Key Points
Question
What clinical, imaging, and thrombus characteristics are associated with recanalization of visible intracranial thrombus in patients with acute ischemic stroke receiving vs not receiving intravenous alteplase?
Findings
In this prospective cohort study of 575 patients with acute ischemic stroke and visible intracranial thrombus on computed tomographic angiography, thrombus recanalization occurred in 30.4% of patients within 6 hours of intravenous alteplase administration, and in 13.3% of patients who did not receive alteplase. More distal thrombus location, greater thrombus permeability, and longer time to recanalization assessment were associated with successful recanalization with intravenous alteplase.
Meaning
Among patients with acute ischemic stroke, clinical and imaging biomarkers were associated with intracranial thrombus recanalization.
Abstract
Importance
Recanalization of intracranial thrombus is associated with improved clinical outcome in patients with acute ischemic stroke. The association of intravenous alteplase treatment and thrombus characteristics with recanalization over time is important for stroke triage and future trial design.
Objective
To examine recanalization over time across a range of intracranial thrombus occlusion sites and clinical and imaging characteristics in patients with ischemic stroke treated with intravenous alteplase or not treated with alteplase.
Design, Setting, and Participants
Multicenter prospective cohort study of 575 patients from 12 centers (in Canada, Spain, South Korea, the Czech Republic, and Turkey) with acute ischemic stroke and intracranial arterial occlusion demonstrated on computed tomographic angiography (CTA).
Exposures
Demographics, clinical characteristics, time from alteplase to recanalization, and intracranial thrombus characteristics (location and permeability) defined on CTA.
Main Outcomes and Measures
Recanalization on repeat CTA or on first angiographic acquisition of affected intracranial circulation obtained within 6 hours of baseline CTA, defined using the revised arterial occlusion scale (rAOL) (scores from 0 [primary occlusive lesion remains the same] to 3 [complete revascularization of primary occlusion]).
Results
Among 575 patients (median age, 72 years [IQR, 63-80]; 51.5% men; median time from patient last known well to baseline CTA of 114 minutes [IQR, 74-180]), 275 patients (47.8%) received intravenous alteplase only, 195 (33.9%) received intravenous alteplase plus endovascular thrombectomy, 48 (8.3%) received endovascular thrombectomy alone, and 57 (9.9%) received conservative treatment. Median time from baseline CTA to recanalization assessment was 158 minutes (IQR, 79-268); median time from intravenous alteplase start to recanalization assessment was 132.5 minutes (IQR, 62-238). Successful recanalization occurred at an unadjusted rate of 27.3% (157/575) overall, including in 30.4% (143/470) of patients who received intravenous alteplase and 13.3% (14/105) who did not (difference, 17.1% [95% CI, 10.2%-25.8%]). Among patients receiving alteplase, the following factors were associated with recanalization: time from treatment start to recanalization assessment (OR, 1.28 for every 30-minute increase in time [95% CI, 1.18-1.38]), more distal thrombus location, eg, distal M1 middle cerebral artery (39/84 [46.4%]) vs internal carotid artery (10/92 [10.9%]) (OR, 5.61 [95% CI, 2.38-13.26]), and higher residual flow (thrombus permeability) grade, eg, hairline streak (30/45 [66.7%]) vs none (91/377 [24.1%]) (OR, 7.03 [95% CI, 3.32-14.87]).
Conclusions and Relevance
In patients with acute ischemic stroke, more distal thrombus location, greater thrombus permeability, and longer time to recanalization assessment were associated with recanalization of arterial occlusion after administration of intravenous alteplase; among patients who did not receive alteplase, rates of arterial recanalization were low. These findings may help inform treatment and triage decisions in patients with acute ischemic stroke.
This cohort study examines baseline clinical and biochemical variables, intracranial thrombus characteristics, and stroke workflow interval times associated with clot recanalization among patients with ischemic stroke treated with intravenous alteplase.
Introduction
Two treatments are now considered standard of care in acute ischemic stroke management: intravenous alteplase administered within 4.5 hours after symptom onset and endovascular therapy initiated within 6 hours of symptom onset.1,2,3 Both treatments aim to achieve early recanalization of an occluded intracranial artery. The magnitude of benefit of both treatments is directly related to the speed at which recanalization is achieved.4,5,6 However, the benefit of intravenous alteplase does not depend on demonstration of an arterial occlusion. Because patients with visible intracranial occlusion on imaging may be candidates for both intravenous alteplase and endovascular therapy, understanding when intravenous alteplase achieves recanalization in patients with visible intracranial occlusions could be useful in determining whether intravenous alteplase alone is sufficient treatment or if the patient should be transported to a comprehensive stroke center for endovascular therapy.
To date, studies examining recanalization with intravenous alteplase have been constrained by technology limitations (such as temporal bone attenuation precluding transcranial Doppler assessment of elderly patients), small sample size, retrospective design, and late assessments of recanalization (24-hour magnetic resonance angiography [MRA] or computed tomography angiography [CTA]).7,8,9,10,11,12 Results from randomized clinical trials are not fully generalizable, given the strict trial inclusion criteria.12,13,14 A recent systematic review recommended that detailed studies are needed to help physicians better predict recanalization with intravenous alteplase.15
The Identifying New Approaches to Optimize Thrombus Characterization for Predicting Early Recanalization and Reperfusion With IV Alteplase and Other Treatments Using Serial CT Angiography study (INTERRSeCT) was a multicenter prospective cohort study that enrolled patients with acute ischemic stroke with intracranial thrombi documented via CTA. The study included patients with acute ischemic stroke and a wide range of clinical presentations, occlusion sites, and thrombus characteristics to identify clinical and imaging variables associated with recanalization with or without intravenous alteplase treatment.
Methods
Study Design and Inclusion Criteria
Local medical ethics committees approved the study. Once a physician identified an eligible patient, written informed consent was provided by the patient or a surrogate. This multicenter prospective cohort study enrolled patients with acute ischemic stroke with visible intracranial occlusion on baseline CTA. The study was conducted at 12 centers (in Canada, South Korea, Spain, the Czech Republic, and Turkey). First enrollment was in March 2010 and final follow-up occurred in March 2016.
Patient eligibility included the following: presentation to the emergency department with symptoms consistent with ischemic stroke 12 hours from last known well, age at least 40 years, and a baseline CTA (before alteplase bolus, if given) with evidence of a symptomatic intracranial thrombus (ie, internal carotid artery [ICA], middle cerebral artery [MCA] proximal segment [proximal M1], MCA distal segment [distal M1], MCA M2 segment [M2], MCA M3 segment [M3], anterior cerebral artery, or posterior cerebral artery). Patients with primary vertebrobasilar artery occlusions were excluded from the study. Patients were also excluded if they had renal impairment (estimated creatinine clearance <60 mL/min); contrast allergy; hypoglycemia (serum glucose <36 mg/dL); or were unlikely to participate in follow-up.
Information collected at baseline included age; sex; baseline National Institutes of Health Stroke Scale score (NIHSS; score ranges from 0-42 with higher scores indicating greater stroke severity); history of hypertension, dyslipidemia, diabetes, smoking, atrial fibrillation, previous stroke/transient ischemic attack (TIA), or coronary artery disease; anticoagulation use; time from last seen well; baseline vital signs; serum glucose level; complete blood cell count; serum creatinine level; international normalized ratio; partial thromboplastin time; and total cholesterol levels. Interval times from symptom onset to presentation in the emergency department, imaging, alteplase bolus, and start of endovascular procedure were also collected.
All patients underwent a head and neck CTA at baseline. Sites were requested to repeat head CTA at 4 (±2) hours after initial CTA unless conventional cerebral angiography was performed within that time frame for diagnostic or neurointerventional purposes. An imaging expert blinded to all clinical information read all images using OsiriX version 3.5. Location of intracranial thrombus was identified on baseline CTA (ICA, proximal M1, distal M1, M2, M3, anterior cerebral artery, or posterior cerebral artery; eFigure 1 in the Supplement).16
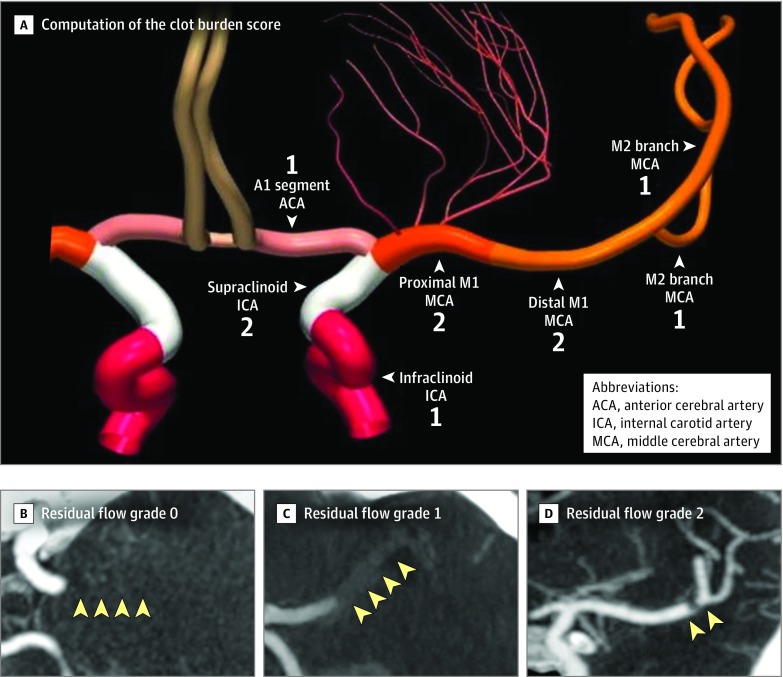
Extent of intracranial thrombus was assessed using the clot burden score (Figure 1A). A score of 0 implies complete occlusion of the ipsilateral anterior circulation vessels; a score of 10 implies no occlusion.17 Permeability of intracranial thrombus to contrast (and therefore blood flow) was assessed using the residual flow grade (Figure 1B).18 Residual flow grade is assessed on the CTA source images (3-mm maximum intensity projections preferred) as follows: grade 0, no contrast permeation of thrombus; grade 1, contrast permeating diffusely through thrombus; and grade 2, tiny hairline lumen or streak of well-defined contrast within the thrombus extending either through its entire length or part of the thrombus.
Figure 1. The Clot Burden Score and Residual Blood Flow in the Assessment of Recanalization in Acute Ischemic Stroke.
A, Computation of the clot burden score, which measures the extent of intracranial thrombus burden in patients with anterior circulation acute ischemic stroke. A score of 0 implies complete occlusion of the ipsilateral anterior circulation vessels; a score of 10 implies no occlusion. The numbers in the figure indicate points assigned to each segment when computing the score. The colors are only to differentiate arterial segments. Two points are subtracted for thrombus found on computed tomographic angiography (CTA) in the supraclinoid internal carotid artery segment and each of the proximal and distal halves of the M1 segment of MCA. One point is subtracted for thrombus seen in the infra-clinoid ICA and A1 segment of ACA and for each affected M2 branch of the middle cerebral artery (MCA).
B-D, Residual flow grade on CTA, a measure of permeability of intracranial thrombus: grade 0 on axial CTA (yellow arrowheads, proximal M1 segment MCA density similar to surrounding brain parenchyma), residual flow grade 1 on axial CTA (yellow arrowheads, distal M1 segment MCA denser than surrounding brain parenchyma), and residual flow grade 2 on coronal CTA (yellow arrowheads, tiny hairline lumen or streak of well-defined contrast within the distal M1 segment MCA thrombus).
Recanalization of intracranial thrombus was assessed using the revised arterial occlusion scale (rAOL) scale on repeat CTA of the head or on first angiographic acquisition of the affected intracranial circulation for endovascular therapy (eTable 1 in the Supplement; modified from the arterial occlusive lesion score).13,14,19 The primary outcome was successful recanalization as defined as rAOL score of 2b or 3 on repeat CTA or conventional cerebral angiogram obtained within 6 hours of initial CTA. The rAOL is scored as follows: 0, primary occlusive thrombus remains same; 1, debulking of proximal part of the thrombus but without any recanalization; 2a, partial or complete recanalization of the primary thrombus with occlusion in major distal vascular branch; 2b, partial or complete recanalization of the primary thrombus with occlusion in minor distal vascular branch, or partial recanalization of the primary thrombus with no thrombus in the vascular tree at or beyond the primary occlusive thrombus; and 3, complete recanalization of the primary occlusive thrombus with no clot in the vascular tree beyond.
Statistical Analyses
Data were assessed for normality using the Shapiro-Wilk test. The association between baseline clinical variables, biochemical parameters, intracranial thrombus imaging characteristics, and acute stroke workflow interval times and the primary outcome (successful recanalization) was assessed using the Wilcoxon rank sum test for nonparametric data and Fisher exact test of proportions for categorical data separately for patients who received intravenous alteplase and patients who did not receive alteplase. The distribution of rAOL score and of time from baseline CTA and intravenous alteplase start to recanalization assessment were plotted. Only variables both statistically associated in bivariable tests with the primary outcome (at P < .05) and deemed clinically relevant were included in multivariable models that assessed variables associated with recanalization both with and without intravenous alteplase (primary analyses). Separate logistic regression models were built for alteplase-treated and untreated patients. Because site of intracranial occlusion and clot burden score both measure extent of thrombus, these 2 variables were not included in the same model. No interaction terms were included in modeling. At each step of multivariable modeling, backward elimination of variables not statistically significant was used to reach to a final parsimonious model.
Adjusted recanalization prevalence over time from the start of intravenous alteplase, stratified by presence or absence of residual flow, was plotted using data from the final logistic regression model derived above that included site of intracranial occlusion as an independent variable instead of the clot burden score. To plot adjusted recanalization prevalence over time from the start of intravenous alteplase to recanalization assessment for each individual intracranial occlusion site, separate logistic regression models for each location of intracranial occlusion site were constructed (secondary analyses). The marginsplot module from Stata was used to generate these plots. Results were reported as odds ratios (ORs) with 95% CIs.
Because missing data for variables included in multivariable modeling were minimal, no imputation technique was used. In post hoc analyses, patient’s geographic region, namely North America (reference) vs Europe and East Asia, respectively, was included as an additional independent variable to analyze any region-specific effects on results. Two-sided P < .05 was considered statistically significant. Secondary analyses were considered exploratory since thresholds for statistical significance were not adjusted for multiple comparisons. All analyses were performed using Stata/MP 14.2 software (StataCorp LP).
Results
After we excluded 17 patients with vertebrobasilar occlusions, 39 patients with baseline CTA to follow-up CTA time longer than 360 minutes, and 5 patients who received intravenous alteplase at a different hospital before being transferred to the enrolling site (all protocol violations), 575 patients were included. These patients were a median age of 72 years (IQR, 63-80 years); 51.5% men; had a median baseline NIHSS score of 14 (IQR, 8-19); and their median time last known well to baseline CTA was 114 minutes (IQR, 74-180 minutes). Less than 5% of data were missing for all variables (Table 1 reports total number of observations for each individual variable except when there were no missing data for that particular variable).
Table 1. Clinical, Imaging, and Stroke Workflow Process Characteristics in Patients Who Received Intravenous Alteplase and Those Who Did Not, Stratified by Recanalization Statusa.
| Variable | All Patients (N = 575) | Received Intravenous Alteplase | Did Not Receive Intravenous Alteplase | ||||
|---|---|---|---|---|---|---|---|
| Successful Recanalization(n = 143) | No Successful Recanalization (n = 327) | P Value | Successful Recanalization (n = 14) | No Successful Recanalization (n = 91) | P Value | ||
| Clinical | |||||||
| Age, median (IQR), y | 72 (63-80) | 73 (64-81) | 72 (61-80) | .28 | 70 (68-76) | 72 (64-81) | .83 |
| Male sex, No. (%) | 296 (51.5) | 76 (53.2) | 161 (49.2) | .48 | 12 (85.7) | 47 (51.6) | .02 |
| Baseline NIHSS score, median (IQR) (n = 574)b | 14 (8-19) | 12 (7-18) | 15 (10-20) | <.001 | 5.5 (2-10) | 13 (6-18) | .005 |
| History, No. (%) | |||||||
| Coronary artery disease | 118 (20.5) | 26 (18.2) | 60 (18.3) | >.99 | 6 (42.9) | 26 (28.6) | .35 |
| Stroke or transient ischemic attack | 93 (16.2) | 24 (16.8) | 46 (14.1) | .48 | 2 (14.3) | 21 (23.1) | .73 |
| Hypertension | 356 (61.9) | 91 (63.6) | 200 (61.2) | .68 | 9 (64.3) | 56 (61.5) | >.99 |
| Dyslipidemia (n = 572) | 189 (33) | 51 (35.7) | 110 (33.9) | .75 | 4 (28.6) | 24 (26.4) | >.99 |
| Antiplatelet use | 201 (35) | 51 (35.7) | 116 (35.5) | >.99 | 6 (42.9) | 28 (30.8) | .37 |
| Atrial fibrillation | 177 (30.8) | 41 (28.7) | 88 (26.9) | .74 | 8 (57.1) | 40 (44) | .40 |
| Anticoagulation | 76 (13.2) | 15 (10.5) | 34 (10.4) | >.99 | 3 (21.4) | 24 (26.4) | >.99 |
| Diabetes | 86 (15) | 16 (11.2) | 59 (18) | .07 | 2 (14.3) | 9 (9.9) | .64 |
| Smoking (n = 562) | 233 (41.5) | 53 (38.4) | 133 (41.6) | .54 | 6 (42.9) | 41 (45.6) | >.99 |
| Heart rate, median (IQR), /min (n = 544) | 76 (66.5-88) | 77 (70-90) | 77.5 (67-86) | .17 | 74 (62-80) | 76 (65-90) | .32 |
| Systolic blood pressure, median (IQR), mm Hg (n = 572) | 147 (132-167) | 150 (133-170) | 148 (134-167) | .16 | 137.5 (136-152) | 140 (126-160) | .32 |
| Diastolic blood pressure, median (IQR), mm Hg (n = 572) | 81 (71-90) | 81 (71-92) | 81 (71-90) | .81 | 86 (71-93) | 80 (70-90) | .55 |
| Blood glucose, median (IQR), mg/dL (n = 574) | 115.3 (104.5-131.5) |
115.3 (104.5-131.5) |
115.3 (102.7-138.7) |
.39 | 109.9 (97.3-145.9) |
111.7 (100.9-127.9) |
.94 |
| Serum creatinine, median (IQR), mg/dL | 0.9 (0.7-1.1) | 0.9 (0.7-1.0) | 0.9 (0.7-1.1) | .84 | 1.1 (1.0-1.2) | 0.9 (0.8-1.1) | .002 |
| Hematocrit, median (IQR), % | 41 (38-44) | 41 (38-44) | 41 (37-44) | .51 | 43 (42-48) | 42 (36-44) | .02 |
| Platelet count, median (IQR), ×109/L (n = 574) | 208.5 (170-254) | 209 (169-255) | 208 (170-257) | .98 | 211 (168-243) | 207 (171-248) | .55 |
| International normalized ratio, median (IQR) (n = 573) | 1 (1-1.1) | 1 (1-1.1) | 1 (1-1.1) | .93 | 1.1 (1-1.3) | 1.1 (1-1.3) | .48 |
| Partial thromboplastin time, median (IQR), s (n = 569) | 27.9 (25-30) | 28 (26-30.5) | 27.3 (24.3-29.6) | .01 | 29.2 (28-34.7) | 27.9 (25.6-30.9) | .08 |
| Total cholesterol, median (IQR), mg/dL (n = 545) | 162.4 (135.3-193.4) |
170.1 (135.3-197.2) |
166.3 (135.3-197.2) |
.56 | 147.0 (139.2-166.3) |
154.7 (131.5-181.7) |
.51 |
| Etiology of ischemic stroke, No. (%) | |||||||
| Extracranial large artery disease | 86 (15.0) | 18 (12.6) | 56 (17.1) | .50 | 2 (14.3) | 10 (11.0) | .22 |
| Intracranial artery disease | 15 (2.6) | 3 (2.1) | 9 (2.7) | 1 (7.1) | 2 (2.2) | ||
| Cardioembolic | 309 (53.7) | 81 (56.6) | 157 (48.0) | 11 (78.6) | 60 (65.9) | ||
| Other causes | 12 (2.1) | 2 (1.4) | 8 (2.4) | 0 | 2 (2.2) | ||
| Undetermined | 153 (26.6) | 39 (27.3) | 97 (29.7) | 0 | 17 (18.7) | ||
| Imaging | |||||||
| Baseline ASPECTS on NCCT, median (IQR) (n = 540)c | 9 (7-10) | 9 (8-10) | 9 (7-9) | .10 | 9 (8-10) | 9 (7-10) | .48 |
| Site of intracranial occlusion at baseline, No. (%) | |||||||
| Internal carotid artery | 104 (18.1) | 10 (7.0) | 82 (25.1) | <.001 | 0 | 12 (13.2) | .05 |
| MCA: proximal M1 segment | 139 (24.2) | 24 (16.8) | 87 (26.6) | 1 (7.1) | 27 (29.7) | ||
| MCA: distal M1 segment | 103 (17.9) | 39 (27.3) | 45 (13.8) | 4 (28.6) | 15 (16.5) | ||
| MCA: M2 segment | 184 (32.0) | 53 (37.1) | 90 (27.5) | 7 (50.0) | 34 (37.4) | ||
| Distal (M3 MCA, ACA, PCA) | 45 (7.8) | 17 (11.9) | 23 (7.0) | 2 (14.3) | 3 (3.3) | ||
| Residual flow within intracranial thrombus, No. (%)d | |||||||
| Grade 0 | 462 (80.3) | 91 (63.6) | 286 (87.5) | <.001 | 9 (64.3) | 76 (83.5) | .13 |
| Grade 1 | 57 (9.9) | 22 (15.4) | 26 (7.9) | 2 (14.3) | 7 (7.7) | ||
| Grade 2 | 56 (9.7) | 30 (20.1) | 15 (4.6) | 3 (21.4) | 8 (8.8) | ||
| Clot burden score, No. (%)e | |||||||
| 0-4 | 149 (25.9) | 13 (9.1) | 114 (34.9) | <.001 | 1 (7.1) | 21 (23.1) | .45 |
| 5-7 | 172 (29.9) | 48 (33.6) | 90 (27.5) | 5 (35.7) | 29 (32.0) | ||
| 8-10 | 254 (44.2) | 82 (57.3) | 123 (37.6) | 8 (57.1) | 41 (45.1) | ||
| Stroke Workflow Process and Times | |||||||
| Time from onset (last known well) to baseline CT angiography, median (IQR), min | 114 (74-180) | 109 (73-170) | 107 (73-156) | .75 | 165.5 (97-312) | 180 (83-334) | .87 |
| Time from baseline CT angiography to intravenous alteplase start, median (IQR), min | 19 (11-28) | 18 (11-30) | 19 (12-28) | .64 | |||
| Endovascular therapy, No. (%) | 243 (42.3) | 19 (13.3) | 176 (53.8) | <.001 | 3 (21.4) | 45 (49.4) | .08 |
| Time from baseline CT angiography to recanalization assessment, median (IQR), min | 158 (79-268) | 245 (154-290) | 115 (70-238) | <.001 | 256 (228-302) | 177 (62-272) | .07 |
| Time from intravenous alteplase start to recanalization assessment, median (IQR), min | 132.5 (62-238) | 221 (141-273) | 92 (50-209) | <.001 | |||
Abbreviations: ACA, anterior cerebral artery; CT, computed tomography; IQR, interquartile range; MCA, middle cerebral artery; NCCT, noncontrast computed tomography; NIHSS, National Institutes of Health Stroke Scale; PCA, posterior cerebral artery.
SI conversion factors: To convert glucose to mmol/L, multiply values by 0.055; to convert creatinine to μmol/L, multiply values by 88.4; to convert cholesterol to mmol/L, multiply values by 0.0259.
Recanalization of intracranial thrombus with intravenous alteplase or not was assessed using the rAOL (revised arterial occlusive lesion) score on repeat head CTA or on first angiographic acquisition of the affected intracranial circulation. Successful recanalization was defined as an rAOL score of 2b or 3. The rAOL score is described in detail in the Methods and eTable 1 in the Supplement.
NIHSS score ranges from 0-42, with higher scores indicating greater stroke severity.
ASPECTS (Alberta Stroke Program Early Computed Tomography Score) measures extent of early ischemic change in the MCA territory on a 0-10 scale. Higher scores indicate smaller extent of early ischemic changes.
Residual flow grade is assessed on CTA source images (3-mm maximum intensity projections preferred) as follows: grade 0, no contrast permeation of thrombus; grade 1, contrast permeating diffusely through thrombus; and grade 2, tiny hairline lumen or streak of well-defined contrast within the thrombus extending either through its entire length or part of the thrombus.
Clot burden score is an ordinal score that measures extent of thrombus within the anterior circulation vessels. A score of 0 implies complete occlusion of the ipsilateral anterior circulation vessels; 10 implies no occlusion.
A total of 275 patients (47.8%) received intravenous alteplase only, 195 (33.9%) received intravenous alteplase plus endovascular thrombectomy, 48 (8.3%) received endovascular thrombectomy alone, and 57 (9.9%) received conservative treatment (eTable 2 and eFigure 2 in the Supplement). Recanalization was assessed in all 575 patients; 332 (57.7%) had recanalization assessed on repeat CTA while 243 (42.3%) had assessment on first angiographic acquisition of the affected intracranial circulation (eTable 1 in the Supplement). Median time from baseline CTA to start of intravenous alteplase was 19 minutes (IQR, 11-28 minutes), median time from baseline CTA to recanalization assessment was 158 minutes (IQR, 79-268 minutes), and median time from start of intravenous alteplase to recanalization assessment was 132.5 minutes (IQR, 62-238 minutes) (Table 1; eFigure 2 in the Supplement). Start of intravenous alteplase to recanalization assessment time stratified by site of intracranial occlusion, clot burden score, and residual flow grades are shown in eFigures 3-5 in the Supplement.
Successful recanalization (rAOL score 2b or 3) was observed in 157 patients (27.3%) (143/470 patients [30.4%] who received intravenous alteplase and 14/105 patients [13.3%] who did not receive intravenous alteplase; difference, 17.1% [95% CI, 10.2%-25.8%]) (Table 1). Cumulative recanalization in patients seen within 8 hours from last known well was 134 of 495 (27.1%) (126/426 [29.6%] in patients who received intravenous alteplase and 8/69 [11.6%] in patients who did not receive intravenous alteplase). Cumulative recanalization within 60 minutes of intravenous alteplase administration in patients with ICA or M1 segment MCA thrombus was 12 of 92 (13%). eFigures 6-8 in the Supplement show the rAOL score in patients who received intravenous alteplase vs those who did not, stratified by intracranial thrombus location, extent (clot burden score), and permeability of intracranial thrombus (residual flow grade).
In multivariable analysis of recanalization with intravenous alteplase (adjusted recanalization prevalence, 23.6% [95% CI, 4.2%-76.9%]), the following factors were associated with recanalization: longer time from alteplase start to recanalization (OR, 1.28 for every 30-minute increase in time [95% CI, 1.18-1.38]), more distal thrombus location, eg, distal M1 middle cerebral artery (39/84 [46.4%]) vs ICA (10/92 [10.9%]) (OR, 5.61 [95% CI, 2.38-13.26]) and higher residual flow grades, eg, hairline streak (30/45 [66.7%]) vs none (91/377 [24.1%]) (OR, 7.03 [95% CI, 3.32-14.87]) (all P < .01) (Table 2).
Table 2. Logistic Regression Models Showing Variables Independently Associated With Successful Recanalization With Intravenous Alteplasea.
| Variable | Patients Achieving Successful Recanalization With Intravenous Alteplase, No./Total (%) | Model 1b | Model 2b | ||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | ||
| Site of intracranial occlusion at baseline | |||||
| Internal carotid artery (reference) | 10/92 (10.9) | 1 [Reference] | |||
| Proximal M1 segment MCA | 24/111 (21.6) | 1.99 (0.83-4.77) | .12 | ||
| Distal M1 segment MCA | 39/84 (46.4) | 5.61 (2.38-13.26) | <.001 | ||
| M2 segment MCA | 53/143 (37.1) | 3.61 (1.62-8.02) | .002 | ||
| Distal (M3 MCA, ACA, and PCA) | 17/40 (42.5) | 5.18 (1.95-13.76) | .001 | ||
| Clot burden scorec | |||||
| 0-4 | 13/127 (10.2) | 1 [Reference] | |||
| 5-7 | 48/138 (34.8) | 3.32 (1.61-6.85) | .001 | ||
| 8-10 | 82/205 (40) | 3.84 (1.93-7.64) | <.001 | ||
| Residual flowd | |||||
| Grade 0 | 91/377 (24.1) | 1 [Reference] | |||
| Grade 1 | 22/48 (45.8) | 2.56 (1.26-5.20) | .009 | 2.7 (1.36-5.36) | .004 |
| Grade 2 | 30/45 (66.7) | 7.03 (3.32-14.87) | <.001 | 6.05 (2.92-12.54) | <.001 |
| Time from intravenous alteplase start to recanalization assessment (for every 30 min) | 1.28 (1.18-1.38) | <.001 | 1.27 (1.18-1.37) | <.001 | |
Abbreviations: ACA, anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery.
Recanalization of intracranial thrombus with intravenous alteplase or not was assessed using the rAOL (revised arterial occlusive lesion) score on repeat head CTA or on first angiographic acquisition of the affected intracranial circulation. Successful recanalization was defined as an rAOL score of 2b or 3. The rAOL score is described in detail in the Methods and eTable 1 in the Supplement.
Models 1 and 2 are similar except for the variables of site of intracranial occlusion at baseline and clot burden score. These variables both measure thrombus extent and therefore were not included in the same model. Other variables included in multivariable modeling included baseline NIHSS, residual flow grade, and time from intravenous alteplase start to recanalization assessment. Only variables with P < .05 are reported in models above.
Clot burden score of 0 implies complete occlusion of the ipsilateral anterior circulation vessels; a score of 10 implies no occlusion.
Residual flow grade is assessed on CTA source images (3-mm maximum intensity projections preferred) as follows: grade 0, no contrast permeation of thrombus; grade 1, contrast permeating diffusely through thrombus; and grade 2, tiny hairline lumen or streak of well-defined contrast within the thrombus extending either through its entire length or part of the thrombus.
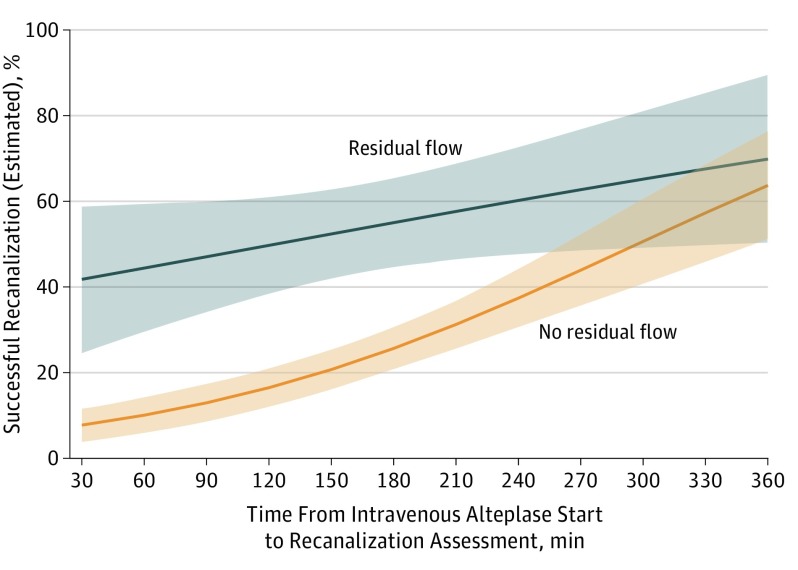
When site of intracranial occlusion was replaced with clot burden score categories, the results were similar (Table 2). Results were similar even after accounting for variance by region (North America, Europe, or East Asia) (eTable 3 in the Supplement). None of the other baseline clinical or imaging variables were associated with recanalization (rAOL score 2b or 3) (Table 1 and Table 2). Estimated successful recanalization over time from intravenous alteplase start to recanalization assessment stratified by presence or absence of residual flow is shown in Figure 2. In multivariable analysis assessing variables associated with recanalization without intravenous alteplase (adjusted recanalization prevalence, 6.7% [95% CI, 1.6%-61%]), only male sex was significantly associated with recanalization (eTable 4 in the Supplement).
Figure 2. Estimated Successful Recanalization From the Start of Intravenous Alteplase to Recanalization Assessment Stratified by Residual Flow (n = 470 Patients).
Results are derived from the logistic regression model reported in Table 2 with residual flow categorized as yes (grade 1 and 2; n = 93) vs no (grade 0; n = 377). The shaded areas indicate 95% CIs. Recanalization of intracranial thrombus with intravenous alteplase was assessed using the revised arterial occlusive lesion (rAOL) score on repeat computed tomographic angiography (CTA) of the head or on first angiographic acquisition of the affected intracranial circulation. Successful recanalization was defined as an rAOL score of 2b or 3. The rAOL score is described in detail in the Methods section and in eTable 1 in the Supplement.
Among patients treated with intravenous alteplase, separate logistic regression models for each individual site of occlusion are shown in eTable 5 in the Supplement. Time from start of intravenous alteplase to recanalization assessment and presence of residual flow (grade 1 and 2) were associated with successful recanalization (rAOL score 2b or 3) in patients with proximal segment MCA (n = 111), distal M1 segment MCA (n = 84), and M2 segment (n = 143) thrombus. Time from start of intravenous alteplase but not residual flow was associated with recanalization in patients with intracranial ICA thrombus (n = 92). Neither of these variables was associated with recanalization with intravenous alteplase in patients with distal (M3 MCA, anterior cerebral artery, posterior cerebral artery) thrombus (n = 40).
Cumulative estimated recanalization over time from start of intravenous alteplase to recanalization assessment stratified by presence or absence of residual flow are shown in Figure 3 for all occlusion sites except distal occlusions (M3 MCA, anterior cerebral artery, posterior cerebral artery) (Figure 3). Because residual flow was not associated with recanalization in patients with intracranial ICA thrombus, cumulative estimated recanalization over time from start of intravenous alteplase to recanalization assessment is shown in Figure 3 for this site of occlusion.
Figure 3. Estimated Recanalization From the Start of Intravenous Alteplase to Recanalization Assessment Stratified by Residual Flow and by Location of Intracranial Thrombus .
Results are derived from individual logistic regression models, each derived from data for that site of intracranial occlusion. The shaded areas indicate 95% CIs. Residual flow is categorized as yes (grade 1 and 2) vs no (grade 0). The numbers of patients are as follows: internal carotid artery, total n = 92; proximal M1 middle cerebral artery (MCA), total n = 111 (residual flow yes = 28, no = 83); distal M1 MCA total n = 84 (residual flow yes = 29, no = 55); and M2 MCA total n = 143 (residual flow yes = 22, no = 121). Presence or absence of residual flow was not associated with recanalization prevalence in patients with intracranial internal carotid artery segment occlusion. Graphs for distal locations (M3 MCA, anterior cerebral artery, and posterior cerebral artery) are not shown because neither residual flow nor time from the start of alteplase to recanalization assessment was significantly associated with recanalization prevalence in that statistical model. Statistical models for each individual site of occlusion are in the Supplement.
Discussion
In this study involving patients with acute ischemic stroke and visible intracranial thrombus on CTA, successful recanalization occurred in 27% of patients overall, including 30% among patients who received intravenous alteplase and 13% among patients who did not receive intravenous alteplase. Among patients who received intravenous alteplase, more distal thrombus location, lower thrombus extent, increased thrombus permeability, and longer time to recanalization assessment were associated with successful recanalization. Among patients not receiving intravenous alteplase, the rate of recanalization was low with only male sex being associated with recanalization. Patients with intracranial ICA thrombi receiving intravenous alteplase also had low rates of recanalization.
The results of this study suggest that recanalization with intravenous alteplase is a continuous process over time. The degree of alteplase recanalization in this study is consistent with prior transcranial Doppler ultrasonography, CTA, and MRA studies that evaluated either early (<2 hour) or late (24-hour) time points.10,20,21 With a plasma half-life of 6 to 7 minutes, alteplase is not likely to be biologically active at 6 hours following administration.22 However, it is possible that the early thrombus debulking effects of alteplase translate to less overall thrombus, allowing endogenous tissue plasminogen activator to complete the remaining lysis required. A prior study using thin-section noncontrast CT demonstrated this early partial debulking effect with thrombus volume reduction in 80% of alteplase-treated patients but complete lysis in only 9.4%.23
These data are relevant to deciding whether to transport patients with stroke to a tertiary stroke center for advanced care in the endovascular therapy era. When transport times are several hours longer to a comprehensive stroke center compared with a primary stroke center, evaluation at a primary stroke center for initial treatment with intravenous alteplase is likely the better option based on reasonable recanalization rates with alteplase over several hours.
There is also a clear need to predict with clinical and imaging criteria which patients are most suitable for transfer to a comprehensive stroke center for endovascular therapy vs who can remain at the primary stroke center. Reasons for futile transfer to a comprehensive stroke center include recanalization and clinical improvement by the time the patient arrives at the comprehensive stroke center and infarct growth during patient transfer.24,25 Patients with factors associated with thrombus responsiveness to alteplase (eg, thrombus permeability) may not require transfer because they will recanalize with intravenous alteplase. These results show that permeable thrombi as assessed using CTA residual flow grade are much more likely to recanalize early with intravenous alteplase (Figure 2 and Figure 3). Recanalization with intravenous alteplase within 2 hours of treatment in this study approaches outcomes achieved with modern endovascular treatment in patients with permeable distal M1 MCA thrombus (Figure 3).
Anticipated treatment effect of any new thrombolytic or ancillary reperfusion therapy will be influenced by thrombus occlusion site and timing of recanalization end point. A recent trial that compared intravenous tenecteplase with alteplase in patients with proximal vessel occlusions reported recanalization rates within an hour of alteplase administration (10%) that are similar to the rates reported in this analysis (13%).26
Thrombus properties such as permeability are also likely to influence the effect of novel reperfusion therapies. Intracranial thrombi permeable to blood flow (residual flow grade 1 or 2) have more surface area exposed to thrombolytic enzyme binding and therefore may be ideal to evaluate with ancillary drug therapies such as argatroban or eptifibitide.27,28 Nonpermeable thrombi recanalize more slowly and less commonly and may require mechanical means to disrupt the thrombus and increase surface area for enzymatic access with therapies such as ultrasound.29 Clinical trial design of novel thrombolytic or ancillary reperfusion treatments might therefore be optimized using the expected recanalization prevalence data from this study.
Limitations
This study has several limitations. First, assessing recanalization status using 2 different imaging modalities (CTA and conventional angiography) is a practical limitation, but given that both tools are “luminal contrast studies,” differences in grading are minimal. Second, patients with proximal intracranial thrombus were more likely to have been reimaged earlier (on first angiographic acquisition of the affected intracranial circulation during endovascular therapy). However, a mixture of comprehensive stroke centers (some with limited access to endovascular therapy) and primary stroke centers as enrolling sites resulted in these data having a range of recanalization assessment times across all occlusion sites, clot burden scores, and residual flow grades (eFigures 2, 3, 4, and 5 in the Supplement). Moreover, recanalization time curves are reported for each occlusion site using data specific to that site of occlusion (Figure 3).
Third, a screening log was not maintained and therefore the total number of patients eligible for intravenous alteplase who had identified intracranial occlusions on CTA is not known. Fourth, small sample size limits the ability to comment on variables associated with alteplase recanalization in patients with distal (M3 MCA, anterior cerebral artery, posterior cerebral artery) occlusions. However, patients with these intracranial occlusions are often not candidates for endovascular therapy. Small sample size also results in wider confidence intervals around the estimates of recanalization in patients with permeable intracranial thrombus (ie, with residual flow).
Fifth, detailed quantitative measurements of intracranial thrombus such as length, volume, surface area, or permeability were not performed. These techniques are still being developed, especially for more distal and branched thrombi. Sixth, collateral status on CTA is difficult to measure in patients with M2 MCA segment or more distal thrombi, especially with single-phase CTA; this variable was therefore not included in analysis.30
Seventh, the duration of data accrual was long and occurred during a time of important advances in the care of patients with ischemic stroke. Eighth, the generalizability of these findings to other settings with different capabilities for acute imaging and stroke care is unknown. Ninth, clinical outcomes data, such as whether the factors related to thrombus recanalization are associated with clinical outcomes, including mortality or functional status, are not reported in the present analysis.
Conclusions
Among patients with acute ischemic stroke, more distal thrombus location, greater thrombus permeability, and longer time to recanalization assessment were associated with recanalization of arterial occlusion after administration of intravenous alteplase, whereas among patients who did not receive alteplase, rates of arterial recanalization were low. These findings may help inform treatment and triage decisions in patients with acute ischemic stroke.
eFigure 1. Location of intracranial thrombus identified using CT angiography.
eFigure 2. Time from intravenous alteplase start or from baseline CTA to recanalization assessment in the different treatment groups in the study, namely intravenous alteplase only, endovascular thrombectomy only, intravenous alteplase + endovascular thrombectomy and conservative treatment.
eFigure 3. Box plots showing time from IV alteplase start to recanalization assessment stratified by site of occlusion on baseline CT angiography.
eFigure 4. Box plots showing time from IV alteplase start to recanalization assessment stratified by clot burden score on baseline CT angiography.
eFigure 5. Box plots showing time from IV alteplase start to recanalization assessment stratified by residual flow grade on baseline CT angiography.
eFigure 6a. The revised Arterial Occlusive Lesion (rAOL) score assessing recanalization with intravenous alteplase stratified by site of occlusion on baseline CT angiography.
eFigure 6b. The revised Arterial Occlusive Lesion (rAOL) score assessing recanalization without intravenous alteplase stratified by site of occlusion on baseline CT angiography.
eFigure 7a. The revised Arterial Occlusive Lesion (rAOL) score assessing recanalization with intravenous alteplase stratified by Clot Burden Score on baseline CT angiography.
eFigure 7b. The revised Arterial Occlusive Lesion (rAOL) score assessing recanalization without intravenous alteplase stratified by Clot Burden Score on baseline CT angiography.
eFigure 8a. The revised Arterial Occlusive Lesion (rAOL) score assessing recanalization with intravenous alteplase stratified by presence or absence of residual flow on baseline CT angiography.
eFigure 8b. The revised Arterial Occlusive Lesion (rAOL) score assessing recanalization without intravenous alteplase stratified by presence or absence of residual flow on baseline CT angiography.
eTable 1. The revised Arterial Occlusive Lesion (rAOL) score.
eTable 2. Clinical and imaging characteristics in different treatment subgroups.
eTable 3. Post hoc analysis of variables independently associated with successful recanalization with intravenous alteplase when accounting for differences by geographic region of patient enrolled.
eTable 4. Logistic regression model in patients not treated with intravenous alteplase with successful recanalization.
eTable 5. Final logistic regression models for each individual intracranial occlusion site (models 1 to 5) showing variables associated with successful recanalization in patients treated with intravenous alteplase.
References
- 1.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 4.Saver JL. Time is brain–quantified. Stroke. 2006;37(1):263-266. doi: 10.1161/01.STR.0000196957.55928.ab [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Fonarow GC, Smith EE, et al. . Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480-2488. doi: 10.1001/jama.2013.6959 [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 7.Alexandrov AV, Burgin WS, Demchuk AM, El-Mitwalli A, Grotta JC. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short-term improvement. Circulation. 2001;103(24):2897-2902. doi: 10.1161/01.CIR.103.24.2897 [DOI] [PubMed] [Google Scholar]
- 8.del Zoppo GJ, Poeck K, Pessin MS, et al. . Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32(1):78-86. doi: 10.1002/ana.410320113 [DOI] [PubMed] [Google Scholar]
- 9.Molina CA, Montaner J, Abilleira S, et al. . Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32(5):1079-1084. doi: 10.1161/01.STR.32.5.1079 [DOI] [PubMed] [Google Scholar]
- 10.Ribo M, Alvarez-Sabín J, Montaner J, et al. . Temporal profile of recanalization after intravenous tissue plasminogen activator: selecting patients for rescue reperfusion techniques. Stroke. 2006;37(4):1000-1004. doi: 10.1161/01.STR.0000206443.96112.d9 [DOI] [PubMed] [Google Scholar]
- 11.Labiche LA, Malkoff M, Alexandrov AV. Residual flow signals predict complete recanalization in stroke patients treated with TPA. J Neuroimaging. 2003;13(1):28-33. doi: 10.1111/j.1552-6569.2003.tb00153.x [DOI] [PubMed] [Google Scholar]
- 12.Demchuk AM, Goyal M, Yeatts SD, et al. ; IMS III Investigators . Recanalization and clinical outcome of occlusion sites at baseline CT angiography in the Interventional Management of Stroke III trial. Radiology. 2014;273(1):202-210. doi: 10.1148/radiol.14132649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 14.Millán M, Remollo S, Quesada H, et al. ; REVASCAT Trial Investigators . Vessel patency at 24 hours and its relationship with clinical outcomes and infarct volume in REVASCAT Trial (Randomized Trial of Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset). Stroke. 2017;48(4):983-989. doi: 10.1161/STROKEAHA.116.015455 [DOI] [PubMed] [Google Scholar]
- 15.Seners P, Turc G, Maïer B, Mas JL, Oppenheim C, Baron JC. Incidence and predictors of early recanalization after intravenous thrombolysis: a systematic review and meta-analysis. Stroke. 2016;47(9):2409-2412. doi: 10.1161/STROKEAHA.116.014181 [DOI] [PubMed] [Google Scholar]
- 16.Goyal M, Menon BK, Krings T, et al. . What constitutes the M1 segment of the middle cerebral artery? J Neurointerv Surg. 2016. doi: 10.1136/neurintsurg-2015-012191 [DOI] [PubMed] [Google Scholar]
- 17.Puetz V, Dzialowski I, Hill MD, et al. ; Calgary CTA Study Group . Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke. 2008;3(4):230-236. doi: 10.1111/j.1747-4949.2008.00221.x [DOI] [PubMed] [Google Scholar]
- 18.Mishra SM, Dykeman J, Sajobi TT, et al. . Early reperfusion rates with IV tPA are determined by CTA clot characteristics. AJNR Am J Neuroradiol. 2014;35(12):2265-2272. doi: 10.3174/ajnr.A4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T; IMS-I Investigators . Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36(11):2400-2403. doi: 10.1161/01.STR.0000185698.45720.58 [DOI] [PubMed] [Google Scholar]
- 20.Bhatia R, Hill MD, Shobha N, et al. . Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41(10):2254-2258. doi: 10.1161/STROKEAHA.110.592535 [DOI] [PubMed] [Google Scholar]
- 21.Zangerle A, Kiechl S, Spiegel M, et al. . Recanalization after thrombolysis in stroke patients: predictors and prognostic implications. Neurology. 2007;68(1):39-44. doi: 10.1212/01.wnl.0000250341.38014.d2 [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Moreton FC, Kalladka D, et al. . Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543-3546. doi: 10.1161/STROKEAHA.115.011290 [DOI] [PubMed] [Google Scholar]
- 23.Kim YD, Nam HS, Kim SH, et al. . Time-dependent thrombus resolution after tissue-type plasminogen activator in patients with stroke and mice. Stroke. 2015;46(7):1877-1882. doi: 10.1161/STROKEAHA.114.008247 [DOI] [PubMed] [Google Scholar]
- 24.Fuentes B, Alonso de Leciñana M, Ximénez-Carrillo A, et al. ; Madrid Stroke Network . Futile interhospital transfer for endovascular treatment in acute ischemic stroke: the Madrid Stroke Network Experience. Stroke. 2015;46(8):2156-2161. doi: 10.1161/STROKEAHA.115.009282 [DOI] [PubMed] [Google Scholar]
- 25.Boulouis G, Siddiqui KA, Lauer A, et al. . Immediate vascular imaging needed for efficient triage of patients with acute ischemic stroke initially admitted to nonthrombectomy centers. Stroke. 2017;48(8):2297-2300. doi: 10.1161/STROKEAHA.117.017607 [DOI] [PubMed] [Google Scholar]
- 26.Campbell BCV, Mitchell PJ, Churilov L, et al. ; EXTEND-IA TNK Investigators . Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi: 10.1056/NEJMoa1716405 [DOI] [PubMed] [Google Scholar]
- 27.Barreto AD, Alexandrov AV, Lyden P, et al. . The argatroban and tissue-type plasminogen activator stroke study: final results of a pilot safety study. Stroke. 2012;43(3):770-775. doi: 10.1161/STROKEAHA.111.625574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adeoye O, Sucharew H, Khoury J, et al. . Combined approach to lysis utilizing eptifibatide and recombinant tissue-type plasminogen activator in acute ischemic stroke-full dose regimen stroke trial. Stroke. 2015;46(9):2529-2533. doi: 10.1161/STROKEAHA.115.010260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexandrov AV, Molina CA, Grotta JC, et al. ; CLOTBUST Investigators . Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351(21):2170-2178. doi: 10.1056/NEJMoa041175 [DOI] [PubMed] [Google Scholar]
- 30.Menon BK, d’Esterre CD, Qazi EM, et al. . Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015;275(2):510-520. doi: 10.1148/radiol.15142256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Location of intracranial thrombus identified using CT angiography.
eFigure 2. Time from intravenous alteplase start or from baseline CTA to recanalization assessment in the different treatment groups in the study, namely intravenous alteplase only, endovascular thrombectomy only, intravenous alteplase + endovascular thrombectomy and conservative treatment.
eFigure 3. Box plots showing time from IV alteplase start to recanalization assessment stratified by site of occlusion on baseline CT angiography.
eFigure 4. Box plots showing time from IV alteplase start to recanalization assessment stratified by clot burden score on baseline CT angiography.
eFigure 5. Box plots showing time from IV alteplase start to recanalization assessment stratified by residual flow grade on baseline CT angiography.
eFigure 6a. The revised Arterial Occlusive Lesion (rAOL) score assessing recanalization with intravenous alteplase stratified by site of occlusion on baseline CT angiography.
eFigure 6b. The revised Arterial Occlusive Lesion (rAOL) score assessing recanalization without intravenous alteplase stratified by site of occlusion on baseline CT angiography.
eFigure 7a. The revised Arterial Occlusive Lesion (rAOL) score assessing recanalization with intravenous alteplase stratified by Clot Burden Score on baseline CT angiography.
eFigure 7b. The revised Arterial Occlusive Lesion (rAOL) score assessing recanalization without intravenous alteplase stratified by Clot Burden Score on baseline CT angiography.
eFigure 8a. The revised Arterial Occlusive Lesion (rAOL) score assessing recanalization with intravenous alteplase stratified by presence or absence of residual flow on baseline CT angiography.
eFigure 8b. The revised Arterial Occlusive Lesion (rAOL) score assessing recanalization without intravenous alteplase stratified by presence or absence of residual flow on baseline CT angiography.
eTable 1. The revised Arterial Occlusive Lesion (rAOL) score.
eTable 2. Clinical and imaging characteristics in different treatment subgroups.
eTable 3. Post hoc analysis of variables independently associated with successful recanalization with intravenous alteplase when accounting for differences by geographic region of patient enrolled.
eTable 4. Logistic regression model in patients not treated with intravenous alteplase with successful recanalization.
eTable 5. Final logistic regression models for each individual intracranial occlusion site (models 1 to 5) showing variables associated with successful recanalization in patients treated with intravenous alteplase.