationally.2,3 However, measles remains endemic in many parts of the world, and persons with measles acquired abroad can transmit measles to susceptible persons in the United States, which can lead to outbreaks. Most of the import-associated cases acquired in the United States are among unvaccinated persons.4
On March 13, 2013, an unvaccinated adolescent returned to New York City, New York, while infectious with measles after visiting London, United Kingdom. This importation led to what had been the largest outbreak of measles in New York City since 1992, before measles was eliminated in the United States. The present evaluation summarizes the outbreak and the associated costs incurred by the New York City Department of Health and Mental Hygiene (DOHMH) in the response and containment of the measles outbreak.
Methods
Case Investigation
The New York City DOHMH Bureau of Immunization received reports from March 22, 2013, through July 2, 2013, of suspected measles cases from health care professionals and by laboratory notification of positive measles diagnostic test results as mandated by the New York City health code and state public health law.5,6,7,8 Case patients residing in New York City were interviewed for clinical symptoms and settings attended during their exposure and infectious periods. Medical records were reviewed. Immunization records were obtained from health care professionals and the New York City population-based immunization information system. Case patients were classified as confirmed based on the 2013 Council of State and Territorial Epidemiologists (CSTE) case definition.9 Because case patient investigations and epidemiologic analyses are conducted as routine DOHMH surveillance and outbreak investigations, institutional approval was not sought for this study. The cost evaluation did not involve case patient information; thus, patient informed consent was unnecessary.
Laboratory Testing
Testing for measles IgM and IgG was performed at commercial laboratories and the New York City DOHMH Public Health Laboratory between March 22, 2013, and July 5, 2013. At the Public Health Laboratory, serum specimens were tested for measles-specific IgM antibodies using an enzyme-linked immunosorbent assay (reference 426060; Wampole). Measles-specific IgG was tested using an enzyme-linked immunosorbent assay (reference 4259000CE; Wampole).
Reverse-transcription polymerase chain reaction (RT-PCR) assays for the detection of measles virus RNA were performed by the Public Health Laboratory. The RNA was extracted from nasopharyngeal swab specimens by using the Biomérieux automated extraction system.10 Measles virus RNA was detected using a real-time RT-PCR assay targeting the measles nucleoprotein gene, as previously described.11
Measles genotyping was performed at the Centers for Disease Control and Prevention for specimens in which measles virus was detected by RT-PCR using a sequencing approach defined previously.12,13,14
Contact Investigation
Persons exposed to measles (contacts) were identified through case patient interviews and follow-up with settings of exposures between March 22, 2013, and July 9, 2013. Contacts were notified by DOHMH about their exposure and educated about symptoms of measles. Presumptive evidence of immunity to measles was assessed through review of the year of birth (before 1957), immunization records documenting measles-containing vaccine, or positive measles IgG titers.1 Contacts without presumptive evidence of immunity were instructed to remain home during their incubation periods while they were at risk of becoming contagious. Postexposure prophylaxis with MMR vaccine within 3 days of initial exposure or immune globulin within 6 days of initial exposure were administered to eligible, nonimmune contacts as indicated.1
Cost Evaluation
Total direct costs were calculated as the sum of inputs (equipment, logistics, and supplies and materials) and personnel time (salary and fringe benefits). Inputs included immunoglobulin and MMR vaccine, courier service, travel, postage, advertising, and laboratory supplies and testing. Personnel time spent on outbreak-related activities was self-reported via a web-based survey disseminated through SurveyMonkey to all 87 staff members involved in the measles outbreak response. The survey took approximately 10 minutes to complete and was sent to staff on November 7, 2013, with reminders through January 29, 2014. Outbreak-related activities were grouped into 4 categories: case patient and contact investigation, administration, laboratory, and community outreach (Table 1). The cost of personnel time was calculated as the hourly wage multiplied by the routine business (compensated) hours worked plus paid overtime and the tabulated fringe benefits. The incremental cost was calculated as total direct costs minus salary and fringe benefits paid to DOHMH staff during routine business hours. In-kind costs (uncompensated hours worked by DOHMH-funded staff and salaries of DOHMH staff who are funded by outside agencies), overhead, and costs to outside medical facilities and patients were not included.
Table 1. Measles Outbreak Activities by Category, Brooklyn, New York, March 13 to June 30, 2013.
| Category | Activity |
|---|---|
| Investigation | Interview case patients and contacts |
| Ascertain medical and immunization records | |
| Coordinate and collect specimens | |
| Arrange prophylaxis | |
| Supervise investigations | |
| Train staff | |
| Develop data analysis and database | |
| Community outreach | Develop press releases, website materials, flyers, and translations |
| Respond to media inquiries | |
| Issue health advisories to alert physicians and enhance surveillance | |
| Generate recall letters to parents of children due for measles vaccination | |
| Conduct immunization audits at schools | |
| Laboratory | Accession, receive, and ship specimens |
| Test specimens | |
| Supervise laboratory testing | |
| Administration | Identify and hire staff to assist |
| Track, monitor, or develop budgets | |
| Process invoices |
Analyses
Descriptive analyses and calculations were performed from August 15, 2013, to August 5, 2014, using SAS, version 9.2 (SAS Institute Inc) and Microsoft Excel.
Results
Case Patient Investigation
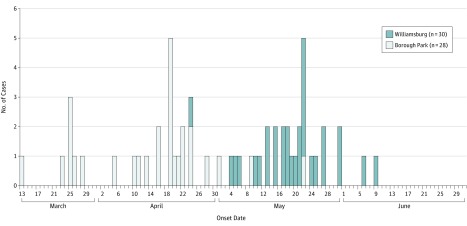
From March 13, 2013, through June 9, 2013, a total of 58 case patients with measles were detected in 2 neighborhoods of Brooklyn, New York, with 6 generations of transmission (Figure). The index case patient presented to a health care professional with symptoms of measles after returning from a trip to London. The case was reported to the New York City DOHMH by a commercial laboratory only once the measles IgM test results had returned positive, 8 days after the clinician initially considered a diagnosis of measles. Two additional case patients resided outside of New York City and were excluded from analyses. Orthodox Jewish persons accounted for 100% of the case patients.
Figure. Numbers of Case Patients With Measles Stratified by Rash Onset Date and Neighborhood of Brooklyn, New York, Between March 13 and June 30, 2013.
The 58 case patients included 1 asymptomatic infant born with measles infection. The date of birth was used as a proxy for onset date in this infant because there was no rash.
Laboratory Testing
Among the 58 case patients, 48 (83%) had laboratory confirmation of measles by positive measles IgM results or by detection of measles virus through RT-PCR analyses; this included 40 of the 41 case patients (98%) who were tested by serologic analyses after rash onset and 31 of the 34 case patients (91%) who were tested by RT-PCR analyses. The remaining 10 case patients (17%) did not have laboratory testing performed after rash onset and were classified as confirmed based on epidemiologic linkage to a confirmed case patient. Measles virus genotype D8, which had been circulating in the United Kingdom during the same period, was identified in 17 case patients. No other genotype was identified among the case patients.
Vaccination Status and Age
The median age of the case patients was 3 years (range, 0-32 years). None of the case patients had documentation of receipt of measles-containing vaccine prior to their presumed exposure to measles virus. Of the 58 case patients, 45 (78%) were at least 12 months old and were unvaccinated owing to parental refusal or intentional delay. Twelve case patients (21%) were less than 12 months old and therefore were too young for routine immunization with measles-containing vaccine. One case patient (1%) was an adult who verbally reported a history of receiving measles-containing vaccine as a child; although no vaccination documentation was available, a high IgG avidity test result obtained at the CDC was consistent with a prior history of vaccination.
Complications
Complications included 1 case of pneumonia and 1 miscarriage. One unvaccinated pregnant woman had rash onset at 37 weeks’ gestation with a live birth at 38 weeks’ gestation. The parents declined immunoglobulin for the newborn. Despite isolation of measles virus from urine and a nasopharyngeal culture on days 1 and 9 of life, indicative of intrauterine exposure, the neonate was asymptomatic per health care professional reports on days 0, 1, and 9 of life and remained asymptomatic per parental report. Corresponding measles virus IgM and IgG were both undetectable on day 1 of life but detectable on day 9 of life. Given the lack of clinical symptoms, this neonate did not meet the CSTE case definition for having measles9; however, the neonate was counted as a case patient given the laboratory findings and epidemiologic linkage.
Transmission
Among the 58 case patients, 41 (71%) were members of 8 extended families. The most common presumed source of transmission was from a relative (30 cases [52%]). Other sources of transmission included building of residence (6 cases [10%]), friend or playmate (4 cases [7%]), health care settings (4 cases [7%]), community gathering (1 case [2%]), and international importation of the index case patient (1 case [2%]). The remaining case patients had unknown sources of infection.
Reporting
Only 28 case patients (48%) visited a medical health care professional who suspected measles and reported the case to the DOHMH at the time of initial clinical suspicion. Another 4 case patients (7%) visited a health care professional at the time of rash illness, but measles was not suspected; 5 case patients (9%) saw a health care professional at the time of their rash, but the cases were not reported to the DOHMH at the initial clinical suspicion. The remaining 21 case patients (36%) did not seek medical care for their rash illness; those case patients were often identified retrospectively through contact tracing of secondary case patients.
Contact Investigations and Control Measures
Many case patients were not placed immediately into airborne isolation owing to delays in clinical suspicion of measles or lack of negative pressure rooms in outpatient practices, or because the case patient presented for care while infectious but prior to rash onset, resulting in exposures in 11 health care facilities. Additional known exposures occurred in schools, an airline flight, residential buildings, households of case patients, and at a wedding.
A total of 3351 exposed contacts were identified, excluding the 58 case patients who developed measles. Among those contacts, 2214 (66%) had evidence of immunity to measles based on receipt of 2 documented doses of measles-containing vaccine, having a positive measles IgG titer, or birth before 1957; 376 contacts (11%) had received 1 dose of measles-containing vaccine; 335 contacts (10%) were susceptible; and immunity status was unknown for 426 contacts (13%). The MMR vaccine was administered within 3 days of initial exposure to 114 contacts who were 6 months or older, and immunoglobulin was administered within 6 days of initial exposure to 77 infants younger than 6 months or to infants aged 6 to 11 months who had not received MMR prophylaxis. Immunoglobulin was not administered to immunocompromised or pregnant persons.
Notifications were sent to health care professionals reminding them to have heightened suspicion for measles, place patients with fever and generalized rash in airborne isolation immediately, perform appropriate diagnostic testing, and report to DOHMH at the time of initial clinical suspicion. Notifications were sent to schools and daycare health care professionals as well as to more than 10 000 families through a community organization. The DOHMH facilitated rapid distribution of MMR vaccine to health care professionals and sent notifications to parents of children who were due for measles vaccine, recalling them to their physicians’ offices. Advertisements were placed in local newspapers and on an established telephone hotline used by the affected community. Educational materials were distributed to attendees at a local health fair that focused on the importance of MMR vaccination and of calling health care professionals promptly if they or their children developed symptoms of measles. Audits of immunization compliance were conducted in schools. The DOHMH also held community briefings with school administrators, pediatricians, religious leaders, and elected officials to reinforce the importance of maintaining high immunization coverage in the community.
During the outbreak period, DOHMH recommended that obstetricians test pregnant women in the affected community for measles immunity and vaccinate susceptible women after delivery. To protect infants during the outbreak, DOHMH expanded vaccination recommendations to include an early dose of MMR vaccine for all children aged 6 to 11 months in the affected communities. Health care professionals were instructed to not consider these doses valid and to repeat the dose at age 12 months, in accordance with Advisory Committee on Immunization Practices recommendations during outbreaks of measles, because the proportion of infants with protective antibody levels following vaccination increases by 12 months of age (eg, 85% at age 9 months vs 95% at age 12 months).1,15 The DOHMH also recommended that the second dose of MMR vaccine be administered early, as soon as 4 weeks after the first dose of MMR vaccine.1
Cost Evaluation
Eighty-seven staff members from 12 bureaus of the DOHMH participated in the outbreak. Responses to the self-reported survey were obtained from all staff (100%). Of the 87 staff members, 41 persons (47%) were from outside the Bureau of Immunization, 75 (86%) were funded by the DOHMH, 59 (68%) performed duties associated with their routine job description, and 16 (18%) performed activities across more than 1 category. The breakdown of hours worked by category was 5862 hours (58%) on investigations, 445 hours (4%) on administrative activities, 2766 hours (28%) on laboratory work, and 981 hours (10%) on community outreach.
In total, 10 054 hours were spent responding to and controlling the outbreak, most of which (8668 hours [86%]) were worked during business hours by DOHMH-paid staff (Table 2). There were 1062 hours (11% of all staff hours) worked by DOHMH-paid staff outside of standard business hours (346 uncompensated hours) or by DOHMH-based staff paid by other agencies (716 hours). The remaining 324 hours (3%) were paid as overtime.
Table 2. Hours Worked by Outbreak Response Category, Brooklyn, New York, March 13 to June 30, 2013.
| Activity Category | Staff, No. | Total No. of Hours | No. of Hours Workeda | ||
|---|---|---|---|---|---|
| During Standard Business Hours | Overtime | In-kindb | |||
| Investigation | 52 | 5862 | 4889 | 179 | 794 |
| Laboratory | 21 | 2766 | 2556 | 123 | 87 |
| Community outreach | 19 | 981 | 818 | 22 | 141 |
| Administration | 13 | 445 | 405 | 0 | 40 |
| Total | 87 | 10 054 | 8668 | 324 | 1062 |
Rounded to the nearest whole number.
Includes uncompensated hours worked by Department of Health and Mental Hygiene (DOHMH)-paid staff outside standard business hours and by DOHMH–based staff paid by other agencies.
Total direct costs were $394 448 ($62 102 for inputs and $332 346 on compensated personnel time) (Table 3). The incremental cost was $73 135, or 19% of the total direct costs. Salaries and fringe benefits combined accounted for $321 313 (97% of personnel costs). Overtime pay totaled $11 033 (3% of personnel costs). The majority (99%) of the cost of inputs was attributed to advertising ($29 425), MMR vaccine ($17 590), laboratory supplies and testing ($9316), and courier service ($4886).
Table 3. Estimated Direct Costs Incurred During Measles Outbreak, Brooklyn, New York, March 13 to June 30, 2013.
| Expense | Cost, $a |
|---|---|
| Input or material | |
| Advertising | 29 425 |
| MMR vaccineb | 17 590 |
| Laboratory supplies and testingc | 9316 |
| Courier service | 4886 |
| Immune globulind | 471 |
| Postagee | 382 |
| Vehicle (mileage)f | 32 |
| Input total | 62 102 |
| Personnel | |
| Salary | 240 091 |
| Fringe benefit | 81 222 |
| Overtime | 11 033 |
| Personnel total | 332 346 |
| Total | 394 448 |
Abbreviation: MMR, measles-mumps-rubella.
All costs rounded to the nearest whole dollar.
Unit cost was $19.33 per MMR dose (910 doses).
Includes collection kits for blood, urine, and nasopharyngeal swabs; reagents for measles reverse-transcription polymerase chain reaction assays and cultures; and serologic tests for antimeasles IgM and IgG.
Unit costs were $47.05 per vial (2 mL) of immune globulin (10 vials).
Unit postage cost was $0.46 per letter sent to 831 parents.
Mileage cost using government vehicles was calculated as $0.24 per mile.
Discussion
This outbreak was fueled by the introduction of measles virus into a small number of families who had previously declined vaccination. The outbreak was prolonged, in part, owing to the spread of measles to infants too young to have been vaccinated and to the delay of vaccination among children. Geographic clustering of persons who refuse or decline vaccination, as observed in this and other outbreaks, has led to outbreaks following importations of a single case of measles.16,17,18,19,20 The insular nature of the affected community and high population-level vaccination coverage in New York City prevented a much larger outbreak, with at least 1-dose MMR coverage of 96.8% (SD, 2.5%) among children aged 19 to 35 months in New York City, as estimated by the 2013 National Immunization Survey.21
Because measles is relatively uncommon in the United States in the postvaccination era, not all health care professionals are familiar with the disease. Prompt recognition of measles is critical, and rapidly isolating suspected case patients in health care facilities may avoid a large number of exposures. Exposures during this and other outbreaks have included settings such as health care facilities, schools, airline flights, and places of residence.17,20 More complete and timely clinician reporting of suspected cases to the New York City DOHMH would have enabled more rapid investigation and implementation of control measures.
The response and containment of the 2013 measles outbreak were resource intensive. The outbreak required assistance from a large number of staff, of whom almost one-third performed duties outside of their routine job descriptions, and almost half worked for a DOHMH bureau other than the Bureau of Immunization, where measles investigations are routinely performed. This required assistance resulted in the redirection of resources away from other public health activities. Personnel costs for this and other outbreaks comprised the majority of expenditures.18,19,22 This outbreak is one of the largest US-based outbreaks with a cost analysis published in the postelimination era. Costs to local and state public health institutions for measles outbreaks in the United States in 2011 were estimated on the basis of documented costs incurred during 4 prior measles outbreaks; estimates ranged from $2685 to $22 375 for small outbreaks, from $47 732 to $208 829 for medium outbreaks, and from $280 829 to $1 640 789 for large outbreaks.17 The cost during the 2013 New York City outbreak was within the range of cost estimates for other large outbreaks.
This outbreak also highlighted the potential consequences of measles infection during pregnancy, with 1 instance of miscarriage and 1 instance of congenital measles. The case patient with congenital measles in this outbreak also emphasized that despite being asymptomatic after birth, newborns with congenital measles can be infectious, potentially requiring prolonged isolation, as evidenced by cultures positive for measles virus. Other reports have also documented case patients with congenital measles that were asymptomatic. For those reports, it is possible that administration of immunoglobulin may have reduced the severity of disease, whereas the present report is the only published one in which immunoglobulin was not administered to an asymptomatic congenital case patient.23,24,25
Limitations
This evaluation has some limitations. The time staff members spent working on outbreak-related tasks was subject to recall bias owing to a lag between the end of the outbreak and survey dissemination as well as to reliance on self-report of hours worked, which may have resulted in an underestimate or an overestimate of public health costs. In addition, the cost figures do not account for in-kind costs or costs to medical facilities, outside agencies, patients, and society and thus underestimate the total cost of the measles outbreak.
Conclusions
Given ongoing outbreaks globally, the United States remains at continued risk for importations of measles resulting in outbreaks. Information from the present evaluation provided the New York City DOHMH with conservative estimates of cost and personnel time for use in future outbreak response planning. The significant burden and consequences of measles outbreaks as well as other public health emergencies underscore the importance of continued support for a robust and flexible public health infrastructure for health departments.
References
- 1.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention . Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-04):1-34. [PubMed] [Google Scholar]
- 2.Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16-17 March 2000. J Infect Dis. 2004;189(suppl 1):S43-S47. doi: 10.1086/377696 [DOI] [PubMed] [Google Scholar]
- 3.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Dietz V; CDC . Vaccination coverage among children aged 19-35 months—United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(39):1065-1071. doi: 10.15585/mmwr.mm6539a4 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Measles—United States, January 1-August 24, 2013. MMWR Morb Mortal Wkly Rep. 2013;62(36):741-743. [PMC free article] [PubMed] [Google Scholar]
- 5.New York City Health Code §11.03; reportable diseases and conditions. http://www1.nyc.gov/assets/doh/downloads/pdf/about/healthcode/health-code-article11.pdf. Accessed June 29, 2017.
- 6.New York City Health Code §13.03; laboratories. http://www1.nyc.gov/assets/doh/downloads/pdf/about/healthcode/health-code-article13.pdf. Accessed June 29, 2017
- 7.New York State Public Health Law Section 2102 of Article 21, Title 1, Communicable diseases; laboratory reports and records. http://public.leginfo.state.ny.us/lawssrch.cgi?NVLWO. Accessed July 7, 2017.
- 8.New York City Department of Health and Mental Hygiene Reporting diseases and conditions. http://www1.nyc.gov/site/doh/providers/reporting-and-services/notifiable-diseases-and-conditions-reporting-central.page. Accessed July 7, 2015.
- 9.Council of State and Territorial Epidemiologists CSTE position statement: measles/rubeola 2013. case definition. https://wwwn.cdc.gov/nndss/conditions/measles/case-definition/2013/. Accessed July 7, 2017.
- 10.Biomérieux. http://www.biomerieux-usa.com. Accessed July 6, 2017.
- 11.Hummel KB, Lowe L, Bellini WJ, Rota PA. Development of quantitative gene-specific real-time RT-PCR assays for the detection of measles virus in clinical specimens. J Virol Methods. 2006;132(1-2):166-173. doi: 10.1016/j.jviromet.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 12.Rota PA, Brown K, Mankertz A, et al. . Global distribution of measles genotypes and measles molecular epidemiology. J Infect Dis. 2011;204(suppl 1):S514-S523. doi: 10.1093/infdis/jir118 [DOI] [PubMed] [Google Scholar]
- 13.Rota PA, Brown KE, Hübschen JM, et al. . Improving global virologic surveillance for measles and rubella. J Infect Dis. 2011;204(suppl 1):S506-S513. doi: 10.1093/infdis/jir117 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Measles virus nomenclature update: 2012. Wkly Epidemiol Rec. 2012;87(9):73-81. [PubMed] [Google Scholar]
- 15.Moss WJ, Griffin DE. Measles. Lancet. 2012;379(9811):153-164. doi: 10.1016/S0140-6736(10)62352-5 [DOI] [PubMed] [Google Scholar]
- 16.Gastañaduy PA, Budd J, Fisher N, et al. . A measles outbreak in an underimmunized Amish community in Ohio. N Engl J Med. 2016;375(14):1343-1354. doi: 10.1056/NEJMoa1602295 [DOI] [PubMed] [Google Scholar]
- 17.Ortega-Sanchez IR, Vijayaraghavan M, Barskey AE, Wallace GS. The economic burden of sixteen measles outbreaks on United States public health departments in 2011. Vaccine. 2014;32(11):1311-1317. doi: 10.1016/j.vaccine.2013.10.012 [DOI] [PubMed] [Google Scholar]
- 18.Parker AA, Staggs W, Dayan GH, et al. . Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med. 2006;355(5):447-455. doi: 10.1056/NEJMoa060775 [DOI] [PubMed] [Google Scholar]
- 19.Sugerman DE, Barskey AE, Delea MG, et al. . Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics. 2010;125(4):747-755. doi: 10.1542/peds.2009-1653 [DOI] [PubMed] [Google Scholar]
- 20.Hall V, Banerjee E, Kenyon C, et al. ; CDC . Measles Outbreak—Minnesota April-May 2017. MMWR Morb Mortal Wkly Rep. 2017;66(27):713-717. doi: 10.15585/mmwr.mm6627a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elam-Evans LD, Yankey D, Singleton JA, Kolasa M; Centers for Disease Control and Prevention (CDC) . National, state, and selected local area vaccination coverage among children aged 19-35 months—United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(34):741-748. [PMC free article] [PubMed] [Google Scholar]
- 22.Dayan GH, Ortega-Sánchez IR, LeBaron CW, Quinlisk MP, Iowa Measles Response Team . The cost of containing one case of measles: the economic impact on the public health infrastructure—Iowa, 2004. Pediatrics. 2005;116(1):e1-e4. doi: 10.1542/peds.2004-2512 [DOI] [PubMed] [Google Scholar]
- 23.Sommerville M, VanDiemen A. Congenital measles [abstract 122]. Infect Dis Health. 2016;21(3):121-122. doi: 10.1016/j.idh.2016.09.027 [DOI] [Google Scholar]
- 24.de la Fuente G, Cherpillod P, Kaiser L, Siegrist CA, Posfay-Barbe KM. Congenital measles: a case report integrating new molecular diagnostic arrays [poster session 267]. Arch Dis Child. 2008;93:267.18356378 [Google Scholar]
- 25.Ozsurekci Y, Kara A, Bayhan C, et al. . Cotreatment of congenital measles with vitamin A and intravenous immunoglobulin. Case Rep Infect Dis. 2014;2014:234545. [DOI] [PMC free article] [PubMed] [Google Scholar]