Key Points
Question
Is mild traumatic brain injury without loss of consciousness associated with an increased risk of dementia diagnosis in veterans?
Findings
In this propensity-matched cohort study of more than 350 000 veterans with and without traumatic brain injuries, mild traumatic brain injury without loss of consciousness was associated with more than a 2-fold increase in the risk of dementia diagnosis, even after adjusting for medical and psychiatric comorbidities.
Meaning
Even mild traumatic brain injuries that do not result in loss of consciousness might have long-term neurodegenerative consequences.
This cohort study examines the association between traumatic brain injury severity, loss of consciousness, and dementia diagnosis in US military veterans.
Abstract
Importance
Traumatic brain injury (TBI) is common in both veteran and civilian populations. Prior studies have linked moderate and severe TBI with increased dementia risk, but the association between dementia and mild TBI, particularly mild TBI without loss of consciousness (LOC), remains unclear.
Objective
To examine the association between TBI severity, LOC, and dementia diagnosis in veterans.
Design, Setting, and Participants
This cohort study of all patients diagnosed with a TBI in the Veterans Health Administration health care system from October 1, 2001, to September 30, 2014, and a propensity-matched comparison group. Patients with dementia at baseline were excluded. Researchers identified TBIs through the Comprehensive TBI Evaluation database, which is restricted to Iraq and Afghanistan veterans, and the National Patient Care Database, which includes veterans of all eras. The severity of each TBI was based on the most severe injury recorded and classified as mild without LOC, mild with LOC, mild with LOC status unknown, or moderate or severe using Department of Defense or Defense and Veterans Brain Injury Center criteria. International Classification of Diseases, Ninth Revision codes were used to identify dementia diagnoses during follow-up and medical and psychiatric comorbidities in the 2 years prior to the index date.
Main Outcomes and Measures
Dementia diagnosis in veterans who had experienced TBI with or without LOC and control participants without TBI exposure.
Results
The study included 178 779 patients diagnosed with a TBI in the Veterans Health Administration health care system and 178 779 patients in a propensity-matched comparison group. Veterans had a mean (SD) age of nearly 49.5 (18.2) years at baseline; 33 250 (9.3%) were women, and 259 136 (72.5%) were non-Hispanic white individuals. Differences between veterans with and without TBI were small. A total of 4698 veterans (2.6%) without TBI developed dementia compared with 10 835 (6.1%) of those with TBI. After adjustment for demographics and medical and psychiatric comobidities, adjusted hazard ratios for dementia were 2.36 (95% CI, 2.10-2.66) for mild TBI without LOC, 2.51 (95% CI, 2.29-2.76) for mild TBI with LOC, 3.19 (95% CI, 3.05-3.33) for mild TBI with LOC status unknown, and 3.77 (95% CI, 3.63-3.91) for moderate to severe TBI.
Conclusions and Relevance
In this cohort study of more than 350 000 veterans, even mild TBI without LOC was associated with more than a 2-fold increase in the risk of dementia diagnosis. Studies of strategies to determine mechanisms, prevention, and treatment of TBI-related dementia in veterans are urgently needed.
Introduction
Traumatic brain injury (TBI) was 1 of the earliest risk factors identified for Alzheimer disease and dementia.1 Although not all studies have found an association,2,3,4,5,6,7 most studies, including several systematic reviews and meta-analyses,8,9 have found that moderate and severe TBI are associated with increased risk or earlier onset of Alzheimer disease and dementia,10,11,12,13,14,15,16,17,18 particularly in those with genetic risk factors, such as 1 or more apolipoproteinE e4 alleles.19,20,21,22 However, the association between mild TBI and dementia remains controversial,8,13 and few studies have specifically examined the effects of mild TBI without loss of consciousness (LOC).4
Mild TBI is extremely common in the general population and is especially so in military personnel. Approximately 2.8 million TBIs occurred in the United States in 2013,23 and approximately 80% of these were mild.24 A recent survey found that 17% of Iraq and Afghanistan troops reported experiencing a mild TBI during deployment and, of these, 59% reported more than 1 mild TBI.25 Most of these are caused by shockwaves from blasts, rather than blunt trauma, and do not necessarily result in LOC.26
There also is growing awareness that mild, repeated TBIs are closely related to chronic traumatic encephalopathy (CTE), a neurodegenerative disease associated with repeated head trauma.27 Recent autopsy studies have identified CTE in professional athletes who participate in American football, boxing, soccer, wrestling, ice hockey, rugby, and baseball.28,29,30,31,32,33 Severity of neuropathology is correlated with number of years of exposure to contact sports, rather than number of concussions, suggesting that subconcussive injuries contribute to disease progression.27,34 Chronic traumatic encephalopathy also has been identified in military veterans exposed to repeated TBIs.35
The objective of this study was to examine the association between TBI and diagnosis of dementia in veterans who receive care in the Veterans Health Administration (VHA) health care system. In particular, we sought to determine whether veterans who experience mild TBI without LOC are more likely to be diagnosed with dementia.
Methods
Study Population
We performed a retrospective cohort study that included all VHA patients who received a TBI diagnosis between October 1, 2001, and September 30, 2014, and a propensity-matched comparison sample. Diagnoses of TBI came from 2 sources: the Comprehensive Traumatic Brain Injury Evaluation (CTBIE) database and National Patient Care Databases (NPCD), which are derived from VHA inpatient and outpatient medical appointments.
All study procedures were approved by institutional review boards at the University of California, San Francisco; San Francisco Veterans Affairs Health Care System; and US Army Medical Research and Materiel Command, Office of Research Protections, Human Research Protection Office. Informed consent was waived because many study participants had died or were no longer receiving care through VHA when these analyses were performed.
The CTBIE database is an accruing national database that began in 2007 and includes Iraq and Afghanistan–era veterans who have separated from military service, enrolled in VHA health care, and received a comprehensive TBI evaluation. Veterans may be referred for a comprehensive TBI evaluation if they screen positive for TBI, are informed prior to screening that they may have sustained a moderate to severe TBI, or report symptoms suggestive of TBI or concussion during a VHA clinical visit. All TBI evaluations are performed by a neurologist or a trained allied health professional, either within VHA or through another facility that was reimbursed by the VA. The CTBIE database includes detailed information on the final determination of TBI status as well as duration of LOC, alteration of consciousness, and posttraumatic amnesia. Veterans who are referred but not evaluated or who receive evaluations that are not captured in the CTBIE database are not included in this study. We identified all Iraq and Afghanistan veterans who received a TBI diagnosis through the CTBIE database from October 2007 to October 2014.
In addition, we identified all other VHA patients who received an inpatient or outpatient TBI diagnosis as part of routine clinical care by using a comprehensive list of International Classification of Diseases, Ninth Revision (ICD-9) codes created by the Defense and Veterans Brain Injury Center and the Armed Forces Health Surveillance Branch for TBI surveillance (2012 criteria).36
For all participants with TBI, we determined the first fiscal year in which a TBI was diagnosed. In addition, as a proxy measure for repeated TBIs, we determined the number of years in which each veteran had at least 1 TBI diagnosis prior to a diagnosis of dementia or data censoring. We hypothesized that a TBI diagnosed in a subsequent year would be more likely to reflect a new event rather than ongoing care for the index event.
To identify a comparison sample of veterans without TBI, we first selected a 2% random sample of all patients who received VHA care from October 1, 2001, to September 30, 2014. We then used propensity matching to select 1 veteran without TBI for each veteran with TBI. We performed propensity score matching with no replacement using nearest-neighbor caliper matching with caliper width of 0.2 SDs of the logit of the propensity score using StataMP, version 15 (64-bit) (StataCorp). Propensity score matching was conducted on the entire sample (both CTBIE and NPCD data) matching the group with any TBI and the group with no TBI using all covariates.
The index date for those with TBI was defined as the date of the most severe TBI. If TBIs were comparable in severity, the index date was defined as the first TBI recorded. For participants without TBI, the index date was defined as the random selection date (between October 1, 2001, and September 30, 2014). Individuals with a dementia diagnosis at the time of the index date or during the 2 previous years were excluded. For all participants, starting with the index date, we extracted dates and diagnoses for all subsequent inpatient and outpatient visits.
TBI Severity
A variety of criteria exist to define TBI severity,37 and a recent study identified more than 50 different definitions of mild TBI.38 Department of Defense coding guidelines from 2010 define mild TBI as TBI with normal structural imaging, an LOC of 0 to 30 minutes, alteration of consciousness lasting from a moment up to 24 hours, and posttraumatic amnesia lasting from 0 to 1 day.39 The Defense and Veterans Brain Injury Center, Armed Forces Health Surveillance Branch, and the Centers for Disease Control have collaborated to develop a standard TBI surveillance case definition using ICD-9 and ICD-10 codes.36
For the current study, we classified the most severe TBI as none, mild, or moderate or severe. We then separated mild TBIs into those without LOC, with LOC, or with LOC status unknown. In patients whose TBI was diagnosed through the CTBIE data, TBI severity was defined using the more stringent DOD criteria.39 In patients whose TBI was diagnosed through ICD-9 codes, TBI severity was defined using Defense and Veterans Brain Injury Center 2012 Criteria (eAppendices 1 and 2 in the Supplement).36 Patients whose TBI severity could not be classified were excluded.
Dementia
Prevalent dementia at baseline was defined using a comprehensive list of ICD-9 codes recommended by the VA Dementia Steering Committee (2016 version; eAppendix 3 in the Supplement). Individuals with a dementia code at the index date or during the previous 2 years were excluded. Incident dementia during the follow-up period was classified using a slightly modified version of the VA Steering Committee ICD-9 codes in which we excluded prion disease (ICD-9 codes 046.11, 046.19, 046.3, and 046.79) and alcohol-induced or drug-induced dementia (ICD-9 codes 291.2 and 292.82).
Other Measures
Demographic information, medical comorbidities, and psychiatric conditions were obtained from the VHA inpatient and outpatient files. Demographic data were based on self-report and included age at index date, sex and race/ethnicity (categorized as non-Hispanic white individuals, non-Hispanic black individuals, Hispanic individuals, or individuals of other or unknown races/ethnicities). In addition, we used zip codes at the index date and US Census data (2000) to classify veterans’ areas of residence into broad educational and income strata. Education was dichotomized as less than or equal to 25% vs more than 25% of the adult population of a given area completing a college education (defined as a bachelor degree or higher). Income was categorized into tertiles of median income for adults younger than 75 years or 75 years or older.
Medical comorbidities and psychiatric disorders were coded as present at baseline if they were coded at the index date or during the previous 2 years using standard ICD-9 codes. Medical comorbidities included diabetes mellitus, hypertension, myocardial infarction, cerebrovascular disease, and peripheral vascular disease. Psychiatric conditions included mood disorder (ie, depression, dysthymia, and bipolar disorder), anxiety, posttraumatic stress disorder, substance use disorder (ie, alcohol or drug use), tobacco use, and sleep disorder (ie, sleep apnea, insomnia, hypersomnia, parasomnia, and circadian rhythm disorders).
Analyses
Baseline characteristics of veterans were compared as a function of TBI using t tests for continuous variables and χ2 analysis for categorical variables. Cumulative incidence of dementia as a function of age and TBI severity was examined graphically. Cox proportional hazards models were used to examine time to dementia diagnosis with censoring at death or last medical encounter and age as the timescale. Models were unadjusted and adjusted in steps for (1) demographics; (2) demographics and medical comorbidities; and (3) demographics, medical comorbidities, and psychiatric disorders. In addition, sensitivity analyses were performed on data stratified by TBI data source (the CTBIE database vs the NPCD) and comparing veterans with TBIs coded in single years vs multiple years. Cox proportional hazards model assumptions were checked for all final models. P values were 2-sided with statistical significance defined as P < .05. Analyses were performed using SAS, version 9.4 (SAS Institute).
Results
Our final cohort included 178 779 veterans who had experienced 1 or more TBIs and a propensity-matched comparison sample of 178 779 veterans who had not had a TBI. A total of 151 354 veterans with TBIs were identified through the NPCD data set only (84.7%), while 12 714 (7.1%) were from the CTBIE data set only; 14 711 (8.2%) were included in both data sets. Overall, TBI severity varied; 17 759 participants (9.9%) with TBI had had a mild TBI without LOC, 23 097 (12.9%) had experienced a mild TBI with LOC, 55 004 (30.8%) had experienced a mild TBI with LOC status unknown, and 82 919 (46.4%) had had a moderate or severe TBI. Of the TBIs identified through the CTBIE data set, 23 196 (84.6%) were mild, compared with 85 955 (51.8%) of those identified through the NPCD data set (eTable in the Supplement). When comparing veterans from the CTBIE data set vs the NPCD data set, the mean (SD) age was 32.9 (8.5) vs 50.6 (18.2) years, respectively.
Veterans with and without TBI were generally well-matched (Table 1). Study participants had a mean (SD) age of 49.5 (18.2) years at their index date; 33 250 (9.3%) were women and the distribution of race/ethnicity was 259 136 non-Hispanic white individuals (72.5%), 57 281 non-Hispanic black individuals (16.0%), 6551 Hispanic individuals (1.8%), and 34 590 individuals of other or unknown races/ethnicities (9.7%). A total of 14 660 individuals (4.1%) had a history of diabetes mellitus; 39 701 (11.1%) had hypertension; 69 394 (19.4%) had a mood disorder, and 38 779 (10.8%) had posttraumatic stress disorder. Study participants were followed up for a mean (SD) of 4.2 (3.4) years until dementia, death, or their most recent clinical visit (whichever occurred first).
Table 1. Baseline Characteristics of 357 558 Veterans With or Without Traumatic Brain Injurya.
| Characteristic | No. (%) | |
|---|---|---|
| Individuals Without TBI (n = 178 779) | Individuals With ≥1 TBI (n = 178 779) | |
| Demographic | ||
| Age, mean (SD), y | 49.95 (18.0) | 49.00 (18.4) |
| Female | 16 835 (9.4) | 16 415 (9.2) |
| Race | ||
| Non-Hispanic white | 130 955 (73.3) | 128 181 (71.7) |
| Non-Hispanic black | 29 475 (16.5) | 27 806 (15.6) |
| Hispanic | 2931 (1.6) | 3620 (2.0) |
| Other or unknown | 15 418 (8.6) | 19 172 (10.5) |
| >25% Residents in zip code college-educated | 93 442 (52.3) | 94 011 (52.6) |
| Median income tertile in zip code | ||
| Low (<$24 632) | 51 753 (29.0) | 49 984 (28.0) |
| Middle ($24 633-$32 541) | 61 449 (34.4) | 61 054 (34.2) |
| High (>$32 452) | 65 577 (36.7) | 67 741 (37.9) |
| Medical comorbidities | ||
| Diabetes mellitus | 7652 (4.2) | 7008 (3.9) |
| Hypertension | 20 104 (11.3) | 19 597 (11.0) |
| Myocardial infarction | 2977 (1.7) | 2643 (1.5) |
| Cerebrovascular disease | 8175 (4.6) | 7445 (4.2) |
| Peripheral vascular disease | 4121 (2.3) | 3603 (2.0) |
| Psychiatric comorbidities | ||
| Mood disorder | 37 262 (20.8) | 32 132 (18.0) |
| Anxiety | 17 898 (10.0) | 15 766 (8.8) |
| Posttraumatic stress disorder | 21 970 (12.3) | 16 809 (9.4) |
| Substance abuse | 15 748 (8.8) | 13 523 (7.6) |
| Smoking or tobacco use | 19 461 (10.9) | 18 353 (10.3) |
| Sleep disorder | 7049 (3.9) | 6497 (3.6) |
Abbreviation: TBI, traumatic brain injury.
Veterans with and without TBI were matched using propensity scores, and differences between groups are not clinically meaningful.
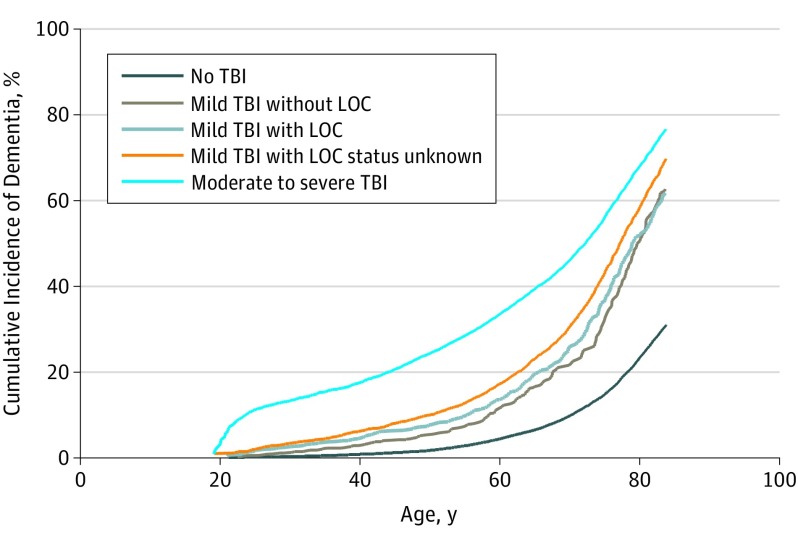
A total of 4698 cases of incident dementia (2.6%) were diagnosed in veterans without TBI, compared with 10 835 cases (6.1%) in those with TBI. After adjustment for age, medical comorbidities, and psychiatric disorders, the adjusted hazard ratio (HR) for dementia diagnosis was 2.36 (95% CI, 2.10, 2.66) for mild TBI without LOC; 2.51 (95% CI, 2.29-2.76) for mild TBI with LOC; 3.19 (95% CI, 3.05-3.33) for mild TBI with LOC status unknown; and 3.77 (95% CI, 3.63-3.91) for moderate to severe TBI (Table 2). The Figure shows that cumulative incidence of dementia based on age at diagnosis increased progressively with TBI severity. The mean (SD) time from index date to dementia diagnosis was 3.6 (3.0) years in those with TBI and 4.8 (3.7) years in those without TBI. Dementia diagnosis occurred an average of 1.5 years earlier in those with TBI vs those without TBI in the NPCD dataset and 1.8 years earlier in the CTBIE data set, with little evidence of difference in time to diagnosis by TBI severity.
Table 2. Unadjusted and Adjusted Risk of Dementia by Traumatic Brain Injury Severity (N = 357 558).
| Model | Individuals Without TBI (n = 178 779) | Individuals With ≥1 TBI (n = 178 779) | Participant Group, Hazard Ratio (95% CI)a | |||
|---|---|---|---|---|---|---|
| Mild TBI Without LOC (n = 17 759) | Mild TBI With LOC (n = 23 097) | Mild TBI With LOC Status Unknown (n = 55 004) | Moderate to Severe TBI (n = 82 919) | |||
| Unadjusted | 1 [Reference] | 3.41 (3.29-3.53) | 2.29 (2.04-2.58) | 2.48 (2.26-2.72) | 3.11 (2.97-3.25) | 3.75 (3.61-3.89) |
| 1b | 1 [Reference] | 3.41 (3.30-3.53) | 2.32 (2.06-2.61) | 2.49 (2.27-2.73) | 3.14 (3.00-3.28) | 3.73 (3.60-3.88) |
| 2c | 1 [Reference] | 3.41 (3.29-3.53) | 2.34 (2.08-2.63) | 2.50 (2.28-2.75) | 3.16 (3.02-3.31) | 3.71 (3.57-3.85) |
| 3d | 1 [Reference] | 3.45 (3.33-3.57) | 2.36 (2.10-2.66) | 2.51 (2.29-2.76) | 3.19 (3.05-3.33) | 3.77 (3.63-3.91) |
Abbreviations: LOC, loss of consciousness; TBI, traumatic brain injury.
No TBI is the reference group; P values in all other cells are <.001.
Model 1 is adjusted for demographic characteristics (sex, race, education, and income).
Model 2 is adjusted for demographic characteristics and medical conditions (diabetes, hypertension, myocardial infarction, cerebrovascular disease, and peripheral vascular disease).
Model 3 is adjusted for demographic characteristics, medical conditions, and psychiatric disorders (mood disorder, anxiety, posttraumatic stress disorder, substance use disorder, tobacco use, and sleep disorder).
Figure. Cumulative Incidence of Dementia by Traumatic Brain Injury (TBI) Severity.
The unadjusted cumulative incidence of dementia (age at dementia diagnosis) is shown as a function of TBI severity. After adjustment for demographics, medical conditions, and psychiatric disorders, there was a dose-response relationship between TBI severity and dementia diagnosis with hazard ratios of 2.36 (95% CI, 2.10-2.66) for mild TBI without loss of consciousness (LOC); 2.51 (95% CI, 2.29-2.76) for mild TBI with LOC; 3.19 (95% CI, 3.05-3.33) for mild TBI with LOC status unknown, and 3.77 (95% CI, 3.63-3.91) for moderate to severe TBI.
Sensitivity analyses yielded similar results. When stratifying based on TBI data source, the adjusted hazard ratios were 2.20 (95% CI, 0.99-4.88) in the CTBIE data set vs 2.27 (95% CI, 2.02-2.55) in the NPCD data set for mild TBI without LOC; 3.20 (95% CI, 1.48-6.90) in the CTBIE data set vs 2.49 (95% CI, 2.31-2.80) in the NPCD data set for mild TBI with LOC; and 5.94 (95% CI, 2.73-12.93) in the CTBIE data set vs 3.44 (95% CI, 3.22-3.57) in the NPCD data set for moderate to severe TBI. All mild TBIs with LOC status unknown were from the NPCD data set. Most patients with TBI had an ICD-9 code for TBI in a single year (81.3%) vs multiple years (18.7%), and the adjusted risk of dementia was similar in both groups (single-year HR, 3.46; 95% CI, 3.34-3.58; multiple-year HR, 3.41; 95% CI, 3.23-3.60). Cox proportional hazards model checks did not reveal any major violations of model assumptions.
Discussion
In this cohort of more than 350 000 veteran patients with and without TBI, we found a dose-response association between TBI severity and dementia diagnosis. Even mild TBI without LOC was associated with more than a 2-fold increase in the risk of receiving a dementia diagnosis. This association remained strong after adjustment for demographics, medical comorbidities, and psychiatric conditions and was consistent in sensitivity analyses. These results confirm prior studies, including a 2008 Institute of Medicine report,8 that have found an association between moderate to severe TBI and risk of dementia.1,8,11,13,21,40 In addition, although prior studies of the association between mild TBI and dementia have been mixed,2,3,4,5,6 our study adds to the weight of evidence suggesting that mild TBI is also associated with increased dementia diagnosis risk.14,15,16,37,41
Our results differ from several recent cohort studies that found no association between self-reported mild TBI and dementia risk.4,5,7 These differences may be because of differences in how TBIs were identified. Self-reported TBIs are likely to be less specific than injuries identified through the medical record, which could potentially bias results toward the null.
We are aware of only 1 prior study that has specifically examined the association between mild TBI without LOC and dementia risk.42 This study used a retrospective, case-control study design in which TBI status was determined in 2233 patients with Alzheimer disease and 14 668 first-degree family members, based on informant interviews and medical record reviews. The study found that TBIs with and without LOCs were both associated with greater odds of dementia, with evidence of a dose-response relationship. This design has several limitations, including the potential for recall bias when classifying TBI status and selection bias based on using first-degree relatives. Our study used a more rigorous cohort design in which TBI and dementia were both ascertained using similar methods in the same study population. In addition, we examined mild TBI with and without LOC.
There are several potential mechanisms that have been proposed to explain the association observed between TBI and dementia.43 First, a TBI can damage brain structure as a direct result of the injury.43 Diffuse axonal injury is common in all severities of TBI.44,45 Autopsy and neuroimaging studies also have shown that a single moderate to severe TBI can cause marked cerebral atrophy 6 months postinjury that may progress for many years. It is possible that these TBI-related brain changes, when combined with dementia-related neuropathological changes, lead to increased risk and earlier onset of dementia symptoms.
Second, TBI may lead directly to neuropathological changes that cause Alzheimer disease, such as deposition of tau in neurofibrillary tangles and amyloid β in plaques. Neurofibrillary tangles are one of the most consistent pathologies observed in CTE.43 Although amyloid β pathology is less consistently observed following TBI, an autopsy study in 39 survivors of TBI found both plaques and tangles in greater density and wider distribution than age-matched, uninjured controls.46 Furthermore, plaques tended to be diffuse in short-term survivors of TBI and fibrillary in long-term survivors of TBI, which is similar to patterns observed in early-stage vs late-stage Alzheimer disease.46
Other neuropathological changes that have been linked with both TBI and dementia include white matter degeneration and neuroinflammation. It also is possible that mechanisms may differ based on TBI severity. For example, mild TBI without LOC may increase dementia risk primarily by accelerating atrophy, while moderate to severe TBI may have a more direct effect on amyloid β and tau concentrations.
An alternative explanation for our results is that dementia diagnoses in these veterans reflect ongoing cognitive and functional impairment associated with their original injuries. For example, a veteran might experience cognitive impairment immediately after TBI, and a clinician might code this as dementia if the patient is still experiencing cognitive impairment that is severe enough to interfere with daily function several years later. If this is occurring, it suggests that even mild TBI without LOC is associated with greater risk of long-term cognitive and functional impairment in these veterans.
Strengths
Our study has several important strengths. First, we performed a longitudinal study in a large cohort, giving us ample power to detect associations and to adjust for a wide range of potential confounders. In particular, our sample size was large enough to examine mild TBI without LOC as a distinct category. Second, we included TBIs diagnosed through either the CTBIE database or VHA inpatient and outpatient records (via the NPCD). The CTBIE database includes TBI evaluations performed outside the VHA system, enabling us to capture a larger number of TBIs and to stratify results using the 2 data sources. Third, we selected our comparison sample using propensity matching to minimize the potential for confounding because of factors that predispose certain veterans to experience TBIs.
Limitations
Several limitations also should be considered when interpreting these results. This was a retrospective study using medical record databases that are based on clinician diagnoses, which do not necessarily reflect consensus definitions for TBI or dementia. This likely resulted in an underdiagnosis of dementia, particularly in the earlier stages. In addition, data on dementia subtypes were limited. All TBIs were diagnosed within the VHA health care system; therefore, results may not generalize to TBIs that do not result in medical care or are treated outside the VHA system. There also was heterogeneity in our working definition of TBI, and we were not able to quantify the number, types, or causes of TBIs experienced. We do not know whether TBIs occurred in military or nonmilitary settings, although it is likely that the CTBIE dataset included primarily deployment-related TBIs. We also did not have information on the history of TBIs. To the extent that misclassification occurred at random, it would tend to bias results toward the null. Additional research is critically needed to determine the mechanisms underlying the association observed between TBI and dementia, including mild TBI without LOC, so that effective treatment and prevention strategies can be developed.
Conclusions
In this large, retrospective cohort study of VHA patients, we observed a dose-response association between TBI severity and dementia diagnosis. Even mild TBI without loss of consciousness was associated with more than a 2-fold increase in the risk of dementia diagnosis after adjusting for demographic factors and medical and psychiatric comorbidities.
eAppendix 1. Traumatic Brain Injury (TBI) Severity Case Definitions.
eAppendix 2. Traumatic Brain Injury (TBI) Severity Classification Using Modified Defense and Veterans Brain Injury Center (DVBIC) 2012 ICD-9 Diagnostic Code List.
eAppendix 3. Dementia ICD-9 Diagnostic Code List.
eTable. Traumatic Brain Injury Case Distribution.
References
- 1.Mortimer JA, van Duijn CM, Chandra V, et al. ; EURODEM Risk Factors Research Group . Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. Int J Epidemiol. 1991;20(suppl 2):S28-S35. [DOI] [PubMed] [Google Scholar]
- 2.Breteler MM, de Groot RR, van Romunde LK, Hofman A. Risk of dementia in patients with Parkinson’s disease, epilepsy, and severe head trauma: a register-based follow-up study. Am J Epidemiol. 1995;142(12):1300-1305. [DOI] [PubMed] [Google Scholar]
- 3.Mehta KM, Ott A, Kalmijn S, et al. . Head trauma and risk of dementia and Alzheimer’s disease: The Rotterdam Study. Neurology. 1999;53(9):1959-1962. [DOI] [PubMed] [Google Scholar]
- 4.Crane PK, Gibbons LE, Dams-O’Connor K, et al. . Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 2016;73(9):1062-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK. Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry. 2013;84(2):177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helmes E, Østbye T, Steenhuis RE. Incremental contribution of reported previous head injury to the prediction of diagnosis and cognitive functioning in older adults. Brain Inj. 2011;25(4):338-347. [DOI] [PubMed] [Google Scholar]
- 7.Weiner MW, Crane PK, Montine TJ, Bennett DA, Veitch DP. Traumatic brain injury may not increase the risk of Alzheimer disease. Neurology. 2017;89(18):1923-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine Long-Term Consequences of Traumatic Brain Injury. Vol 7 Washington, DC: the National Academies Press; 2008. Gulf War and Health. [PubMed] [Google Scholar]
- 9.Perry DC, Sturm VE, Peterson MJ, et al. . Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg. 2016;124(2):511-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayeux R, Ottman R, Maestre G, et al. . Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology. 1995;45(3 pt 1):555-557. [DOI] [PubMed] [Google Scholar]
- 11.Schofield PW, Tang M, Marder K, et al. . Alzheimer’s disease after remote head injury: an incidence study. J Neurol Neurosurg Psychiatry. 1997;62(2):119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemetz PN, Leibson C, Naessens JM, et al. . Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. Am J Epidemiol. 1999;149(1):32-40. [DOI] [PubMed] [Google Scholar]
- 13.Plassman BL, Havlik RJ, Steffens DC, et al. . Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55(8):1158-1166. [DOI] [PubMed] [Google Scholar]
- 14.Wang HK, Lin SH, Sung PS, et al. . Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry. 2012;83(11):1080-1085. [DOI] [PubMed] [Google Scholar]
- 15.Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One. 2013;8(5):e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83(4):312-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71(12):1490-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu SF, Chiu WT, Lin HW, Chiang YH, Liou TH. Hazard ratio and repeat injury for dementia in patients with and without a history of traumatic brain injury: a population-based secondary data analysis in Taiwan. Asia Pac J Public Health. 2016;28(6):519-527. [DOI] [PubMed] [Google Scholar]
- 19.Luukinen H, Viramo P, Herala M, et al. . Fall-related brain injuries and the risk of dementia in elderly people: a population-based study. Eur J Neurol. 2005;12(2):86-92. [DOI] [PubMed] [Google Scholar]
- 20.Sundström A, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, Nyberg L. Increased risk of dementia following mild head injury for carriers but not for non-carriers of the APOE epsilon4 allele. Int Psychogeriatr. 2007;19(1):159-165. [DOI] [PubMed] [Google Scholar]
- 21.LoBue C, Wadsworth H, Wilmoth K, et al. . Traumatic brain injury history is associated with earlier age of onset of Alzheimer disease. Clin Neuropsychol. 2017;31(1):85-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes JP, Logue MW, Sadeh N, et al. . Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain. 2017;140(3):813-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor CA, Bell JM, Breiding MJ, Xu L. traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faul M, Coronado V. Epidemiology of traumatic brain injury. Handb Clin Neurol. 2015;127:3-13. [DOI] [PubMed] [Google Scholar]
- 25.Wilk JE, Herrell RK, Wynn GH, Riviere LA, Hoge CW. Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: association with postdeployment symptoms. Psychosom Med. 2012;74(3):249-257. [DOI] [PubMed] [Google Scholar]
- 26.Champion HR, Holcomb JB, Young LA. Injuries from explosions: physics, biophysics, pathology, and required research focus. J Trauma. 2009;66(5):1468-1477. [DOI] [PubMed] [Google Scholar]
- 27.McKee AC, Alosco ML, Huber BR. Repetitive head impacts and chronic traumatic encephalopathy. Neurosurg Clin N Am. 2016;27(4):529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mez J, Daneshvar DH, Kiernan PT, et al. . Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA. 2017;318(4):360-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omalu BI, DeKosky ST, Hamilton RL, et al. . Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 2006;59(5):1086-1092. [DOI] [PubMed] [Google Scholar]
- 30.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(1):128-134. [DOI] [PubMed] [Google Scholar]
- 31.Ling H, Morris HR, Neal JW, et al. . Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol. 2017;133(3):337-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omalu BI, Fitzsimmons RP, Hammers J, Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J Forensic Nurs. 2010;6(3):130-136. [DOI] [PubMed] [Google Scholar]
- 33.McKee AC, Daneshvar DH, Alvarez VE, Stein TD. The neuropathology of sport. Acta Neuropathol. 2014;127(1):29-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montenigro PH, Alosco ML, Martin BM, et al. . Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J Neurotrauma. 2017;34(2):328-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein LE, Fisher AM, Tagge CA, et al. . Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4(134):134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armed Forces Health Surveillance Branch Surveillance case definitions. https://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Epidemiology-and-Analysis/Surveillance-Case-Definitions. Published 2016. Accessed January 5, 2017.
- 37.Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci. 2015;66(pt B):75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kristman VL, Borg J, Godbolt AK, et al. . Methodological issues and research recommendations for prognosis after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. 2014;95(3)(suppl):S265-S277. [DOI] [PubMed] [Google Scholar]
- 39.Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury Department of Defense coding guidance for traumatic brain injury fact sheet. https://health.mil/About-MHS/Defense-Health-Agency/Research-and-Development. Published September 2010. Accessed March 14, 2018.
- 40.Sayed N, Culver C, Dams-O’Connor K, Hammond F, Diaz-Arrastia R. Clinical phenotype of dementia after traumatic brain injury. J Neurotrauma. 2013;30(13):1117-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guskiewicz KM, Marshall SW, Bailes J, et al. . Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57(4):719-726. [DOI] [PubMed] [Google Scholar]
- 42.Guo Z, Cupples LA, Kurz A, et al. . Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54(6):1316-1323. [DOI] [PubMed] [Google Scholar]
- 43.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol. 2013;9(4):211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15(1):49-59. [DOI] [PubMed] [Google Scholar]
- 45.Adams JH, Jennett B, Murray LS, Teasdale GM, Gennarelli TA, Graham DI. Neuropathological findings in disabled survivors of a head injury. J Neurotrauma. 2011;28(5):701-709. [DOI] [PubMed] [Google Scholar]
- 46.Johnson VE, Stewart W, Smith DH. Widespread τ and amyloid-β pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22(2):142-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Traumatic Brain Injury (TBI) Severity Case Definitions.
eAppendix 2. Traumatic Brain Injury (TBI) Severity Classification Using Modified Defense and Veterans Brain Injury Center (DVBIC) 2012 ICD-9 Diagnostic Code List.
eAppendix 3. Dementia ICD-9 Diagnostic Code List.
eTable. Traumatic Brain Injury Case Distribution.