Key Points
Question
Is levofloxacin effective in preventing bacteremia in children receiving intensive chemotherapy or undergoing hematopoietic stem cell transplants?
Findings
In this randomized clinical trial that included 195 children with acute leukemia and 418 children undergoing stem cell transplants, the likelihood of bacteremia in those receiving levofloxacin prophylaxis compared with no prophylaxis was 21.9% vs 43.4% for patients with acute leukemia (a significant difference) and 11.0% vs 17.3% for patients undergoing stem cell transplantation (nonsignificant difference).
Meaning
Levofloxacin prophylaxis significantly reduced the risk of bacteremia in children with acute leukemia receiving intensive chemotherapy but not in those undergoing stem cell transplantation.
Abstract
Importance
Bacteremia causes considerable morbidity among children with acute leukemia and those undergoing hematopoietic stem cell transplantation (HSCT). There are limited data on the effect of antibiotic prophylaxis in children.
Objective
To determine the efficacy and risks of levofloxacin prophylaxis in children receiving intensive chemotherapy for acute leukemia or undergoing HSCT.
Design, Setting, and Participants
In this multicenter, open-label, randomized trial, patients (6 months-21 years) receiving intensive chemotherapy were enrolled (September 2011-April 2016) in 2 separate groups—acute leukemia, consisting of acute myeloid leukemia or relapsed acute lymphoblastic leukemia, and HSCT recipients—at 76 centers in the United States and Canada, with follow-up completed September 2017.
Interventions
Patients with acute leukemia were randomized to receive levofloxacin prophylaxis for 2 consecutive cycles of chemotherapy (n = 100) or no prophylaxis (n = 100). Those undergoing HSCT were randomized to receive levofloxacin prophylaxis during 1 HSCT procedure (n = 210) or no prophylaxis (n = 214).
Main Outcomes and Measures
The primary outcome was the occurrence of bacteremia during 2 chemotherapy cycles (acute leukemia) or 1 transplant procedure (HSCT). Secondary outcomes included fever and neutropenia, severe infection, invasive fungal disease, Clostridium difficile–associated diarrhea, and musculoskeletal toxic effects.
Results
A total of 624 patients, 200 with acute leukemia (median [interquartile range {IQR}] age, 11 years [6-15 years]; 46% female) and 424 undergoing HSCT (median [IQR] age, 7 years [3-14]; 38% female), were enrolled. Among 195 patients with acute leukemia, the likelihood of bacteremia was significantly lower in the levofloxacin prophylaxis group than in the control group (21.9% vs 43.4%; risk difference, 21.6%; 95% CI, 8.8%-34.4%, P = .001), whereas among 418 patients undergoing HSCT, the risk of bacteremia was not significantly lower in the levofloxacin prophylaxis group (11.0% vs 17.3%; risk difference, 6.3%; 95% CI, 0.3%-13.0%; P = .06). Fever and neutropenia were less common in the levofloxacin group (71.2% vs 82.1%; risk difference, 10.8%; 95% CI, 4.2%-17.5%; P = .002). There were no significant differences in severe infection (3.6% vs 5.9%; risk difference, 2.3%; 95% CI, −1.1% to 5.6%; P = .20), invasive fungal disease (2.9% vs 2.0%; risk difference, −1.0%; 95% CI, −3.4% to 1.5%, P = .41), C difficile–associated diarrhea (2.3% vs 5.2%; risk difference, 2.9%; 95% CI, −0.1% to 5.9%; P = .07), or musculoskeletal toxic effects at 2 months (11.4% vs 16.3%; risk difference, 4.8%; 95% CI, −1.6% to 11.2%; P = .15) or at 12 months (10.1% vs 14.4%; risk difference, 4.3%; 95% CI, −3.4% to 12.0%; P = .28) between the levofloxacin and control groups.
Conclusions and Relevance
Among children with acute leukemia receiving intensive chemotherapy, receipt of levofloxacin prophylaxis compared with no prophylaxis resulted in a significant reduction in bacteremia. However, there was no significant reduction in bacteremia for levofloxacin prophylaxis among children undergoing HSCT.
This open-label randomized clinical trial involving pediatric patients with acute leukemia receiving intensive chemotherapy or undergoing hematopoietic stem cell transplantation compares the rate of bacteremia among those who received levofloxacin prophylaxis with those who did not.
Introduction
Bacteremia is a leading cause of morbidity and mortality in children receiving intensive chemotherapy for acute myeloid leukemia (AML) and relapsed acute lymphoblastic leukemia (ALL) and for those undergoing hematopoietic stem cell transplantation (HSCT).1,2,3 The use of prophylactic antibiotics in adult patients with neutropenia secondary to cancer therapy is supported by meta-analyses of randomized trials demonstrating decreased risk of infections confirmed by blood cultures and death.4 Data describing prophylactic antibiotics in children with cancer are limited to several small single-group observational studies.5,6,7,8,9,10
The adoption of antibacterial prophylaxis is tempered by potential negative consequences including Clostridium difficile–associated diarrhea, bacterial resistance, and musculoskeletal toxicities.11,12,13 A randomized trial involving children is required to understand the benefits and risks of prophylaxis. The primary objective was to determine whether prophylactic levofloxacin during neutropenia decreased the risk of bacteremia in children with acute leukemia or undergoing HSCT. The secondary objectives included the evaluation of potential adverse effects and outcomes associated with prophylaxis.
Methods
Study Design
This was a multicenter, randomized, open-label phase 3 trial (ACCL0934) conducted by the Children’s Oncology Group (COG). The protocol and all amendments were approved by the National Cancer Institute’s Central Institutional Review Board and the institutional review boards at each of the 76 participating institutions (the protocol and statistical analysis plan are available in Supplement 1). Written informed consent and assent (if appropriate) were obtained from participants or their guardians.
Patients
Patients aged 6 months to 21 years were enrolled into 2 groups: (1) the acute leukemia group, in which patients were anticipated to receive at least 2 consecutive cycles of intensive chemotherapy and (2) the HSCT group, in which patients were anticipated to receive a myeloablative autologous or allogeneic HSCT. Eligible leukemia diagnoses included any AML (de novo, relapsed, or secondary AML, and acute leukemia of ambiguous lineage treated with standard AML therapy), or relapsed ALL. Intensive chemotherapy and myeloablative HSCT were defined as regimens anticipated to cause neutropenia for more than 7 days. Additional eligibility criteria were adequate renal function and Eastern Cooperative Oncology Group performance status of 2 or less. Exclusion criteria were previous participation in this trial, allergy to quinolones, chronic active arthritis, known pathological prolongation of the QTc, pregnant or breast feeding, and treatment with systemic antibacterial agents with the exception of Pneumocystis jiroveci prophylaxis. Race/ethnicity data were collected according to the National Institutes of Health inclusion policy. These categorizations were determined by institutional investigators based on fixed categories.
Randomization and Blinding
Randomization was performed separately in the acute leukemia and HSCT groups. Patients were randomized 1:1 at enrollment to receive levofloxacin prophylaxis (intervention group) or no antibiotic prophylaxis (control group). The allocation sequence was generated by the COG trial management system and the sequence was concealed to all investigators, clinicians, and participants. Stratification factors were AML vs relapsed ALL and autologous vs allogeneic HSCT among the patients with acute leukemia and those undergoing HSCT, respectively. Randomization was conducted in block sizes of 4; block size was not available in the protocol and was not disclosed to the study personnel, sites investigators, research associates, or participants. The study was open label, so clinicians and participants were aware of treatment allocation.
Procedures
Supportive care was dictated by each institution’s policies. Available to each site were the COG-endorsed guideline for the management of fever and neutropenia14 that includes a strong recommendation to obtain blood cultures at the onset of fever and neutropenia. Specimens were processed and reported by local microbiology laboratories.
Intervention Group
For children with acute leukemia, levofloxacin was administered during 2 consecutive cycles of chemotherapy starting on day 1 of systemic therapy. The study was amended on December 16, 2013, to start levofloxacin on day 3 to facilitate patient recruitment. For children undergoing HSCT, levofloxacin was administered during 1 transplant procedure starting on day negative 2 from stem cell infusion. Prophylaxis continued until the first of the following events occurred: absolute neutrophil count (ANC) of more than 200/μL after nadir, day 60, or initiation of the next chemotherapy cycle. Levofloxacin was held if parenteral antibacterial therapy was administered for any reason, such as empirical antibiotics for fever and neutropenia. Levofloxacin dosing for those older than 6 months to those younger than 5 years was 10 mg/kg twice daily and for those older than 5 years was 10 mg/kg once daily (maximum, 750 mg/d). Levofloxacin was given orally or intravenously (if not tolerated orally) with the same dose and schedule.
Control Group
No levofloxacin prophylaxis was administered.
Evaluations
The infection observation period was identical for both groups. For patients with acute leukemia, it began on day 1 (preamendment) or day 3 (postamendment) of each chemotherapy cycle. For patients undergoing HSCT, it began on day negative 2 prior to stem cell infusion. The infection observation period ended when the first of the following events occurred: ANC of more than 200 cells/μL after nadir, day 60, or initiation of the next cycle of chemotherapy.
During the infection observation period, all positive sterile-site bacterial cultures were submitted in addition to data related to secondary outcomes. Potential covariates collected were duration of ANC of less than 200 cells/μL, administration of prophylactic granulocyte-colony stimulating factor, central venous catheter ethanol or antibacterial lock therapy, chlorhexidine bathing, and systemic steroid exposure.
Data for predefined musculoskeletal disorders were collected at baseline and at 2 and 12 months following completion of the final infection observation period. Musculoskeletal assessment was performed by an individual blinded to treatment allocation with a data collection tool focused on tendinopathy, arthritis, arthralgia, and gait abnormalities.
Antibiotic susceptibility of all sterile site isolates was collected from institutional clinical laboratory reports. An ancillary study with optional participation described the change in resistance patterns for specific pathogens colonizing the intestinal tract by randomized groups. Perirectal or stool specimens were collected at baseline and at completion of each infection observation period using Dacron-tipped culture swabs and were shipped to a central laboratory where each swab was streaked onto colistin nalidixic acid agar and then onto MacConkey agar. Plates were incubated at 37°C for 24 to 48 hours. Susceptibility of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa isolates to cefepime, imipenem, and levofloxacin, and susceptibility of Streptococcus mitis to cefepime, levofloxacin, and penicillin were determined using Clinical Laboratories Standards Institute criteria.15
In terms of adverse events, all Common Terminology Criteria for Adverse Events (CTCAE)16 grade 4 nonhematological and grade 5 (fatal) events were submitted from enrollment to 30 days following the final infection observation period.
Outcomes
Primary Outcome
The primary outcome was centrally adjudicated true bacteremia. All positive sterile-site bacterial cultures were reviewed by a panel of 3 committee members (S.A., B.T.F., A.H.G., or L.S.) blinded to treatment allocation. Contaminant bacteria were defined as single occurrences of organisms included on the National Healthcare Safety Network–Centers for Disease Control and Prevention list of common commensals.17 The common commensal list was modified to exclude viridans group streptococci,18 given their known association with sepsis syndrome in this population. Multiple positive cultures for common commensals were considered true bacteremia events if the second culture occurred on the same or subsequent day. True bacteremia was defined as bacteremia not meeting contaminant criteria.
Secondary Outcomes
Secondary outcomes included (1) fever and neutropenia, (2) severe infection and death from bacterial infection, (3) invasive fungal disease, (4) C difficile–associated diarrhea, (5) prespecified musculoskeletal conditions, and (6) resistance patterns of bacterial isolates from all sterile-site cultures and from perirectal or stool swabs.
Fever and neutropenia was defined as ANC of less than 1000 cells/μL and a single temperature of 38.3°C or higher or a sustained temperature of 38°C or higher for more than 1 hour. Severe infection was defined as any CTCAE grade 4 or 5 infection or infestation including bacterial, nonbacterial, and clinically documented infections. Invasive fungal disease was defined as microbiologically or pathologically proven disease.19 C difficile–associated diarrhea was defined as an assay that tested positive for C difficile concurrent with grade 2 or higher diarrhea by CTCAE criteria. Predefined musculoskeletal conditions were arthralgia, arthritis, gait disturbance, tendinopathy, and tendon rupture. In terms of antibiotic resistance, intermediate susceptibility was classified as resistant for analysis. A specimen that did not yield 1 of the 4 organisms evaluated on culture was considered to lack resistance for that organism.
Post hoc Outcomes
Post hoc outcomes were (1) total days of fever of 38°C or higher, (2) days of hospitalization, (3) days of antifungal therapy, (4) positive C difficile assay results, and (5) total antibiotic exposure days and any exposure stratified by antibiotic class or spectrum of activity (gram-positive agents, aminoglycosides, third- or fourth-generation cephalosporins, and antibiotics commonly used for empirical therapy for fever and neutropenia).
Adverse Events
Serious adverse events were defined as any grade 4 toxicity that was unexpected and possibly, probably, or definitely related to levofloxacin and all grade 5 toxicities.
Statistical Analysis
The study was designed and powered for separate analyses of the acute leukemia and HSCT groups for the primary outcome. Power calculations assumed a baseline risk of true bacteremia of 30% in the control group and a minimal clinically important difference of 50% relative risk reduction (15% risk reduction) based on the findings of a large study of levofloxacin prophylaxis in adult patients with cancer who had received intensive chemotherapy.20 Assuming 84% power and 2-sided α of .05, 266 evaluable patients would need to be enrolled to each of group. The protocol was amended in March 2014 to increase the planned enrollment of the HSCT group based on interim data released from the data and safety monitoring committee that the observed bacteremia rate in the HSCT control group was lower than anticipated. The revised calculation assumed 20% true bacteremia rate in the control group and 80% power resulting in a total anticipated sample size of 400 evaluable patients undergoing HSCT. Consequently, planned enrollment was 266 patients with acute leukemia and 400 patients undergoing HSCT (666 total).
All analyses were modified intention to treat for which ineligible patients and patients who withdrew consent prior to the start of the first infection observation period were not included. Missing data were handled using available case analysis.
Primary Analysis
The proportion of patients with at least 1 true bacteremia episode during the infection observation period(s) was compared between randomized groups using the χ2 test.
Interim monitoring of the primary outcome was performed twice when approximately one-third and two-thirds of patients (separately for acute leukemia and HSCT groups) were enrolled and evaluated. Efficacy monitoring boundary values were based on Lan-DeMets α spending function alpha t2. The spending all α method21 was used to adjust the P values for the final analysis (.046 and .038 for acute leukemia and HSCT groups, respectively). To determine if the treatment effect differed between groups, logistic regression was conducted with the 2 groups combined and a test for interaction was performed between treatment and patient group.
Secondary Analyses
Patients with acute leukemia and those undergoing HSCT were combined into a single cohort for analyses of all secondary outcomes. Given that there was no adjustment for multiple comparison, the secondary analyses were considered exploratory. Fever and neutropenia, severe infection, invasive fungal disease, C difficile–associated diarrhea, and predefined musculoskeletal conditions were binary variables consisting of at least 1 occurrence of these events in either of the 2 chemotherapy cycles or the 1 HSCT procedure (or at 2 and 12 months for musculoskeletal conditions).
The effects of levofloxacin on these outcomes were assessed via the Wald test from logistic regression models adjusted for group (acute leukemia vs HSCT). No prophylaxis was the reference level. The proportion of patients who developed new resistance to specific agents in bacteria identified from the perirectal or stool swabs was compared between groups using the χ2 test or Fisher exact test. For these analyses, a 2-sided P value <.05 was considered statistically significant.
Post hoc Analyses
For the primary outcome, Poisson regression compared the number of true bacteremia events in the 2 study groups accounting for exposure days during the infection observation period(s) as the offset. The model assumed that events occurred independently and the event rate was constant. Mixed-effects modeling of the primary outcome with site as a random effect was also conducted.
Post hoc outcomes were total days of fever, days of hospitalization, days of antifungal therapy, C difficile–positive test result, total exposure days, and any exposure to antibiotics stratified by antibiotic class or spectrum of activity. The effects of levofloxacin on these outcomes were assessed via the Wald test from logistic regression models (for binary outcomes) and Poisson regression models (for duration outcomes), adjusted for group (acute leukemia vs HSCT). No prophylaxis was the reference level. For these analyses, a 2-sided P value <.05 was considered statistically significant.
Results
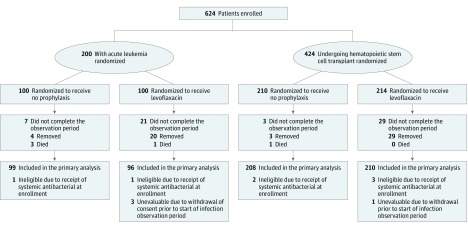
A total of 624 patients, 200 with acute leukemia and 424 undergoing HSCT, were enrolled and randomized between September 2011 and April 2016 (Figure). Data current to September 30, 2017, were used in this article. By this date, all patients had completed the final study end point, the musculoskeletal 12-month posttherapy evaluation. Seven patients were ineligible because they had received systemic antibiotics at the time of enrollment and 4 were unevaluable because of withdrawal of consent prior to the start of the first infection observation period. In October 2016, the COG data and safety monitoring committee recommended early termination of the study because the second planned interim analysis of the acute leukemia group demonstrated efficacy of levofloxacin prophylaxis. At the time, the target enrollment to the HSCT group had been reached. Thus, 195 patients with acute leukemia (96 randomized to levofloxacin and 99 to control group) and 418 patients undergoing HSCT (210 randomized to levofloxacin and 208 randomized to control group) were included in the modified intention-to-treat analyses.
Figure. Study Participation and Flow Through the Triala.
aNo information regarding those approached was available. The total number of patients assessed for participation and reasons for exclusion were not collected.
Characteristics of all eligible patients are described in Table 1. Within each group, baseline characteristics of the intervention and control patients were similar. Further details regarding diagnosis, chemotherapy, HSCT conditioning and graft-vs-host-disease prophylaxis are in eTables 1 and 2 in Supplement 2. The mean (SD) duration of levofloxacin for the prophylaxis and no prophylaxis groups were 14.6 (1.0) and 0.3 (0.2) days per 30 days at risk for the acute leukemia group and 13.8 (0.8) and 0.4 (0.2) days per 30 days at risk for the HSCT group.
Table 1. Baseline Characteristics and Covariates With Potential to Affect Risk of Infection of the Study Patients by Group (N = 617).
| Baseline Characteristics | No. (%) of Patients | |||
|---|---|---|---|---|
| Acute Leukemia (n = 198) | Hematopoietic Stem Cell Transplantation (n = 419) | |||
| Levofloxacin (n = 99) | No Prophylaxis (n = 99) | Levofloxacin (n = 211) | No Prophylaxis (n = 208) | |
| Underlying diagnosis and treatment group | ||||
| Acute myeloid leukemiaa | 65 (65.7) | 63 (63.6) | ||
| Relapsed acute lymphoblastic leukemia | 34 (34.3) | 36 (36.4) | ||
| Autologous transplant | 79 (37.4) | 78 (37.5) | ||
| Allogeneic transplant | 132 (62.6) | 130 (62.5) | ||
| Age, median (IQR), y | 12 (7-16) | 11 (4-15) | 7 (3-15) | 8 (3.5-14.0) |
| Male | 60 (60.6) | 54 (54.5) | 132 (62.6) | 127 (61.1) |
| Race | ||||
| White | 64 (64.6) | 70 (70.7) | 150 (71.1) | 143 (68.8) |
| Black | 12 (12.1) | 12 (12.1) | 23 (10.9) | 33 (15.9) |
| Asian | 5 (5.1) | 3 (3.0) | 17 (8.1) | 14 (6.7) |
| Other or unknown | 18 (18.2) | 14 (14.1) | 21 (10) | 18 (8.7) |
| Ethnicity | ||||
| Non-Hispanic | 68 (68.9) | 81 (81.8) | 138 (65.4) | 163 (78.4) |
| Hispanic | 28 (28.3) | 18 (18.2) | 59 (28) | 38 (18.3) |
| Unknown | 3 (3.0) | 0 | 14 (6.6) | 7 (3.4) |
| Neutropeniab | ||||
| Days to ANC >200 μL, median (IQR) | 17 (14-22) | 17 (10-24) | 12 (7-17) | 11 (7-17) |
| ANC never < 500 μL | 2 (2) | 1 (1) | 9 (4.3) | 2 (1) |
| ANC never >200 μL post nadir | 4 (4) | 2 (2) | 11 (5.2) | 2 (1) |
| Supportive care interventionsb | ||||
| Prophylactic G-CSF, No./total (%) | 21/96 (21.9) | 30/99 (30.3) | 129/210 (61.4) | 134/208 (64.4) |
| Central venous–catheter ethanol or antibacterial lock therapy, No./total (%)c | 4/83 (4.8) | 13/89 (14.6) | 6 /171 (3.5) | 6/170 (3.5) |
| Chlorhexidine bathing, No./total (%)c | 8/83 (9.6) | 24/89 (27.0) | 38/171 (22.2) | 48/170 (28.2) |
| Exposure to systemic steroid | 28 (28.3) | 31 (31.3) | 101 (47.9) | 100 (48.1) |
| Steroids for >5 d, consecutively | 21 (21.2) | 28 (28.3) | 35 (16.6) | 39 (18.8) |
Abbreviations: ANC, absolute neutrophil count; G-CSF, granulocyte colony–stimulating factor; IQR, interquartile range.
Includes patients with leukemia of ambiguous lineage treated with standard acute myeloid leukemia therapy.
Neutropenia information and supportive care intervention data are events that occurred after randomization.
Collection of central line–lock therapy and chlorhexidine bathing was added in an amendment dated February 11, 2013, and thus, available from 172 patients with acute leukemia and 341 patients undergoing hematopoietic stem cell transplant.
Primary Outcome
Of the 195 patients with acute leukemia, 14 (7%) were observed for only 1 cycle of chemotherapy, whereas 181 (93%) were observed for 2 cycles. Among all 195 patients with acute leukemia, the likelihood of bacteremia was significantly lower in the levofloxacin prophylaxis group than in the control group (21.9% vs 43.4%; risk difference, 21.6%; 95% CI, 8.8%-34.4%; P = .001) (Table 2). For patients undergoing HSCT, there was no significant difference in the likelihood of bacteremia in the levofloxacin prophylaxis group compared with the control group (11.0% vs 17.3%; risk difference, 6.3; 95% CI, 0.3-13.0; P = .06).
Table 2. Comparison of Bacteremia Incidence per Patient During the Infection Observation Period and Bacteremia Rate per 1000 Patient-Days Between Randomized Groups for Acute Leukemia and HSCT Groups (N = 613).
| Bacteremia Incidence, No./Total (%) | Risk Difference, % (95% CI) | Risk Ratio (95% CI) | P Value | ||
|---|---|---|---|---|---|
| Levofloxacin | No Prophylaxis | ||||
| Primary Analysisa | |||||
| Total acute leukemia | 21/96 (21.9) | 43/99 (43.4) | 21.6 (8.8-34.4) | 0.50 (0.32-0.78) | .001 |
| AML | 15/64 (23.4) | 25/63 (39.7) | 16.3 (0.3-32.2) | 0.59 (0.35-1.01) | .05 |
| Relapsed ALL | 6/32 (18.8) | 18/36 (50.0) | 31.2 (10.1-52.5) | 0.38 (0.17-0.83) | .007 |
| Total HSCT | 23/210 (11.0) | 36/208 (17.3) | 6.3 (0.3-13.0) | 0.63 (0.39-1.03) | .06 |
| Autologous | 3/79 (3.8) | 9/78 (11.5) | 7.7 (5.1-16.0) | 0.33 (0.09-1.17) | .07 |
| Allogeneic | 20/131 (15.3) | 27/130 (20.8) | 5.5 (3.8-14.8) | 0.74 (0.43-1.24) | .25 |
| Post hoc Analysisb | |||||
| Bacteremia Rate/1000 Patient-Days (95% CI) | Adjusted Rate Ratio (95% CI)c | ||||
| Total acute leukemia | 4.9 (3.3-7.3)c | 9.4 (7.1-12.3)c | 4.3 (1.3-7.4) | 0.52 (0.32-0.85) | .008 |
| Person-days of observation, No. | 5327 | 5973 | |||
| Total HSCT | 5.3 (3.5-8.0)c | 10.0 (6.6-14.8)c | 5.2 (1.1-9.3) | 0.53 (0.32-0.88) | .02 |
| Person-days of observation, No. | 4042 | 3766 | |||
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; HSCT, hematopoietic stem cell transplant.
Each patient could only contribute 1 bacteremia outcome; this was a comparison of the proportion of patients having at least 1 bacteremia event during the infection observation period.
Each patient could contribute more than 1 bacteremia event.
Rates were adjusted for AML vs relapsed ALL in the total acute leukemia analysis and for autologous vs allogeneic in the total HSCT analysis.
There was no significant interaction between group (acute leukemia vs HSCT) and assigned treatment (levofloxacin vs no levofloxacin) (odds ratio, 0.62; 95% CI, 0.27-1.44; P = .27). When all patients were combined, levofloxacin significantly reduced the likelihood of bacteremia (risk difference, 11.4%; 95% CI, 5.1%-17.6%, P < .001).
Among the 123 bacteremia episodes, there were 136 organisms identified (eTable 3 in Supplement 2). Viridans group streptococci represented the most common cultured organisms in both the acute leukemia and HSCT groups.
Secondary Outcomes
Table 3 shows that patients in the levofloxacin prophylaxis group were less likely to have fever and neutropenia (71.2% vs 82.1%; risk difference, 10.8%; 95% CI, 4.2%-17.5%; P = .002) without a significant difference in the likelihood of having severe infection (3.6% vs 5.9%; risk difference, 2.3%; 95% CI, −1.1% to 5.6%; P = .20). There were no deaths attributed to bacterial infection in either group. There were no significant differences in invasive fungal disease (2.9% vs 2.0%; risk difference, −1.0%; 95% CI, −3.4% to 1.5%%; P = .41), C difficile–associated diarrhea (2.3% vs 5.2%; risk difference, 2.9%; 95% CI, −0.1% to 5.9%; P = .07) or musculoskeletal toxic effects at 2 months (11.4% vs 16.3%; risk difference, 4.8%; 95% CI, −1.6% to 11.2%; P = .15) or 12 months (10.1% vs 14.4%; risk difference, 4.3%; 95% CI, −3.4% to 12.0%; P = .28) between the levofloxacin and control groups.
Table 3. Secondary Outcomes for All Patients by Allocation Among All Patients.
| Levofloxacin (n = 306)a | No Prophylaxis (n = 307)a | Risk or Rate Difference (95% CI) | Adjusted OR (95% CI)b | P Value | |
|---|---|---|---|---|---|
| Secondary outcomes, No. (%) | |||||
| Fever and neutropenia | 218 (71.2) | 252 (82.1) | 10.8 (4.2 to 17.5) | 0.54 (0.37 to 0.79) | .002 |
| Severe infectionc | 11 (3.6) | 18 (5.9) | 2.3 (−1.1 to 5.6) | 0.60 (0.28 to 1.30) | .20 |
| Invasive fungal disease | 9 (2.9) | 6 (2.0) | −1.0 (−3.4 to 1.5) | 1.55 (0.54 to 4.43) | .41 |
| C difficile–associated diarrhead | 7 (2.3) | 16 (5.2) | 2.9 (−0.1 to 5.9) | 0.43 (0.17 to 1.05) | .07 |
| Any musculoskeletal condition, No. (%)e | |||||
| Baseline | 18 (5.9) | 30/300 (10.0) | 4.1 (−0.3 to 8.4) | 0.57 (0.31 to 1.05) | .07 |
| 2 mo | 23 (11.4) | 39/240 (16.3) | 4.8 (−1.6 to 11.2) | 0.66 (0.38 to 1.15) | .15 |
| 12 mo | 13 (10.1) | 21/146 (14.4) | 4.3 (−3.4 to 12.0) | 0.67 (0.32 to 1.40) | .28 |
Abbreviations: C difficile, Clostridium difficile; OR, odds ratio.
For duration end points, estimates are mean number of days per 30 days at risk during the infection observation period.
Effect of levofloxacin evaluated by logistic regression for dichotomous end points (and shown as the adjusted OR) and by Poisson regression for duration end points (and shown as the adjusted RR); analyses were adjusted for acute leukemia vs hematopoietic stem cell transplant.
Defined as any grade 4 or 5 Common Terminology Criteria for Adverse Events (CTCAE) v 4.0 infections and includes clinically documented nonbacterial and bacterial infections.
Defined as a positive C difficile test and documentation of grade 2 or higher diarrhea by CTCAE criteria.
Musculoskeletal conditions included at least 1 occurrence of arthralgia, arthritis, gait abnormality or tendinopathy; a patient may have more than 1 condition; data are for patients that were assessed. Not all patients were assessed at each time point. The total number of patients in the levofloxacin group at baseline was 303; at 2 months, 201; and at 12 months, 129. The total number of patients in the no prophylaxis group at baseline was 300; at 2 months, 240; and at 12 months, 146.
Susceptibility testing results for levofloxacin were available for a minority of bacteremia isolates. In the levofloxacin prophylaxis group, 5 of 25 viridans group streptococcal isolates were tested for levofloxacin sensitivity and all 5 showed resistance, whereas in the control group, 8 of 39 viridans group streptococcal isolates were tested and all 8 were sensitive. In the levofloxacin prophylaxis group, 3 of 9 E coli, Pseudomonas species or Klebsiella species isolates were tested for levofloxacin sensitivity and all 3 showed resistance. In the control group, 9 of 21 of these 3 organisms were tested for levofloxacin sensitivity; 7 were sensitive and 2 were resistant.
Table 4 shows the acquisition of resistance from baseline to follow-up for selected perirectal or stool swab organisms. The proportion with newly detected resistance to any of the selected pathogens was low and not significantly different between the levofloxacin prophylaxis and control groups for either patients with acute leukemia (5 of 43 vs 7 of 45; risk difference, 3.9; 95% CI, −10.4 to 18.2; P = .59) or those undergoing HSCT (4 of 118 vs 4 of 120, risk difference, 0.6; 95% CI, −4.5 to 4.6; P = .98).
Table 4. Development of New Resistance to Specific Agents in Bacteria Colonizing the Stool.
| Any Resistance Developing in Colonizing Organismsa | No. (%) of Patients | |||
|---|---|---|---|---|
| Acute Leukemia | Hematopoietic Stem Cell Transplant | |||
| Levofloxacin (n = 43) | No Prophylaxis (n = 45) | Levofloxacin (n = 118) | No Prophylaxis (n = 120) | |
| Levofloxacin | 4 (9.3) | 4 (8.9) | 2 (1.7) | 1 (0.8) |
| Cefepime | 1 (2.3) | 4 (8.9) | 3 (2.5) | 3 (2.5) |
| Imipenem | 0 (0) | 3 (6.7) | 1 (0.9) | 0 (0) |
| Penicillin | 3 (7.0) | 1 (2.2) | 3 (2.5) | 3 (2.5) |
Newly detected resistance to levofloxacin, cefepime, imipenem, or penicillin among Streptococcus mitis, Escherichia coli, Klebsiella pneumoniae, or Pseudomonas aeruginosa isolates in follow-up stool specimens compared with baseline. The total number represent those with evaluable stool samples at baseline and at follow-up. All 4 organisms were assessed for levofloxacin and cefepime resistance. Penicillin resistance was only assessed for S mitis isolates, and imipenem resistance was only assessed for E coli, K pneumoniae, and P aeruginosa isolates.
Post hoc Analyses and Outcomes
To account for time at risk, a post hoc analysis of the primary outcome using Poisson regression was completed. Patients with acute leukemia without a second cycle of chemotherapy were censored at the completion of their first chemotherapy cycle observation period. Among the patients with acute leukemia, levofloxacin prophylaxis significantly reduced the likelihood of bacteremic episodes (4.9 vs 9.4 bacteremias per 1000 patient-days; rate difference, 4.3; 95% CI, 1.3-7.4; P = .008). Among the patients undergoing HSCT, levofloxacin prophylaxis also significantly reduced the likelihood of bacteremic episodes (5.3 vs 10.0 bacteremias per 1000 patient days; rate difference, 5.2; 95% CI, 1.1-9.3; P = .02) (Table 2).
The comparison of bacteremia incidence with mixed-effects modeling with site as a random effect had results similar to the primary analysis. In this analysis, the estimated bacteremia probability for patients with acute leukemia receiving levofloxacin vs no prophylaxis was 21.0% vs 43.8%, respectively (risk difference, 22.8%; 95% CI, 12.1%-38.8%, P = .002). For patients undergoing HSCT the estimated bacteremia probability for patients randomized to levofloxacin vs no prophylaxis was 9.0% vs 14.5%, respectively (risk difference, 5.5%; 95% CI, 0.3%-55.5%, P = .07).
In terms of post hoc outcomes, the mean duration of fever was significantly lower with levofloxacin prophylaxis (4.0 days; 95% CI, 3.6-4.6 vs 5.1 days; 95% CI, 4.6-5.6; rate difference, 1.0; 95% CI, 0.6-1.3; P = .004) (Table 5), whereas the mean duration of hospitalization was not significantly different between groups (26.1 days; 95% CI, 24.9-27.3 vs 25.7 days, 95% CI 24.4-27.1; rate difference, 0.3; 95% CI, −0.4 to 1.1; P = .97). The mean duration of antifungal therapy was similar between the levofloxacin prophylaxis and no prophylaxis groups (18.9 days; 95% CI, 17.6-20.4 vs 18.6 days; 95% CI, 17.3-20.1; rate difference, 0.3; 95% CI, −0.4 to 1.0; P = >.99). Patients in the levofloxacin group were less likely to have a positive test result for C difficile (7.8% vs 14.0%; risk difference, 6.2; 95% CI, 1.3-11.1; P = .02) than those in the control group.
Table 5. Post hoc Outcomes for All Patients by Allocation Among All Patients.
| Outcomes | Mean (SE) Days per 30 Patient-Daysa | Risk or Rate Difference (95% CI) | Adjusted RR (95% CI)b | Adjusted OR (95% CI)b | P Value | |
|---|---|---|---|---|---|---|
| Levofloxacin | No Prophylaxis | |||||
| Total No. of observation days | 9369 | 9739 | ||||
| Fever | 4.0 (0.3) | 5.1 (0.3) | 1.0 (0.6 to 1.3) | 0.79 (0.67 to 0.93) | .004 | |
| Hospitalization | 26.1 (0.6) | 25.7 (0.7) | 0.3 (−0.4 to 1.1) | 1.00 (0.94 to 1.06) | .97 | |
| Antifungal therapy | 18.9 (0.7) | 18.6 (0.7) | 0.3 (−0.4 to 1.0) | 1.00 (0.91 to 1.10) | >.99 | |
| C difficile positive test, No. (%) | 24 (7.8) | 43 (14.0) | 6.2 (1.3 to 11.1) | 0.52 (0.31 to 0.89) | .02 | |
| Antibiotic Days and Any Exposure by Antibiotic Class or Spectrum of Activityc | ||||||
| Gram-positive agentsd | 5.3 (0.4) | 6.1 (0.5) | 0.8 (0.4 to 1.1) | 0.87 (0.70 to 1.06) | .17 | |
| Any exposure, No. (%) | 180 (58.8) | 202 (65.8) | 7.0 (−0.7 to 14.6) | 0.74 (0.53 to 1.03) | .08 | |
| Aminoglycosidese | 1.2 (0.2) | 2.3 (0.3) | 1.1 (0.9 to 1.3) | 0.49 (0.33 to 0.73) | .001 | |
| Any exposure, No. (%) | 70 (22.9) | 109 (35.5) | 12.6 (5.5 to 19.8) | 0.54 (0.38 to 0.77) | .001 | |
| Third- or fourth-generation cephalosporinsf | 5.3 (0.5) | 7.1 (0.5) | 1.9 (1.5 to 2.3) | 0.74 (0.60 to 0.92) | .006 | |
| Any exposure, No. (%) | 141 (46.1) | 184 (59.9) | 13.9 (6.0 to 21.7) | 0.56 (0.40 to 0.78) | .001 | |
| Antibiotics commonly used for empirical therapy for fever and neutropeniag | 9.6 (0.5) | 13.1 (0.6) | 3.5 (3.0 to 4.0) | 0.72 (0.63 to 0.83) | <.001 | |
| Any exposure, No. (%) | 210 (68.6) | 263 (85.7) | 17.0 (10.5 to 23.6) | 0.36 (0.24 to 0.55) | <.001 | |
Abbreviations: OR, odds ratio; RR, rate ratio.
For duration end points, the estimates are mean number of days per 30 days at risk during the infection observation period.
Effect of levofloxacin evaluated by logistic regression for dichotomous end points (and shown as the adjusted OR) and by Poisson regression for duration end points (and shown as the adjusted RR); analyses were adjusted for acute leukemia vs hematopoietic stem cell transplantation.
Exposure to antibiotic during the infection observation period.
Gram-positive agents defined as vancomycin, linezolid, daptomycin or quinupristin/dalfopristin.
Aminoglycosides defined as amikacin, gentamicin or tobramycin.
Third- or fourth-generation cephalosporins defined as cefepime, ceftazidime, ceftriaxone, or cefotaxime.
Antibiotics commonly used for empirical therapy for fever and neutropenia defined as imipenem, meropenem, cefepime, ceftazidime, or piperacillin-tazobactam.
Table 5 also shows both total duration of exposure and any exposure to aminoglycosides, third- and fourth-generation cephalosporins and antibiotics commonly used for empirical therapy for fever and neutropenia were lower in the levofloxacin group compared with the no prophylaxis group.
Adverse Events
Grade 4 nonhematologic and all grade 5 adverse event rates were low (<5% in all categories) and were similar in the patients in the levofloxacin and no prophylaxis groups. There were 23 serious adverse events reported in 8 patients. Eight of these were considered unrelated to levofloxacin and 3 were considered unlikely to be related to levofloxacin. The remaining adverse events occurred in 2 patients. A patient had grade 4 acute kidney injury, capillary leak, pulmonary edema, and respiratory failure considered probably related to HSCT and possibly related to levofloxacin. A second patient had grade 4 agitation, anxiety, confusion, delirium, delusion, hallucinations, personality change, and psychosis. These changes were considered possibly related to levofloxacin and possibly related to an anxiety disorder complicated by insomnia in the context of HSCT. No deaths were considered related to levofloxacin.
Discussion
In this multicenter randomized trial, levofloxacin prophylaxis significantly reduced the likelihood of bacteremia in children being treated for AML or relapsed ALL whereas levofloxacin prophylaxis did not significantly reduce the likelihood of bacteremia in children undergoing HSCT. The observed risk reduction was similar to findings from adult studies.20
Bacteremia was chosen as the primary outcome because it is associated with sepsis, infection-related mortality and increased health care utilization. Consequently, this outcome is meaningful to both clinicians and patients. While bacteremia may also delay subsequent cancer-direct therapy, this end point was not collected because the decision to initiate the next cycle of chemotherapy is influenced by many factors. Levofloxacin prophylaxis was effective at reducing the risk of bacteremia among patients with acute leukemia but not among patients undergoing HSCT. It is possible that the effect was similar in the 2 groups but that there was reduced power to detect a significant difference related to fewer events among patients undergoing HSCT. Fewer events may be explained, in part, by a shorter duration of neutropenia in the HSCT group. This hypothesis is supported by the lack of statistical interaction by treatment and group and the similar effect size in the post hoc Poisson regression, which takes into account time at risk. However, it is also plausible that the leukemia and HSCT groups had different supportive care measures or were infected with pathogens that had differential sensitivity to levofloxacin resulting in different efficacy of levofloxacin in the 2 groups.
The finding that positive C difficile test results were less common among levofloxacin prophylaxis recipients builds on a similar observation noted in a single-center study involving pediatric patients with ALL.9 Exposure to antibacterial agents has been previously identified as a risk factor for C difficile–associated diarrhea.22,23 The decrease in positive C difficile tests may be due to less therapeutic antimicrobial exposure in the prophylaxis group.
Acquisition of antibiotic resistance is an important potential negative consequence of antibiotic prophylaxis.24 In this short-term follow-up period, increased acquisition of resistance in S mitis, E coli, K pneumonia, and P aeruginosa isolates was not found. It is possible that an effect of prophylaxis on resistance was counterbalanced by a decrease in therapeutic antibiotic exposures.
Pathogens detected during bacteremia events for patients randomized to prophylaxis were frequently resistant to levofloxacin. Adult studies have described an increase in fluoroquinolone resistance among pathogens identified during breakthrough bacteremia.25 These findings suggest that patients receiving levofloxacin prophylaxis who develop fever and neutropenia should not receive an empirical antibiotic regimen that relies on fluoroquinolones.
Quinolone exposure is associated with risks of musculoskeletal adverse effects including tendinitis and tendinopathy.13 Unique to children is the concern for quinolone-related arthropathy. Arthropathy was described in juvenile beagles exposed to fluoroquinolones.26 A study of the musculoskeletal toxicity assessed by parent report in children randomized to receive levofloxacin or an alternate antibiotic for otitis media or community-acquired pneumonia found that musculoskeletal disorders (primarily arthralgia) were significantly more common in patients treated with levofloxacin at 1 year after exposure, although in longer-term follow-up, the symptoms had resolved.27,28 In this study, systematic assessment for musculoskeletal conditions did not identify a difference in the reported rates of musculoskeletal conditions between randomized groups, although the study was not powered to detect a significant difference.
Levofloxacin prophylaxis decreased the likelihood of bacteremia among patients with acute leukemia. However, there were still many viridans group streptococcal and gram-negative bacteremias in the prophylaxis group. This finding suggests that levofloxacin prophylaxis will not eliminate the risk of these important infections. Failure to eliminate infections may be related to spectrum of activity, lack of absorption in those receiving oral levofloxacin, or poor adherence. Other interventions will likely need to be combined with levofloxacin prophylaxis to further decrease bacteremia risk.
This study evaluated prophylaxis in pediatric patients receiving therapy for AML or relapsed ALL or undergoing HSCT. Further study is required to assess the utility of levofloxacin prophylaxis on the largest group of pediatric patients with leukemia, those undergoing primary therapy for ALL.
Limitations
This study has several limitations. First, the study was open-label. Knowledge of allocation could have affected patient care decisions. Second, this study evaluated short-term prophylaxis and the effect of longer-term prophylaxis requires further study. Third, detection of resistant bacteria colonizing the stool included a limited number of pathogens, and the evaluation of levofloxacin sensitivity results for pathogens isolated from blood cultures was completed on a minority of isolates. Fourth, invasive fungal disease was defined as microbiologically or pathologically proven infection. Episodes of possible fungal disease based on clinical or radiographic findings were not included; thus, the frequency of invasive fungal disease may have been underestimated. This potential for reduced capture of invasive fungal disease could have limited the detection of an association between levofloxacin and this outcome. Fifth, because the cohort of patients with acute leukemia was terminated early for efficacy, the effect of levofloxacin may have been overestimated. Sixth, fixed-block size and unblinded allocation can lead to biased participant selection. However, personnel at enrolling centers and study committee members were not aware of block size, thus minimizing this risk.
Conclusions
Among children with acute leukemia receiving intensive chemotherapy, receipt of levofloxacin prophylaxis compared with no prophylaxis resulted in a significant reduction in bacteremia. However, there was no significant reduction in bacteremia for levofloxacin prophylaxis among children undergoing HSCT.
Trial Protocol
eTable 1. Diagnosis of all eligible patients undergoing autologous or allogeneic stem cell transplant by allocation
eTable 2. Chemotherapy, conditioning and graft versus host disease prophylaxis by allocation of all eligible patients
eTable 3. Organisms causing bacteremia by allocation
eTable 4. Clinical characteristics of patients evaluable and not evaluable for acquisition of resistance for selected intestinal organisms
References
- 1.Ammann RA, Laws HJ, Schrey D, et al. Bloodstream infection in paediatric cancer centres—leukemia and relapsed malignancies are independent risk factors. Eur J Pediatr. 2015;174(5):675-686. doi: 10.1007/s00431-015-2525-5 [DOI] [PubMed] [Google Scholar]
- 2.Leather HL, Wingard JR. Bacterial Infections. Thomas’ Hematopoietic Cell Transplantation. 5th ed Chichester, UK: John Wiley & Sons Ltd; 2015. [Google Scholar]
- 3.Sung L, Buxton A, Gamis A, Woods WG, Alonzo TA. Life-threatening and fatal infections in children with acute myeloid leukemia: a report from the Children’s Oncology Group. J Pediatr Hematol Oncol. 2012;34(1):e30-e35. doi: 10.1097/MPH.0b013e31822817a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gafter-Gvili A, Fraser A, Paul M, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev. 2012;1:CD004386. [DOI] [PubMed] [Google Scholar]
- 5.Yousef AA, Fryer CJ, Chedid FD, Abbas AA, Felimban SK, Khattab TM. A pilot study of prophylactic ciprofloxacin during delayed intensification in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;43(6):637-643. doi: 10.1002/pbc.20065 [DOI] [PubMed] [Google Scholar]
- 6.Kurt B, Flynn P, Shenep JL, et al. Prophylactic antibiotics reduce morbidity due to septicemia during intensive treatment for pediatric acute myeloid leukemia. Cancer. 2008;113(2):376-382. doi: 10.1002/cncr.23563 [DOI] [PubMed] [Google Scholar]
- 7.Inaba H, Gaur AH, Cao X, et al. Feasibility, efficacy, and adverse effects of outpatient antibacterial prophylaxis in children with acute myeloid leukemia. Cancer. 2014;120(13):1985-1992. doi: 10.1002/cncr.28688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sulis ML, Blonquist TM, Stevenson KE, et al. Effectiveness of antibacterial prophylaxis during induction chemotherapy in children with acute lymphoblastic leukemia. Pediatric Blood Cancer. 2018;65(5):e26952. [DOI] [PubMed] [Google Scholar]
- 9.Wolf J, Tang L, Flynn PM, et al. Levofloxacin prophylaxis during induction therapy for pediatric acute lymphoblastic leukemia. Clin Infect Dis. 2017;65(11):1790-1798. doi: 10.1093/cid/cix644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeh TC, Liu HC, Hou JY, et al. Severe infections in children with acute leukemia undergoing intensive chemotherapy can successfully be prevented by ciprofloxacin, voriconazole, or micafungin prophylaxis. Cancer. 2014;120(8):1255-1262. doi: 10.1002/cncr.28524 [DOI] [PubMed] [Google Scholar]
- 11.Alexander S, Nieder M, Zerr DM, Fisher BT, Dvorak CC, Sung L. Prevention of bacterial infection in pediatric oncology: what do we know, what can we learn? Pediatr Blood Cancer. 2012;59(1):16-20. doi: 10.1002/pbc.23416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehrnbecher T, Sung L. Anti-infective prophylaxis in pediatric patients with acute myeloid leukemia. Expert Rev Hematol. 2014;7(6):819-830. doi: 10.1586/17474086.2014.965140 [DOI] [PubMed] [Google Scholar]
- 13.FDA Drug Safety Communication FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. May 12, 2016. Accessed August 23, 2018.
- 14.Lehrnbecher T, Phillips R, Alexander S, et al. ; International Pediatric Fever and Neutropenia Guideline Panel . Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. J Clin Oncol. 2012;30(35):4427-4438. doi: 10.1200/JCO.2012.42.7161 [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- 16.National Cancer Institute. CREP Cancer Therapy Evaluation Program web page. Common terminology criteria for adverse events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. Updated March 1, 2018. Accessed August 17, 2018.
- 17.National Healthcare Safety Network. Centers for Disease Control and Prevention web page. Central line–associated bloodstream infection (CLABSI) and non-central line–associated bloodstream infection. https://www.cdc.gov/nhsn/acute-care-hospital/clabsi/index.html. 2017. Accessed August 23, 2018.
- 18.Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev. 2002;15(4):613-630. doi: 10.1128/CMR.15.4.613-630.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Pauw B, Walsh TJ, Donnelly JP, et al. ; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group . Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813-1821. doi: 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucaneve G, Micozzi A, Menichetti F, et al. ; Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) Infection Program . Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353(10):977-987. doi: 10.1056/NEJMoa044097 [DOI] [PubMed] [Google Scholar]
- 21.Proschan MA, Lan KK, Wittes G, Turk J. Statistical Monitoring of Clinical Trials: A Unified Approach. New York, NY: Springer; 2006. [Google Scholar]
- 22.Vardakas KZ, Trigkidis KK, Boukouvala E, Falagas ME. Clostridium difficile infection following systemic antibiotic administration in randomised controlled trials: a systematic review and meta-analysis. Int J Antimicrob Agents. 2016;48(1):1-10. doi: 10.1016/j.ijantimicag.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 23.Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile infection in children. JAMA Pediatr. 2013;167(6):567-573. doi: 10.1001/jamapediatrics.2013.441 [DOI] [PubMed] [Google Scholar]
- 24.Bow EJ. Fluoroquinolones, antimicrobial resistance and neutropenic cancer patients. Curr Opin Infect Dis. 2011;24(6):545-553. doi: 10.1097/QCO.0b013e32834cf054 [DOI] [PubMed] [Google Scholar]
- 25.See I, Freifeld AG, Magill SS. Causative organisms and associated antimicrobial resistance in healthcare-associated, central line-associated bloodstream infections from oncology settings, 2009-2012. Clin Infect Dis. 2016;62(10):1203-1209. doi: 10.1093/cid/ciw113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaad UB. Will fluoroquinolones ever be recommended for common infections in children? Pediatr Infect Dis J. 2007;26(10):865-867. doi: 10.1097/INF.0b013e3180cc20e4 [DOI] [PubMed] [Google Scholar]
- 27.Noel GJ, Bradley JS, Kauffman RE, et al. Comparative safety profile of levofloxacin in 2523 children with a focus on four specific musculoskeletal disorders. Pediatr Infect Dis J. 2007;26(10):879-891. doi: 10.1097/INF.0b013e3180cbd382 [DOI] [PubMed] [Google Scholar]
- 28.Bradley JS, Kauffman RE, Balis DA, et al. Assessment of musculoskeletal toxicity 5 years after therapy with levofloxacin. Pediatrics. 2014;134(1):e146-e153. doi: 10.1542/peds.2013-3636 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Diagnosis of all eligible patients undergoing autologous or allogeneic stem cell transplant by allocation
eTable 2. Chemotherapy, conditioning and graft versus host disease prophylaxis by allocation of all eligible patients
eTable 3. Organisms causing bacteremia by allocation
eTable 4. Clinical characteristics of patients evaluable and not evaluable for acquisition of resistance for selected intestinal organisms