Abstract
This study examines the use of pharmacologic and mechanical prophylaxis in hospitalized patients at low and high risk for venous thromboembolism.
National guidelines1 recommend objective risk stratification for venous thromboembolism (VTE) in hospitalized medical patients. The Padua Prediction Score risk assessment model2 is recommended to categorize patients as high or low risk. The Michigan Hospital Medicine Safety Consortium (HMS), a statewide quality collaborative aimed at preventing adverse events in hospitalized medical patients, collects detailed data on VTE risk factors, prophylactic treatment, and outcomes. Using data from the HMS,3 we sought to determine whether patients in this cohort were receiving appropriate VTE prophylaxis.
Methods
Patients admitted to a non–intensive care medicine unit for 2 or more days were eligible for inclusion; data were collected through a standardized process at each hospital. Using the Padua Prediction Score risk assessment model,2 we categorized patients on admission as low or high risk for VTE events. For high-risk patients, contraindications to pharmacologic prophylaxis were assessed. Excessive VTE prophylaxis was defined as pharmacologic or mechanical prophylaxis for low-risk patients, pharmacologic prophylaxis for high-risk patients with a contraindication to anticoagulation, and the combination of pharmacologic and mechanical prophylaxis in all cases. Underuse of VTE prophylaxis was defined as no pharmacologic prophylaxis in high-risk patients without a contraindication to anticoagulation and no prophylaxis (pharmacologic or mechanical) in high-risk patients with a contraindication to anticoagulation. Appropriate pharmacologic prophylaxis included any of the following on day 1 and/or day 2 of the index hospitalization: heparin, 5000 U twice daily; heparin, 5000 U 3 times daily; heparin, 7500 U 3 times daily (for morbid obesity); enoxaparin, 40 mg/d; enoxaparin, 30 mg/d (for creatinine clearance <30 mL/min); enoxaparin, 30 mg twice daily; dalteparin, 5000 U daily; or fondaparinux 2.5 mg/d. The HMS-defined contraindications include any of the following: bleeding present upon hospital admission; intracranial hemorrhage within the past year; other hemorrhage within the last 6 months; coagulopathy, hemophilia, or other significant bleeding disorder; and platelet levels lower than 50 × 103/μL (to convert to × 109 per liter, multiply by 1.0). Hospitals were rank ordered according to rates of excess VTE prophylaxis across low- and high-risk patients and rates of underuse of VTE prophylaxis in high-risk patients. Rank of hospital excess use and underuse of VTE prophylaxis were compared using a Pearson correlation coefficient.
Excessive VTE prophylaxis was assessed using descriptive variables. Odds ratios, 95% CIs, and P values for excess prophylaxis were calculated using logistic generalized estimating equation models, accounting for hospital clustering. Findings were considered significant at P < .05. Because the purpose of the HMS is to measure and improve the quality of existing medical practice, this project received a “not regulated” status by the University of Michigan Medical School institutional review board.
Results
Between January 1, 2015, and December 21, 2016, data were collected on 44 775 eligible patients across 52 Michigan hospitals. Mean (SD) patient age was 64.7 (18.4) years; 24 742 (55.3%) were women. The mean (SD) length of hospital stay was 4.4 (4.6) days (median, 3.0 days). Of the eligible patients, 32 549 were assessed as low-risk for VTE, whereas 1804 were at high risk with a contraindication for pharmacologic prophylaxis and 10 422 were at high risk without a contraindication for pharmacologic prophylaxis.
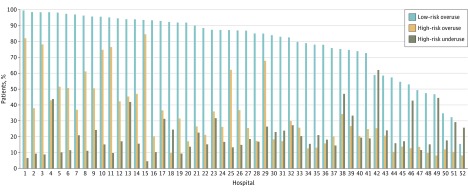
Excess prophylaxis was used in 25 367 low-risk patients (77.9%), 3366 high-risk patients (32.3%) without a contraindication to prophylaxis, and 485 high-risk patients (26.9%) with a contraindication to pharmacologic prophylaxis (Table). The odds of receiving excess prophylaxis for low- vs high-risk patients was 3.26 (95% CI, 2.36-4.51; P < .001). The rate of excess VTE prophylaxis varied between hospitals, with rates ranging from 8.2% to 84.6% in high-risk patients (mean [SD], 32.8% [21.4%]) and from 15.5% to 99.6% in low-risk patients (mean [SD], 79.7% [19.8%]) (Figure).
Table. Excess Prophylaxis Use by Risk Class in 44 775 Patients.
| Group | Excess Prophylaxis, No. (%) | OR (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Overall | Mechanical | Pharmacologic | Pharmacologic and Mechanical | |||
| Padua Prediction Score category2,a | ||||||
| Low risk (n = 32 549) | 25 367 (77.9) | 6783 (20.8) | 9950 (30.6) | 8634 (26.5) | 3.26 (2.36-4.51) | .001 |
| High risk (n = 12 226) | 3851 (31.5) | NA | 221 (1.8) | 3630 (29.7) | 1 [Reference] | |
| With contraindications (n = 1804)b | 485 (26.9) | NA | 221 (12.3) | 264 (14.6) | NA | NA |
| Without contraindications (n = 10 422)b | 3366 (32.3) | NA | NA | 3366 (32.3) | NA | NA |
Abbreviations: NA, not applicable; OR, odds ratio.
Padua risk score elements: active cancer, prior venous thromboembolism, reduced mobility, known thrombophilia, recent trauma and/or surgery, age 70 y or older, heart and/or respiratory failure, acute myocardial infarction or ischemic stroke, acute infection and/or rheumatologic disorder, body mass index 30 or higher (calculated as weight in kilograms divided by height in meters squared), and hormonal treatment. High risk, 4 points or more; low risk, less than 4 points.
Contraindications to pharmacologic prophylaxis. The Hospital Medicine Safety Consortium–defined contraindications include any of the following: bleeding present upon hospital admission; intracranial hemorrhage within the past year; other hemorrhage within the last 6 mo; coagulopathy, hemophilia, or other significant bleeding disorder; platelet level lower than 50 × 103/μL (to convert to × 109 per liter, multiply by 1.0).
Figure. Excess Use of Venous Thromboembolism Prophylaxis in Low- and High-Risk Patients and Underuse in High-Risk Patients.
Padua Prediction Score model2 used to categorize patients by risk. Mean excess use rate in low-risk patients, 79.7%; mean excess use rate in high risk patients, 32.8%; and mean underuse rate, 21.3%.
In the 12 226 high-risk patients, VTE prophylaxis was underused in 2693 individuals (22.0%) and was more prevalent in those without a contraindication to pharmacologic prophylaxis (2467 [23.7%] vs 226 [12.5%]; P < .001). As with low-risk patients, rates of VTE prophylaxis underuse in high-risk patients varied by hospital (range, 4.6%-62.1%; mean [SD], 21.3% [11.7%]) (Figure). Hospitals with higher rates of excessive VTE prophylaxis had lower rates of prophylaxis underuse in high-risk patients (Pearson correlation coefficient, −0.48; 95% CI, −0.66 to −0.23; P < .001).
Discussion
In this study, anticoagulant use among low-risk patients (18 584 [57.1%]) was the most important contributor to excess use of VTE prophylaxis. Excess use of mechanical VTE prophylaxis (almost exclusively sequential compression devices) was common among both low-risk patients (15 417 [47.4%]) and high-risk patients without a contraindication to anticoagulation (3366 [32.3%]). When combining low- and high-risk patients, the total rate of excessive VTE prophylaxis was 65.3%.
The 22.0% rate of underuse of VTE prophylaxis was significantly lower than the 65.3% rate of excessive prophylaxis and is lower than the rates of underuse previously described in medical patients.4 Efforts aimed at improving VTE prophylaxis at local, regional, and national levels have been successful. However, most interventions have focused on increasing overall rates of prophylaxis rather than overall appropriateness. Although overall rates have improved, the unintended consequence may be excess administration of VTE prophylaxis among low-risk patients.
The major drawback to pharmacologic overprophylaxis is major bleeding.5 Patient discomfort, potential risk of falls and impaired mobility with mechanical prophylaxis, medication cost, and risk for heparin-induced thrombocytopenia are additional concerns.
Limitations of this study include its observational design subject to inherent biases. Furthermore, this analysis did not incorporate VTE events, so it is unknown whether 1 specific VTE prophylaxis strategy was superior to another.
After years of promoting aggressive VTE prophylaxis strategies for hospitalized patients, renewed effort to scale back—or “deimplement”—this practice in low-risk patients may be necessary.6 Discontinuing conventional practices, however, can be difficult, even in the presence of newer compelling data.
References
- 1.Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in non-surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice. Chest. 2012;141(2 suppl):e195S-e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbar S, Noventa F, Rossetto V, et al. . A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450-2457. [DOI] [PubMed] [Google Scholar]
- 3.Flanders SA, Greene MT, Grant P, et al. . Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AT, Tapson VF, Bergmann JF, et al. ; ENDORSE Investigators . Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371(9610):387-394. [DOI] [PubMed] [Google Scholar]
- 5.Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. [DOI] [PubMed] [Google Scholar]
- 6.Rothberg MB. Venous thromboembolism prophylaxis for medical patients: who needs it? JAMA Intern Med. 2014;174(10):1585-1586. [DOI] [PubMed] [Google Scholar]