Key Points
Question
Are poor combined health behaviors and factors associated with similar increases in risk of incident cardiovascular disease and diabetes among individuals with low, intermediate, and high genetic risk?
Findings
In this population-based cohort study of 339 003 individuals, health behaviors were associated with incident cardiovascular disease and diabetes within and across genetic risk groups.
Meaning
Adherence to multiple ideal health behaviors and factors is inversely associated with the risk of incident cardiovascular disease and diabetes.
This population-based cohort study of UK Biobank data investigates the association of combined health behaviors and factors within genetic risk groups for coronary artery disease, atrial fibrillation, stroke, hypertension, and type 2 diabetes among UK adults.
Abstract
Importance
Genetic and lifestyle factors both contribute to the risk of developing cardiovascular disease, but whether poor health behaviors are associated with similar increases in risk among individuals with low, intermediate, or high genetic risk is unknown.
Objective
To investigate the association of combined health behaviors and factors within genetic risk groups with coronary artery disease, atrial fibrillation, stroke, hypertension, and type 2 diabetes as well as to investigate the interactions between genetic risk and lifestyle.
Design, Setting, and Participants
The UK Biobank cohort study includes more than 500 000 participants aged 40 to 70 years who were recruited from 22 assessment centers across the United Kingdom from 2006 to 2010. A total of 339 003 unrelated individuals of white British descent with available genotype and matching genetic data and reported sex were included in this study from the UK Biobank population-based sample. Individuals were included in the analyses of 1 or more new-onset diseases. Data were analyzed from April 2006 to March 2015.
Main Outcomes and Measures
Risks of new-onset cardiovascular disease and diabetes associated with genetic risk and combined health behaviors and factors. Genetic risk was categorized as low (quintile 1), intermediate (quintiles 2-4), or high (quintile 5). Within each genetic risk group, the risks of incident events associated with ideal, intermediate, or poor combined health behaviors and factors were investigated and compared with low genetic risk and ideal lifestyle.
Results
Of 339 003 individuals, 181 702 (53.6%) were female, and the mean (SD) age was 56.86 (7.99) years. During follow-up, 9771 of 325 133 participants (3.0%) developed coronary artery disease, 7095 of 333 637 (2.1%) developed atrial fibrillation, 3145 of 332 971 (0.9%) developed stroke, 11 358 of 234 651 (4.8%) developed hypertension, and 4379 of 322 014 (1.4%) developed diabetes. Genetic risk and lifestyle were independent predictors of incident events, and there were no interactions for any outcome. Compared with ideal lifestyle in the low genetic risk group, poor lifestyle was associated with a hazard ratio of up to 4.54 (95% CI, 3.72-5.54) for coronary artery disease, 5.41 (95% CI, 4.29-6.81) for atrial fibrillation, 4.68 (95% CI, 3.85-5.69) for hypertension, 2.26 (95% CI, 1.63-3.14) for stroke, and 15.46 (95% CI, 10.82-22.08) for diabetes in the high genetic risk group.
Conclusions and Relevance
In this large contemporary population, genetic composition and combined health behaviors and factors had a log-additive effect on the risk of developing cardiovascular disease. The relative effects of poor lifestyle were comparable between genetic risk groups. Behavioral lifestyle changes should be encouraged for all through comprehensive, multifactorial approaches, although high-risk individuals may be selected based on the genetic risk.
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality and morbidity worldwide and is driven by both genetic and lifestyle factors.1 Previous studies have shown that modifiable health behaviors and factors, including smoking, physical activity, diet, and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), have strong associations with both the risk of developing CVD2,3,4,5 as well as other long-term diseases6 and mortality.7,8 To tackle the CVD burden in the United States, the American Heart Association has formulated a guideline for improving behavioral and nonbehavioral lifestyle factors.9 This guideline aims to reduce CVD burden and improve cardiovascular health by 20% by 2020. The guideline considered smoking, BMI, physical activity, and diet as health behaviors and factors.9 Total cholesterol level, blood pressure, and fasting plasma glucose level were considered as nonbehavioral factors.9
In 2016, Khera et al10 showed that genetic variants and lifestyle behavior conjointly increased the risk of coronary artery disease (CAD). Individuals with poor health behaviors were at nearly 2-fold higher risk of CAD compared with individuals with ideal health behaviors but similar genetic risk (GR). Moreover, genetic and lifestyle factors independently contributed to the risk of developing CAD.10
Genome-wide association studies have been successful in identifying genetic variants associated with a range of cardiovascular phenotypes, including CAD, atrial fibrillation (AF), stroke, hypertension, and risk factors of CVD, such as type 2 diabetes. Whether the interplay between behavioral lifestyle and GR that was observed for CAD is a universal principle applicable in other CVDs and diabetes remains to be elucidated. It is also unknown if there is an interaction at play between behavioral lifestyle and GR.
This study primarily aimed to investigate whether poor modifiable health behaviors and factors were associated with similar increases in risk of incident CVD and diabetes among individuals with low, intermediate, or high GR in the UK Biobank study. The secondary aim was to investigate possible interactions between health behaviors and factors and GR.
Methods
UK Biobank Participants
The UK Biobank study design and population have been described in detail previously.11 In brief, the UK Biobank study started in 2006 and, until 2010, recruited more than 500 000 participants aged 40 to 70 years from the general population at 22 assessment centers throughout the United Kingdom. Participants provided information on lifestyle and other potentially health-related aspects through extensive baseline questionnaires, interviews, and physical measurements. Furthermore, blood samples were collected for genotyping.12 All participants provided written informed consent for the study.12 The UK Biobank study has approval from the North West Multi-center Research Ethics Committee.13 UK Biobank data are available for researchers after acceptance of a research proposal to the UK Biobank. The present study was conducted under application number 12006 of the UK Biobank resource.
Genotyping and Imputation
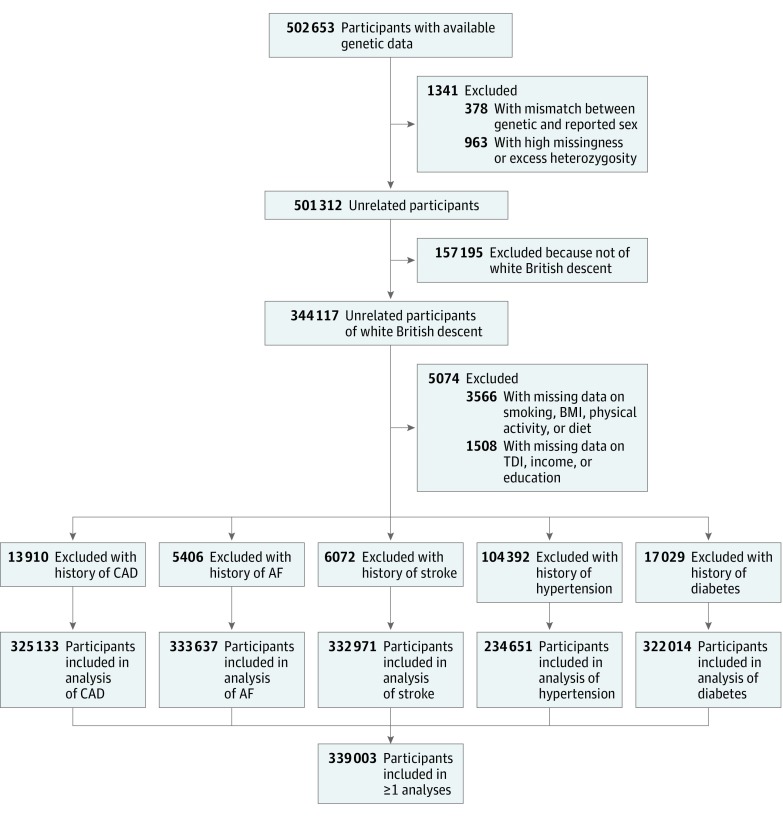
The genotyping process and arrays used in the UK Biobank study have been described elsewhere in more detail.14 Briefly, participants were genotyped using the custom UK Biobank Lung Exome Variant Evaluation Axiom (Affymetrix; n = 49 949), which includes 807 411 single-nucleotide polymorphisms (SNPs), or the UK Biobank Axiom array (Affymetrix; n = 452 713), which includes 820 967 SNPs.15 The arrays have insertion and deletion markers with more than 95% common content.14,15 Imputed genotype data were provided by UK Biobank, based on merged UK10K and 1000 Genomes phase 3 panels.16 We only considered participants of white British descent. Participants were excluded if there was no genotype or if there was a mismatch between genetic and reported sex (n = 378). Furthermore, related participants were pruned based on lowest missingness to create a maximal independent set of 344 117 unrelated individuals. Figure 1 shows a flowchart of the study sample selection.
Figure 1. Flowchart for the Selection of the Analyzed Study Sample From the UK Biobank Study.
AF indicates atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; TDI, Townsend Deprivation Index.
Polygenic Score
Polygenic risk scores were created following an additive model for CAD, AF, stroke, hypertension, and diabetes separately, as previously described.17 In short, the number of alleles (0, 1 or 2) for each individual was summed after multiplication with the effect size between the SNP and disease of interest. Effect sizes of SNP–disease associations were based on previously published genome-wide association studies. For CAD, 169 SNPs were used18; for AF, 25 SNPs19; for stroke, 11 SNPs20; for hypertension, 107 SNPs21,22,23,24,25,26; and for diabetes, 38 SNPs27,28,29,30 (eTables 1-5 in the Supplement). If multiple effect sizes were reported in a study, those estimated in the largest sample size were used (eg, the combined replication and discovery phase). Effect sizes were not considered for the polygenic score if they were estimated with UK Biobank data to avoid potential overestimation. Single-nucleotide polymorphisms were excluded if they were missing in UK Biobank data. Because some studies reported multiple correlated variants in the same locus, independent SNPs were selected based on the highest reported P value by using the linkage disequilibrium clumping procedure (at R2 < 0.01) implemented in PLINK version 1.9 (https://www.cog-genomics.org/plink2).
Health Behaviors and Factors
The American Heart Association 2020 Strategic Impact Goal guideline was used to define ideal, intermediate, and poor categories for smoking, BMI, and physical activity for each participant.9 For defining an ideal or poor diet, we used a more recent definition of ideal intake of dietary components for cardiovascular health.31 The eMethods in the Supplement includes the definitions of smoking, BMI, and physical activity, and eTable 6 in the Supplement includes the definitions and variables used for diet components. Overall lifestyle was subsequently categorized into ideal (having at least 3 ideal lifestyle factors), poor (having at least 3 poor lifestyle factors), or intermediate (all other combinations).
Townsend Deprivation Index, Years in Education, and Income
We single-inverse normalized the skewed Townsend Deprivation Index (TDI) variable—an area-based proxy measure for socioeconomic status composed of data on car ownership, household overcrowding, household owner-occupation, and unemployment32—provided by UK Biobank. Years spent in education were calculated based on the standardized International Standard Classification of Education of the United Nations Educational, Scientific and Cultural Organization, based on an earlier report.33 Average annual household income was self-reported.
Ascertainment of Disease Prevalence and Incidence
Definitions used to define incident and prevalent outcomes are presented in eTable 7 in the Supplement. We used self-reported diagnoses and medication and Hospital Episode Statistics data, as previously described.34 Participants with prevalent disease were excluded per outcome (Figure 1).
Statistical Analyses
Multivariable Cox regression analyses were performed to test the association of GR and lifestyle groups with incident events of CAD, AF, stroke, hypertension, and diabetes. We determined whether participants were at high (quintile 5), intermediate (quintile 2-4), or low (quintile 1) GR for each outcome, as previously described.10,35,36 Hazard ratios (HRs) with 95% confidence intervals were calculated between lifestyle categories in each GR group and the reference group (ideal lifestyle with low GR). Cox regression analyses were adjusted for age at inclusion, sex, genotyping chip, the first 30 principal components (to adjust for population structure), years in education, TDI, and income. The population-attributable fraction, an estimate of the proportion of events that would have been prevented if all individuals would have been in the ideal lifestyle category,37 was calculated. Finally, we tested for interactions between lifestyle and the quantitative GR for each outcome. To maximize the likelihood of reporting true findings, we set the α at .005 instead of .0538 and used Bonferroni correction to adjust for multiple testing. We considered 2-sided P values less than .001 (P value of less than .005 divided by the number of tests, ie, .005/5) statistically significant. P values less than .01 (ie, .05/5) were considered of suggestive significance. All analyses were performed using Stata version 13 (StataCorp).
Results
Population Characteristics
From the 344 117 unrelated individuals with available genotypes, 3566 participants were excluded because of missing data on smoking, BMI, physical activity, or diet, and 1508 were excluded because of missing data on TDI, income, or education. Participants with prevalent disease were excluded per outcome (Figure 1), leaving 325 133 participants for the analyses of CAD, 333 637 for AF, 332 971 for stroke, 234 651 for hypertension, and 322 014 for diabetes. After these exclusions, a total of 339 003 white British participants remained for 1 or more of the current analyses. The mean (SD) age was 56.86 (7.99) years, and 181 702 (53.6%) were female. In total, 68 666 individuals (20.3%) had ideal overall lifestyle, 252 557 (74.5%) had intermediate overall lifestyle, and 17 780 (5.2%) had poor overall lifestyle. Baseline characteristics are provided in Table 1, and characteristics per outcome are presented in eTables 8-12 in the Supplement. In general, individuals with poor lifestyle had higher blood pressure and BMI and fewer years spent in education. During a median (interquartile range) follow-up of 6.2 (5.5-6.7) years for new-onset disease, 9771 participants (3.0%) developed CAD, 7095 (2.1%) developed AF, 3145 (0.9%) developed stroke, 11 358 (4.8%) developed hypertension, and 4379 (1.4%) developed diabetes. Individuals with poor lifestyle generally experienced higher incidence of all outcomes with increasing GR (Table 2).
Table 1. Baseline Characteristics.
| Characteristic | No. (%) |
|---|---|
| Total, No. | 339 003 |
| Age, mean (SD), y | 56.86 (7.99) |
| Female | 181 702 (53.6) |
| Blood pressure, mean (SD), mm Hg | |
| Systolic | 133.75 (17.93) |
| Diastolic | 82.16 (8.53) |
| Smoking | |
| Ideal (never or stopped >1 y ago) | 185 843 (54.8) |
| Intermediate (stopped <1 y ago) | 119 372 (35.2) |
| Poor (current smoker) | 33 788 (10.0) |
| Body mass indexa | |
| Mean (SD) | 27.44 (4.70) |
| Ideal (18.5-24.9) | 111 281 (32.8) |
| Intermediate (25-29.9) | 145 730 (43.0) |
| Poor (≥30) | 81 992 (24.2) |
| Physical activity | |
| Ideal (regular physical activity) | 229 342 (67.7) |
| Intermediate (some physical activity) | 84 358 (24.9) |
| Poor (no regular physical activity) | 25 303 (7.5) |
| Diet | |
| Ideal (adequate intake of >5 dietary components) | 47 246 (13.9) |
| Poor (inadequate intake of >5 dietary components) | 291 757 (86.1) |
| Lifestyle | |
| Ideal (≥3 ideal factors) | 68 666 (20.3) |
| Intermediate (all other combinations) | 252 557 (74.5) |
| Poor (≥3 poor factors) | 17 780 (5.2) |
| Years in education, mean (SD) | 14.75 (4.81) |
| Income, £b | |
| <18 000 | 63 738 (18.8) |
| 18 000-30 999 | 75 419 (22.2) |
| 31 000-51 999 | 77 640 (22.9) |
| 52 000-100 000 | 60 695 (17.9) |
| >100 000 | 15 763 (4.6) |
| Unknown | 45 748 (13.5) |
Body mass index calculated as weight in kilograms divided by height in meters squared.
To convert from pound sterling to US dollar, multiply by 1.32712.
Table 2. Total Participantsa and Incident Events per End Point in Each Genetic and Lifestyle Group.
| Lifestyle | Low Genetic Risk Group | Intermediate Genetic Risk Group | High Genetic Risk Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ideal | Intermediate | Poor | Ideal | Intermediate | Poor | Ideal | Intermediate | Poor | |
| CAD | |||||||||
| Participants, No. | 13 648 | 49 079 | 3264 | 40 632 | 145 208 | 9869 | 13 113 | 47 144 | 3176 |
| New-onset events, No. (%) | 175 (1.3) | 1170 (2.4) | 157 (4.8) | 660 (1.6) | 4490 (3.1) | 583 (5.9) | 291 (2.2) | 2023 (4.3) | 222 (7.0) |
| IRb | 2.11 | 3.93 | 7.98 | 2.67 | 5.11 | 9.83 | 3.66 | 7.14 | 11.67 |
| PT, y | 82 942 | 297 390 | 19 681 | 247 088 | 877 887 | 59 318 | 79 520 | 283 392 | 19 022 |
| AF | |||||||||
| Participants, No. | 13 496 | 49 804 | 3466 | 41 154 | 150 668 | 10 527 | 13 302 | 47 830 | 3390 |
| New-onset events, No. (%) | 121 (0.9) | 761 (1.5) | 80 (2.3) | 544 (1.3) | 3150 (2.1) | 343 (3.3) | 301 (2.3) | 1610 (3.4) | 185 (5.5) |
| IRb | 1.47 | 2.51 | 3.76 | 2.17 | 3.44 | 5.35 | 3.74 | 5.57 | 9.06 |
| PT, y | 82 288 | 303 394 | 21 290 | 250 938 | 916 124 | 64 172 | 80 501 | 289 147 | 20 415 |
| Stroke | |||||||||
| Participants, No. | 15 135 | 55 864 | 3839 | 39 126 | 142 751 | 9803 | 13 727 | 49 325 | 3401 |
| New-onset events, No. (%) | 94 (0.6) | 491 (0.9) | 56 (1.5) | 267 (0.7) | 1368 (1.0) | 169 (1.7) | 103 (0.8) | 538 (1.1) | 59 (1.7) |
| IRb | 1.02 | 1.44 | 2.37 | 1.12 | 1.57 | 2.81 | 1.23 | 1.79 | 2.83 |
| PT, y | 92 489 | 341 153 | 23 587 | 238 912 | 872 155 | 60 196 | 83 920 | 301 334 | 20 873 |
| Hypertension | |||||||||
| Participants, No. | 11 323 | 36 193 | 2046 | 33 583 | 102 563 | 5607 | 10 666 | 31 045 | 1625 |
| New-onset events, No. (%) | 243 (2.1) | 1602 (4.4) | 167 (8.2) | 966 (2.9) | 5454 (5.3) | 552 (9.8) | 331 (3.1) | 1869 (6.0) | 174 (10.7) |
| IRb | 3.54 | 7.35 | 13.75 | 4.76 | 8.88 | 16.66 | 5.15 | 10.08 | 18.09 |
| PT, y | 68 739 | 217 818 | 12 142 | 203 145 | 614 409 | 33 136 | 64 320 | 185 346 | 9616 |
| Diabetes | |||||||||
| Participants, No. | 14 074 | 50 521 | 3439 | 40 339 | 144 261 | 9339 | 12 609 | 44 670 | 2762 |
| New-onset events, No. (%) | 38 (0.3) | 506 (1.0) | 133 (3.9) | 143 (0.4) | 2034 (1.4) | 420 (4.5) | 65 (0.5) | 887 (2.0) | 153 (5.5) |
| IRb | 0.44 | 1.64 | 6.33 | 0.58 | 2.31 | 7.4 | 0.84 | 3.26 | 9.12 |
| PT, y | 86 253 | 308 800 | 20 996 | 246 742 | 880 283 | 56 775 | 77 018 | 271 921 | 16 769 |
Abbreviations: AF, atrial fibrillation; CAD, coronary artery disease; IR, incidence rate; PT, person-time.
All individuals with prevalent disease were excluded per end point.
Incidence rates are provided per 1000 person-years.
Associations of GR With Incident CVD and Diabetes
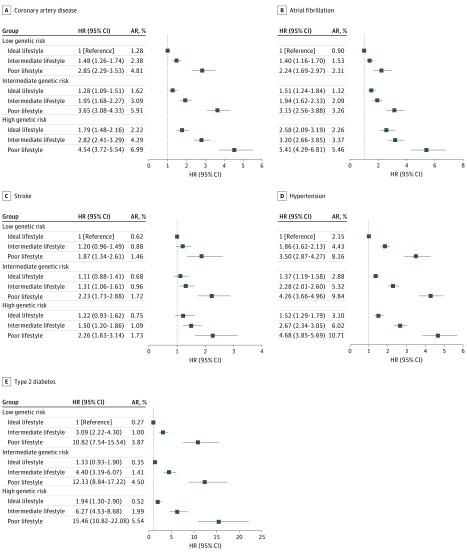
eTable 13 in the Supplement presents the HRs of participants at intermediate and high GR compared with low GR and shows that higher GR was associated with higher risk of incident CVD and diabetes during follow-up. The analyses were adjusted for age, sex, genotyping chip, the first 30 principal components, years in education, TDI, income, and lifestyle. High GR was associated with a higher risk of incident CAD (HR, 1.86; 95% CI, 1.74-1.98; P < .001), incident AF (HR, 2.33; 95% CI, 2.16-2.52; P < .001), incident stroke (HR, 1.24; 95% CI, 1.12-1.38; P = 6.9 × 10−5), incident hypertension (HR, 1.44; 95% CI, 1.36-1.53; P < .001), and incident diabetes (HR, 1.91; 95% CI, 1.74-2.10; P < .001). Intermediate GR of stroke was not associated with increased risk of incident events compared with low GR (HR, 1.10; 95% CI, 1.01-1.21; P = .03).
Associations of Lifestyle and GR With Incident CVD and Diabetes
Across higher GR groups, ideal and poor lifestyle were associated with higher absolute risks of incident events, but poor lifestyle was associated with similar increases in risk compared with ideal lifestyle (Figure 2). A similar trend was observed for individual lifestyle factors (eTable 14 in the Supplement). The highest risks were observed among individuals with high GR and poor lifestyle. Compared with ideal lifestyle and low GR, adherence to poor lifestyle and having a high GR was associated with higher risk of CAD (HR, 4.54; 95% CI, 3.72-5.54; P < .001), AF (HR, 5.41; 95% CI, 4.29-6.81; P < .001), stroke (HR, 2.26; 95% CI, 1.63-3.14; P < .001), hypertension (HR, 4.68; 95% CI, 3.85-5.69; P < .001), and diabetes (HR, 15.46; 95% CI, 10.82-22.08; P < .001). Compared with low GR, intermediate GR of stroke was not associated with increased risk of events (absolute risk, 0.94%), and the associations with lifestyle were similar in both GR groups (Figure 2). Furthermore, high compared with low GR of stroke among individuals with ideal lifestyle was not associated with increased risk (HR, 1.22; 95% CI, 0.93-1.62; P = .15). However, for CAD and diabetes, higher GR did increase the risk of events, and ideal lifestyle in the intermediate and high GR groups was similarly not or only suggestively associated with increased risk compared with ideal lifestyle and low GR (Figure 2). Unlike stroke, poor lifestyle was associated with much higher risks of CAD and, in particular, diabetes in the high GR group compared with poor lifestyle in the low GR group. After excluding individuals with systolic blood pressure of 130 mm Hg or greater and/or diastolic blood pressure of 80 mm Hg or greater at baseline (n = 147 037), poor lifestyle remained associated with increased risk of new-onset hypertension compared with ideal lifestyle in the same GR group (eFigure 1 and eTable 15 in the Supplement). However, intermediate and high GR compared with low GR of hypertension among individuals with ideal lifestyle was not associated with increased risk of new-onset events. As a sensitivity analysis, we calculated the HR for all outcomes in equally sized tertiles of GR, but the results remained essentially unchanged (eTable 16 in the Supplement). Additionally, we calculated correlations between individual lifestyle factors as well as with years in education, income, and TDI and found only mild to moderate correlations (eTable 17 in the Supplement).
Figure 2. Genetic and Lifestyle Risk of Cardiovascular Diseases and Diabetes.
Hazard ratios (HRs) are provided with 95% CIs. The vertical line indicates the reference value of 1. AR indicates absolute risk.
GR × Lifestyle Interactions and Sex Differences
No significant interactions were found between behavioral lifestyle and GR of any outcome (eTable 18 in the Supplement). The minimal interaction effects that would have been detected with 80% power in our population are presented per outcome in eTable 19 in the Supplement. Also, no interactions were identified between the GR of hypertension and lifestyle among individuals with baseline systolic blood pressure less than 130 mm Hg and/or diastolic blood pressure less than 80 mm Hg (GR × intermediate lifestyle: coefficient = 1.01; P = .54; GR × poor lifestyle: coefficient = 0.98; P = .72). Risks of new-onset events associated with lifestyle in the GR groups were also tested by sex, but the results were not markedly different among men and women (eFigures 2-6 in the Supplement).
Population-Attributable Fractions
Since there were no interactions between lifestyle and GR, the population-attributable fraction was calculated regardless of GR. For CAD, 37% (95% CI, 33-41) of new-onset events during follow-up might have been prevented if all individuals would have adhered to ideal lifestyle; for AF, 25% (95% CI, 19-30); for stroke, 19% (95% CI, 10-27); for hypertension, 44% (95% CI, 40-47); and for diabetes, 72% (95% CI, 68-76). The fractions were higher when taking into account only individuals with poor lifestyle who would have adhered to ideal lifestyle and ranged from 51% for stroke to 90% for diabetes (eTable 20 in the Supplement).
Discussion
In this large community-based population of more than 339 000 individuals, high GR was associated with increased risk of new-onset CVD and diabetes events independent of lifestyle. Within and across GR groups, adherence to poor behavioral lifestyle was associated with increased risk of CVD and diabetes. No interaction effects were observed between GR and lifestyle. For diabetes, the effects of lifestyle on disease development were the strongest. Ideal lifestyle returned the risk of incident diabetes toward the referent in any GR subgroup, but poor lifestyle was associated with 15-fold higher risk in the high GR group. This study shows that genetic composition and lifestyle have a log-additive effect on the risk of developing disease and that the relative effects of poor lifestyle are comparable between GR groups.
Comparison With Previous Studies
To our knowledge, this study is the first to report the associations of combined health behaviors and factors in different GR groups for AF, stroke, hypertension, and diabetes. The effects of combined health behaviors and factors across GR groups are in line with a previous report for CAD,10 which studied a smaller population of 55 685 participants with 5103 (9.2%) new-onset events. The general risk patterns associated with lifestyle and GR were similar in both studies. However, the present study suggests the HR associated with poor lifestyle and high GR may be 1.3-fold (95% CI, 1.25-1.34) higher compared with the previous report. Compared with the previous report,10 the present study included more SNPs associated with CAD (169 vs 50) to increase power for estimating the GR. Furthermore, information on lifestyle behaviors and factors were collected uniformly for all participants in the UK Biobank study, whereas each of the 4 cohorts included in the previous report used different methods to collect this data. Earlier studies have also indicated beneficial effects of adherence to healthy lifestyle for CVD4,5,39,40,41 and diabetes.6,42 However, these studies did not consider the genetic burden of the diseases and mostly looked at individual behavioral and nonbehavioral lifestyle variants rather than combined health behaviors and factors, as was done in the current analyses.
Future Perspectives for Using GR in Patient Selection and Decision Making
The current analyses show that behavioral lifestyle had no interactions with GR and that poor lifestyle was associated with similar effects compared with ideal lifestyle within the same GR group. For diabetes, it has previously been shown that there were no interactions between individual behavioral lifestyle factors and GR.43 These findings indicate the strong potential benefits of adherence to multiple ideal behavioral lifestyle factors regardless of GR. Therefore, preventive policies should promote stricter adherence to multiple ideal behavioral lifestyle factors (eg, eliminating smoking, eating a healthy diet, maintaining a healthy weight, and engaging in regular physical activity) for all.
Challenges in Communicating GR
Challenges remain in communicating individual GR for outcomes such that it is understandable and interpretable by the general population.44,45 Knowledge of GR may lead individuals to believe they are destined to develop diseases regardless of their lifestyle and may insufficiently motivate behavior changes.45 One study (n = 65) indicated that knowledge of GR for CAD did not lead to a change in lipids compared with individuals who did not know their GR (n = 35), although modest beneficial effects were observed for weight loss and physical activity.46 A study of 207 participants found that disclosure of GR for CAD led participants to search for more information on CAD and discuss their risk with others.47 However, these participants were generally well-educated, and the quality of information found was unknown. Alternatively, the GR could remain undisclosed but be included as a factor in risk prediction models. However, the effects of communicating the risk indicated in existing CVD risk prediction models are similarly limited.48 Research in larger cohorts is needed to investigate whether GR should be disclosed and, if so, which method is most effective and whether this knowledge encourages individuals to undergo stricter and earlier lifestyle intervention. Furthermore, understandable and reliable information on diseases and possible preventive measures should become easily available for patients.
Strengths and Limitations
To our knowledge, this study is the first to investigate the associations and interaction of combined modifiable health behaviors and factors across GR subgroups of CAD, AF, stroke, hypertension, and diabetes simultaneously while adjusting for various demographic confounders. Major strengths of this study were the large sample size, the prospective design of the UK Biobank study, and the low significance threshold that accounted for multiple testing and increased the likelihood of reporting true and reproducible findings.
This study has limitations. A limitation that should be considered is that causality between the health behaviors and factors and diseases cannot be inferred from the observational study design. Therefore, the population-attributable fractions should be interpreted with caution. Furthermore, SNPs contributing to the polygenic risk scores may also have pleiotropic effects on lifestyle factors. A third limitation is that data on physical activity, smoking, and diet were self-reported. Also, accuracy of Hospital Episode Statistics data are only known for some outcomes,49,50 and incident cases of hypertension and diabetes may have been missed if they were diagnosed and treated in outpatient settings and not reported during a follow-up visit to the assessment center, which may have introduced some ascertainment bias. However, possible measurement and classification errors are likely biased toward the null and would underestimate the risk associated with poor health behaviors and factors. A fourth limitation is that the present analyses were performed only in individuals of white British descent, which may reduce the generalizability of the results to other racial/ethnic groups. Furthermore, changes in behavioral lifestyle factors over time were not taken into account in the present analyses. Future research is needed to investigate the effects of behavioral and nonbehavioral lifestyle changes over time on the risk associated with incident and recurrent events within GR groups. Finally, as increasingly more genetic variants are identified,51 the variance explained by genetics and GR estimates will improve. Similarly, improved monitoring of lifestyle factors, eg, physical activity, will allow more accurate risk estimates for lifestyle.
Conclusions
In conclusion, poor behavioral lifestyle was a strong incremental risk factor of new-onset CVD and diabetes in this large cohort. This study showed that GR and combined health behaviors and factors have a log-additive effect on the risk of new-onset diseases but that there were no interactions between these risk factors. Behavioral lifestyle changes should be encouraged for all through comprehensive multifactorial approaches, although high-risk individuals may be selected based on their GR.
eMethods. Behavioral lifestyle factor definitions in the UK Biobank study.
eTable 1. Single-nucleotide polymorphisms used to build the genetic risk score for coronary artery disease.
eTable 2. Single-nucleotide polymorphisms used to build the genetic risk score for atrial fibrillation.
eTable 3. Single-nucleotide polymorphisms used to build the genetic risk score for stroke.
eTable 4. Single-nucleotide polymorphisms used to build the genetic risk score for hypertension (based on systolic blood pressure).
eTable 5. Single-nucleotide polymorphisms used to build the genetic risk score for type 2 diabetes.
eTable 6. Diet component definitions used in the UK Biobank study.
eTable 7. Disease definitions used in the UK Biobank study.
eTable 8. Baseline characteristics per lifestyle category and genetic risk group of coronary artery disease.
eTable 9. Baseline characteristics per lifestyle category and genetic risk group of atrial fibrillation.
eTable 10. Baseline characteristics per lifestyle category and genetic risk group of stroke.
eTable 11. Baseline characteristics per lifestyle category and genetic risk group of hypertension.
eTable 12. Baseline characteristics per lifestyle category and genetic risk group of type 2 diabetes.
eTable 13. Genetic risk of cardiovascular diseases and diabetes.
eTable 14. Associations of individual lifestyle factors with cardiovascular diseases and diabetes across genetic risk groups.
eTable 15. Genetic and lifestyle risk of hypertension in individuals with baseline systolic blood pressure less than 130 mm Hg and/or diastolic blood pressure less than 80 mm Hg.
eTable 16. Genetic and lifestyle risk of cardiovascular diseases and diabetes in equally sized tertiles of genetic risk.
eTable 17. Correlations between individual lifestyle factors and covariates.
eTable 18. Genetic risk × lifestyle interactions.
eTable 19. Minimal detectable interaction effect between genetic risk × lifestyle.
eTable 20. Population-attributable fraction per behavioral lifestyle group.
eFigure 1. Risk of incident hypertension in individuals with baseline systolic blood pressure less than 130 mm Hg and/or diastolic blood pressure less than 80 mm Hg.
eFigure 2. Risk of incident coronary artery disease associated with genetic risk and lifestyle stratified by sex.
eFigure 3. Risk of incident atrial fibrillation associated with genetic risk and lifestyle stratified by sex.
eFigure 4. Risk of incident stroke associated with genetic risk and lifestyle stratified by sex.
eFigure 5. Risk of incident hypertension associated with genetic risk and lifestyle stratified by sex.
eFigure 6. Risk of incident diabetes associated with genetic risk and lifestyle stratified by sex.
eReferences.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117-171. doi: 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378(9799):1297-1305. doi: 10.1016/S0140-6736(11)60781-2 [DOI] [PubMed] [Google Scholar]
- 3.Huxley RR, Misialek JR, Agarwal SK, et al. Physical activity, obesity, weight change, and risk of atrial fibrillation: the Atherosclerosis Risk in Communities Study. Circ Arrhythm Electrophysiol. 2014;7(4):620-625. doi: 10.1161/CIRCEP.113.001244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343(1):16-22. doi: 10.1056/NEJM200007063430103 [DOI] [PubMed] [Google Scholar]
- 5.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 2006;114(2):160-167. doi: 10.1161/CIRCULATIONAHA.106.621417 [DOI] [PubMed] [Google Scholar]
- 6.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790-797. doi: 10.1056/NEJMoa010492 [DOI] [PubMed] [Google Scholar]
- 7.Celis-Morales CA, Lyall DM, Welsh P, et al. Association between active commuting and incident cardiovascular disease, cancer, and mortality: prospective cohort study. BMJ. 2017;357:j1456. doi: 10.1136/bmj.j1456 [DOI] [PubMed] [Google Scholar]
- 8.Celis-Morales CA, Lyall DM, Anderson J, et al. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 UK-Biobank participants. Eur Heart J. 2017;38(2):116-122. doi: 10.1093/eurheartj/ehw249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Hong Y, Labarthe D, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586-613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 10.Khera AV, Emdin CA, Drake I, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375(24):2349-2358. doi: 10.1056/NEJMoa1605086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UK Biobank UK Biobank: protocol for a large-scale prospective epidemiological resource. http://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf. Accessed December 15, 2015.
- 13.UK Biobank UK Biobank ethics and governance framework. https://www.ukbiobank.ac.uk/wp-content/uploads/2011/05/EGF20082.pdf. Accessed December 15, 2015.
- 14.Bycroft C, Freeman C, Petkova D, et al. Genome-wide genetic data on ~500,000 UK Biobank participants [published online July 20, 2017]. bioRxiv. doi: 10.1101/166298 [DOI] [Google Scholar]
- 15.UK Biobank Genotyping and quality control of UK Biobank, a large-scale, extensively phenotyped prospective resource. https://biobank.ctsu.ox.ac.uk/crystal/docs/genotyping_qc.pdf. Accessed December 15, 2015.
- 16.Marchini J; UK Biobank UK Biobank phasing and imputation documentation. https://biobank.ctsu.ox.ac.uk/crystal/docs/impute_ukb_v1.pdf. Accessed August 18, 2017.
- 17.Verweij N, Eppinga RN, Hagemeijer Y, van der Harst P. Identification of 15 novel risk loci for coronary artery disease and genetic risk of recurrent events, atrial fibrillation and heart failure. Sci Rep. 2017;7(1):2761. doi: 10.1038/s41598-017-03062-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikpay M, Goel A, Won HH, et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47(10):1121-1130. doi: 10.1038/ng.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christophersen IE, Rienstra M, Roselli C, et al. ; METASTROKE Consortium of the ISGC; Neurology Working Group of the CHARGE Consortium; AFGen Consortium . Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet. 2017;49(6):946-952. doi: 10.1038/ng.3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik R, Chauhan G, Traylor M, et al. ; AFGen Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium; International Genomics of Blood Pressure (iGEN-BP) Consortium; INVENT Consortium; STARNET; BioBank Japan Cooperative Hospital Group; COMPASS Consortium; EPIC-CVD Consortium; EPIC-InterAct Consortium; International Stroke Genetics Consortium (ISGC); METASTROKE Consortium; Neurology Working Group of the CHARGE Consortium; NINDS Stroke Genetics Network (SiGN); UK Young Lacunar DNA Study; MEGASTROKE Consortium; MEGASTROKE Consortium . Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50(4):524-537. doi: 10.1038/s41588-018-0058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehret GB, Ferreira T, Chasman DI, et al. ; CHARGE-EchoGen consortium; CHARGE-HF consortium; Wellcome Trust Case Control Consortium . The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat Genet. 2016;48(10):1171-1184. doi: 10.1038/ng.3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann TJ, Ehret GB, Nandakumar P, et al. Genome-wide association analyses using electronic health records identify new loci influencing blood pressure variation. Nat Genet. 2017;49(1):54-64. doi: 10.1038/ng.3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surendran P, Drenos F, Young R, et al. ; CHARGE-Heart Failure Consortium; EchoGen Consortium; METASTROKE Consortium; GIANT Consortium; EPIC-InterAct Consortium; Lifelines Cohort Study; Wellcome Trust Case Control Consortium; Understanding Society Scientific Group; EPIC-CVD Consortium; CHARGE+ Exome Chip Blood Pressure Consortium; T2D-GENES Consortium; GoT2DGenes Consortium; ExomeBP Consortium; CHD Exome+ Consortium . Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat Genet. 2016;48(10):1151-1161. doi: 10.1038/ng.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren HR, Evangelou E, Cabrera CP, et al. ; International Consortium of Blood Pressure (ICBP) 1000G Analyses; BIOS Consortium; Lifelines Cohort Study; Understanding Society Scientific group; CHD Exome+ Consortium; ExomeBP Consortium; T2D-GENES Consortium; GoT2DGenes Consortium; Cohorts for Heart and Ageing Research in Genome Epidemiology (CHARGE) BP Exome Consortium; International Genomics of Blood Pressure (iGEN-BP) Consortium; UK Biobank CardioMetabolic Consortium BP working group . Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat Genet. 2017;49(3):403-415. doi: 10.1038/ng.3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Kraja AT, Smith JA, et al. ; CHD Exome+ Consortium; ExomeBP Consortium; GoT2DGenes Consortium; T2D-GENES Consortium; Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia; CKDGen Consortium . Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat Genet. 2016;48(10):1162-1170. doi: 10.1038/ng.3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato N, Loh M, Takeuchi F, et al. ; BIOS-consortium; CARDIo GRAMplusCD; LifeLines Cohort Study; InterAct Consortium . Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat Genet. 2015;47(11):1282-1293. doi: 10.1038/ng.3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dupuis J, Langenberg C, Prokopenko I, et al. ; DIAGRAM Consortium; GIANT Consortium; Global BPgen Consortium; Anders Hamsten on behalf of Procardis Consortium; MAGIC investigators . New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105-116. doi: 10.1038/ng.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voight BF, Scott LJ, Steinthorsdottir V, et al. ; MAGIC investigators; GIANT Consortium . Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42(7):579-589. doi: 10.1038/ng.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeggini E, Scott LJ, Saxena R, et al. ; Wellcome Trust Case Control Consortium . Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40(5):638-645. doi: 10.1038/ng.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris AP, Voight BF, Teslovich TM, et al. ; Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44(9):981-990. doi: 10.1038/ng.2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187-225. doi: 10.1161/CIRCULATIONAHA.115.018585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Townsend P. Deprivation. J Soc Policy. 1987;16(2):125-146. doi: 10.1017/S0047279400020341 [DOI] [Google Scholar]
- 33.Okbay A, Beauchamp JP, Fontana MA, et al. ; LifeLines Cohort Study . Genome-wide association study identifies 74 loci associated with educational attainment. Nature. 2016;533(7604):539-542. doi: 10.1038/nature17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Said MA, Eppinga RN, Lipsic E, Verweij N, van der Harst P. Relationship of arterial stiffness index and pulse pressure with cardiovascular disease and mortality. J Am Heart Assoc. 2018;7(2):e007621. doi: 10.1161/JAHA.117.007621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385(9984):2264-2271. doi: 10.1016/S0140-6736(14)61730-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tada H, Shiffman D, Smith JG, et al. Twelve-single nucleotide polymorphism genetic risk score identifies individuals at increased risk for future atrial fibrillation and stroke. Stroke. 2014;45(10):2856-2862. doi: 10.1161/STROKEAHA.114.006072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization Metrics: population attributable fraction (PAF). http://www.who.int/healthinfo/global_burden_disease/metrics_paf/en/. Accessed August 12, 2017.
- 38.Benjamin DJ, Berger JO, Johannesson M, et al. Redefine statistical significance. Nat Hum Behav. 2018;2(1):6-10. doi: 10.1038/s41562-017-0189-z [DOI] [PubMed] [Google Scholar]
- 39.Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366(4):321-329. doi: 10.1056/NEJMoa1012848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Effoe VS, Carnethon MR, Echouffo-Tcheugui JB, et al. The American Heart Association ideal cardiovascular health and incident type 2 diabetes mellitus among blacks: the Jackson Heart Study. J Am Heart Assoc. 2017;6(6):e005008. doi: 10.1161/JAHA.116.005008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogunmoroti O, Oni E, Michos ED, et al. Life’s simple 7 and incident heart failure: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017;6(6):e005180. doi: 10.1161/JAHA.116.005180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the Cardiovascular Health Study. Arch Intern Med. 2009;169(8):798-807. doi: 10.1001/archinternmed.2009.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langenberg C, Sharp SJ, Franks PW, et al. Gene-lifestyle interaction and type 2 diabetes: the EPIC InterAct case-cohort study. PLoS Med. 2014;11(5):e1001647. doi: 10.1371/journal.pmed.1001647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shefer G, Silarova B, Usher-Smith J, Griffin S. The response to receiving phenotypic and genetic coronary heart disease risk scores and lifestyle advice: a qualitative study. BMC Public Health. 2016;16(1):1221. doi: 10.1186/s12889-016-3867-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollands GJ, French DP, Griffin SJ, et al. The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ. 2016;352:i1102. doi: 10.1136/bmj.i1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knowles JW, Zarafshar S, Pavlovic A, et al. Impact of a genetic risk score for coronary artery disease on reducing cardiovascular risk: a pilot randomized controlled study. Front Cardiovasc Med. 2017;4:53. doi: 10.3389/fcvm.2017.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown SN, Jouni H, Marroush TS, Kullo IJ. Effect of disclosing genetic risk for coronary heart disease on information seeking and sharing: the MI-GENES Study (Myocardial Infarction Genes). Circ Cardiovasc Genet. 2017;10(4):e001613. doi: 10.1161/CIRCGENETICS.116.001613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karmali KN, Persell SD, Perel P, Lloyd-Jones DM, Berendsen MA, Huffman MD. Risk scoring for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;3:CD006887. doi: 10.1002/14651858.CD006887.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodfield R, Grant I, Sudlow CL; UK Biobank Stroke Outcomes Group; UK Biobank Follow-Up and Outcomes Working Group . Accuracy of electronic health record data for identifying stroke cases in large-scale epidemiological studies: a systematic review from the UK Biobank Stroke Outcomes Group. PLoS One. 2015;10(10):e0140533. doi: 10.1371/journal.pone.0140533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrett E, Shah AD, Boggon R, et al. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ. 2013;346:f2350. doi: 10.1136/bmj.f2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. 2018;122(3):433-443. doi: 10.1161/CIRCRESAHA.117.312086 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Behavioral lifestyle factor definitions in the UK Biobank study.
eTable 1. Single-nucleotide polymorphisms used to build the genetic risk score for coronary artery disease.
eTable 2. Single-nucleotide polymorphisms used to build the genetic risk score for atrial fibrillation.
eTable 3. Single-nucleotide polymorphisms used to build the genetic risk score for stroke.
eTable 4. Single-nucleotide polymorphisms used to build the genetic risk score for hypertension (based on systolic blood pressure).
eTable 5. Single-nucleotide polymorphisms used to build the genetic risk score for type 2 diabetes.
eTable 6. Diet component definitions used in the UK Biobank study.
eTable 7. Disease definitions used in the UK Biobank study.
eTable 8. Baseline characteristics per lifestyle category and genetic risk group of coronary artery disease.
eTable 9. Baseline characteristics per lifestyle category and genetic risk group of atrial fibrillation.
eTable 10. Baseline characteristics per lifestyle category and genetic risk group of stroke.
eTable 11. Baseline characteristics per lifestyle category and genetic risk group of hypertension.
eTable 12. Baseline characteristics per lifestyle category and genetic risk group of type 2 diabetes.
eTable 13. Genetic risk of cardiovascular diseases and diabetes.
eTable 14. Associations of individual lifestyle factors with cardiovascular diseases and diabetes across genetic risk groups.
eTable 15. Genetic and lifestyle risk of hypertension in individuals with baseline systolic blood pressure less than 130 mm Hg and/or diastolic blood pressure less than 80 mm Hg.
eTable 16. Genetic and lifestyle risk of cardiovascular diseases and diabetes in equally sized tertiles of genetic risk.
eTable 17. Correlations between individual lifestyle factors and covariates.
eTable 18. Genetic risk × lifestyle interactions.
eTable 19. Minimal detectable interaction effect between genetic risk × lifestyle.
eTable 20. Population-attributable fraction per behavioral lifestyle group.
eFigure 1. Risk of incident hypertension in individuals with baseline systolic blood pressure less than 130 mm Hg and/or diastolic blood pressure less than 80 mm Hg.
eFigure 2. Risk of incident coronary artery disease associated with genetic risk and lifestyle stratified by sex.
eFigure 3. Risk of incident atrial fibrillation associated with genetic risk and lifestyle stratified by sex.
eFigure 4. Risk of incident stroke associated with genetic risk and lifestyle stratified by sex.
eFigure 5. Risk of incident hypertension associated with genetic risk and lifestyle stratified by sex.
eFigure 6. Risk of incident diabetes associated with genetic risk and lifestyle stratified by sex.
eReferences.