Key Points
Question
What are the dynamics and pathogenesis of white matter hyperintensities in patients with reversible cerebral vasoconstriction syndrome?
Findings
In this cohort study including 65 patients, white matter hyperintensity loads peaked in the third week postonset and fell to a minimum in the fourth week, which correlated strongly with the severity of vasoconstriction and carotid pulsatility index.
Meaning
The dynamic white matter hyperintensities in patients with reversible cerebral vasoconstriction syndrome may be attributed to regional hypoperfusion and transmission of high pulsatile flow to microcirculation.
This cohort study investigates the spatiotemporal distribution and pathomechanisms of white matter hyperintensities in patients with reversible cerebral vasoconstriction syndrome.
Abstract
Importance
White matter hyperintense lesions (WMHs) are highly prevalent in patients with reversible cerebral vasoconstriction syndrome (RCVS); however, their characteristics and underlying pathophysiology are unclear.
Objective
To investigate the spatiotemporal distribution and pathomechanisms of WMHs in patients with RCVS.
Design, Setting, and Participants
We prospectively recruited patients with RCVS over a 3-year period from January 2010 through December 2012 from the headache center or emergency department of Taipei Veterans General Hospital, Taipei, Taiwan, a 2947-bed national medical center. In total, 85 patients with RCVS were approached, of whom 4 declined to participate, 5 declined follow-up scans, 6 were lost to follow-up, and 5 had suboptimal images. Patients received serial isotropic 3-dimension fluid-attenuated inversion recovery sequence imaging (1-mm slice thickness) with a 3-T magnetic resonance imaging machine as well as transcranial and extracranial color-coded sonography on registration and during follow-ups (at 1 and 2 months, with variations adapting to clinical condition). Data were analyzed from January 2015 to May 2017.
Main Outcomes and Measures
The fluid-attenuated inversion recovery lesion segmentation toolbox was used to segment WMHs automatically. The WMHs were classified as periventricular or deep and were segmented into 13 anatomical locations. The neuroimaging scientists who executed the program were blinded to clinical information. Vascular parameters, including the Lindegaard index (vasoconstriction severity), pulsatility index, and resistance index of the internal carotid artery, were independently collected for comparison.
Results
Sixty-five patients with RCVS completed the study and underwent a total of 162 magnetic resonance imaging examinations. Of the 65 included patients, 58 (89%) were women, and the mean (SD) age was 50.1 (8.9) years. The total mean (SD) WMH load peaked at 3.2 (4.4) cm3 in the third week postonset and fell to 0.8 (0.6) cm3 in the fourth week. White matter hyperintensities were predominantly frontal and periventricular. White matter hyperintensity load correlated strongly with Lindegaard index during the second week of the disease course (r = 0.908; P < .001) and also correlated with the pulsatility index and resistance index of the internal carotid artery.
Conclusions and Relevance
White matter hyperintensities in patients with RCVS have a dynamic temporal evolution that parallels disease severity. The finding of partially reversible WMHs deserves attention and should be known by clinicians taking care of patients with RCVS. White matter hyperintensities in RCVS may be attributed, at least partially, to regional hypoperfusion and impaired dampening capacity to central pulsatile flow.
Introduction
Reversible cerebral vasoconstriction syndrome (RCVS) is a neurovascular disorder characterized by abrupt, severe (mostly thunderclap) headaches and reversible segmental vasoconstriction of cerebral arteries.1,2,3 Headaches in RCVS usually repetitively occur in 2-week to 3-week periods after disease onset, whereas the vasoconstrictions might take up to 3 months to resolve.4,5 Up to one-third of patients with RCVS experience complications, such as posterior reversible encephalopathy syndrome (PRES), ischemic stroke, intracranial (ie, cortical, intracerebral, or subdural) hemorrhage,4,5,6,7,8,9,10 or cervical artery dissection.11 White matter hyperintensities (WMHs),5,12 hyperintense vessels on fluid-attenuated inversion recovery (FLAIR) imaging,12,13 or leakage of the blood-brain barrier14 might be the only imaging findings in patients without overt vasoconstrictions on initial angiographic studies,5,13 making diagnosis challenging in some cases.
Although WMHs on neuroimaging are more commonly seen than ischemic or hemorrhagic complications in patients with RCVS,5,12 to our knowledge, there have not yet been any studies that have focused on investigating their nature. The anatomical distribution of these WMHs has not been well characterized. However, in a preliminary study,12 we observed an apparent tendency for WMHs to be located in brain regions supplied by anterior circulation. The dynamic changes and pathomechanism associated with these WMHs also remain to be elucidated. In particular, it is not known whether RCVS-associated WMHs reflect lesions that are similar to the white matter lesions observed in other neurological disorders and aging15,16,17,18,19,20 or have their own disease-specific characteristics. Because WMHs have been known to predict increased risks of stroke, dementia, and death,19 imaging changes are frequently asked by both patients and clinicians. It is important to know if the WMHs lesions in patients with RCVS could accumulate or disappear with time, leading to significant burden of the brain. Our goals in this study were 2-fold. First, we investigated the spatial distribution and temporal evolution of WMHs in patients with RCVS. Second, we explored the possible pathogenesis of these WMHs by analyzing whether their loads correlated with vascular parameters.
Methods
Participants and Clinical Settings
The study protocol was approved by the Institutional Review Board of Taipei Veterans General Hospital. All participants provided written informed consent before entering the study. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki.
We recruited patients with RCVS prospectively from the headache center or emergency department of Taipei Veterans General Hospital, Taipei, Taiwan, a 2947-bed national medical center, over a 3-year period from January 2010 through December 2012. Reversible cerebral vasoconstriction syndrome diagnosis was based on the criteria we reported previously (Box).21 These criteria were based on the essential diagnostic elements of RCVS proposed by Calabrese et al1 and the diagnostic criteria of “benign (or reversible) angiopathy of the central nervous system” put forth by the International Classification of Headache Disorders, second edition (Code 6.7.3),22 with the exception of duration criterion D, which states that the headache (and neurological deficits, if present) should resolve spontaneously within 2 months.21 We confirmed retrospectively that all these patients fulfilled the proposed criteria for “headaches attributed to RCVS” in the International Classification of Headache Disorders, third edition, beta version (code 6.7.3).23
Box. Diagnostic Criteria of Reversible Cerebral Vasoconstriction Syndrome (RCVS) and Investigations for Differential Diagnosis.
Diagnostic Criteria of RCVS
At least 2 abrupt, severe (thunderclap) headaches, with or without focal neurological deficits
Vasoconstrictions demonstrated on magnetic resonance angiography
Reversibility of vasoconstrictions, as demonstrated by at least 1 follow-up magnetic resonance angiography within 3 mo
Aneurysmal subarachnoid hemorrhage or other intracranial disorders ruled out by appropriate investigations for differential diagnosis, but complications of RCVS, including posterior reversible encephalopathy syndrome, ischemic stroke, intracerebral hemorrhage, or cortical subarachnoid hemorrhage, are allowed
Investigations for Differential Diagnosis
History taking and neurological examinations to characterize headache features, identify red flags of secondary headaches, and look for secondary causes of RCVS
Laboratory testing to exclude secondary causes of hypertension (including serum and urine catecholamines, thyroid function, adrenocorticotropic hormone/cortisol, aldosterone, and pre-renin activities) as well as autoimmune diseases, especially markers for vasculitis
Magnetic resonance imaging, including magnetic resonance angiography and venography, to exclude secondary causes of thunderclap headaches, to exclude central nervous system vasculitis and atherosclerosis, and to evaluate reversibility of vascular stenosis
Extracranial and transcranial duplex sonography to exclude atheroma and dissection and to evaluate reversibility of vascular stenosis
Cerebrospinal fluid studies to exclude secondary causes of thunderclap headaches and to exclude vasculitis or other infectious or inflammatory processes
Diagnostic Evaluations, Treatment, and Follow-ups
The diagnostic procedures and interventions administered to the patients have been detailed elsewhere and are summarized in the Box.5,6,21 Briefly, within the first 2 days after the patient was seen, all clinical, neuroimaging, and laboratory investigations were performed to confirm cerebral vasoconstrictions and to exclude all other possible causes of thunderclap headaches, especially aneurysmal subarachnoid hemorrhage. Patients underwent oral (30-60 mg/4 hours) or intravenous (0.5-2 mg/hour) nimodipine therapy with close blood pressure monitoring immediately on being diagnosed with RCVS. Sequential magnetic resonance angiography and transcranial color-coded sonography were performed to ensure the reversibility of vasoconstrictions. All patients were followed up with regularly at the headache clinics until there were no more thunderclap headache attacks and marked improvement or complete resolution of the vasoconstrictions.
Quantification of WMHs
In addition to the conventional magnetic resonance imaging (MRI) sequences routinely obtained for patients with RCVS,21 we obtained isotropic 3-dimension FLAIR sequences with a 1-mm slice thickness using a 3-T MRI machine (MR750; GE Medical Systems) to enable identification of WMHs at the time of study enrollment and at follow-ups (Figure 1A). The follow-up scans were performed 1 and/or 2 months after entering the study, with variations of up to 1 week, depending on the patients’ clinical conditions and MRI machine availability. Sagittal isotropic 3-dimension FLAIR images were acquired with a fast spin-echo sequence with inversion recovery preparation and variable refocusing flip angles (repetition time/echo time = 6000/128 milliseconds; inversion time = 1864 milliseconds; field of view = 256 × 256 × 180; matrix size = 512 × 512; slice thickness = 1 mm). All images were acquired parallel to the anterior commissure–posterior commissure line. Each participant’s head was immobilized with cushions to minimize motion artifacts during image acquisition.
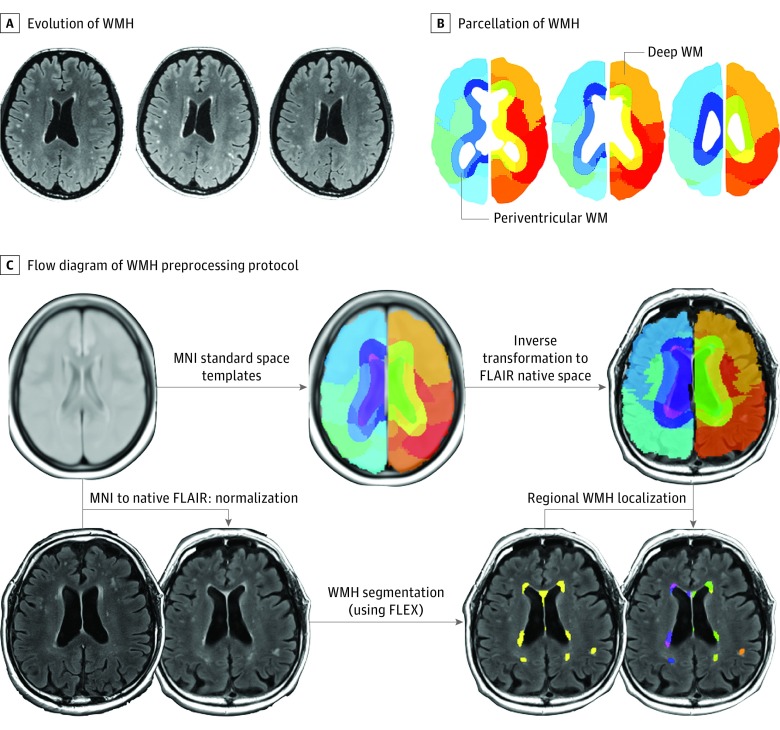
Figure 1. Segmentation of White Matter Hyperintensity Lesions (WMH) in Patients With Reversible Cerebral Vasoconstriction Syndrome.
A, Typical evolution of WMH in a patient with reversible cerebral vasoconstriction syndrome. Left panel, day 16; middle panel, day 29; right panel, day 84. B, Parcellation of WMH into periventricular (≤13 mm from ventricles) and deep (>13 mm from ventricles) lesions. C, The flow diagram of the WMH preprocessing protocol used in this study. The isotropic 3-dimension fluid-attenuated inversion recovery (FLAIR) images were used to perform automatic WMH segmentation with the FLAIR lesion segmentation toolbox (FLEX). An inverse transformation matrix derived from the nonlinear registration between native T2 images and FLAIR templates in MNI standard space was used to transform the brain atlas. Individual brain atlases were used for WMH localization.
To increase the accuracy of subsequent WMH segmentation with FLAIR imaging, we performed skull stripping using the Brain Extraction Tool24 on FLAIR images directly to remove any nonbrain tissue component and thereby obtain a brain mask. The skull-stripped FLAIR images were then used as initial inputs for automatic WMH segmentation procedures using similar protocols we adopted previously (Figure 1B and C).25 Before WMH segmentation, the nonparametric nonuniform intensity normalization approach26 (iterations = 150; stop threshold = 0.0001; distance = 55 mm) was applied on skull-stripped FLAIR images to account for intensity nonuniformity.
White matter hyperintensities were segmented automatically in skull-stripped intensity-normalized 3-dimension FLAIR images with the FLAIR lesion segmentation toolbox.27 We used a 4-step automated protocol: (1) noise-reduction filtering; (2) removal of clearly hyperintense voxels; (3) 2-class fuzzy C-means clustering; and (4) thresholding to segment probable WMHs. White matter hyperintensity segmented images were viewed in the same dimension as the native space FLAIR images.
To investigate potential correlations between clinical characteristics, severity of vasoconstriction, and regional WMH volume, we analyzed periventricular (≤13 mm from ventricles) and deep (>13 mm from ventricles) WMHs separately28 and divided the brain into 13 subregions based on major anatomical segments: bilateral frontal lobes, parietal lobes, limbic areas, subcortical areas, temporal lobes, occipital lobes, the brainstem, and the cerebellum. The anatomical divisions were delineated using the Wake Forest University PickAtlas (Figure 1B and C).29
Because high-resolution T1-weighted images were not available for all participants, we used the FLAIR template provided online (https://brainder.org/download/flair/) as the reference image for registering patients’ native space FLAIR images into MNI standard space. Each inverse transformation matrix from the MNI standard space to native space was calculated by nonlinear spatial normalization implemented in Statistical Parametric Mapping (SPM8; Wellcome Institute of Neurology) and then applied to the parcellation atlas to generate atlases in individual native space. The resultant individual brain atlases were applied to patient-corresponded WMH. Total WMH volumes were then determined for each location and corrected for total intracranial volume. Of note, any lesions attributable to ischemic stroke, intracerebral hemorrhage, or PRES were removed manually from the analysis. Because PRES lesions could also appear hyperintense on FLAIR imaging, PRES lesions with the signal changes on apparent diffusion coefficient mapping and diffusion-weighted imaging judged by an experienced neuroradiologist (J.F.L.) were excluded from final analysis. The volumes of the PRES lesions were markedly larger in comparison with those of the WMHs that we studied (eFigure 1 in the Supplement). All WMH quantification procedures were performed and cross-validated by investigators (K.H.C., C.C.H., and Y.H.H.) blinded to clinical data and magnetic resonance angiography findings.
Calculation of Vascular Parameters
Mean flow velocities of the middle cerebral artery (MCA) and the Lindegaard index (LI), calculated by dividing the mean flow velocity of the MCA by the mean flow velocity of the ipsilateral distal extracranial internal carotid artery (ICA), were taken as representations of vasoconstriction severity, as previously described.4 We also evaluated several additional vascular parameters for hypothesis generation in this exploratory study. The pulsatility index (PI) and resistance index (RI) of the ICA and MCA (including distal and proximal portion of the first segment of the MCA [M1], separated by a distance of 20 mm) were used to evaluate intracranial vascular compliance and resistance. Because the PI in large cerebral arteries decreases from proximal to distal30 and can be affected by the location and length of vasoconstriction, we calculated the mean PI in the distal and proximal M1 segments of both MCAs.14 The mean RI in the distal and proximal M1 segments of both MCAs was calculated in a similar way. The mean RI ratio along M1 (ie, distal RI to proximal RI), which has been shown to reflect the pulsation of penetrating arteries ramified from M1 (ie, the major blood supplier of periventricular and subcortical white matter) and to be associated with aging-related WMH progression, was also analyzed.14
Statistical Analysis
The primary outcome of this study was the spatial and temporal distribution of WMHs in the brains of patients with RCVS. The secondary outcome was to explore the association between vascular parameters and WMH loads. Descriptive statistics are presented as means and standard deviations, medians and ranges, or numbers and percentages. t Test, Fisher exact test, or χ2 test were used for comparisons between groups when appropriate. Paired t test was used to compare the WMH loads of the first scan with the the final scan. The Shapiro-Wilk test was used to test for normality. Pearson correlational analysis was used to examine relationships between WMH loads and clinical parameters. Time trend curves for WMH volume and LI were derived by the distance-weighted least squares method, which fit the smoothing curves by the following procedure: a polynomial (second-order) regression was calculated for each value on the X-variable scale (days from disease onset) to determine the corresponding Y value (WMH volume or LI) such that the influence of individual data points on the regression (ie, the weight) decreased with their distance from the particular X value.31 All calculated P values were 2-tailed; statistical significance was defined as a P value less than .05. Because the hypothesis-generating part of this study was exploratory, we did not correct for multiple comparisons. The analyses were performed with SPSS Statistics software package version 23.0 (IBM) or Statistica 10 (TIBCO Software).
Results
Participants
The screening process of participants is summarized in eFigure 2 in the Supplement. In total, 110 patients with 2 or more thunderclap headaches were screened, 4 were identified to have other secondary causes, and 21 were diagnosed as having probable RCVS. Among the 85 patients with RCVS approached, 4 declined to participate, 5 declined follow-up scans, 6 were lost to follow-up, and 5 had suboptimal images. In total, 65 patients with RCVS (58 women [89%]; mean [SD] age, 50.1 [8.9] years) completed the study and underwent a total of 162 MRI examinations (eTable 1 in the Supplement). Thirty-three patients received 2 scans, and 32 received 3 scans. Seven patients (11%) had a history of hypertension, and 28 (43%) exhibited blood pressure surges during headache attacks. Sixteen patients (25%) had a history of migraine. None of the patients had diabetes or coronary artery disease. Twenty-nine women (50%) were postmenopausal, and 3 (5%) were receiving hormone therapy at RCVS onset. Six patients (9%) had some possible secondary causes or associated conditions, including ingestion of pseudoephedrine (n = 3) or sibutramine (n = 1), massive blood transfusion (n = 1), and right vertebral artery dissection (n = 1). Of these patients, 3 had PRES, one of whom also had intracerebral hemorrhage. One of these patients had ischemic stroke, and another had cortical subarachnoid hemorrhage.
WMH Characteristics
The mean (SD) duration of the first image from disease onset in this cohort was 12.2 (7.0) days. In almost all cases (64 patients [98.5%]), WMHs were observed on the initial isotropic 3-dimension FLAIR imaging scan. None of these lesions were gadolinium-enhancing lesions or had restricted diffusion in diffusion-weighted images. The WMHs were located predominantly in the frontal lobe (40% to 52%) and in periventricular areas (68% to 80%) at all time points; WMHs were scarce in the brainstem and cerebellum (Figure 2) (eTable 2 in the Supplement).
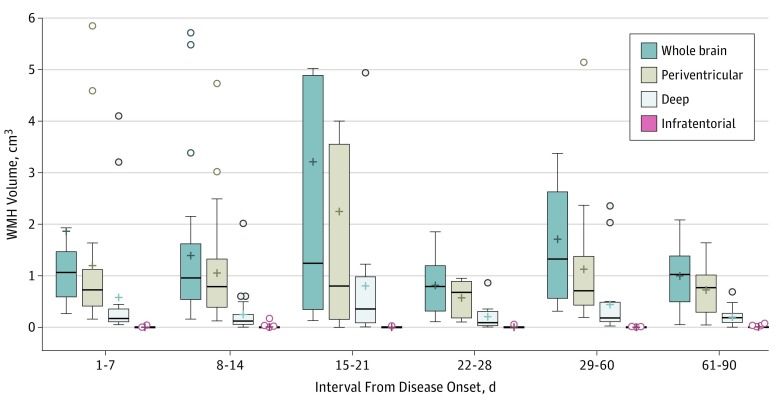
Figure 2. Comparisons of White Matter Hyperintensity Lesion Loads in Different Time Intervals From Disease Onset.
The horizontal lines within the box indicate the median, and the + indicates the mean. The error bars indicate the 1.5 interquartile range of the lower and upper quartiles. The open circle indicates outliers. The following outliers with a volume greater than 6 cm3 are not shown in the figure: day 1-7: whole brain, 8.06 cm3 and 10.35 cm3; day 15-21: whole brain, 16.33 cm3; periventricular, 11.39 cm3; and day 29-60: whole brain, 6.24 cm3.
The total WMH volume was dynamic over the disease course (Figure 3). Maximal WMH volume (mean [SD] volume, 3.05 [4.35] cm3) was noted during the third week after headache onset. A similar change in volume dynamics was observed for periventricular but not deep WMHs specifically (Figure 2) (eTable 2 in the Supplement). With respect to anatomical segments, WMHs in frontal, parietal, and limbic areas exhibited dynamic changes similar to total WMHs (ie, maximal in the third week), whereas the dynamic changes, owing to less lesion volume, were not statistically significant in temporal, occipital, or subcortical areas. When periventricular and deep WMHs within each anatomical location were analyzed separately, we found that periventricular frontal and limbic WMHs retained the aforementioned temporal trend (ie, maximum volume in the third week). Among deep WMHs, only those in the parietal lobe exhibited similar dynamic changes over time (Figure 2) (eTable 2 in the Supplement). When comparing the WMH loads on the final MRI scans with their corresponding initial scans in all individuals, there was a significant reduction of WMH volume (mean [SD] reduction, −0.66 [1.31] cm3; 95% CI, −0.99 to −0.34; P < .001).
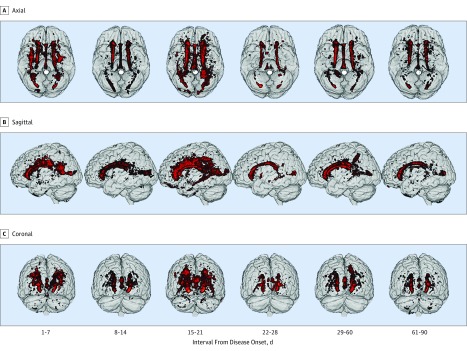
Figure 3. Anatomical Distribution and Temporal Evolution of White Matter Hyperintensity Lesions in Patients With Reversible Cerebral Vasoconstriction Syndrome.
The distribution of white matter hyperintensity lesions is demonstrated in axial (A), sagittal (B), and coronal (C) planes.
Exploratory Correlational Analyses Between WMH Volume and Vascular Parameters
The temporal trend of total WMH load (ie, summed WMH volume) was similar to that of vasoconstriction severity, as indicated by LI (eFigure 3 in the Supplement). Considering the disease course in its entirety, there was a significant correlation between total WMH load and LI for periventricular WMHs (r = 0.236; P < .001) but not deep WMHs (r = 0.059; P = .39) (Table). In the second week, the correlation between LI and total WMH load was very strong (r = 0.906; P < .001) and could be found for both periventricular and deep white matter. The WMH load–LI correlation remained remarkable in the third week (r = 0.640; P = .008) but became marginal when the WMHs were divided into periventricular and deep subgroups (Table).
Table. Correlation Between Lindegaard Index (LI) and the Volume of White Matter Hyperintensity Lesions (WMHs) in Different Periods of the Disease Course.
| Characteristic | Duration From Headache Onset, d | |||||
|---|---|---|---|---|---|---|
| 1-7 | 8-14 | 15-21 | 22-28 | 29-60 | 61-90 | |
| No. of scans | 18 | 28 | 21 | 23 | 35 | 36 |
| Whole brain, r | 0.014 | 0.906 | 0.640 | 0.312 | 0.333 | −0.155 |
| P value | .87 | <.001 | .008 | .22 | .18 | .44 |
| Periventricular white matter, r | 0.031 | 0.908 | 0.496 | 0.384 | 0.406 | −0.131 |
| P value | .90 | <.001 | .051 | .13 | .01 | .51 |
| Deep white matter, r | 0.051 | 0.806 | 0.454 | −0.018 | 0.017 | −0.153 |
| P value | .84 | <.001 | .08 | .09 | .95 | .45 |
| Brainstem and cerebellum, r | 0.116 | 0.311 | −0.030 | 0.019 | 0.003 | −0.156 |
| P value | .65 | .26 | .91 | .94 | .99 | .44 |
For the entire disease course, both periventricular and deep WMH volumes correlated with PI and RI for the ICA, with a particularly strong correlation in periventricular frontal white matter. Similar to PI and RI for the ICA, the mean PI in the distal and proximal M1 correlated with the volume of WMH majorly for the periventricular WMH; the mean RI in the distal and proximal M1 segment had a similar trend of association with periventricular WMH, but the association was weaker than the mean PI in the distal and proximal M1. The mean RI ratio along M1 did not correlate with WMH volumes in any specific anatomical location (eTable 3 in the Supplement).
Correlation Between Clinical Parameters and WMHs
All examined clinical parameters, including age, sex, preexisting hypertension, migraine, menopausal status, types or numbers of headache triggers, blood pressure surge, numbers and durations of thunderclap headaches prior to sample collection, and accompanying symptoms, such as nausea, vomiting, and photophobia or phonophobia, were not associated with WMH volume (eTable 4 in the Supplement). Because the sample sizes in different time intervals were limited, only the WMH loads at the first scan were used to correlate with clinical parameters.
Discussion
Based on our implementation of high-field, high-resolution isotropic 3-dimension FLAIR imaging with a novel WMH segmentation technique, we developed a systemic characterization of the spatial distribution and temporal evolution of WMHs in patients with RCVS. We found that the WMHs in RCVS waxed and waned in a dynamic pattern distinct from that seen in other physiological processes (ie, aging) and pathological white matter disorders. Although our study design did not allow us to address the long-term outcomes, our longitudinal results indicate that these RCVS-related lesions could at least partially be reversible within the 3-month observation period. This finding deserves attention and should be known by both clinicians and patients.
Dynamic changes in WMH volumes, especially periventricular WMH volumes, resembled the temporal trend in vasoconstrictions, which we determined previously by transcranial color-coded sonography and magnetic resonance angiography.4,5 This parallel indicates that vasoconstriction of major cerebral arteries may contribute to the rapid development and resolution of WMHs in RCVS. Although our study could not confirm causality directly, the strong association between WMH load and LI, especially during the second (r = 0.906) and third (r = 0.640) weeks after disease onset, suggests that WMH load during this period could be a disease severity marker that allows us to visualize the progression of disease. Interestingly, this dramatic wax-and-wane phenomenon of WMHs within a period of only a few weeks has not been demonstrated in other neurovascular disorders. Although our study did not include a control group, 1 previous study32 that involved a large cohort of similarly aged community residents (aged 44 to 48 years; n = 428) provides a tentative basis for comparison. The mean WMH load in that cohort was 0.278 cm3, which is less than a third of the nadir WMH load (1.00 cm3) and less than a tenth of the peak WMH load (3.05 cm3) observed in this group of patients with RCVS. However, it should be noted that the size of the same lesion measured by the 2 different imaging techniques might not be completely the same.
Periventricular WMHs accounted for 68% to 80% of the total WMH load (variance along disease course) in RCVS, with periventricular frontal WMHs accounting for roughly half of all observed WMHs. This anatomical distribution is distinct from the WMHs in patients with migraine, in which deep white matter rather than periventricular white matter is more frequently affected.17 The spatial distribution of WMHs in these patients was, relatively, more similar to that in elderly individuals, particularly with small ischemic vascular diseases,33,34 suggesting that it might be consequent, at least partially, to hypoperfusion because periventricular white matter is known to lack collateral circulation and to be vulnerable to ischemic events related to local or systemic perturbations in circulation.33,34 However, the different spatial distribution of WMHs observed here vs in patients with ischemic stroke or PRES suggests that hypoperfusion is unlikely the sole cause. It is possible that the dampening capacity of MCA to extracranial pulsatile blood flow was overwhelmed despite increased impedance attributed to vasoconstriction, which imposes excessive pulse pressure to the perforating arteries ramifying from the M1 stem and may eventually lead to increased microvascular damage, increased vascular permeability, and impaired solute clearance via the perivascular glymphatic system.
Strengths and Limitations
This study had several strengths, including a well-characterized cohort with diagnoses ascertained by headache experts using validated criteria, a large quantity of high-resolution images obtained from the same high-field MRI machine with a standardized protocol, and automatic and bias-free methods of WMH quantification and segmentation. This study also had limitations. First, all of the patients received nimodipine treatment, which could have altered the clinical course. Based on a prior study,35 our practice was to treat RCVS with nimodipine therapy, which may differ from practices at other institutions and may have influenced these data. Importantly, with judicious dose monitoring and adjustment, none of the patients experienced hypotension during the therapeutic course, excluding the possibility that pathogenic hypoperfusion was caused by the medication. Second, we did not correct our P values for multiple comparisons considering the exploratory nature of the study, the small sample sizes after subgrouping, and the possibility of overcorrection owing to high collinearity of the studied vascular parameters. These analyses involving multiple comparisons might be underpowered, and the significance derived from these findings should be interpreted with caution. Furthermore, the lack of consistency in the repeated scans obtained in different follow-up periods makes it difficult to draw firm conclusions about the influence of clinical covariates. The heterogeneity of the interval between the first image and disease onset might also have affected findings. Future studies are needed to investigate to validate our findings.
Conclusions
White matter hyperintensity lesions in RCVS have a disease-specific dynamic spatiotemporal evolution that parallels severity of vasoconstrictions. The pathogenesis of WMHs in patients with RCVS may be associated with regional hypoperfusion, impaired dampening capacity of cerebral arteries or arterioles to central pulsatile flow, and microvascular damage. White matter hyperintensities in patients with RCVS are partially reversible, which should be known by both clinicians and patients.
eTable 1. Characteristics of the current cohort (n = 65).
eTable 2. Spatial and temporal distribution of white matter hyperintensity lesions during the disease course.
eTable 3. Exploratory analyses for the correlation between volume of white matter hyperintensity lesions and vascular parameters throughout the disease course.
eTable 4. Correlation between clinical variables and the volume of white matter hyperintense lesions (in the initial MRI).
eFigure 1. Box plot showing the distribution of the sizes of lesions identified on the first isotropic 3-dimension fluid-attenuated inversion recovery images.
eFigure 2. Diagram of patient screening and enrollment.
eFigure 3. Relationship between the white matter hyperintensity lesion load and the severity of vasoconstriction indicated by the Lindegaard index along the disease course.
References
- 1.Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146(1):34-44. [DOI] [PubMed] [Google Scholar]
- 2.Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012;11(10):906-917. [DOI] [PubMed] [Google Scholar]
- 3.Chen SP, Fuh JL, Wang SJ. Reversible cerebral vasoconstriction syndrome: current and future perspectives. Expert Rev Neurother. 2011;11(9):1265-1276. [DOI] [PubMed] [Google Scholar]
- 4.Chen SP, Fuh JL, Chang FC, Lirng JF, Shia BC, Wang SJ. Transcranial color doppler study for reversible cerebral vasoconstriction syndromes. Ann Neurol. 2008;63(6):751-757. [DOI] [PubMed] [Google Scholar]
- 5.Chen SP, Fuh JL, Wang SJ, et al. Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol. 2010;67(5):648-656. [DOI] [PubMed] [Google Scholar]
- 6.Chen SP, Fuh JL, Lirng JF, Chang FC, Wang SJ. Recurrent primary thunderclap headache and benign CNS angiopathy: spectra of the same disorder? Neurology. 2006;67(12):2164-2169. [DOI] [PubMed] [Google Scholar]
- 7.Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome: a prospective series of 67 patients. Brain. 2007;130(pt 12):3091-3101. [DOI] [PubMed] [Google Scholar]
- 8.Ducros A, Fiedler U, Porcher R, Boukobza M, Stapf C, Bousser MG. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke. 2010;41(11):2505-2511. [DOI] [PubMed] [Google Scholar]
- 9.Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68(8):1005-1012. [DOI] [PubMed] [Google Scholar]
- 10.Topcuoglu MA, Singhal AB. Hemorrhagic reversible cerebral vasoconstriction syndrome: features and mechanisms. Stroke. 2016;47(7):1742-1747. [DOI] [PubMed] [Google Scholar]
- 11.Mawet J, Boukobza M, Franc J, et al. Reversible cerebral vasoconstriction syndrome and cervical artery dissection in 20 patients. Neurology. 2013;81(9):821-824. [DOI] [PubMed] [Google Scholar]
- 12.Chen SP, Fuh JL, Lirng JF, Wang SJ. Hyperintense vessels on flair imaging in reversible cerebral vasoconstriction syndrome. Cephalalgia. 2012;32(4):271-278. [DOI] [PubMed] [Google Scholar]
- 13.Singhal AB, Topcuoglu MA, Fok JW, et al. Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Ann Neurol. 2016;79(6):882-894. [DOI] [PubMed] [Google Scholar]
- 14.Lee WJ, Jung KH, Ryu YJ, et al. Progression of cerebral white matter hyperintensities and the associated sonographic index. Radiology. 2017;284(3):824-833. [DOI] [PubMed] [Google Scholar]
- 15.Dinia L, Bonzano L, Albano B, et al. White matter lesions progression in migraine with aura: a clinical and MRI longitudinal study. J Neuroimaging. 2013;23(1):47-52. [DOI] [PubMed] [Google Scholar]
- 16.Kruit MC, van Buchem MA, Hofman PA, et al. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291(4):427-434. [DOI] [PubMed] [Google Scholar]
- 17.Palm-Meinders IH, Koppen H, Terwindt GM, et al. Structural brain changes in migraine. JAMA. 2012;308(18):1889-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuller LH, Arnold AM, Longstreth WT Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28(9):1307-1315. [DOI] [PubMed] [Google Scholar]
- 19.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11(3):157-165. [DOI] [PubMed] [Google Scholar]
- 21.Chen SP, Fuh JL, Lirng JF, Wang YF, Wang SJ. Recurrence of reversible cerebral vasoconstriction syndrome: a long-term follow-up study. Neurology. 2015;84(15):1552-1558. [DOI] [PubMed] [Google Scholar]
- 22.Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(suppl 1):9-160. [DOI] [PubMed] [Google Scholar]
- 23.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang CC, Liu ME, Chou KH, et al. Effect of BDNF Val66Met polymorphism on regional white matter hyperintensities and cognitive function in elderly males without dementia. Psychoneuroendocrinology. 2014;39:94-103. [DOI] [PubMed] [Google Scholar]
- 26.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87-97. [DOI] [PubMed] [Google Scholar]
- 27.Gibson E, Gao F, Black SE, Lobaugh NJ. Automatic segmentation of white matter hyperintensities in the elderly using FLAIR images at 3T. J Magn Reson Imaging. 2010;31(6):1311-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry. 2008;64(4):273-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233-1239. [DOI] [PubMed] [Google Scholar]
- 30.Zarrinkoob L, Ambarki K, Wåhlin A, et al. Aging alters the dampening of pulsatile blood flow in cerebral arteries. J Cereb Blood Flow Metab. 2016;36(9):1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLain DH. Drawing contours from arbitrary data points. Comput J. 1974;17(4):318-324. doi: 10.1093/comjnl/17.4.318 [DOI] [Google Scholar]
- 32.Wen W, Sachdev PS, Li JJ, Chen X, Anstey KJ. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44-48. Hum Brain Mapp. 2009;30(4):1155-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63(2):246-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holland CM, Smith EE, Csapo I, et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39(4):1127-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu SR, Liao YC, Fuh JL, Lirng JF, Wang SJ. Nimodipine for treatment of primary thunderclap headache. Neurology. 2004;62(8):1414-1416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of the current cohort (n = 65).
eTable 2. Spatial and temporal distribution of white matter hyperintensity lesions during the disease course.
eTable 3. Exploratory analyses for the correlation between volume of white matter hyperintensity lesions and vascular parameters throughout the disease course.
eTable 4. Correlation between clinical variables and the volume of white matter hyperintense lesions (in the initial MRI).
eFigure 1. Box plot showing the distribution of the sizes of lesions identified on the first isotropic 3-dimension fluid-attenuated inversion recovery images.
eFigure 2. Diagram of patient screening and enrollment.
eFigure 3. Relationship between the white matter hyperintensity lesion load and the severity of vasoconstriction indicated by the Lindegaard index along the disease course.