This randomized clinical trial characterizes the metabolic effects of first exposure to antipsychotics in youths with disruptive behavior disorders using criterion standard assessments of body composition and tissue-specific insulin sensitivity.
Key Points
Question
What is the effect of first exposure to antipsychotics on adiposity and insulin sensitivity in youths?
Findings
In this randomized clinical trial, 144 youths aged 6 to 18 years with disruptive behavior disorders who were randomized to receive aripiprazole, olanzapine, or risperidone experienced clinically significant increases in total and abdominal adiposity during 12 weeks of treatment. Increases were greater for olanzapine vs risperidone or aripiprazole; decreases in insulin sensitivity and improvements in behavior were also noted.
Meaning
Antipsychotic medications are commonly used in children for the treatment of disruptive behavior disorders, but potential benefits should be carefully weighed against the risk for adverse changes in total and abdominal adiposity and insulin sensitivity, known contributors to the development of early-onset type 2 diabetes, cardiovascular disease, and other illnesses associated with premature morbidity and mortality.
Abstract
Importance
Antipsychotic medications are commonly used to treat nonpsychotic disruptive behavioral disorders in youths.
Objective
To characterize the metabolic effects of first exposure to antipsychotics in youths using criterion standard assessments of body composition and insulin sensitivity.
Design, Setting, and Participants
This randomized clinical trial recruited antipsychotic-naive youths aged 6 to 18 years in the St Louis, Missouri, metropolitan area who were diagnosed with 1 or more psychiatric disorders and clinically significant aggression and in whom antipsychotic treatment was considered. Participants were enrolled from June 12, 2006, through November 10, 2010. Enrolled participants were randomized (1:1:1) to 1 of 3 antipsychotics commonly used in children with disruptive behavioral disorders and evaluated for 12 weeks. Data were analyzed from January 17, 2011, through August 9, 2017.
Interventions
Twelve weeks of treatment with oral aripiprazole (n = 49), olanzapine (n = 46), or risperidone (n = 49).
Main Outcomes and Measures
Primary outcomes included percentage total body fat measured by dual-energy x-ray absorptiometry (DXA) and insulin sensitivity in muscle measured via hyperinsulinemic clamps with stable isotopically labeled tracers. Secondary outcomes included abdominal adiposity measured by magnetic resonance imaging (MRI) and adipose and hepatic tissue insulin sensitivity measured via clamps with tracers.
Results
The intention-to-treat sample included 144 participants (98 males [68.1%]; mean [SD] age, 11.3 [2.8] years); 74 (51.4%) were African American, and 43 (29.9%) were overweight or obese at baseline. For the primary outcomes, from baseline to week 12, DXA percentage total body fat increased by 1.18% for risperidone, 4.12% for olanzapine, and 1.66% for aripiprazole and was significantly greater for olanzapine than risperidone or aripiprazole (time by treatment interaction P < .001). From baseline to week 12, insulin-stimulated change in glucose rate of disappearance increased by 2.30% for risperidone and decreased by 29.34% for olanzapine and 30.26% for aripiprazole, with no significant difference across medications (time by treatment interaction, P < .07). This primary measure of insulin sensitivity decreased significantly during 12 weeks in the pooled study sample (effect of time, F = 17.38; P < .001). For the secondary outcomes from baseline to week 12, MRI measured abdominal fat increased, with subcutaneous fat increase significantly greater for olanzapine than risperdone or aripiprazole (time by treatment, P = .003). Behavioral improvements occurred with all treatments.
Conclusions and Relevance
Adverse changes in adiposity and insulin sensitivity were observed during 12 weeks of antipsychotic treatment in youths, with the greatest fat increases on olanzapine. Such changes, likely attributable to treatment, may be associated with risk for premature cardiometabolic morbidity and mortality. The results inform risk-benefit considerations for antipsychotic use in youths.
Trial Registration
ClinicalTrials.gov identifier: NCT00205699
Introduction
Treatment with antipsychotics is associated with risks for weight gain, type 2 diabetes (T2D), and related conditions, including incident diabetes in children.1,2,3,4 Although antipsychotics are first-line treatments for conditions like pediatric-onset schizophrenia, they are more commonly prescribed off-label for attention-deficit/hyperactivity disorder and disruptive behavior disorders,5 with greater use in publicly insured youths.6 Although youths with these most commonly treated disorders might benefit from antipsychotic treatment, careful consideration of risks and benefits is warranted, particularly risks such as overweight, obesity, and insulin resistance, for which childhood onset can have greater effects on risk for T2D and cardiovascular disease compared with adult onset.7,8
Prior studies of antipsychotics in children9,10,11 have relied on weight-based surrogate or indirect measures of adiposity (eg, weight, body mass index [BMI] percentile or z score) and insulin sensitivity (eg, fasting plasma insulin or triglyceride levels), commonly in the context of secondary analyses among children who were not antipsychotic naive. These studies have been interpreted to suggest that antipsychotics adversely affect adiposity and insulin sensitivity. That we know of, direct measures of adiposity and insulin sensitivity have not been used together as primary outcomes in any published studies of prospective randomized antipsychotic treatment in youth, limiting understanding of key treatment-induced risks. Acute treatment-induced changes in insulin sensitivity have been reported in adults.12 Of importance, antipsychotic-induced weight increases may not be fully interpretable without a direct measure of body composition.13 Direct measurement of treatment effects on adiposity is critical, because increased adiposity, particularly abdominal adiposity, is a known contributor to insulin resistance in adults and children.14,15,16
Dual-energy x-ray absorptiometry (DXA) and magnetic resonance imaging (MRI) provide validated quantification of adiposity in children.17,18 The hyperinsulinemic-euglycemic clamp method provides validated measures of insulin sensitivity in muscle, liver, and adipose tissue.19,20,21 This study is, to our knowledge, the first randomized, prospective clinical trial designed to test the hypothesis that antipsychotic treatment adversely effects adiposity and insulin sensitivity in previously antipsychotic-naive youths using criterion standard measures of primary outcomes. We hypothesized that all tested antipsychotics would produce adverse effects on adiposity and insulin sensitivity, supporting current US Food and Drug Administration class labeling of antipsychotics for metabolic risk, and that adverse effects would be greater with olanzapine.
Methods
Study Population
Participants were antipsychotic-naive youths aged 6 to 18 years with 1 or more Axis I DSM IV-TR diagnosis22 and clinically significant aggression defined by a score of at least 18 on the Irritability subscale of the Aberrant Behavior Checklist (scores range from 0 to 45, with higher scores indicating greater symptom severity).23 Recruitment targeted youths from the St Louis, Missouri, metropolitan area whose clinicians and parents had already decided to initiate antipsychotic treatment, so that study participation offered safety monitoring beyond the scope of clinical practice. All participant screening, study procedures, and data collection were conducted at Washington University School of Medicine in St Louis. The study was approved by the institutional review board of Washington University in St Louis, and all participants or parents or guardians provided written informed consent or assent as required.
The trial protocol is found in Supplement 1. Recruitment for the study took place from June 12, 2006, through November 10, 2010 (eFigure 1 in Supplement 2). Treatment with at least 4 weeks of stable doses of stimulants, atomoxetine hydrochloride, α-adrenergic agents, and selective serotonin reuptake inhibitors was allowed to increase study generalizability. No exclusions were based on sex, race/ethnicity, with targeted enrollment reflecting the sex distribution of males to females for externalizing disorders (ie, 2.5:1).24 Race/ethnicity was determined by self-report and parent or guardian report using open-ended questions.
Exclusions included substance use disorders and an IQ of less than 70; more than 1 week of lifetime antipsychotic exposure; attention-deficit/hyperactivity disorder, depression, or anxiety without a prior trial of stimulant or selective serotonin reuptake inhibitor treatment; and diabetes (fasting plasma glucose level ≥126 mg/dL [to convert to millimoles per liter, multiply by 0.0555] or hemoglobin A1c level ≥6.5% [to convert to a proportion of total hemoglobin, multiply by 0.01]).
Clinical Evaluation and Psychiatric Symptom Assessment
Detailed medical history, anthropomorphic measurements (height, weight, and waist circumference), vital signs (blood pressure, temperature, and heart rate), clinical laboratory tests (fasting metabolic panel, complete blood cell count, thyrotropin level, fasting plasma lipid panel, hemoglobin A1c level, and high-sensitivity C-reactive protein level), and resting 12-lead electrocardiography were obtained for all participants. Pubertal status was assessed using the Duke Pubertal Status Questionnaire.25 Safety monitoring, including laboratory, anthropomorphic, and electrocardiographic measures and vital signs, was conducted at baseline and 6 and 12 weeks.
Consensus diagnoses were determined by semistructured Missouri Assessment of Genetics Interview for Children26 and assessment by board-certified child psychiatrists (including G.E.N.). Youths with untreated or undertreated psychiatric conditions were referred back to treating clinicians for first-line medication trials (eg, stimulants or antidepressants). Symptom severity was assessed at baseline and 12 weeks with the Aberrant Behavior Checklist,23 Clinical Global Impression Severity and Improvement subscales,27 and the Child Global Assessment Scale.28,29 Adverse events were rated at baseline and 6 and 12 weeks using a 33-item adverse event reporting scale (eFigure 2 in Supplement 2), the Abnormal Involuntary Movements Scale,30 the Barnes Akathisia Scale,31 and the Simpson Angus Scale.32
Randomization and Treatment Administration
Randomization was generated by the study statistician (K.B.S.) in blocks of 10, implemented by the study coordinator (J.A.S.), and stratified by age (6-12 vs 13-18 years) to allow exploratory analysis of age effects, with open-label treatment assignment. Group composition was monitored for sex, race/ethnicity, and concurrent use of stimulant medication. The Clinical Global Impression scale and Child Global Assessment Scale raters were blinded to randomization and not involved in treatment. Antipsychotics for this project were chosen to evaluate outcomes in adiposity and insulin sensitivity, with use in child populations supported by current literature and/or prescribing patterns indicating increasing use in children. At study initiation, olanzapine and risperidone were the most frequently prescribed antipsychotics for aggression in children, with aripiprazole use increasing. We used flexible titration of antipsychotic doses based on clinical response and tolerability, aiming for minimally effective doses,33,34,35 with terminal doses attained by 6 weeks. Adherence to the medication regimen was facilitated at follow-up visits through self-report and reports by a parent or a guardian, with pill counts as needed.
Primary and Secondary Outcomes
The per-protocol primary outcome measures were to evaluate antipsychotic treatment effects over 12 weeks on total body fat as well as insulin sensitivity at muscle (glucose disposal). Secondary outcomes included effects over 12 weeks on abdominal fat and insulin sensitivity at liver (glucose production) and adipose tissue (lipolysis). Clinical and routine laboratory follow up was scheduled at 6 months. In this article, we report the 12 week findings of the effects on body fat and insulin sensitivity. The 6-month outcomes will be reported elsewhere.
Study Procedures
The percentage total body fat and total lean body mass were determined by DXA (QDR 1000 w; Hologic, Inc)36 at baseline and 6 and 12 weeks. Abdominal 1.5-T MRI scans (Siemens) were performed at baseline and 12 weeks to quantify subcutaneous and visceral abdominal adiposity37 (eMethods in Supplement 2). All participants presented to the research unit at approximately 6 am after an overnight fast starting at 10 pm (including medications) and underwent a single-stage hyperinsulinemic-euglycemic clamp procedure (40 mU × m−2 × min−1) at baseline and 12 weeks using tracer infusion of 6,6-2H2–labeled glucose to measure glucose uptake in muscle (glucose rate of disappearance) and hepatic glucose production (glucose rate of appearance) and 1,1,2,3,3-2H5–labeled glycerol to measure lipolytic rate (glycerol rate of appearance). The clamp procedure is based on well-established methods previously performed in nonpsychiatric pediatric samples (eMethods in Supplement 2).19,38,39
Insulin-stimulated percentage change for glucose and glycerol kinetics (glucose rates of appearance and disappearance and glycerol rate of appearance) was calculated as the absolute value of the difference between the kinetic rate calculated during insulin-stimulated vs basal conditions. The resulting value was divided by the basal condition and multiplied by 100%. For all insulin sensitivity measures, exploratory analyses corrected for (nonsignificant) variation in clamped insulin concentrations by dividing each measure by the mean clamped insulin concentration. Exploratory analyses were conducted with whole-body insulin sensitivity, calculated as [(D20 mL/h ×0.2/60) × (0.2/60)] × [1000/participant DXA fat percentage], where D20 represents the rate of infusion and 0.2 accounts for urinary glucose loss, to provide commonly reported values in milligrams per kilogram per minute.19,40
Statistical Analysis
Data were analyzed from January 17, 2011, through August 9, 2017. Primary and secondary analyses for adiposity and insulin sensitivity outcomes were conducted using intention-to-treat analyses and SPSS software (version 24; IBM, Inc), including all randomized participants. Primary outcomes were change in DXA-measured adiposity (DXA percentage total fat) and clamp-derived insulin sensitivity in muscle (percentage change in the glucose rate of disappearance), with secondary outcomes of change in MRI-measured adiposity and percentage change in glucose and glycerol rates of appearance. Primary analysis for change in DXA percentage total fat used a likelihood-based mixed-effects model with time (0, 6, and 12 weeks) and medication group as independent variables and Toeplitz covariance structure specified, based on Bayesian information criteria. The primary outcome analysis for insulin sensitivity, as well as the secondary outcome analyses for adiposity and insulin sensitivity, used repeated-measures analyses of covariance (ANCOVA) with baseline values of the dependent variables as the covariate (to address potential baseline influence on outcomes) and time and treatment condition as independent factors, with testing for time by treatment condition as well as covariate interactions. When time by treatment interactions were significant, contrasts were used to test comparisons of interest; when not significant, treatment condition was removed from the model to calculate the main effect of time and any interactions. The models for MRI abdominal fat were run with compartment (subcutaneous vs visceral) as an additional 2-level factor to test for time by compartment and time by treatment by compartment interactions. Exploratory analyses tested whether the effects of age, stimulant use, sex, and race/ethnicity altered primary results, with the primary analyses rerun with an additional 2- or 3-level factor (eg, yes/no stimulant); these exploratory analyses were corrected for multiple tests (Bonferroni adjustment, where 0.05/4 = 0.0125). Other exploratory analyses used ANCOVA as above (weeks 0 and 12) to support interpretation of primary and secondary measures (eg, DXA percentage lean tissue, clamped insulin concentration) or for clinical context (eg, BMI percentile, clinical laboratory test results, and psychiatric symptoms), with analysis of variance used to test the effect of time within individual treatment groups. Effect sizes (Cohen d) were calculated for primary and secondary outcomes.
The original enrollment target was based on power analyses to detect between-group differences in DXA-measured whole-body adiposity and clamp-measured whole-body insulin sensitivity (approximating muscle insulin sensitivity) using projected screen failure and dropout rates. Data from first-exposure studies suggested differential increases in body weight across drugs, with weight gain predominantly accounted for by increases in adiposity.41,42 Expected mean (SD) percentage body fat was 20% (10%) at baseline with projected increases above baseline during the 12-week study of approximately 10% (4%) for olanzapine, 5% (4%) for risperidone, and 0 (4%) for aripiprazole. Based on this expectation, the power for a 2-sided test at a significance level of P = .05 to compare treatment groups on change in DXA percentage fat was determined to be greater than 0.9 using a sample size of 80 per group. Prior data on likely between-group differences in the glucose disappearance rate were unavailable. Thus, we applied the same 2-sided test and significance level with a target sample size of 80 per group and determined this would allow detection of a between-group difference in the mean disappearance rate equal to an SD of 0.5 with a power of 0.88.
Results
Participant Characteristics
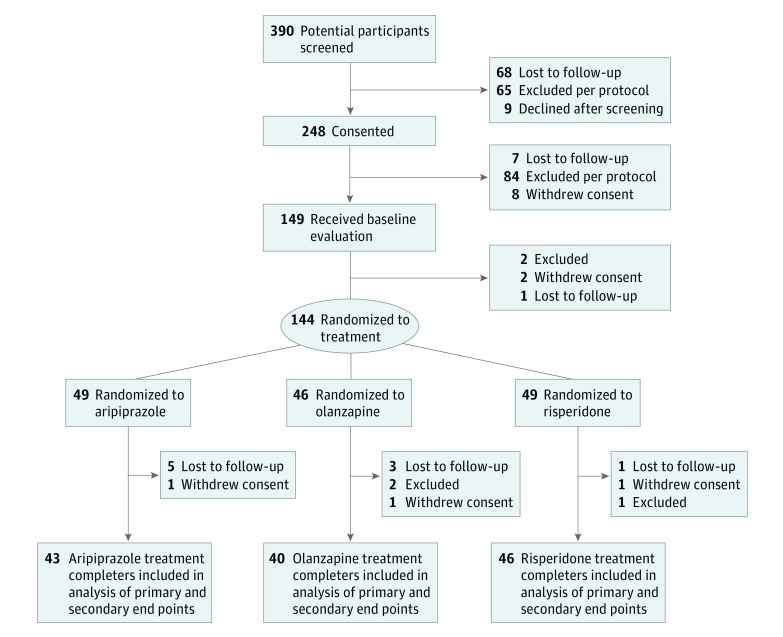
The intention-to-treat sample included 144 participants (98 males [68.1%] and 46 females [31.9%]; mean [SD] age, 11.4 [2.8] years). Seventy-four participants (51.4%) were African American. Study flow and demographics of the intention-to-treat sample are presented in Figure 1 and Table 1, respectively. Mean (SD) final antipsychotic doses were representative of pediatric practice patterns and below the doses typically used to treat psychosis (risperidone, 1.0 [0.6] mg; olanzapine, 6.3 [3.2] mg; aripiprazole, 6.0 [4.5] mg).33,34,35 Eighty participants (55.6%) had a primary diagnosis of attention-deficit/hyperactivity disorder with irritability and aggression insufficiently responsive to prior therapy (eFigure 3 in Supplement 2). Clinically and statistically significant improvements in irritability, aggression, and overall symptoms occurred during treatment, with similar results across medications (eTable 3 in Supplement 2). Seventy-seven of 127 participants (60.6%) reported at least 1 school suspension in the 12 months before the study; suspensions decreased by 43% during the study.
Figure 1. CONSORT Diagram.
Table 1. Baseline Demographic and Clinical Characteristics of the Intention-to-Treat Sample.
| Baseline Variable | Treatment Groupa | |||
|---|---|---|---|---|
| Pooled (n = 144) | Risperidone (n = 49) | Olanzapine (n = 46) | Aripiprazole (n = 49) | |
| Demographic Variables | ||||
| Age, mean (SD), y | 11.4 (2.8) | 11.3 (3.0) | 11.1 (2.5) | 11.6 (2.9) |
| Aged 6-11 y | 89 (61.8) | 29 (59.2) | 30 (65.2) | 30 (61.2) |
| Female | 46 (31.9) | 13 (26.5) | 18 (39.1) | 15 (30.6) |
| Race/ethnicity | ||||
| White | 65 (45.1) | 24 (49.0) | 22 (47.8) | 19 (38.8) |
| Hispanic | 4 (2.8) | 2 (4.1) | 1 (2.2) | 1 (2.0) |
| Clinical Variables | ||||
| Primary diagnosis | ||||
| ADHD | 80 (55.6) | 28 (57.1) | 23 (50.0) | 29 (59.2) |
| Disruptive behavior disorder | 32 (22.2) | 8 (16.3) | 11 (23.9) | 13 (26.5) |
| Mood disorder | 16 (11.1) | 7 (14.3) | 5 (10.9) | 4 (8.2) |
| Autism spectrum disorder | 10 (6.9) | 3 (6.1) | 4 (8.7) | 3 (6.1) |
| Psychosis | 4 (2.8) | 2 (4.1) | 2 (4.3) | 0 |
| Obsessive-compulsive disorder | 1 (0.7) | 0 | 1 (2.2) | 0 |
| Tourette syndrome | 1 (0.7) | 1 (2.0) | 0 | 0 |
| Use of stimulants | 72 (50.0) | 29 (59.2) | 21 (45.7) | 22 (44.9) |
| Use of SSRIs | 17 (11.8) | 8 (16.3) | 5 (10.9) | 4 (8.2) |
| Self-reported first-degree relative with blood glucose level problems | 16 (11.1) | 5 (10.2) | 9 (19.6) | 2 (4.1) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; SSRI, selective serotonin reuptake inhibitors.
Unless otherwise indicated, data are expressed as number (percentage) of participants.
Outcomes
Adiposity
The primary outcome of mean DXA percentage total body fat increased significantly during 12 weeks for all study treatments (Figure 2, Table 2, and eFigure 4 in Supplement 2), with larger increases for olanzapine. A significant time by treatment condition interaction was observed for DXA percentage total fat (F = 6.17; P < .001) (Table 2 lists only weeks 0 and 12 data for comparability), with greater mean (SD) increases for olanzapine (4.12% [3.10%]) compared with risperidone (1.81% [95% CI, 0.91%-2.71%]; t = 4.05; P < .001; Cohen d = 0.74) or aripiprazole (1.66% [95% CI, 0.86-2.46%]; t = 4.34; P < .001; Cohen d = 0.85).
Figure 2. Change in Adiposity During Initial Antipsychotic Exposure in the Study Participants.
A, Baseline and 6- and 12-week mean percentage total body fat measured by dual-energy x-ray absorptiometry (DXA) for each group treated with risperidone, olanzapine, or aripiprazole. B, Baseline and 12-week end points of mean subcutaneous and visceral fat measured using magnetic resonance imaging (MRI) for each treatment group. Error bars indicate SE.
aIndicates time by treatment condition.
bIndicates time by treatment condition by compartment.
Table 2. Change in Primary Outcome Variables Over Timea.
| Variable | Treatment Group | Time by Treatment Value | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risperidone | Olanzapine | Aripiprazole | |||||||||||||||
| Week 0 | Week 12 | Δ (95% CI) | F Value (df) | P Value | Week 0 | Week 12 | Δ (95% CI) | F Value (df) | P Value | Week 0 | Week 12 | Δ (95% CI) | F Value (df) | P Value | F Value (df) | P Value | |
| Primary Outcomes | |||||||||||||||||
| DXA total body fat, % | 26.43 (10.82) |
28.24 (10.92) |
1.81 (0.91 to 2.71) |
15.63 (1, 45) |
<.001 | 24.45 (10.52) |
28.57 (10.82) |
4.12 (3.16 to 5.08) |
70.73 (1, 39) |
<.001 | 27.13 (11.14) |
28.79 (11.27) |
1.66 (0.86 to 2.46) |
16.54 (1, 41) |
<.001 | 6.17 (4, 196.22) |
<.001 |
| Insulin-stimulated change in glucose rate of disappearance, % | 165.28 (77.35) |
167.58 (98.28) |
2.30 (−24.04 to 28.64) |
0.03 (1, 38) |
.87 | 169.84 (83.11) |
140.50 (93.07) |
−29.34 (−58.53 to −0.15) |
3.88 (1, 32) |
.06 | 156.48 (82.18) |
126.22 (73.48) |
−30.26 (−50.55 to −9.97) |
8.55 (1, 39) |
.006 | 2.70 (2, 108) |
.07 |
| Secondary Outcomes | |||||||||||||||||
| MRI subcutaneous fat, cm2 | 127.87 (129.29) |
146.08 (132.81) |
18.21 (10.24 to 26.18) |
20.06 (1, 29) |
<.001 | 85.97 (67.98) |
120.23 (86.91) |
34.27 (23.81 to 44.73) |
41.21 (1, 25) |
<.001 | 107.78 (89.11) |
123.63 (95.44) |
15.84 (9.03 to 22.65) |
20.82 (1, 29) |
<.001 | 6.44 (2, 82) |
.003 |
| MRI visceral fat, cm2 | 26.20 (19.56) |
33.06 (24.87) |
6.85 (2.92 to 10.78) |
11.66 (1, 29) |
.002 | 20.39 (20.87) |
31.11 (20.02) |
10.73 (5.16 to 16.30) |
14.23 (1, 25) |
.001 | 26.83 (24.40) |
38.87 (34.95) |
12.04 (6.63 to 17.45) |
19.05 (1, 29) |
<.001 | 1.27 (2, 82) |
.29 |
| Insulin-stimulated change in glucose rate of appearance, % | 83.23 (11.27) |
80.72 (8.91) |
−2.50 (−4.89 to −0.11) |
4.23 (1, 38) |
.05 | 82.42 (10.04) |
75.85 (19.19) |
−6.57 (−11.06 to −2.08) |
8.22 (1, 32) |
.007 | 82.57 (12.28) |
79.30 (10.47) |
−3.27 (−6.14 to −0.40) |
4.99 (1, 39) |
.03 | 1.80 (2, 108) |
.17 |
| Insulin-stimulated change in glycerol rate of appearance, % | 56.23 (13.76) |
52.58 (15.58) |
−3.65 (−9.13 to 1.83) |
1.71 (1, 37) |
.20 | 56.21 (15.49) |
47.92 (18.08) |
−8.29 (−16.17 to −0.41) |
4.25 (1, 30) |
.05 | 50.45 (15.43) |
52.16 (13.37) |
1.70 (−3.57 to 6.97) |
0.40 (1, 38) |
.53 | 1.33 (2, 104) |
.27 |
Abbreviations: DXA, dual-energy x-ray absorptiometry; MRI, magnetic resonance imaging.
Unless otherwise indicated, data are expressed as mean (SD).
The secondary outcome of abdominal fat measured by MRI increased significantly in visceral and subcutaneous compartments, with a similar mean increase in visceral fat during all treatments (6.85 [95% CI, 2.92-10.78] cm2 for risperidone; 10.73 [95% CI, 5.16-16.30] cm2 for olanzapine; and 12.04 [95% CI, 6.63-17.45] cm2 for aripiprazole), but greater subcutaneous mean fat increase with olanzapine (34.27 [95% CI, 23.81-44.73] cm2) compared with risperidone (18.21 [95% CI, 10.24-26.18] cm2) and aripiprazole (15.84 [95% CI, 9.03-22.65] cm2) (Figure 2, Table 2, and eFigure 4 in Supplement 2). A significant time by compartment by treatment condition interaction (F = 8.60; P < .001) was observed, explained by a significant time by treatment condition interaction for subcutaneous (F = 6.44; P = .003) but not visceral (F = 1.27; P = .29) fat (Table 2), with olanzapine increasing subcutaneous fat more than risperidone (t = 3.02; P = .003; Cohen d = 0.65) or aripiprazole (t = 3.26; P = .002; Cohen d = 0.78).
Baseline rates of overweight (BMI of 85th-94th percentiles) and obesity (BMI≥95th percentile) among those who completed the study were 17 of 129 (13.2%) and 23 of 129 (17.8%), respectively, similar to rates in the intention-to-treat sample (20 of 144 [13.9%] and 23 of 144 [16.0%], respectively), and similar to rates in the general population.43,44 At 12 weeks, 27 of 129 (20.9%) and 33 of 129 (25.6%) met criteria for overweight or obesity (46.5% combined), respectively.
Insulin Sensitivity
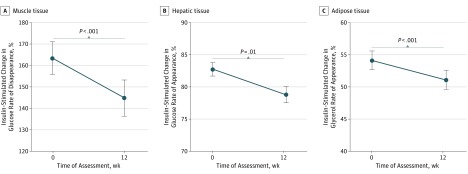
Reductions in insulin sensitivity from baseline—larger in those with higher baseline sensitivity (ie, time by baseline interaction)—were observed in the pooled study sample for the primary outcome of change during 12 weeks in the insulin-stimulated glucose rate of disappearance (F = 17.38; P < .001; Cohen d = 0.22), as well as for the secondary outcomes of glucose rate of appearance (F = 6.25; P = .01; Cohen d = 0.32), and glycerol rate of appearance (F = 59.65; P < .001; Cohen d = 0.20) (Figure 3 and eFigure 5 in Supplement 2). Changes in insulin sensitivity measured by rates of glucose disappearance and appearance and glycerol appearance did not differ significantly across treatment groups (Table 2).
Figure 3. Insulin Sensitivity During Initial Antipsychotic Exposure in the Study Participants.
Data are represented by baseline and 12-week mean values for insulin-stimulated percentage change in glucose rates of disappearance (A) and appearance (B) and glycerol rate of appearance (C) for the total study sample. Error bars indicate SE.
Exploratory Outcomes
Change values and exploratory analyses for clinical variables are provided in eTable 3 in Supplement 2. Psychiatric symptoms improved significantly, but small adverse changes in mean glucose levels and some lipid and hepatic measures were observed. No participants developed diabetes or dyslipidemia during the study; 9 developed impaired fasting glucose levels (100-125 mg/dL) by the end point (2 in the olanzapine, 5 in the risperidone, and 2 in the aripiprazole groups).
The introduction of age group, Tanner stage, stimulant use, sex, or race/ethnicity into the models for our primary outcomes did not change the significance of reported results or yield relevant interactions, with 2 exceptions. Age group was associated with differences in the magnitude of change in DXA percentage total body fat (time × age: F = 7.97; P < .001), with younger children experiencing greater increases in percentage total body fat. A trend-level interaction between time and treatment condition for glucose rate of disappearance became statistically significant (F = 3.74; P = .03) with the addition of age group into the model. However, this finding was not stable when Tanner stage was substituted for age in that model, in which case the interaction returned to a trend-level result.
Adverse Events
Treatment-related adverse events were similar to those in US prescribing information (eTable 1 in Supplement 2). No significant effects of time or time by treatment group interactions for neurologic adverse events were measured by the Abnormal Involuntary Movements Scale, Barnes Akathisia Scale, or Simpson Angus Scale. The clamp procedure was well tolerated, with no study discontinuations due to adverse effects associated with the procedure (eTable 2 in Supplement 2).
Discussion
The results of this randomized clinical trial that used criterion standard measures of adiposity and insulin sensitivity indicate that 12 weeks of antipsychotic treatment with olanzapine in previously antipsychotic-naive youths produces greater adverse effects on DXA-measured whole-body adiposity—with no larger psychiatric symptom benefit—compared with treatment with risperidone or aripiprazole. Similar greater increases in subcutaneous abdominal adiposity were observed with olanzapine compared with risperidone or aripiprazole. In contrast to stable or progressive reductions in percentage body fat observed in the general pediatric population across the age range in this study,45 increases in whole-body and abdominal adiposity were observed in all treatment groups.
Reductions from baseline in insulin sensitivity in 1 or more tissues were also observed in each group, with the pooled study sample indicating reduced sensitivity in all tissues. Although the absence of a placebo group prevents experimental clarity on whether within-group changes are attributable to antipsychotic treatment, no prior evidence suggests that rapid-onset, adverse changes in adiposity and insulin sensitivity are associated with normal childhood or untreated disruptive behavioral disorders. Of interest, these adverse effects occurred at low doses and with medications categorized as lower, as well as higher, metabolic risk. Of note, all treatment groups had similar worsening of MRI-measured visceral abdominal adiposity, a constrained compartment, with greater adverse effects of olanzapine observed in the more expandable subcutaneous space. Based on prior observations of progressive antipsychotic-induced weight gain during 1 year or more with some medications and with no evidence that adverse metabolic effects of antipsychotics routinely reverse with long-term treatment, the present study likely underestimates longer-term treatment effects.
Substantial off-label use of antipsychotics in pediatric populations with nonpsychotic disorders and prior reports of treatment-related risk for hyperglycemia2,3 and weight gain11 using surrogate measures of body composition such as the BMI z score10,11 established the need for direct assessment of antipsychotic treatment effects on adiposity and insulin sensitivity in children. Results from direct assessments of treatment effects on adiposity and insulin sensitivity can be interpreted using knowledge of the contribution of adiposity and insulin resistance to the risk for development of T2D and cardiovascular disease,14 prevalent conditions in psychiatric populations.1 Risk for T2D commonly involves increased adiposity with increased hepatic fat and circulating lipid levels, eventually leading to decreases in insulin sensitivity,46 such that reliably measured changes in insulin sensitivity may lag behind changes in adiposity. The study sample was highly representative of youths who receive antipsychotic treatment, including the most commonly treated diagnoses,6 frequent stimulant use (for which no protective metabolic effect was observed), appropriate racial/ethnic and sex diversity, and baseline rates of overweight and obesity matching those of the general pediatric population.43,44 The inclusion of antipsychotic-naive participants allow observed changes to be better attributed to assigned medications and avoided metabolic effects associated with switching between antipsychotics, for example weight and lipid improvements that can occur when switching from higher- to lower-risk medications.47 In this study, the combined rates of overweight and obesity increased in the 129 participants who completed the study from the general population rate of 31.0% (40 participants) to 46.5% (60 participants) in just 12 weeks. Finally, the use of validated measures of adiposity and insulin sensitivity increase our understanding of treatment effects relevant to the pathophysiologic features of T2D, contributing to the explanation of previously reported increases in incident T2D in antipsychotic-treated youth.2
Limitations
The study was 12 weeks in duration, shorter than the long-term treatment received by many patients. For feasibility and ethical reasons, we had no placebo group, and treatment assignment was open-label with the exception of psychiatric ratings, limiting the interpretation of results. With the exception of the planned exploratory analyses of age, stimulant, sex, and race/ethnicity on primary results, we did not correct for multiple tests. This study did not collect measures of activity, nutrition, cortisol, or antipsychotic levels. Based on commonly observed within- and between-participant variance in insulin sensitivity, including variability during puberty, the power to detect medication-induced changes in insulin sensitivity may have been limited. Power calculations targeted a larger sample than was achieved owing to higher-than-expected screen failures, but low (approximately 10%) postrandomization attrition and stronger-than-expected effects allowed detection of within-group effects on insulin sensitivity in the pooled sample if not between-group differences across medications. To limit participant burden, a single- rather than 2-stage clamp was used, which may have reduced the ability to characterize hepatic vs peripheral insulin sensitivity.
Conclusions
The present results inform risk-benefit considerations for the use of antipsychotics in children and adolescents. Adverse effects on adiposity—greater for olanzapine—and on 1 or more tissue-related measures of insulin sensitivity were associated with all medications tested, suggesting that in youths, the decision to use an antipsychotic in the first place may be as important as the decision to select lower-risk medications. Future studies should explore clinical indicators of risk. Although strong evidence motivates the use of antipsychotic medications for certain conditions (eg, childhood schizophrenia), most pediatric antipsychotic use is for off-label treatment of nonpsychotic, disruptive behavior disorders.5,6 The potential psychiatric benefits of antipsychotic use in this population, evident in this trial and others, should be carefully weighed against the potential for childhood onset of abdominal obesity and insulin resistance that—compared with adult onset—further increases long-term risk for T2D, cardiovascular disease, and related conditions.7
Trial Protocol
eFigure 1. Study Recruitment and Enrollment Timeline
eFigure 2. Adverse Events Reporting Scale
eFigure 3. Diagnostic Makeup of Intention-to-Treat (ITT) Sample
eFigure 4. DEXA and MRI Change in Adiposity During Initial Antipsychotic Exposure in Youths
eFigure 5. Change in in Insulin Sensitivity
eMethods. Protocol Synopsis
eTable 1. Adverse Events Associated With Study Medication
eTable 2. Adverse Events Associated With Clamp Procedure
eTable 3. Change in All Primary, Secondary and Clinical Outcome Variables Over Time
eReferences.
References
- 1.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93. doi: 10.2165/00023210-200519001-00001 [DOI] [PubMed] [Google Scholar]
- 2.Morrato EH, Nicol GE, Maahs D, et al. Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med. 2010;164(4):344-351. doi: 10.1001/archpediatrics.2010.48 [DOI] [PubMed] [Google Scholar]
- 3.Bobo WV, Cooper WO, Stein CM, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry. 2013;70(10):1067-1075. doi: 10.1001/jamapsychiatry.2013.2053 [DOI] [PubMed] [Google Scholar]
- 4.Rubin DM, Kreider AR, Matone M, et al. Risk for incident diabetes mellitus following initiation of second-generation antipsychotics among Medicaid-enrolled youths. JAMA Pediatr. 2015;169(4):e150285. doi: 10.1001/jamapediatrics.2015.0285 [DOI] [PubMed] [Google Scholar]
- 5.Olfson M, King M, Schoenbaum M. Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry. 2015;72(9):867-874. doi: 10.1001/jamapsychiatry.2015.0500 [DOI] [PubMed] [Google Scholar]
- 6.Crystal S, Olfson M, Huang C, Pincus H, Gerhard T. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770-w781. doi: 10.1377/hlthaff.28.5.w770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876-1885. doi: 10.1056/NEJMoa1010112 [DOI] [PubMed] [Google Scholar]
- 8.Abbasi A, Juszczyk D, van Jaarsveld CHM, Gulliford MC. Body mass index and incident type 1 and type 2 diabetes in children and young adults: a retrospective cohort study. J Endocr Soc. 2017;1(5):524-537. doi: 10.1210/js.2017-00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeza I, Vigo L, de la Serna E, et al. The effects of antipsychotics on weight gain, weight-related hormones and homocysteine in children and adolescents: a 1-year follow-up study. Eur Child Adolesc Psychiatry. 2016;26(1):35-46. [DOI] [PubMed] [Google Scholar]
- 10.Moreno C, Merchán-Naranjo J, Alvarez M, et al. Metabolic effects of second-generation antipsychotics in bipolar youth: comparison with other psychotic and nonpsychotic diagnoses. Bipolar Disord. 2010;12(2):172-184. doi: 10.1111/j.1399-5618.2010.00797.x [DOI] [PubMed] [Google Scholar]
- 11.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773. doi: 10.1001/jama.2009.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62(9):3232-3240. doi: 10.2337/db13-0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404-2412. doi: 10.1016/S0140-6736(15)00308-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reaven GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes. 1988;37(12):1595-1607. doi: 10.2337/diab.37.12.1595 [DOI] [PubMed] [Google Scholar]
- 15.Berenson GS, Srinivasan SR, Bao W, Newman WP III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults: the Bogalusa Heart Study. N Engl J Med. 1998;338(23):1650-1656. doi: 10.1056/NEJM199806043382302 [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes. 2002;51(1):204-209. doi: 10.2337/diabetes.51.1.204 [DOI] [PubMed] [Google Scholar]
- 17.Eisenmann JC, Heelan KA, Welk GJ. Assessing body composition among 3- to 8-year-old children: anthropometry, BIA, and DXA. Obes Res. 2004;12(10):1633-1640. doi: 10.1038/oby.2004.203 [DOI] [PubMed] [Google Scholar]
- 18.Lockner DW, Heyward VH, Baumgartner RN, Jenkins KA. Comparison of air-displacement plethysmography, hydrodensitometry, and dual X-ray absorptiometry for assessing body composition of children 10 to 18 years of age. Ann N Y Acad Sci. 2000;904:72-78. doi: 10.1111/j.1749-6632.2000.tb06423.x [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214-E223. [DOI] [PubMed] [Google Scholar]
- 20.Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144(1):47-55. doi: 10.1016/j.jpeds.2003.09.045 [DOI] [PubMed] [Google Scholar]
- 21.Conte C, Fabbrini E, Kars M, Mittendorfer B, Patterson BW, Klein S. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care. 2012;35(6):1316-1321. doi: 10.2337/dc11-1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 23.Aman M, Singh N.. Aberrant Behavior Checklist (ABC): Community Supplementary Manual. East Aurora, NY: Slosson Educational Publications; 1994. [Google Scholar]
- 24.Maughan B, Rowe R, Messer J, Goodman R, Meltzer H. Conduct disorder and oppositional defiant disorder in a national sample: developmental epidemiology. J Child Psychol Psychiatry. 2004;45(3):609-621. doi: 10.1111/j.1469-7610.2004.00250.x [DOI] [PubMed] [Google Scholar]
- 25.Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66(6):918-920. [PubMed] [Google Scholar]
- 26.Todd RD, Joyner CA, Heath AC, Neuman RJ, Reich W. Reliability and stability of a semistructured DSM-IV interview designed for family studies. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1460-1468. doi: 10.1097/00004583-200312000-00013 [DOI] [PubMed] [Google Scholar]
- 27.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education and Welfare; 1976. [Google Scholar]
- 28.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry. 1983;40(11):1228-1231. doi: 10.1001/archpsyc.1983.01790100074010 [DOI] [PubMed] [Google Scholar]
- 29.Bird HR, Canino G, Rubio-Stipec M, Ribera JC. Further measures of the psychometric properties of the Children’s Global Assessment Scale. Arch Gen Psychiatry. 1987;44(9):821-824. doi: 10.1001/archpsyc.1987.01800210069011 [DOI] [PubMed] [Google Scholar]
- 30.Burti L, Parolin A, Zanotelli R. Tardive dyskinesia. AIMS (Abnormal Involuntary Movement Scale) as a diagnostic and research tool [in Italian]. Minerva Med. 1981;72(42):2829-2836. [PubMed] [Google Scholar]
- 31.Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676. doi: 10.1192/bjp.154.5.672 [DOI] [PubMed] [Google Scholar]
- 32.Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11-19. doi: 10.1111/j.1600-0447.1970.tb02066.x [DOI] [PubMed] [Google Scholar]
- 33.Zyprexa (olanzapine) [package insert]. Indianapolis, IN: Eli Lilly & Co; 2008.
- 34.Risperdal (risperidone) [package insert]. Titusville, NJ: Janssen Pharmaceutica Products ; 2008.
- 35.Abilify (aripiprazole) [package insert]. Rockville, MD: Otsuka Pharmaceutical Co, Ltd; 2008.
- 36.Jensen MD, Kanaley JA, Roust LR, et al. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc. 1993;68(9):867-873. doi: 10.1016/S0025-6196(12)60695-8 [DOI] [PubMed] [Google Scholar]
- 37.Abate N, Burns D, Peshock RM, Garg A, Grundy SM. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res. 1994;35(8):1490-1496. [PubMed] [Google Scholar]
- 38.Arslanian SA, Kalhan SC. Correlations between fatty acid and glucose metabolism: potential explanation of insulin resistance of puberty. Diabetes. 1994;43(7):908-914. doi: 10.2337/diab.43.7.908 [DOI] [PubMed] [Google Scholar]
- 39.Vitola BE, Deivanayagam S, Stein RI, et al. Weight loss reduces liver fat and improves hepatic and skeletal muscle insulin sensitivity in obese adolescents. Obesity (Silver Spring). 2009;17(9):1744-1748. doi: 10.1038/oby.2009.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462-1470. doi: 10.2337/diacare.22.9.1462 [DOI] [PubMed] [Google Scholar]
- 41.Baptista T, De Mendoza S, Beaulieu S, Bermúdez A, Martinez M. The metabolic syndrome during atypical antipsychotic drug treatment: mechanisms and management. Metab Syndr Relat Disord. 2004;2(4):290-307. doi: 10.1089/met.2004.2.290 [DOI] [PubMed] [Google Scholar]
- 42.Lieberman JA, Tollefson G, Tohen M, et al. ; HGDH Study Group . Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160(8):1396-1404. doi: 10.1176/appi.ajp.160.8.1396 [DOI] [PubMed] [Google Scholar]
- 43.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549-1555. doi: 10.1001/jama.295.13.1549 [DOI] [PubMed] [Google Scholar]
- 44.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303(3):242-249. doi: 10.1001/jama.2009.2012 [DOI] [PubMed] [Google Scholar]
- 45.Ellis KJ, Shypailo RJ, Abrams SA, Wong WW. The reference child and adolescent models of body composition: a contemporary comparison. Ann N Y Acad Sci. 2000;904:374-382. doi: 10.1111/j.1749-6632.2000.tb06486.x [DOI] [PubMed] [Google Scholar]
- 46.Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11(9):351-356. doi: 10.1016/S1043-2760(00)00323-4 [DOI] [PubMed] [Google Scholar]
- 47.Newcomer JW, Weiden PJ, Buchanan RW. Switching antipsychotic medications to reduce adverse event burden in schizophrenia: establishing evidence-based practice. J Clin Psychiatry. 2013;74(11):1108-1120. doi: 10.4088/JCP.12028ah1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Study Recruitment and Enrollment Timeline
eFigure 2. Adverse Events Reporting Scale
eFigure 3. Diagnostic Makeup of Intention-to-Treat (ITT) Sample
eFigure 4. DEXA and MRI Change in Adiposity During Initial Antipsychotic Exposure in Youths
eFigure 5. Change in in Insulin Sensitivity
eMethods. Protocol Synopsis
eTable 1. Adverse Events Associated With Study Medication
eTable 2. Adverse Events Associated With Clamp Procedure
eTable 3. Change in All Primary, Secondary and Clinical Outcome Variables Over Time
eReferences.