This systemic review and meta-analysis examines the association between hypertensive disorders of pregnancy and risk of neurodevelopmental disorders in offspring.
Key Points
Question
What are the pooled estimates from existing literature examining the association between hypertensive disorders of pregnancy and neurodevelopmental disorders in offspring?
Findings
Pooled estimates from this systematic review and meta-analysis of 61 studies suggest that exposure to hypertensive disorders of pregnancy is associated with a small yet statistically significant increase in the odds of autism spectrum disorder and attention-deficit/hyperactivity disorder in offspring compared with no exposure.
Meaning
Increased developmental screening of infants exposed to hypertensive disorders of pregnancy could allow for early intervention, which in turn may improve neurodevelopmental outcome.
Abstract
Importance
Although research suggests an association between hypertensive disorders of pregnancy (HDP) and autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), and other neurodevelopmental disorders in offspring, consensus is lacking. Given the increasing prevalence of hypertension in pregnancy, it is important to examine the association of HDP with neurodevelopmental outcome.
Objective
To synthesize the published literature on the association between HDP and risk of neurodevelopmental disorders in offspring in a systematic review and meta-analysis.
Data Sources
On the basis of a preprepared protocol, a systematic search of PubMed, CINAHL, Embase, PsycINFO, and Web of Science was performed from inception through June 7, 2017, supplemented by hand searching of reference lists.
Study Selection
Two investigators independently reviewed titles, abstracts, and full-text articles. English-language cohort and case-control studies were included in which HDP and neurodevelopmental disorders were reported.
Data Extraction and Synthesis
Data extraction and quality appraisal were performed independently by 2 reviewers. Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines were followed throughout.
Main Outcomes and Measures
Random-effects meta-analyses of estimated pooled odds ratios (ORs) for HDP and ASD and for HDP and ADHD. Stand-alone estimates were reported for all other neurodevelopmental disorders.
Results
Of 1166 studies identified, 61 unique articles met inclusion criteria. Twenty studies reported estimates for ASD. Eleven of these (including 777 518 participants) reported adjusted estimates, with a pooled adjusted OR of 1.35 (95% CI, 1.11-1.64). Ten studies reported estimates for ADHD. Six of these (including 1 395 605 participants) reported adjusted estimates, with a pooled adjusted OR of 1.29 (95% CI, 1.22-1.36). Subgroup analyses according to type of exposure (ie, preeclampsia or other HDP) showed no statistically significant differences for ASD or ADHD. Thirty-one studies met inclusion criteria for all other neurodevelopmental disorders. Individual estimates reported for these were largely inconsistent, with few patterns of association observed.
Conclusions and Relevance
Exposure to HDP may be associated with an increase in the risk of ASD and ADHD. These findings highlight the need for greater pediatric surveillance of infants exposed to HDP to allow early intervention that may improve neurodevelopmental outcome.
Introduction
The International Society for the Study of Hypertension in Pregnancy classifies hypertensive disorders of pregnancy (HDP) into the following categories: chronic hypertension (essential/secondary), white-coat hypertension, masked hypertension, transient gestational hypertension, gestational hypertension, and preeclampsia (de novo or superimposed on chronic hypertension).1 Hypertensive disorders of pregnancy affect 5% to 15% of all pregnancies and therefore are among the most common prenatal complications.2,3 Hypertensive disorders of pregnancy create adverse in utero conditions that can alter fetal development and may increase the risk of long-term vascular, cognitive, and psychiatric sequelae in offspring.4,5,6,7 Neurodevelopmental disorders, including autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD), are a group of conditions with onset during the developmental period that can impair personal, social, academic, or occupational functioning.8,9 Genetics are widely accepted to play a key role.10,11 Familial coaggregation implies shared genetic risk factors,12 but environmental factors may contribute to their etiology.13,14,15 A study conducted using Swedish National Registries estimated that environmental contribution of ASD ranges from 17% to 50%,10,16 highlighting the importance of identifying the environmental factors that contribute to the risk of neurodevelopmental disorders in offspring.
Overall, epidemiologic evidence examining the association between HDP and neurodevelopmental disorders remains largely inconsistent,17,18,19,20,21,22 and residual or unmeasured confounding is of particular concern in the literature.23,24,25,26,27,28 Given the increasing prevalence of HDP, partially owing to increasing levels of obesity, metabolic syndrome, and advanced maternal age,3,4 collating the existing evidence of the association of HDP with neurodevelopmental outcome is timely. The objective of this study was to synthesize the available published literature on the association between HDP and the risk of neurodevelopmental disorders in offspring in a systematic review and meta-analysis.
Methods
Data Sources and Search Strategy
The systematic review required studies with the following: a population of pregnant women and their children, exposure to HDP, a comparison group with no HDP, and primary outcome of ASD or ADHD, with secondary outcomes consisting of neurodevelopmental and other behavioral or cognitive outcomes. The protocol for this systematic review and meta-analysis was registered on PROSPERO, the international prospective register of systematic reviews (CRD42017068258), and subsequently published.29
In accordance with Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines,30 one of us (G.M.M.) conducted a systematic literature search in PubMed, CINAHL, Embase, PsycINFO, and Web of Science electronic databases from inception through June 7, 2017. Search terms relating to HDP, ASD, ADHD, and other neurodevelopmental disorders were combined according to the principles of Boolean logic (using AND, OR, or NOT) and using Medical Subject Headings, for example, (preeclampsia OR gestational hypertension) AND (autism spectrum disorder OR attention-deficit/hyperactivity disorder OR neurodevelopmental disorder). The full search strategy is included in eTable 1 in the Supplement. Results were limited to English-language studies with human participants. No restrictions were placed on publication date, location of study, or age of participants. Searches of the electronic databases were supplemented by hand searching the reference lists of included studies for further potentially eligible studies, and authors were contacted when a conference proceeding only was located. We also conducted a post hoc search of PubMed adding the keywords perinatal complication OR prenatal complication OR obstetric* complication to the search strategy.
Study Selection
Two of us (G.M.M. and A.S.K.) independently reviewed the titles and abstracts of all studies, obtaining full texts when necessary. When consensus on eligibility could not be achieved, a third reviewer (G.W.O.K.) was involved in the discussion. Eligibility criteria for inclusion in the systematic review included the following: cohort, case-control, or cross-sectional studies in which a diagnosis of HDP was reported and neurodevelopmental disorders were the outcome of interest; the association between HDP and neurodevelopmental disorders were part of the main objective of the study (including studies that investigated other perinatal risk factors in addition to HDP); data were from an original study and HDP was confirmed through medical records and/or physician-diagnosed self-reporting; peer-reviewed literature only; and neurodevelopmental and other behavioral or cognitive outcomes only (motor disorders were included in the search strategy to capture studies that include these outcomes).
Data Extraction
Two reviewers (G.M.M. and G.W.O.K.) independently extracted data from eligible studies using a standardized data collection form. Information extracted included author, year of publication, study design, definition of exposure and outcome used, sample size, confounders adjusted for (if any), and crude and adjusted estimates. Any discrepancies were resolved by consensus with a third reviewer (A.S.K.). Authors of 2 studies were contacted for further information, with a reply received from one.
Bias and Quality Assessment
Publication bias was evaluated by visually assessing a funnel plot and Egger test for asymmetry of the funnel plot, by which 10 or more studies were included in the meta-analysis.31 Quality assessment of included studies was conducted by 2 reviewers (G.M.M. and M.M.) independently using an appropriate quality assessment tool and agreed on subsequently. Any discrepancies were resolved by a third reviewer (A.S.K.). We used a bias classification tool32 consisting of a checklist to assess 6 types of bias most often associated with observational studies (selection, exposure, outcome, confounding, analytic, and attrition). Study bias was classified as minimal, low, moderate, high, or not reported for each type of bias. An overall likelihood of bias based on the total of the 6 types of bias was then measured and reported. For example, selection bias was minimized if the sample was taken from a “consecutive unselected population,” whereas selection bias was categorized as high if “sample selection was ambiguous and the sample was not likely representative.”32(p146)
Statistical Analysis
Data were analyzed using Review Manager software (version 5.3; http://community.cochrane.org/tools/review-production-tools/revman-5/revman-5-download), and the Egger test was conducted in Stata/MP software (version 14.2; StataCorp). Random-effects meta-analyses were performed to calculate overall pooled estimates of the association between combined HDP, preeclampsia, and HDP excluding preeclampsia and the outcomes of ASD and ADHD using the generic inverse variance method. The generic inverse variance method allowed studies that do not report raw data to be included in the meta-analyses.33 Partially adjusted estimates, as a result of matching, were included as crude results, and studies that adjusted for confounders in the analysis phase were included as adjusted results. Forest plots were used to display crude and adjusted estimates, with adjustment based on the definition outlined in each identified study. Studies that provided a crude and adjusted estimate and studies that adjusted for similar variables were analyzed separately (eFigures 1-4 in the Supplement).
The following subgroup and sensitivity analyses were decided a priori: type of HDP (preeclampsia and other HDP), study design, location, income level of country, study quality, and measurement of exposure and outcome data. A post hoc subgroup analysis was performed according to length of follow-up.
Results
Search Results
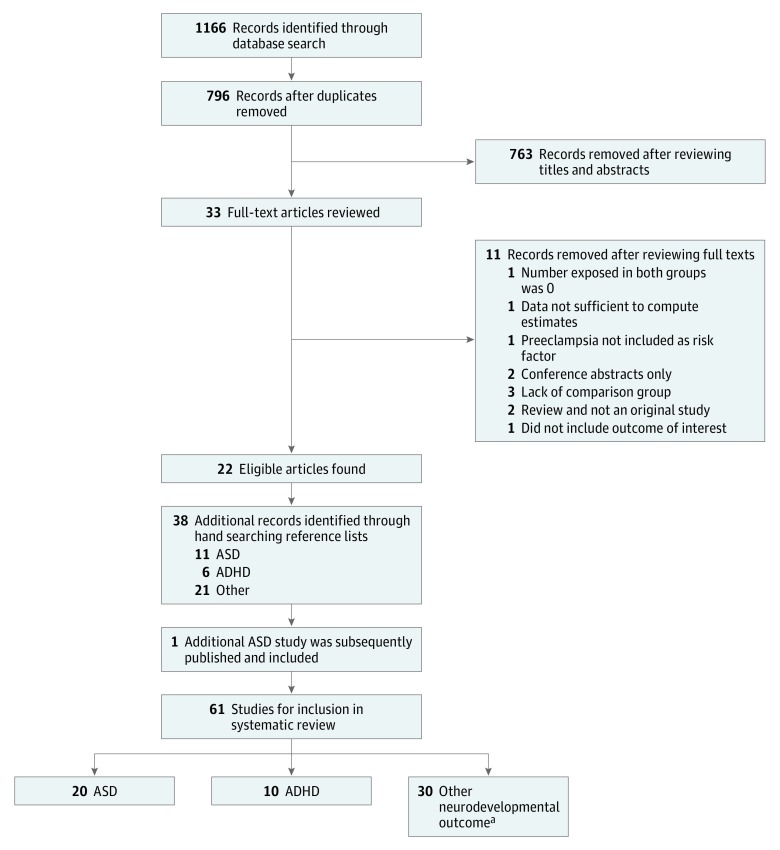
The original search produced 796 unique results after removal of duplicates (Figure 1). Of these, 33 full-text articles were reviewed after screening of titles and abstracts. Eleven articles were excluded because they did not meet the inclusion criteria. Reasons for exclusion are outlined in Figure 1. This process resulted in 22 eligible articles. After reviewing reference lists, 38 additional articles were identified. One additional study of ASD was subsequently published and included in the review. A total of 61 unique articles were included in the systematic review, including 20 for ASD20,21,26,27,28,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48 (8 identified from the original search, 11 from reference lists, and 1 subsequently published), 10 for ADHD17,18,19,23,24,25,49,50,51,52 (4 identified from the original search and 6 from reference lists), and 31 for other neurodevelopmental outcomes53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83 (10 identified from the original search and 21 from reference lists).
Figure 1. Flow Diagram of Studies Selected for Inclusion in the Systematic Review.
ADHD indicates attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder.
aAn additional 6 studies are also included for ASD and 1 for ADHD.
Characteristics of Studies Included in the Systematic Review
A summary of included studies that report ASD and ADHD is available in eTables 2 and 3 in the Supplement. A summary of studies that report other neurodevelopmental outcomes (including cognitive functioning/developmental delay, behavioral outcomes, and intellectual disability), along with main findings, is available in eTable 4 in the Supplement.
Results of the Meta-analyses
ASD: Primary Analysis
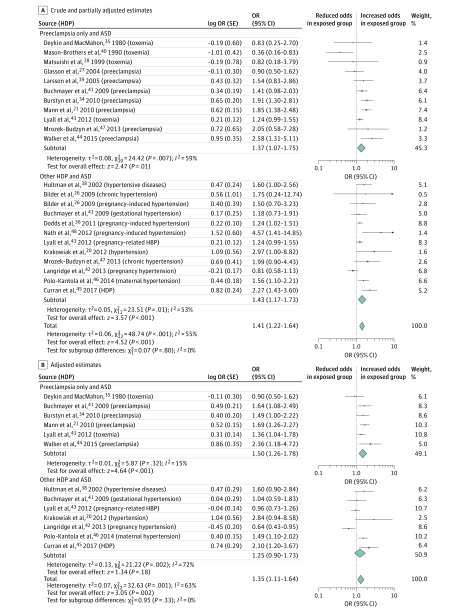
A total of 20 studies20,21,26,27,28,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48 were identified in which a diagnosis of HDP was reported and ASD was the outcome of interest. The prevalence of HDP among cohort studies of ASD ranged from 1.3% to 9.1% (mean, 6.2%; median, 6.9%; interquartile range [IQR], 3.6%-8.9%). Twenty-three estimates from 19 unique studies20,21,26,27,28,34,35,36,38,39,40,41,42,43,44,45,46,47,48 included crude estimates, and 13 estimates from 11 unique studies20,21,34,35,38,41,42,43,44,45,46 included adjusted estimates and were included in the meta-analysis (including 777 518 participants). Figure 2A displays crude and partially adjusted estimates (as a result of matching), producing a pooled odds ratio (OR) of 1.41 (95% CI, 1.22-1.64). A subgroup analysis examining the association of preeclampsia with ASD and other HDP with ASD separately resulted in ORs of 1.37 (95% CI, 1.07-1.75) and 1.43 (95% CI, 1.17-1.73), respectively (test for subgroup differences, P = .80).
Figure 2. Forest Plot for Studies of the Association of Hypertensive Disorders of Pregnancy (HDP) With Autism Spectrum Disorder (ASD).
Odds ratios (ORs) are calculated using random-effects analysis. The generic inverse variance method was used to include studies that do not report raw data. Diamonds indicate effect size; size of markers, 95% CI. HBP indicates high blood pressure.
Adjusted estimates reduced the overall HDP-ASD estimate to 1.35 (95% CI, 1.11-1.64) (Figure 2B). Subgroup analysis examining the association of preeclampsia with ASD resulted in an OR of 1.50 (95% CI, 1.26-1.78), whereas the association of other HDP with ASD produced a nonsignificant OR of 1.25 (95% CI, 0.90-1.73). However, the test for subgroup differences does not indicate a statistically significant difference (P = .33).
ADHD: Primary Analysis
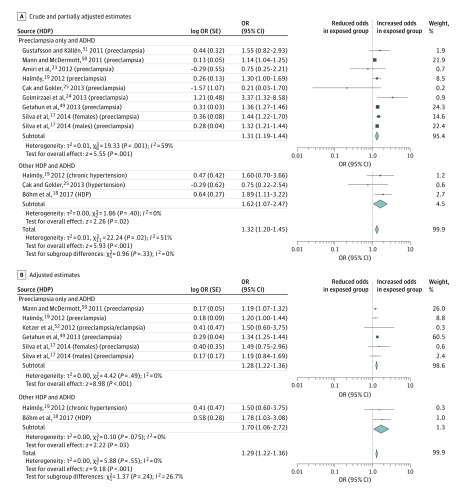
Ten studies17,18,19,23,24,25,49,50,51,52 were identified in which a diagnosis of HDP was reported and ADHD was the outcome of interest. The prevalence of HDP among cohort studies of ADHD ranged from 0.1% to 20.8%, (mean, 7.8%; median, 5.5%; IQR, 2.4%-15.1%). Twelve estimates from 9 unique studies17,18,19,23,24,25,49,50,51 included crude results examining the association of HDP with ADHD, and 8 estimates from 6 unique studies17,18,19,49,50,52 included adjusted estimates (including 1 395 605 participants) (Figure 3A). Crude pooled estimates produced an OR of 1.32 (95% CI, 1.20-1.45). In subgroup analysis examining the association of preeclampsia with ADHD only, the OR was 1.31 (95% CI, 1.19-1.44), and the OR for the association between other HDP and ADHD was 1.62 (95% CI, 1.07-2.47) (P = .33).
Figure 3. Forest Plot for Studies of the Association of Hypertensive Disorders of Pregnancy (HDP) With Attention-Deficit/Hyperactivity Disorder (ADHD) .
Odds ratios (ORs) are calculated using random-effects analysis. The generic inverse variance method was used to include studies that do not report raw data. Diamonds indicate effect size; size of markers, 95% CI.
Adjusted estimates remained relatively unchanged (Figure 3B) (overall pooled OR, 1.29; 95% CI, 1.22-1.36). Results of the subgroup analysis examining the association of preeclampsia with ADHD (OR, 1.28; 95% CI, 1.22-1.36), and of other HDP with ADHD (OR, 1.70; 95% CI, 1.06-2.72) were not significantly different (P = .24).
Other Neurodevelopmental Outcomes
Owing to varying outcome measures, assessment methods, and summary scales used, pooling results of these studies was not appropriate. Therefore, we reported stand-alone estimates for 31 unique studies (plus 7 studies that were also included as ASD or ADHD outcomes) examining the association between HDP and neurodevelopmental, cognitive, or behavioral outcomes. A summary of the main findings of these studies is available in eTable 4 in the Supplement. Overall, results were largely inconsistent; however, a few patterns of association emerged. For example, 6 of 10 studies53,54,55,56,57,58 suggest a positive association between preeclampsia and cognitive impairment within specific populations, whereas 4 of 5 studies37,42,59,60 suggest a potential association between HDP and intellectual disability.
Bias and Heterogeneity
Visual inspection of the funnel plot including adjusted studies only did not indicate publication bias (eFigure 5 in the Supplement) (Egger test, P = .43; 95% CI, −1.8 to 4.0). Moderate heterogeneity for ASD (I2 = 63%) and low heterogeneity for ADHD (I2 = 0) were found on the basis of adjusted estimates. Heterogeneity among ASD studies was possibly attributable to differences in confounder adjustments, because heterogeneity reduced to 0 when studies that adjusted for maternal age, maternal smoking, and parity and/or birth order were analyzed separately (eFigure 3 in the Supplement). Most studies were classified as having low or moderate risk of bias (eTables 5-7 in the Supplement).
ASD: Subgroup/Sensitivity Analysis
The Table shows pooled estimates for all studies that reported crude estimates, adjusted estimates, and adjusted estimates according to the category of HDP for ASD and ADHD. Results of the following subgroup analysis are also outlined in the Table.
Table. Subgroup Meta-analyses for HDP-ASD and HDP-ADHD Associations.
| Variable | No. of Studies (No. of Estimates) | No. of Participants | No. of Outcomes | OR (95% CI) | I2, % | Test for Subgroup Differences P Value |
|---|---|---|---|---|---|---|
| Autism Spectrum Disorder | ||||||
| Overall unadjusted | 19 (23) | 941 285 | 9331 | 1.41 (1.22-1.64) | 55 | .80a |
| Overall adjustedb | 11 (13) | 777 518 | 6866 | 1.35 (1.11-1.64) | 63 | .33a |
| Category of HDPb | ||||||
| Preeclampsia | 6 (6) | 378 991 | 4254 | 1.50 (1.26-1.78) | 15 | .33 |
| Other | 7 (7) | 472 268 | 4621 | 1.25 (0.90-1.73) | 72 | |
| Study designb | ||||||
| Case-control | 6 (7) | 16 975 | 3812 | 1.47 (1.18-1.84) | 21 | .41 |
| Cohort | 5 (6) | 760 543 | 3054 | 1.26 (0.93-1.70) | 78 | |
| Locationb | ||||||
| North America | 6 (7) | 372 527 | 3555 | 1.39 (1.09-1.77) | 59 | <.001 |
| Europe | 4 (5) | 28 010 | 2859 | 1.53 (1.26-1.87) | 0 | |
| Australia | 1 (1) | 376 981 | 452 | 0.64 (0.43-0.95) | NA | |
| Study qualityc | ||||||
| Minimal to low risk of bias | 14 (16) | 873 772 | 8041 | 1.39 (1.17-1.65) | 47 | .46 |
| Moderate to high risk of bias | 5 (7) | 67 513 | 1263 | 1.18 (0.81-1.74) | 69 | |
| Exposure measurementb | ||||||
| Self-reported | 4 (5) | 81 242 | 2026 | 1.54 (1.07-2.22) | 68 | .39 |
| Medical records | 7 (8) | 696 276 | 4840 | 1.27 (0.99-1.64) | 65 | |
| Outcome measurementb | ||||||
| Maternal-reported | 2 (3) | 79 543 | 992 | 1.32 (0.91-1.91) | 72 | .86 |
| Medical records | 9 (10) | 697 975 | 5874 | 1.37 (1.07-1.75) | 63 | |
| Length of follow-up, yb | ||||||
| ≤7 | 5 (5) | 102 838 | 1823 | 1.71 (1.23-2.38) | 41 | .09 |
| ≤21 | 6 (8) | 674 680 | 5043 | 1.22 (0.98-1.52) | 64 | |
| Attention-Deficit/Hyperactivity Disorder | ||||||
| Overall unadjusted | 9 (12) | 1 428 209 | 37 635 | 1.32 (1.20-1.45) | 48 | .33a |
| Overall adjustedb | 6 (8) | 1 395 605 | 37 128 | 1.29 (1.22-1.36) | 0 | .24a |
| Category of HDPb | ||||||
| Preeclampsia | 5 (6) | 1 382 105 | 36 962 | 1.28 (1.22-1.36) | 0 | .24 |
| Other | 2 (2) | 1 185 896 | 2489 | 1.70 (1.06-2.72) | 0 | |
| Study designb | ||||||
| Case-control | 3 (4) | 124 988 | 26 728 | 1.34 (1.25-1.43) | 0 | .08 |
| Cohort | 3 (4) | 1 270 617 | 10 400 | 1.21 (1.10-1.32) | 0 | |
| Locationb | ||||||
| North America | 2 (2) | 166 399 | 21 524 | 1.27 (1.13-1.43) | 70 | .99 |
| Europe | 2 (3) | 1 185 896 | 2489 | 1.26 (1.06-1.49) | 0 | |
| Other | 2 (3) | 43 310 | 13 115 | 1.27 (0.95-1.70) | 0 | |
| Study qualityc | ||||||
| Minimal to low risk of bias | 7 (9) | 1 427 617 | 37 365 | 1.29 (1.22-1.36) | 0 | .57 |
| Moderate to high risk of bias | 3 (4) | 840 | 394 | 0.95 (0.32-2.76) | 67 | |
| Exposure measurementb | ||||||
| Self-reported | 2 (2) | 13 748 | 290 | 1.70 (1.06-2.72) | 0 | .24 |
| Medical records | 4 (6) | 1 381 857 | 36 838 | 1.28 (1.22-1.36) | 0 | |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; HDP, hypertensive disorders of pregnancy; NA, not applicable; OR, odds ratio.
Calculated using test for subgroup differences between preeclampsia and other HDP.
Includes all studies that adjusted for confounders in the analysis phase.
Includes all studies.
Six case-control studies20,35,38,41,44,46 (7 estimates) resulted in a pooled OR of 1.47 (95% CI, 1.18-1.84). Five cohort studies21,34,42,43,45 (6 estimates) provided an overall nonsignificant OR of 1.26 (95% CI, 0.93-1.70) (P = .41). Six studies20,21,34,35,43,44 (7 adjusted estimates) were from North America (OR, 1.39; 95% CI, 1.09-1.77), 4 studies38,41,45,46 were from Europe (5 estimates) (OR, 1.53; 95% CI, 1.26-1.87), and 1 study42 was from Australia (OR, 0.64; 95% CI, 0.43-0.95) (P < .001). All ASD studies were conducted in high-income countries. Fourteen studies20,21,26,27,28,34,36,38,39,41,42,44,45,46 (16 estimates) were assessed as having minimal to low risk of bias, resulting in a significant OR of 1.39 (95% CI, 1.17-1.65). Five studies35,40,43,47,48 (7 estimates) were assessed as having moderate to high risk of bias, resulting in a nonsignificant OR of 1.18 (95% CI, 0.81-1.74) (P = .46). Four studies20,43,44,45 that relied on self-reported measurements of HDP produced 5 adjusted estimates, resulting in a pooled OR of 1.54 (95% CI, 1.07-2.22). The pooled OR observed among the 7 studies21,34,35,38,41,42,46 (with 8 estimates) that relied on medical records for confirmation of HDP was 1.27 (95% CI, 0.99-1.64) (P = .39). Two studies43,45 (with 3 adjusted estimates) used maternal reporting of ASD and resulted in an OR of 1.32 (95% CI, 0.91-1.91). However, individual point estimates for these studies ranged from 0.96 to 2.10. Pooled results were similar among the 9 studies20,21,34,35,38,41,42,44,46 (10 estimates) that used medical records to determine ASD status in the offspring, with an OR of 1.37 (95% CI, 1.07-1.75) (P = .86). Five studies20,21,35,44,45 had a potential 2 to 7 years of follow-up with a pooled OR of 1.71 (95% CI, 1.23-2.38), and 6 studies34,38,41,42,43,46 (8 estimates) had a potential 2 to 21 years follow-up with a pooled OR of 1.22 (95% CI, 0.98-1.52) (P = .09).
ADHD: Subgroup/Sensitivity Analysis
Three case-control studies17,49,52 (with 4 adjusted estimates) were identified with a pooled OR of 1.34 (95% CI, 1.25-1.43), whereas 3 cohort studies18,19,50 (4 adjusted estimates) resulted in a pooled OR of 1.21 (95% CI, 1.10-1.32) (P = .08). Two studies49,50 (2 estimates) were from North America (OR, 1.27; 95% CI, 1.13-1.43), 2 studies18,19 (3 estimates) were from Europe (OR, 1.26; 95% CI, 1.06-1.49), and 2 studies17,52 (3 estimates) were from other locations (OR, 1.27; 95% CI, 0.95-1.70) (P = .99). Five studies17,18,19,49,50 (7 adjusted estimates) were conducted in high-income countries compared with 1 study conducted in an upper- to middle-income country.52 Results of a sensitivity analysis including results from high-income countries only did not change the pooled results (OR, 1.29; 95% CI, 1.22-1.36). Seven studies17,18,19,49,50,51,52 (9 estimates) were assessed as having minimal or low risk of bias. The pooled estimate for these studies was significant (OR, 1.29; 95% CI, 1.22-1.36). A pooled estimate for the 3 studies23,24,25 (4 estimates) assessed as having moderate to high risk of bias was nonsignificant (OR, 0.95; 95% CI, 0.32-2.76) (P = .57). Two studies18,52 (2 estimates) used self-reporting of HDP status, resulting in a pooled OR of 1.70 (95% CI, 1.06-2.72), whereas pooled results of 4 studies17,19,49,50 (6 estimates) using medical records to obtain exposure status resulted in a pooled estimate of 1.28 (95% CI, 1.22-1.36) (P = .24).
Five studies17,19,49,50,52 (7 adjusted estimates) used medical records to measure ADHD status in offspring, and 1 study18 used maternal reporting. However, results of a sensitivity analysis (including medical records only) did not change pooled results (OR, 1.28; 95% CI, 1.22-1.36).
Discussion
The aim of this systematic review and meta-analysis was to synthesize the published literature on the association between HDP and the risk of neurodevelopmental disorders in offspring. Three principal findings resulted. First, our adjusted pooled results indicate that exposure to HDP was associated with a 35% increased odds of ASD compared with nonexposure. Results of a subgroup analysis examining an association between preeclampsia and ASD in isolation provided an OR of 1.50, whereas the association between other HDP and ASD was nonsignificant, with an OR of 1.25. Although subgroup analysis may suggest that the type of HDP may play a role in determining the association with neurodevelopmental outcome, subgroup differences were not statistically significant.
Second, adjusted pooled results suggest that offspring exposed to HDP in utero were 30% more likely to have ADHD compared with unexposed offspring. Examination of an association between preeclampsia and ADHD in isolation did not change the estimate, whereas the odds of ADHD was increased by 70% in association with other HDP. However, this subgroup difference was not statistically significant.
These reported effect sizes are similar to those of other obstetric risk factors for ASD. For example, cesarean delivery is associated with a 23% to 26% increased odds of ASD, and advancing maternal age (>35 years) is associated with a 30% increased odds of ASD84,85,86; breech presentation and Apgar score of less than 7 may increase the risk of ADHD by 14% and 30%, respectively.87
Third, literature examining the association between HDP and other neurodevelopmental, cognitive, or behavioral outcomes remains inconsistent (eTable 4 in the Supplement). Some patterns of association were observed between preeclampsia and cognitive impairment when confined to specific populations, such as infants with growth restriction, preterm birth, and low birth weight.53,54,55,56,57,58 Similarly, the epidemiologic evidence examined suggests a potential association between HDP and intellectual disability.37,42,59,60 However, methodologic differences among studies, particularly differences in population and outcome assessment methods, may partially explain the overall lack of consistent findings.
Although this study is suggestive of an association between HDP and neurodevelopmental disorders in offspring, it is difficult to rule out the possibility that antihypertensive medication used during pregnancy may be associated with adverse effects in offspring.88 However, several potential mechanisms have been proposed in attempts to explain the association between HDP and neurodevelopmental outcome. For example, placental dysfunction, associated with HDP, may result in reduced placental perfusion and oxidative stress.89 In turn, suboptimal nutrient and oxygen availability for the fetus, attributable to placental insufficiency, may affect the developing brain, increasing the risk of a poor neurodevelopmental outcome.6,7,21,44
Maternal inflammation may also play a key role. Results of a population-based study in Finland90,91 with data from more than 1 million pregnancies showed that increased levels of the inflammatory biomarker C-reactive protein associated with preeclampsia was significantly associated with a 43% increased risk of autism in offspring when maternal C-reactive protein levels in the highest and lowest quintiles were compared. Fewer hypotheses have been suggested that address the biological mechanisms of ADHD specifically; however, similar mechanisms may be involved.18,92,93
The literature examining HDP and ASD is suggestive of a small increase in the risk of ASD in offspring exposed to HDP21,36,41,43,44,45,46,48; however, some studies20,26,34,38,39,41,47 fail to meet statistical significance. In contrast, other studies27,28,35,37,43 suggest a protective association between HDP and ASD, with only 2 of these40,42 reaching statistical significance. Similarly, the literature alludes to a positive association between HDP and ADHD, with some studies18,24,49,50 indicating significant associations and others17,19,51,52 producing nonsignificant positive estimates. In comparison, 2 HDP-ADHD studies23,25 suggest reduced odds of ADHD in HDP-exposed offspring. However, neither study reached statistical significance or controlled for potential confounders.
Strengths and Limitations
This systematic review had several strengths. It was based on a preprepared protocol, and MOOSE guidelines were followed throughout.30 It included a comprehensive search of 5 relevant databases, supplemented by hand searching the reference lists of included studies for additional potentially eligible studies.
However, several limitations, including those of the current literature, should be noted. Results were limited to English-language studies, potentially leading to relevant, non–English language studies being overlooked. Although the full search strategy was published along with the protocol, it may have been lacking in keywords, such as perinatal complication OR prenatal complication OR obstetric* complication, because hand searching the reference lists identified a larger number of relevant studies compared with searching the electronic databases. Therefore, we conducted a post hoc search of PubMed, adding these words to the search strategy. Although this search increased the number of hits retrieved 5-fold, identifying more eligible studies than the original search strategy, no new studies were identified in the process.
Sample size calculations are lacking in the literature examining the associations of HDP with ASD and ADHD, and therefore the studies may lack statistical power. For example, 5 of 20 studies of ASD26,28,35,47,48 and 3 of 10 studies of ADHD19,23,25 had fewer than 10 exposed cases. Validated questionnaires were not always used to obtain exposure and outcome status, potentially introducing misclassification bias,18,20,43,44,45,52 whereas varying HDP and ASD or ADHD diagnostic criteria may increase clinical heterogeneity between studies.94
Finally, residual or unmeasured confounding is of particular concern in observational studies, and therefore important confounding factors may not always be considered or available.95 Most of the studies included in our meta-analyses identified potential confounders a priori on the basis of previous literature, and only 2 studies20,44 appear to have aided this method with directed acyclic graphs to evaluate and assess suspected confounding.96 Other studies in our review, however, failed to control for confounding or did not provide justification for included confounders. Only 1 study of ASD45 and 1 study of ADHD49 controlled for a combination of key variables, such as maternal age, socioeconomic status, ethnic origin, and family history of mental illness. Separately, only 3 studies of ASD34,38,42 and 1 study of ADHD17 adjusted for gestational age and birthweight, all of which attenuated toward the null after adjustment. Therefore, although an apparent association exists between HDP and ASD and between HDP and ADHD, future research examining the association between HDP and neurodevelopmental outcomes needs to identify a comprehensive set of confounders to assess whether this association is causal or attributable to residual or unmeasured confounding. Furthermore, this research focused specifically on ASD, ADHD, and other neurodevelopmental, behavioral, or cognitive outcomes. Future research could explore the association between HDP and mental disorders not included in this review to gain a greater understanding of the specificity of the effects of HDP.
Conclusions
Our systematic review indicates that exposure to HDP may be associated with an increase in the risk of ASD and ADHD. If the observed associations are causal, they highlight the potential need for increased developmental screening of HDP-exposed infants to allow early intervention, which may improve neurodevelopmental outcome. However, before more definitive conclusions can be reached, more robust research is needed that addresses key limitations in the literature.
eTable 1. Search Strategy for Each Electronic Database
eTable 2. Summary of Characteristics of ASD Studies Included
eTable 3. Summary of Characteristics of ADHD Studies Included
eTable 4. Summary of HDP and Other Neurodevelopmental Disorders
eTable 5. Level of Bias in ASD Studies
eTable 6. Level of Bias in ADHD Studies
eTable 7. Level of Bias in Other Neurodevelopmental Outcome Studies
eFigure 1. ASD Studies With Crude and Adjusted Estimates
eFigure 2. ADHD Studies With Crude and Adjusted Estimates
eFigure 3. ASD Studies That Adjust for Maternal Age and Smoking and Parity/Birth Order
eFigure 4. ADHD Studies That Adjust for Maternal Age and Educational Level
eFigure 5. Funnel Plot of Published HDP and ASD Studies
eReferences.
References
- 1.Brown MA, Magee LA, Kenny L, et al. ; International Society for the Study of Hypertension in Pregnancy (ISSHP) . The hypertensive disorders of pregnancy: ISSHP classification, diagnosis and management recommendations for international practice 2018 [published online May 22, 2018]. Preg Hypertens. doi: 10.1016/j.preghy.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 2.Olson-Chen C, Seligman NS. Hypertensive emergencies in pregnancy. Crit Care Clin. 2016;32(1):29-41. [DOI] [PubMed] [Google Scholar]
- 3.Gillon TER, Pels A, von Dadelszen P, MacDonell K, Magee LA. Hypertensive disorders of pregnancy: a systematic review of international clinical practice guidelines. PLoS One. 2014;9(12):e113715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakim J, Senterman MK, Hakim AM. Preeclampsia is a biomarker for vascular disease in both mother and child: the need for a medical alert system. Int J Pediatr. 2013;2013:953150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis EF, Lazdam M, Lewandowski AJ, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552-e1561. [DOI] [PubMed] [Google Scholar]
- 6.Nomura Y, John RM, Janssen AB, et al. Neurodevelopmental consequences in offspring of mothers with preeclampsia during pregnancy: underlying biological mechanism via imprinting genes. Arch Gynecol Obstet. 2017;295(6):1319-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinheiro TV, Brunetto S, Ramos JG, Bernardi JR, Goldani MZ. Hypertensive disorders during pregnancy and health outcomes in the offspring: a systematic review. J Dev Orig Health Dis. 2016;7(4):391-407. [DOI] [PubMed] [Google Scholar]
- 8.Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387(10024):1240-1250. [DOI] [PubMed] [Google Scholar]
- 9.Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383(9920):896-910. [DOI] [PubMed] [Google Scholar]
- 10.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311(17):1770-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akutagava-Martins GC, Rohde LA, Hutz MH. Genetics of attention-deficit/hyperactivity disorder: an update. Expert Rev Neurother. 2016;16(2):145-156. [DOI] [PubMed] [Google Scholar]
- 12.Ghirardi L, Brikell I, Kuja-Halkola R, et al. The familial co-aggregation of ASD and ADHD: a register-based cohort study. Mol Psychiatry. 2018;23(2):257-262. doi: 10.1038/mp.2017.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D, Volk H, Girirajan S, et al. The joint effect of air pollution exposure and copy number variation on risk for autism. Autism Res. 2017;10(9):1470-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaafsma SM, Gagnidze K, Reyes A, et al. Sex-specific gene-environment interactions underlying ASD-like behaviors. Proc Natl Acad Sci U S A. 2017;114(6):1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandin S, Lichtenstein P, Kuja-Halkola R, Hultman C, Larsson H, Reichenberg A. The heritability of autism spectrum disorder. JAMA. 2017;318(12):1182-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva D, Colvin L, Hagemann E, Bower C. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;133(1):e14-e22. [DOI] [PubMed] [Google Scholar]
- 18.Böhm S, Curran EA, Kenny LC, O’Keeffe GW, Murray D, Khashan AS. The effect of hypertensive disorders of pregnancy on the risk of ADHD in the offspring [published online January 1, 2017]. J Atten Disord. doi:10.1177/1087054717690230 [DOI] [PubMed] [Google Scholar]
- 19.Halmøy A, Klungsøyr K, Skjærven R, Haavik J. Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71(5):474-481. [DOI] [PubMed] [Google Scholar]
- 20.Krakowiak P, Walker CK, Bremer AA, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129(5):e1121-e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann JR, McDermott S, Bao H, Hardin J, Gregg A. Pre-eclampsia, birth weight, and autism spectrum disorders. J Autism Dev Disord. 2010;40(5):548-554. [DOI] [PubMed] [Google Scholar]
- 22.Tuovinen S, Eriksson JG, Kajantie E, Räikkönen K. Maternal hypertensive pregnancy disorders and cognitive functioning of the offspring: a systematic review. J Am Soc Hypertens. 2014;8(11):832-847.e1. [DOI] [PubMed] [Google Scholar]
- 23.Amiri S, Malek A, Sadegfard M, Abdi S. Pregnancy-related maternal risk factors of attention-deficit hyperactivity disorder: a case-control study. ISRN Pediatr. 2012;2012:458064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golmirzaei J, Namazi S, Amiri S, et al. Evaluation of attention-deficit hyperactivity disorder risk factors. Int J Pediatr. 2013;2013:953103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Çak HT, Gokler B. Attention deficit hyperactivity disorder and associated perinatal risk factors in preterm children. Turk Pediatr Ars. 2013;48(4):315-322. [Google Scholar]
- 26.Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics. 2009;123(5):1293-1300. [DOI] [PubMed] [Google Scholar]
- 27.Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, Hallmayer JF. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry. 2004;61(6):618-627. [DOI] [PubMed] [Google Scholar]
- 28.Matsuishi T, Yamashita Y, Ohtani Y, et al. Brief report: incidence of and risk factors for autistic disorder in neonatal intensive care unit survivors. J Autism Dev Disord. 1999;29(2):161-166. [DOI] [PubMed] [Google Scholar]
- 29.Maher GM, O’Keeffe GW, Kenny LC, Kearney PM, Dinan TG, Khashan AS. Hypertensive disorders of pregnancy and risk of neurodevelopmental disorders in the offspring: a systematic review and meta-analysis protocol. BMJ Open. 2017;7(10):e018313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 31.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 32.McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A; Knowledge Synthesis Group . Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):138-148. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. http://handbook-5-1.cochrane.org. Updated March 2011. Accessed July 6, 2017.
- 34.Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can. 2010;30(4):125-134. [PubMed] [Google Scholar]
- 35.Deykin EY, MacMahon B. Pregnancy, delivery, and neonatal complications among autistic children. Am J Dis Child. 1980;134(9):860-864. [DOI] [PubMed] [Google Scholar]
- 36.Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord. 2011;41(7):891-902. [DOI] [PubMed] [Google Scholar]
- 37.Eaton WW, Mortensen PB, Thomsen PH, Frydenberg M. Obstetric complications and risk for severe psychopathology in childhood. J Autism Dev Disord. 2001;31(3):279-285. [DOI] [PubMed] [Google Scholar]
- 38.Hultman CM, Sparén P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology. 2002;13(4):417-423. [DOI] [PubMed] [Google Scholar]
- 39.Larsson HJ, Eaton WW, Madsen KM, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol. 2005;161(10):916-925. [DOI] [PubMed] [Google Scholar]
- 40.Mason-Brothers A, Ritvo ER, Pingree C, et al. The UCLA–University of Utah epidemiologic survey of autism: prenatal, perinatal, and postnatal factors. Pediatrics. 1990;86(4):514-519. [PubMed] [Google Scholar]
- 41.Buchmayer S, Johansson S, Johansson A, Hultman CM, Sparén P, Cnattingius S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics. 2009;124(5):e817-e825. [DOI] [PubMed] [Google Scholar]
- 42.Langridge AT, Glasson EJ, Nassar N, et al. Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS One. 2013;8(1):e50963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyall K, Pauls DL, Spiegelman D, Ascherio A, Santangelo SL. Pregnancy complications and obstetric suboptimality in association with autism spectrum disorders in children of the Nurses’ Health Study II. Autism Res. 2012;5(1):21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr. 2015;169(2):154-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curran EA, O’Keeffe GW, Looney AM, et al. Exposure to hypertensive disorders of pregnancy increases the risk of autism spectrum disorder in affected offspring [published online October 3, 2017]. Mol Neurobiol. doi: 10.1007/s12035-017-0794-x [DOI] [PubMed] [Google Scholar]
- 46.Polo-Kantola P, Lampi KM, Hinkka-Yli-Salomäki S, Gissler M, Brown AS, Sourander A. Obstetric risk factors and autism spectrum disorders in Finland. J Pediatr. 2014;164(2):358-365. [DOI] [PubMed] [Google Scholar]
- 47.Mrozek-Budzyn D, Majewska R, Kieltyka A. Prenatal, perinatal and neonatal risk factors for autism: study in Poland. Cent Eur J Med. 2013;8(4):424-430. [Google Scholar]
- 48.Nath S, Roy R, Mukherjee S. Perinatal complications associated with autism: a case control study in a neurodevelopment and early intervention clinic. J Indian Med Assoc. 2012;110(8):526-529. [PubMed] [Google Scholar]
- 49.Getahun D, Rhoads GG, Demissie K, et al. In utero exposure to ischemic-hypoxic conditions and attention-deficit/hyperactivity disorder. Pediatrics. 2013;131(1):e53-e61. [DOI] [PubMed] [Google Scholar]
- 50.Mann JR, McDermott S. Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school-aged children? J Atten Disord. 2011;15(8):667-673. [DOI] [PubMed] [Google Scholar]
- 51.Gustafsson P, Källén K. Perinatal, maternal, and fetal characteristics of children diagnosed with attention-deficit–hyperactivity disorder: results from a population-based study utilizing the Swedish Medical Birth Register. Dev Med Child Neurol. 2011;53(3):263-268. [DOI] [PubMed] [Google Scholar]
- 52.Ketzer CR, Gallois C, Martinez AL, Rohde LA, Schmitz M. Is there an association between perinatal complications and attention-deficit/hyperactivity disorder-inattentive type in children and adolescents? Rev Bras Psiquiatr. 2012;34(3):321-328. [DOI] [PubMed] [Google Scholar]
- 53.Many A, Fattal A, Leitner Y, Kupferminc MJ, Harel S, Jaffa A. Neurodevelopmental and cognitive assessment of children born growth restricted to mothers with and without preeclampsia. Hypertens Pregnancy. 2003;22(1):25-29. [DOI] [PubMed] [Google Scholar]
- 54.Morsing E, Maršál K. Pre-eclampsia: an additional risk factor for cognitive impairment at school age after intrauterine growth restriction and very preterm birth. Early Hum Dev. 2014;90(2):99-101. [DOI] [PubMed] [Google Scholar]
- 55.Cheng S-W, Chou H-C, Tsou K-I, Fang L-J, Tsao P-N. Delivery before 32 weeks of gestation for maternal pre-eclampsia: neonatal outcome and 2-year developmental outcome. Early Hum Dev. 2004;76(1):39-46. [DOI] [PubMed] [Google Scholar]
- 56.Leversen KT, Sommerfelt K, Rønnestad A, et al. Prediction of neurodevelopmental and sensory outcome at 5 years in Norwegian children born extremely preterm. Pediatrics. 2011;127(3):e630-e638. [DOI] [PubMed] [Google Scholar]
- 57.Szymonowicz W, Yu VY. Severe pre-eclampsia and infants of very low birth weight. Arch Dis Child. 1987;62(7):712-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson S, Evans TA, Draper ES, et al. Neurodevelopmental outcomes following late and moderate prematurity: a population-based cohort study. Arch Dis Child Fetal Neonatal Ed. 2015;100(4):F301-F308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffith MI, Mann JR, McDermott S. The risk of intellectual disability in children born to mothers with preeclampsia or eclampsia with partial mediation by low birth weight. Hypertens Pregnancy. 2011;30(1):108-115. [DOI] [PubMed] [Google Scholar]
- 60.Salonen JT, Heinonen OP. Mental retardation and mother’s hypertension during pregnancy. J Ment Defic Res. 1984;28(pt 1):53-56. [DOI] [PubMed] [Google Scholar]
- 61.Barker DJ, Edwards JH. Obstetric complications and school performance. Br Med J. 1967;3(5567):695-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gray PH, O’Callaghan MJ, Mohay HA, Burns YR, King JF. Maternal hypertension and neurodevelopmental outcome in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;79(2):F88-F93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawlor DA, Batty GD, Morton SM, et al. Early life predictors of childhood intelligence: evidence from the Aberdeen children of the 1950s study. J Epidemiol Community Health. 2005;59(8):656-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leitner Y, Harel S, Geva R, Eshel R, Yaffo A, Many A. The neurocognitive outcome of IUGR children born to mothers with and without preeclampsia. J Matern Fetal Neonatal Med. 2012;25(11):2206-2208. [DOI] [PubMed] [Google Scholar]
- 65.Leonard H, de Klerk N, Bourke J, Bower C. Maternal health in pregnancy and intellectual disability in the offspring: a population-based study. Ann Epidemiol. 2006;16(6):448-454. [DOI] [PubMed] [Google Scholar]
- 66.McCowan LM, Pryor J, Harding JE. Perinatal predictors of neurodevelopmental outcome in small-for-gestational-age children at 18 months of age. Am J Obstet Gynecol. 2002;186(5):1069-1075. [DOI] [PubMed] [Google Scholar]
- 67.Robinson M, Mattes E, Oddy WH, et al. Hypertensive diseases of pregnancy and the development of behavioral problems in childhood and adolescence: the Western Australian Pregnancy Cohort Study. J Pediatr. 2009;154(2):218-224. [DOI] [PubMed] [Google Scholar]
- 68.Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Pre-eclampsia and offspring’s blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstet Gynaecol. 1991;98(10):1009-1014. [DOI] [PubMed] [Google Scholar]
- 69.Spinillo A, Iasci A, Capuzzo E, Egbe TO, Colonna L, Fazzi E. Two-year infant neurodevelopmental outcome after expectant management and indicated preterm delivery in hypertensive pregnancies. Acta Obstet Gynecol Scand. 1994;73(8):625-629. [DOI] [PubMed] [Google Scholar]
- 70.Spinillo A, Montanari L, Gardella B, Roccio M, Stronati M, Fazzi E. Infant sex, obstetric risk factors, and 2-year neurodevelopmental outcome among preterm infants. Dev Med Child Neurol. 2009;51(7):518-525. [DOI] [PubMed] [Google Scholar]
- 71.Tuovinen S, Eriksson JG, Kajantie E, et al. Maternal hypertensive disorders in pregnancy and self-reported cognitive impairment of the offspring 70 years later: the Helsinki Birth Cohort Study. Am J Obstet Gynecol. 2013;208(3):200.e1-200.e9. [DOI] [PubMed] [Google Scholar]
- 72.Tuovinen S, Räikkönen K, Kajantie E, et al. Hypertensive disorders in pregnancy and cognitive decline in the offspring up to old age. Neurology. 2012;79(15):1578-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tuovinen S, Räikkönen K, Kajantie E, et al. Hypertensive disorders in pregnancy and intellectual abilities in the offspring in young adulthood: the Helsinki Birth Cohort Study. Ann Med. 2012;44(4):394-403. [DOI] [PubMed] [Google Scholar]
- 74.Whitehouse AJ, Robinson M, Newnham JP, Pennell CE. Do hypertensive diseases of pregnancy disrupt neurocognitive development in offspring? Paediatr Perinat Epidemiol. 2012;26(2):101-108. [DOI] [PubMed] [Google Scholar]
- 75.Winer EK, Tejani NA, Atluru V, DiGiuseppe R, Borofsky LG. Four- to seven-year evaluation in two groups of small-for-gestational age infants. Am J Obstet Gynecol. 1982;143(4):425-429. [DOI] [PubMed] [Google Scholar]
- 76.Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Health of children born to mothers who had preeclampsia: a population-based cohort study. Am J Obstet Gynecol. 2009;201(3):269.e1-269.e10. [DOI] [PubMed] [Google Scholar]
- 77.Ehrenstein V, Rothman KJ, Pedersen L, Hatch EE, Sørensen HT. Pregnancy-associated hypertensive disorders and adult cognitive function among Danish conscripts. Am J Epidemiol. 2009;170(8):1025-1031. [DOI] [PubMed] [Google Scholar]
- 78.Heikura U, Hartikainen AL, Nordström T, Pouta A, Taanila A, Järvelin MR. Maternal hypertensive disorders during pregnancy and mild cognitive limitations in the offspring. Paediatr Perinat Epidemiol. 2013;27(2):188-198. [DOI] [PubMed] [Google Scholar]
- 79.Love ER, Crum J, Bhattacharya S. Independent effects of pregnancy induced hypertension on childhood development: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2012;165(2):219-224. [DOI] [PubMed] [Google Scholar]
- 80.Schlapbach LJ, Ersch J, Adams M, Bernet V, Bucher HU, Latal B. Impact of chorioamnionitis and preeclampsia on neurodevelopmental outcome in preterm infants below 32 weeks gestational age. Acta Paediatr. 2010;99(10):1504-1509. [DOI] [PubMed] [Google Scholar]
- 81.Silveira RC, Procianoy RS, Koch MS, Benjamin ACW, Schlindwein CF. Growth and neurodevelopment outcome of very low birth weight infants delivered by preeclamptic mothers. Acta Paediatr. 2007;96(12):1738-1742. [DOI] [PubMed] [Google Scholar]
- 82.Warshafsky C, Pudwell J, Walker M, Wen SW, Smith G. A prospective assessment of neurodevelopment in children following a pregnancy complicated by severe preeclampsia. Paediatr Child Health. 2015;20(5):e40-e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Many A, Fattal-Valevski A, Leitner Y. Neurodevelopmental and cognitive assessment of 6-year-old children born growth restricted. Int J Gynaecol Obstet. 2005;89(1):55-56. [DOI] [PubMed] [Google Scholar]
- 84.Curran EA, O’Neill SM, Cryan JF, et al. Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Child Psychol Psychiatry. 2015;56(5):500-508. [DOI] [PubMed] [Google Scholar]
- 85.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128(2):344-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sandin S, Hultman CM, Kolevzon A, Gross R, MacCabe JH, Reichenberg A. Advancing maternal age is associated with increasing risk for autism: a review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2012;51(5):477-486.e1. [DOI] [PubMed] [Google Scholar]
- 87.Zhu T, Gan J, Huang J, Li Y, Qu Y, Mu D. Association between perinatal hypoxic-ischemic conditions and attention-deficit/hyperactivity disorder: a meta-analysis. J Child Neurol. 2016;31(10):1235-1244. [DOI] [PubMed] [Google Scholar]
- 88.Bánhidy F, Acs N, Puhó EH, Czeizel AE. The efficacy of antihypertensive treatment in pregnant women with chronic and gestational hypertension: a population-based study. Hypertens Res. 2010;33(5):460-466. [DOI] [PubMed] [Google Scholar]
- 89.Spencer RN, Carr DJ, David AL. Treatment of poor placentation and the prevention of associated adverse outcomes: what does the future hold? Prenat Diagn. 2014;34(7):677-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rebelo F, Schlüssel MM, Vaz JS, et al. C-reactive protein and later preeclampsia: systematic review and meta-analysis taking into account the weight status. J Hypertens. 2013;31(1):16-26. [DOI] [PubMed] [Google Scholar]
- 91.Brown AS, Sourander A, Hinkka-Yli-Salomäki S, McKeague IW, Sundvall J, Surcel H-M. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry. 2014;19(2):259-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rätsep MT, Paolozza A, Hickman AF, et al. Brain structural and vascular anatomy is altered in offspring of pre-eclamptic pregnancies: a pilot study. AJNR Am J Neuroradiol. 2016;37(5):939-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schneider M, Retz W, Coogan A, Thome J, Rösler M. Anatomical and functional brain imaging in adult attention-deficit/hyperactivity disorder (ADHD)—a neurological view. Eur Arch Psychiatry Clin Neurosci. 2006;256(1):i32-i41. [DOI] [PubMed] [Google Scholar]
- 94.Wardenaar KJ, de Jonge P. Diagnostic heterogeneity in psychiatry: towards an empirical solution. BMC Med. 2013;11(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Flanders WD, Klein M, Darrow LA, et al. A method for detection of residual confounding in time-series and other observational studies. Epidemiology. 2011;22(1):59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin W. Making valid causal inferences from observational data. Prev Vet Med. 2014;113(3):281-297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Strategy for Each Electronic Database
eTable 2. Summary of Characteristics of ASD Studies Included
eTable 3. Summary of Characteristics of ADHD Studies Included
eTable 4. Summary of HDP and Other Neurodevelopmental Disorders
eTable 5. Level of Bias in ASD Studies
eTable 6. Level of Bias in ADHD Studies
eTable 7. Level of Bias in Other Neurodevelopmental Outcome Studies
eFigure 1. ASD Studies With Crude and Adjusted Estimates
eFigure 2. ADHD Studies With Crude and Adjusted Estimates
eFigure 3. ASD Studies That Adjust for Maternal Age and Smoking and Parity/Birth Order
eFigure 4. ADHD Studies That Adjust for Maternal Age and Educational Level
eFigure 5. Funnel Plot of Published HDP and ASD Studies
eReferences.