Key Points
Question
What are the clinical features of Zika virus–associated Guillain-Barré syndrome?
Findings
In this analysis of public health surveillance data from 123 patients with Guillain-Barré syndrome with and without evidence of Zika virus infection identified through public health surveillance in Puerto Rico, clinical features more frequent among cases with evidence of Zika virus infection were facial weakness, facial paresthesia, dysphagia, shortness of breath, elevated protein levels in cerebrospinal fluid, admission to the intensive care unit, and need for mechanical ventilation, as well as (at 6 months) excessive or inadequate tearing, difficulty drinking, and self-reported substantial pain.
Meaning
Zika virus–associated Guillain-Barré syndrome had higher morbidity during acute-phase neuropathy and manifested more frequent acute and residual cranial neuropathy.
Abstract
Importance
The pathophysiologic mechanisms of Guillain-Barré syndrome (GBS) associated with Zika virus (ZIKV) infection may be indicated by differences in clinical features.
Objective
To identify specific clinical features of GBS associated with ZIKV infection.
Design, Setting, and Participants
During the ZIKV epidemic in Puerto Rico, prospective and retrospective strategies were used to identify patients with GBS who had neurologic illness onset in 2016 and were hospitalized at all 57 nonspecialized hospitals and 2 rehabilitation centers in Puerto Rico. Guillain-Barré syndrome diagnosis was confirmed via medical record review using the Brighton Collaboration criteria. Specimens (serum, urine, cerebrospinal fluid, and saliva) from patients with GBS were tested for evidence of ZIKV infection by real-time reverse transcriptase–polymerase chain reaction; serum and cerebrospinal fluid were also tested by IgM enzyme-linked immunosorbent assay. In this analysis of public health surveillance data, a total of 123 confirmed GBS cases were identified, of which 107 had specimens submitted for testing; there were 71 patients with and 36 patients without evidence of ZIKV infection. Follow-up telephone interviews with patients were conducted 6 months after neurologic illness onset; 60 patients with and 27 patients without evidence of ZIKV infection participated.
Main Outcomes and Measures
Acute and long-term clinical characteristics of GBS associated with ZIKV infection.
Results
Of 123 patients with confirmed GBS, the median age was 54 years (age range, 4-88 years), and 68 patients (55.3%) were male. The following clinical features were more frequent among patients with GBS and evidence of ZIKV infection compared with patients with GBS without evidence of ZIKV infection: facial weakness (44 [62.0%] vs 10 [27.8%]; P < .001), dysphagia (38 [53.5%] vs 9 [25.0%]; P = .005), shortness of breath (33 [46.5%] vs 9 [25.0%]; P = .03), facial paresthesia (13 [18.3%] vs 1 [2.8%]; P = .03), elevated levels of protein in cerebrospinal fluid (49 [94.2%] vs 23 [71.9%]; P = .008), admission to the intensive care unit (47 [66.2%] vs 16 [44.4%]; P = .03), and required mechanical ventilation (22 [31.0%] vs 4 [11.1%]; P = .02). Six months after neurologic illness onset, patients with GBS and evidence of ZIKV infection more frequently reported having excessive or inadequate tearing (30 [53.6%] vs 6 [26.1%]; P = .03), difficulty drinking from a cup (10 [17.9%] vs 0; P = .03), and self-reported substantial pain (15 [27.3%] vs 1 [4.3%]; P = .03).
Conclusions and Relevance
In this study, GBS associated with ZIKV infection was found to have higher morbidity during the acute phase and more frequent cranial neuropathy during acute neuropathy and 6 months afterward. Results indicate GBS pathophysiologic mechanisms that may be more common after ZIKV infection.
This analysis of public health surveillance data identifies specific clinical features and pathophysiologic mechanisms of Guillain-Barré syndrome associated with Zika virus infection among patients in Puerto Rico.
Introduction
Guillain-Barré syndrome (GBS) is an uncommon autoimmune condition, characterized by progressive, bilateral weakness and diminished reflexes.1 It has been associated with infectious agents, including Campylobacter jejuni,2 and (less frequently) with vaccines.1 The global annual GBS incidence is estimated at 1.1 to 1.8 cases per 100 000 population, increasing with age and varying by sex and geographic region.3 Mortality rates among patients with GBS in North America and Europe vary from 3% to 7%,1 with death most often resulting from respiratory failure, autonomic dysfunction, or deep vein thrombosis.4 In Puerto Rico, the annual GBS incidence before the introduction of Zika virus (ZIKV) was estimated at 1.7 cases per 100 000 population, and in-hospital mortality was estimated at 4%.5
Increased GBS incidence during ZIKV epidemics has provided a unique opportunity to further delineate the clinical features and pathophysiology of GBS.6,7,8,9,10,11 The Puerto Rico Department of Health (PRDH), in San Juan, reported the first case of ZIKV disease in December 2015. Staff at the Adult’s University Hospital (San Juan) suspected antecedent ZIKV infection in a patient with GBS who was seen in January 2016; specimen testing by the PRDH and the US Centers for Disease Control and Prevention (CDC), San Juan, Puerto Rico, detected evidence of ZIKV infection.12 Thereafter, emergency public health surveillance was implemented to prospectively identify GBS cases and test patient specimens for evidence of infection with ZIKV, dengue virus (DENV), and chikungunya virus (CHIKV).13,14 To characterize GBS associated with ZIKV infection and lend insights into pathophysiologic mechanisms, we analyzed the clinical features of patients with GBS in Puerto Rico with vs without evidence of ZIKV infection who had neurologic illness onset during 2016.
Methods
GBS Case Identification
This investigation was reviewed by the CDC human participants’ research advisors and was determined to be nonresearch emergency public health activities; patient informed consent was not required to report suspected cases. In February 2016, a passive GBS surveillance system was implemented to prospectively identify GBS cases in Puerto Rico13,14; in October 2016, reporting of suspected and fatal GBS cases was made compulsory.15 Health care professionals were requested to report suspected GBS cases using a case report form along with serum specimens (eFigure 1 in the Supplement); if available, urine, cerebrospinal fluid (CSF), and saliva specimens were also submitted. During February through April 2017, all 57 nonspecialized hospitals and 2 rehabilitative inpatient care centers in Puerto Rico provided lists of patients hospitalized during 2016 who had an International Statistical Classification of Health Related Problems and Diseases, Tenth Revision, diagnostic code for GBS (code G61.0) in their medical record.
Arbovirus Diagnostic Testing
Patient specimens were tested using a real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) Trioplex assay to detect evidence of current infection with ZIKV, DENV, and CHIKV.16 Serum and CSF specimens were also tested by IgM enzyme-linked immunosorbent assay (ELISA) to detect anti-ZIKV, anti-DENV, and anti-CHIKV IgM antibodies.17,18,19 Cases with evidence of ZIKV infection were positive for ZIKV by rRT-PCR and/or IgM ELISA. Cases without evidence of ZIKV infection were negative or equivocal for ZIKV in all specimens by both rRT-PCR and IgM ELISA. Only results from specimens collected within 2 months before neurologic illness onset or during hospitalization for neurologic illness were considered. The database for the Puerto Rico Passive Arboviral Disease Surveillance System was queried for laboratory test results.
Confirmation of GBS Diagnosis and Clinical Data Collection
Medical record review was conducted to confirm GBS diagnosis for all suspected GBS cases reported via passive surveillance and cases retrospectively identified by diagnostic code that had hospital stays of at least 3 days and no alternative diagnosis of neurologic illness. Guillain-Barré syndrome diagnosis was confirmed using the Brighton Collaboration criteria,20 which include data on clinical presentation, CSF laboratory results, and electrophysiologic findings. Confirmed GBS cases met the Brighton Collaboration criteria levels 1 through 3 or criteria for Miller-Fisher syndrome phenotype.20 Cases that did not meet these criteria and had no alternative final diagnosis were considered suspected GBS cases. Medical record reviews were performed after hospital discharge or more than 28 days after neurologic illness onset. Collected data included demographic characteristics, place of residence, acute antecedent illness, in-hospital infectious disease testing, clinical course of neurologic illness, medical interventions, electrodiagnostic study and imaging results, and hospital discharge status. Cytoalbuminologic dissociation was defined as a total CSF white blood cell count less than 50 cells/μL and a CSF protein concentration of at least 45 mg/dL. Modified Rankin Scale score and Hughes Disability Score were calculated for disability at clinical nadir.21,22
Assessment of Long-term Disability
Long-term disability data approximately 6 months after neurologic illness onset were collected through telephone interview. Oral informed consent was obtained before interview. Patients were considered lost to follow-up after 5 telephone calls at different times of the day without establishing contact. The administered questionnaire used the modified Rankin Scale, Overall Disability Sum Score,23 and Facial Disability Index.24 Self-reported data on substantial fatigue and pain at the time of interview and poor mental status within the past 30 days were also collected.
Statistical Analysis
Frequencies were calculated for demographic, laboratory, clinical, and long-term disability data. Pearson χ2 test, Fisher exact test, and χ2 partitioning were used to compare categorical variables between confirmed GBS cases with vs without evidence of ZIKV infection. Median 2-sample test was used to compare continuous variables. P < .05 was considered statistically significant. The 2016 US Census Bureau population estimate for Puerto Rico (3 411 307 residents) was used to calculate the annual incidence in Puerto Rico and by public health region.25 Population data were stratified by persons 50 years or older vs persons younger than 50 years.
Results
Case Identification, Arbovirus Diagnostic Test Findings, and Epidemiologic Characteristics
Health care professionals reported a total of 135 suspected GBS cases with neurologic illness onset in 2016, of which 98 (72.6%) were confirmed GBS cases, 8 (5.9%) were suspected GBS cases, and 29 (21.5%) were noncases (eFigure 2 in the Supplement). A total of 181 individual patients were identified by diagnostic code, of whom 100 (55.2%) had been reported through passive surveillance. Nine of 181 (5.0%) were excluded from additional evaluation given hospital stays less than 3 days (n = 3) and discharge diagnosis (n = 9). After medical record review of the remaining 72 previously unreported patients, 25 (34.7%) were confirmed GBS cases, 4 (5.6%) were suspected GBS cases, and 43 (59.7%) were noncases.
Combined, 123 confirmed GBS cases were identified. Their median age was 54 years (age range, 4-88 years), and 68 patients (55.3%) were male. A total of 107 confirmed GBS cases (87.0%) had at least one specimen submitted for arbovirus testing, of which 71 (66.4%) had evidence of ZIKV infection; 28 (39.4%) of these had at least one specimen that was positive by rRT-PCR (eTable 1 in the Supplement). Of confirmed GBS cases with evidence of ZIKV infection, 15 (21.1%), 42 (59.2%), and 14 (19.7%) met the Brighton Collaboration criteria levels 1, 2, and 3, respectively; no cases met criteria for Miller-Fisher syndrome phenotype (Table 1). This distribution did not differ from that of confirmed GBS cases without evidence of ZIKV infection. Two confirmed GBS cases had detectable anti-CHIKV IgM antibodies, one of which also had ZIKV nucleic acid detected by rRT-PCR. No confirmed GBS cases had DENV or CHIKV nucleic acid detected by rRT-PCR or had detectable anti-DENV IgM antibodies in the absence of detection of anti-ZIKV IgM antibodies. Among the 12 suspected GBS cases, 10 had specimens submitted for arbovirus testing. Five suspected cases had evidence of ZIKV infection; 1 had at least one specimen positive by rRT-PCR. No suspected GBS cases had anti-DENV IgM antibodies in the absence of anti-ZIKV IgM antibodies or any evidence of CHIKV infection.
Table 1. Demographic and Clinical Characteristics of Acute Neurologic Illness Among Patients With Confirmed Guillain-Barré Syndrome With vs Without Evidence of Zika Virus Infection, Puerto Rico, 2016.
| Variable | With Evidence of Zika Virus Infection (n = 71) |
Without Evidence of Zika Virus Infection (n = 36) |
P Value |
|---|---|---|---|
| Age, y | |||
| Median (range) | 55 (21-88) | 49 (4-83) | .25 |
| Sex, No. (%) | |||
| Female | 37 (52.1) | 10 (27.8) | .02 |
| Brighton Collaboration Criteria level, No. (%) | |||
| 1 | 15 (21.1) | 6 (16.7) | .75 |
| 2 | 42 (59.2) | 24 (66.7) | |
| 3 | 14 (19.7) | 6 (16.7) | |
| Duration, median (range), d | |||
| Antecedent illness to neurologic illness onset | 7 (0-21) | 7 (0-15) | .90 |
| Neurologic illness onset to clinical nadir | 7 (2-27) | 9 (1-28) | .23 |
| Neurologic illness onset to lumbar puncture | 9 (1-92) | 8 (1-34) | .65 |
| Hospitalization | 12 (4-94) | 10 (2-90) | .40 |
| Antecedent illness, No. (%) | |||
| Acute antecedent illness | 57 (80.3) | 26 (72.2) | .33 |
| Rash | 36 (50.7) | 3 (8.3) | <.001 |
| Fever | 28 (39.4) | 13 (36.1) | .74 |
| Muscle pain | 13 (18.3) | 5 (13.9) | .56 |
| Arthralgia | 13 (18.3) | 1 (2.8) | .03 |
| Conjunctivitis | 10 (14.1) | 1 (2.8) | .09 |
| Diarrhea | 9 (12.7) | 8 (22.2) | .20 |
| Chills | 7 (9.9) | 3 (8.3) | >.99 |
| Coughing | 3 (4.2) | 6 (16.7) | .06 |
| Rhinorrhea | 0 | 5 (13.9) | .004 |
| Signs and symptoms of neurologic illness, No. (%) | |||
| Hyporeflexia or areflexia | 71 (100) | 36 (100) | NA |
| Leg weakness | 68 (95.8) | 36 (100) | .55 |
| Leg parethesia | 56 (78.9) | 25 (69.4) | .28 |
| Arm weakness | 52 (73.2) | 21 (58.3) | .12 |
| Facial weakness | 44 (62.0) | 10 (27.8) | <.001 |
| Arm paresthesia | 39 (54.9) | 16 (44.4) | .31 |
| Dysphagia | 38 (53.5) | 9 (25.0) | .005 |
| Shortness of breath | 33 (46.5) | 9 (25.0) | .03 |
| Dysarthria | 27 (38.0) | 9 (25.0) | .18 |
| Gait ataxia | 23 (32.4) | 13 (36.1) | .70 |
| Dysautonomia | 13 (18.3) | 7 (19.4) | .89 |
| Facial paresthesia | 13 (18.3) | 1 (2.8) | .03 |
| Medical complications, No./Total No. (%)a | |||
| Tracheostomy | 6/38 (15.8) | 1/24 (4.2) | .23 |
| Sepsis | 5/38 (13.2) | 1/24 (4.2) | .39 |
| Gastrostomy | 5/38 (13.2) | 0/24 | .15 |
| Pneumonia | 4/38 (10.5) | 2/24 (8.3) | >.99 |
| Shock | 1/38 (2.6) | 1/24 (4.2) | >.99 |
| CSF analysis, No./Total No. (%)b | |||
| WBC count <50 cells/μLc | 52/52 (100) | 28/30 (93.3) | .13 |
| Protein ≥45 mg/dL | 49/52 (94.2) | 23/32 (71.9) | .008 |
| Cytoalbuminologic dissociationc | 49/52 (94.2) | 19/30 (63.3) | <.001 |
| Electrophysiologic findings, No./Total No. (%)d | |||
| AIDP subtype | 16/19 (84.2) | 6/14 (42.9) | .06 |
| Acute motor axonal neuropathy subtype | 2/19 (10.5) | 3/14 (21.4) | |
| Acute motor-sensory axonal neuropathy subtype | 1/19 (5.3) | 2/14 (14.3) | |
| Abnormal findings possibly associated with GBS | 0/19 | 1/14 (7.1) | |
| Normal study results | 0/19 | 2/14 (14.3) | |
| Medical interventions, No. (%) | |||
| Admitted to hospital | 71 (100) | 36 (100) | NA |
| Treated with intravenous IgG | 69 (97.2) | 32 (88.9) | .18 |
| Admitted to intensive care unit | 47 (66.2) | 16 (44.4) | .03 |
| Required mechanical ventilation | 22 (31.0) | 4 (11.1) | .02 |
| Disability at clinical nadir, median (range) | |||
| Modified Rankin Scale score | 5 (2-6) | 5 (3-6) | .39 |
| Hughes Disability Score | 4 (1-6) | 4 (2-6) | .09 |
| Clinical outcome, No. (%) | |||
| Discharged home | 32 (45.1) | 22 (61.1) | .12 |
| Transferred to rehabilitation center or skilled nursing facility | 35 (49.3) | 11 (30.6) | |
| Persistent comatose state >12 mo | 2 (2.8) | 0 | |
| Died in hospital | 2 (2.8) | 3 (8.3) |
Abbreviations: AIDP, acute inflammatory demyelinating polyneuropathy; CSF, cerebrospinal fluid; GBS, Guillain-Barré syndrome; NA, not applicable; WBC, white blood cell.
Does not include 33 cases with and 12 cases without evidence of Zika virus infection for which data on complications were not collected.
Does not include 19 cases with and 4 cases without evidence of ZIKV infection for which CSF analysis was not performed.
Does not include 2 cases without evidence of ZIKV infection for which no WBC results from CSF analysis were available.
Does not include 52 cases with and 22 cases without evidence of Zika virus infection for which electrodiagnostic study was not performed or study results were not available.
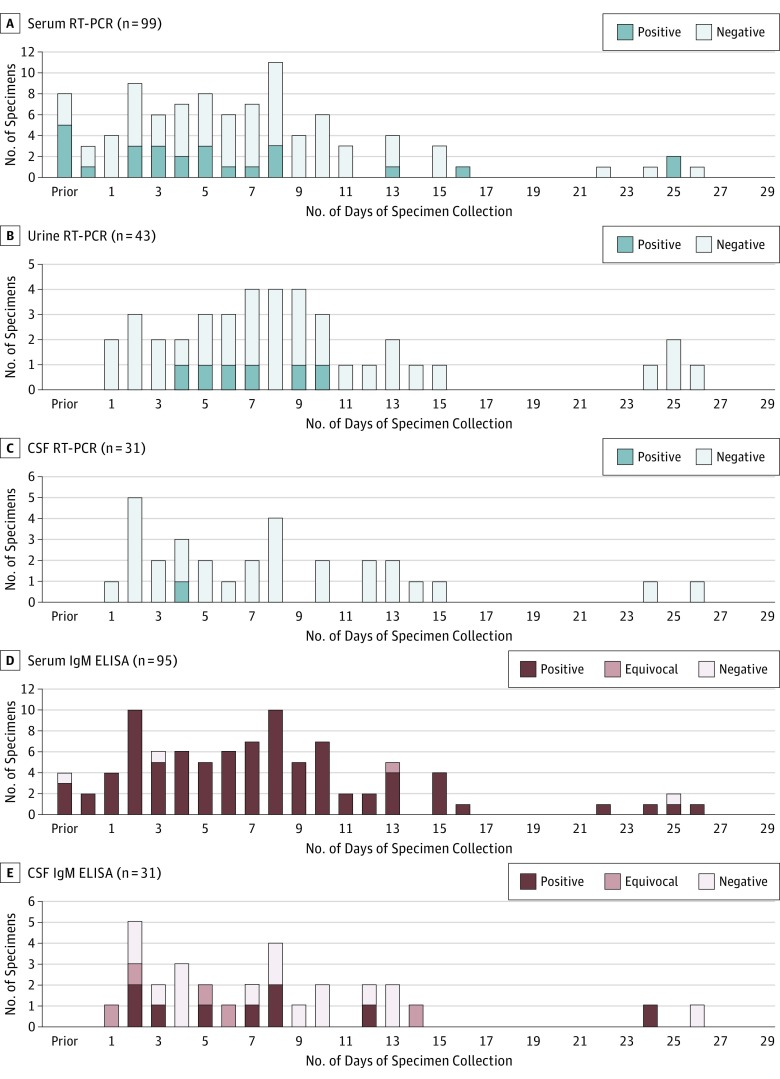
Among the 71 confirmed GBS cases with evidence of ZIKV infection, a total of 107 serum specimens, 43 urine specimens, 32 CSF specimens, and 18 saliva specimens were submitted and had available ZIKV rRT-PCR and/or IgM ELISA test results (Figure 1 and eTable 2 in the Supplement). For specimens collected during days 0 through 9 after neurologic illness onset, ZIKV nucleic acid was detected by rRT-PCR in 17 (26.2%) of 65 serum specimens, 5 (18.5%) of 27 urine specimens, and 1 (5.0%) of 20 CSF specimens. For specimens collected at least 10 days after neurologic illness onset, ZIKV nucleic acid was detected by rRT-PCR in 5 of 26 (19.2%) serum specimens and 1 of 16 (6.3%) urine specimens but in none of 11 CSF specimens. Zika virus nucleic acid was not detected in any saliva specimens. Of specimens with ELISA results, anti-ZIKV IgM antibodies were detected in 90 of 95 (94.7%) serum specimens and 9 of 31 (29.0%) CSF specimens (Figure 1).
Figure 1. Detection of Zika Virus RNA and Anti–Zika Virus IgM Antibodies in Body Fluids From Patients With Guillain-Barré Syndrome, According to the Number of Days After Neurologic Illness Onset, Puerto Rico, 2016.
A-C, Real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) results. D and E, Enzyme-linked immunosorbent assay (ELISA) results. CSF indicates cerebrospinal fluid.
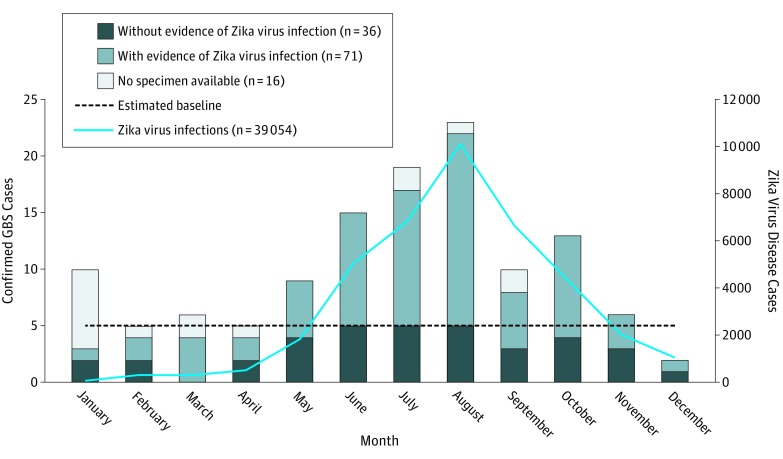
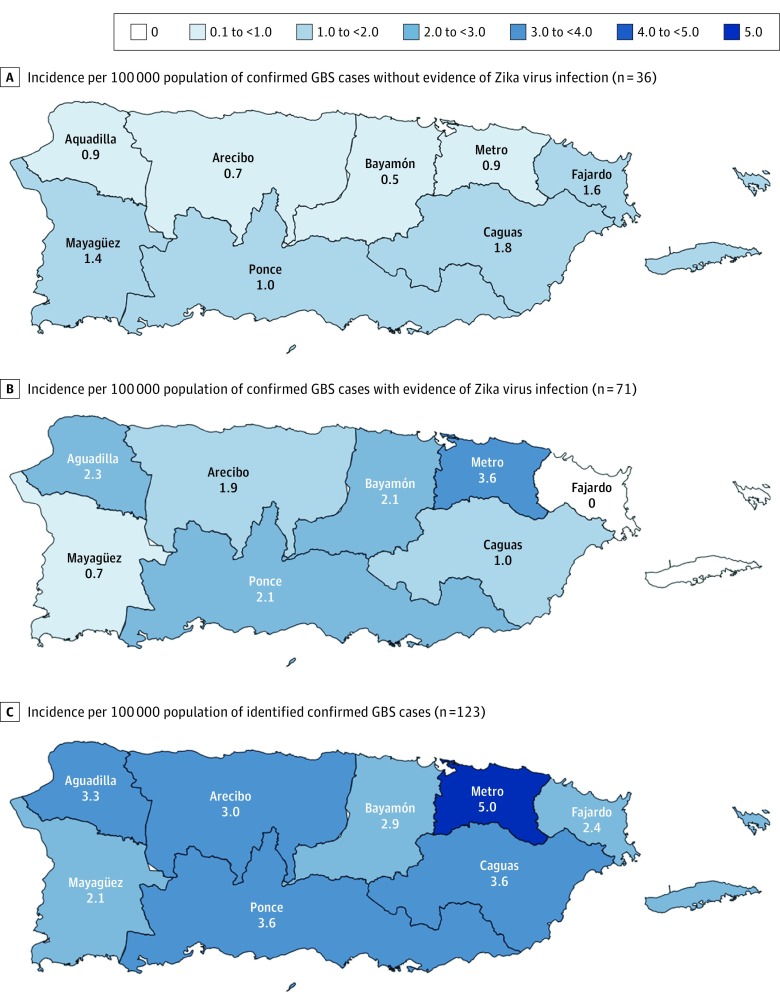
Compared with confirmed GBS cases without evidence of ZIKV infection, those with evidence of ZIKV infection did not differ by age but were more frequently female (Table 1). The monthly GBS case count by date of neurologic illness onset increased during the first half of the year, peaked in August, and decreased thereafter, mirroring identified ZIKV disease cases (Figure 2). The incidence of GBS in 2016 was 2.1-fold higher than the estimated annual baseline incidence (3.5 vs 1.7 cases per 100 000 population), and the incidence in August was 4.8-fold greater than the estimated baseline. Confirmed GBS cases were identified in all 8 public health regions (Figure 3). The San Juan metropolitan area had the highest proportion of cases and annual incidence (5.0 cases per 100 000 population) (Figure 3C). The incidence of confirmed GBS cases was higher among individuals 50 years or older than among individuals younger than 50 years (6.4 vs 2.1 cases per 100 000 population; P < .001).
Figure 2. Confirmed Guillain-Barré Syndrome Cases With and Without Evidence of Zika Virus Infection and Zika Virus Disease Cases by Month of Symptom Onset, Puerto Rico, 2016.
GBS indicates Guillain-Barré syndrome.
Figure 3. Incidence of Guillain-Barré Syndrome per 100 000 Population Among Patients With vs Without Evidence of Zika Virus Infection by Public Health Region, Puerto Rico, 2016.
A-C, Confirmed Guillain-Barré syndrome (GBS) cases were identified in all 8 public health regions.
Acute Clinical Characteristics of Patients With GBS and Evidence of ZIKV Infection
Although patients with GBS with vs without evidence of ZIKV infection did not differ by frequency of reported antecedent illness, those with evidence of ZIKV infection more frequently reported antecedent rash and arthralgia (Table 1). Patients with vs without evidence of ZIKV infection did not differ by the median time from antecedent illness to neurologic illness onset.
All patients with confirmed GBS were hospitalized, with no difference between those with vs without evidence of ZIKV infection by duration of hospitalization, certain medical complications, or proportion that received intravenous immunoglobulin (Table 1). Patients with evidence of ZIKV infection more frequently were admitted to the intensive care unit and required mechanical ventilation. In-hospital deaths did not differ between patients with vs without evidence of ZIKV.
Compared with patients with GBS without evidence of ZIKV infection, those with evidence of ZIKV infection more frequently had facial weakness, dysphagia, shortness of breath, and facial paresthesia (Table 1). Frequency of other signs and symptoms of neurologic illness did not differ between patients with vs without evidence of ZIKV infection. Among 84 patients with GBS for whom CSF was analyzed, patients with evidence of ZIKV infection more frequently had abnormally elevated protein levels and cytoalbuminologic dissociation; there was no difference in terms of the time between neurologic symptom onset and lumbar puncture. Of 19 patients with and 14 patients without evidence of ZIKV infection who had electrophysiologic studies performed and results available, the overall difference by result was not significant; however, a larger proportion of patients with evidence of ZIKV infection had the acute inflammatory demyelinating polyneuropathy (AIDP) variant of GBS. At clinical nadir, the median modified Rankin Scale score and Hughes Disability Score did not differ between patients with vs without evidence of ZIKV infection.
Long-term Disability of Patients With GBS and Evidence of ZIKV Infection
Data on disability 6 months after onset of neurologic illness were available for 60 patients (84.5%) with and 27 patients (75.0%) without evidence of ZIKV infection (Table 2). The median disability scores did not differ between patients with vs without evidence of ZIKV infection. However, patients with evidence of ZIKV infection more frequently reported ongoing facial disability, including excessive or inadequate tearing and difficulty drinking from a cup. Patients with evidence of ZIKV infection also more frequently reported substantial pain at the time of interview but not substantial fatigue or poor mental status. An additional 7 deaths were identified through follow-up interviews, 3 of which were patients with evidence of ZIKV infection.
Table 2. Disability 6 Months After Neurologic Illness Onset Among Patients With Guillain-Barré Syndrome With vs Without Evidence of Zika Virus Infection, Puerto Rico, 2016.
| Variable | With Evidence of Zika Virus Infection (n = 60) |
Without Evidence of Zika Virus Infection (n = 27) |
P Value |
|---|---|---|---|
| Facial Disability Indexa | |||
| Median score (range) | 95 (25-100) | 100 (55-100) | .13 |
| Disability, No./Total No. (%) | |||
| Brushing teeth | 8/56 (14.3) | 3/23 (13.0) | >.99 |
| Excessive or inadequate tearing | 30/56 (53.6) | 6/23 (26.1) | .03 |
| Speaking | 15/56 (26.8) | 3/23 (13.0) | .19 |
| Difficulty drinking from a cup | 10/56 (17.9) | 0/23 | .03 |
| Chewing food | 12/56 (21.4) | 1/23 (4.3) | .09 |
| Overall disability sum scorea | |||
| Median score (range) | 3 (0-12) | 3 (0-12) | .95 |
| Disability, No./Total No. (%) | |||
| Washing hair | 16/56 (28.6) | 5/23 (21.7) | .53 |
| Turning keys | 14/56 (25.0) | 5/23 (21.7) | .76 |
| Using utensils | 14/56 (25.0) | 6/23 (26.1) | .92 |
| Using buttons | 15/56 (26.8) | 7/23 (30.4) | .74 |
| Dressing | 17/56 (30.4) | 4/23 (17.4) | .24 |
| Any difficulty walking | 27/56 (48.2) | 12/23 (52.2) | .75 |
| Able to walk 10 m without an aid | 32/56 (57.1) | 16/23 (69.6) | .30 |
| Need support to walk 10 m | |||
| Need 1 crutch to walk 10 m | 9/56 (16.1) | 2/23 (8.7) | .64 |
| Need 2 crutches to walk 10 m | 7/56 (12.5) | 1/23 (4.3) | |
| Need wheelchair to walk 10 m | 8/56 (14.3) | 4/23 (17.4) | |
| For those needing wheelchair, can stand and walk 1 m with help | 6/8 (75.0) | 0/4 | .06 |
| For those needing wheelchair, can make purposeful movement with legs | 8/8 (100) | 4/4 (100) | |
| Modified Rankin Scale | |||
| Median score (range) | 2 (0-6) | 2 (0-6) | .47 |
| Score, No. (%) | NA | ||
| 0 | 6 (10.0) | 4 (14.8) | |
| 1 | 20 (33.3) | 6 (22.2) | |
| 2 | 15 (25.0) | 6 (22.2) | |
| 3 | 9 (15.0) | 2 (7.4) | |
| 4 | 2 (3.3) | 2 (7.4) | |
| 5 | 5 (8.3) | 3 (11.1) | |
| 6 | 3 (5.0) | 4 (14.8) | |
| Additional variables, No./Total No. (%)a,b | |||
| Self-reported substantial fatigue | 16/55 (29.1) | 3/23 (13.0) | .13 |
| Self-reported substantial pain | 15/55 (27.3) | 1/23 (4.3) | .03 |
| Self-reported poor mental status ≥1 d in the past monthc | 18/50 (36.0) | 5/22 (22.7) | .27 |
Abbreviation: NA, not applicable.
Does not include 1 patient with evidence of Zika virus infection in a comatose state, as well as 3 patients with and 4 patients without evidence of Zika virus infection who died before the 6-month disability follow-up.
Does not include 1 patient with evidence of Zika virus infection who declined to answer additional variable questions.
Does not include an additional 5 patients with and 1 patient without evidence of Zika virus infection interviewed before implementation of the question on mental health status.
Discussion
Prospective and retrospective case identification of patients with GBS with and without evidence of ZIKV infection allowed for the clinical characterization of GBS associated with ZIKV. As reported elsewhere,6,7,26 the GBS incidence increased above the estimated baseline concurrent to the 2016 ZIKV epidemic in Puerto Rico; however, the increased incidence was lower than the estimated increase of 3.2 to 5.1 times the baseline.27 Early reports from countries affected by ZIKV facilitated rapid implementation of rigorous laboratory-based case definitions for evidence of ZIKV infection and standardized GBS case definitions, which likely contributed to more accurate epidemiologic data. Concordant with GBS epidemiology, the GBS incidence was higher among those 50 years or older. Unexpectedly, patients with GBS and evidence of ZIKV infection were more frequently female, mirroring the higher incidence of ZIKV disease among this sex in Puerto Rico and elsewhere.28,29,30,31
Timing of specimen collection was important in detecting ZIKV nucleic acid by rRT-PCR among 39.4% (28 of 71) of patients with GBS and evidence of ZIKV infection. Most specimens with confirmatory results were collected within 9 days of neurologic illness onset. Compared with urine specimens, a higher proportion of serum specimens had positive ZIKV rRT-PCR results, with the added benefit of also being able to detect anti-ZIKV antibodies. Results are consistent with those reported from Brazil32 and further suggest that ZIKV RNA persistence was similar among patients with ZIKV disease and those who developed GBS after ZIKV infection. For patients with GBS, adding the median times from antecedent illness to neurologic illness onset (7 days) and from neurologic illness onset to specimen collection (9 days), the time from antecedent illness to specimen collection was roughly 16 days. This is comparable to patients with ZIKV disease, in whom ZIKV RNA is detectable in serum specimens for a median of 14 days after onset of ZIKV disease.33
With regard to antecedent illness, patients with GBS and evidence of ZIKV infection more frequently reported rash and arthralgia but did not differ in terms of the median time from antecedent illness to neurologic illness onset (7 days). The median time to neurologic illness onset for patients with GBS and evidence of ZIKV infection was similar to what has been reported elsewhere.6,7 Furthermore, before the introduction of ZIKV, patients with GBS in Puerto Rico also had a median time to neurologic illness onset of 7 days.5 Therefore, the median time to neurologic illness onset was similar for patients with GBS associated with ZIKV and those triggered by other etiologies.
Although all identified patients with GBS were hospitalized, patients with evidence of ZIKV infection had more severe morbidity (ie, more frequently were admitted to the intensive care unit and required mechanical ventilation) and differential long-term outcomes (ie, higher frequency of excessive or inadequate tearing, difficulty drinking from a cup, and self-reported substantial pain). Nonetheless, disability scores at clinical nadir, medical complications, and long-term disability scores did not differ between patients with vs without evidence of ZIKV infection; in-hospital mortality rates also did not differ and were within the expected range for Puerto Rico.5 Therefore, although acute morbidity may be more severe for patients with GBS and evidence of ZIKV infection, mortality and patient prognosis are similar to GBS associated with other etiologies.
This investigation also lends insights into pathophysiologic mechanisms of GBS triggered by ZIKV. Most ZIKV infections are asymptomatic31,34; however, inversely, 80.3% (57 of 71) of patients herein with GBS and evidence of ZIKV infection reported an antecedent illness. Furthermore, patients with GBS and evidence of ZIKV more frequently had abnormally elevated levels of protein in CSF and cytoalbuminologic dissociation, indicating a heightened immune response compared with GBS triggered by other etiologies. Future research should investigate risk factors for developing GBS after ZIKV infection, as well as for developing GBS triggered by ZIKV compared with other etiologies. The AIDP variant of GBS was predominant among patients with evidence of ZIKV infection, as indicated by electrophysiologic results elsewhere10 and by phenotypic evidence from a postmortem investigation of a Puerto Rico patient with GBS and evidence of ZIKV infection.11 Specific mechanisms leading to anti-ZIKV antibody–mediated demyelination should be investigated, particularly to identify specific target antigens.35 Finally, although clinically similar to patients with GBS but without evidence of ZIKV infection, those with evidence of ZIKV infection more frequently had acute and residual cranial neuropathy. This suggests that the immune response to ZIKV infection may more regularly target cranial nerves compared with GBS triggered by other etiologies.7,9,11,36,37 Research should examine anti-ZIKV antibody targets, such as antigens that are specific to or overexpressed in cranial nerves.
Limitations
These findings are subject to several limitations. First, patients with GBS may have been missed, including patients who did not seek medical attention, mild and fatal cases without clinical suspicion of GBS, and suspected GBS cases without sufficient documented clinical evidence to meet the Brighton Collaboration criteria. However, a separate analysis of GBS case identification completeness found that an estimated 97% of all confirmed GBS cases with neurologic illness onset during 2016 were identified.14 Second, ZIKV infection among patients with GBS could have been misclassified. Cross-reactivity with DENV by IgM ELISA could have led to an overestimation of patients having GBS with evidence of ZIKV infection; however, ZIKV was the predominant arbovirus circulating in 2016 in Puerto Rico.28,38 Conversely, false-negative laboratory results could have led to an underestimation. Furthermore, specimens were not available for 13.1% (16 of 123) of confirmed GBS cases, some of which may have been infected with ZIKV. Third, disability was assessed using self-reported information collected via telephone interview, whereas clinical assessment, including electrophysiologic studies, could have provided more accurate disability data. Fourth, apart from laboratory testing for ZIKV, DENV, and CHIKV, other etiologic triggers were not systematically identified because of constraints in public health surveillance. Additional laboratory testing was dependent on medical orders, and data collection was limited to results available in patients’ medical records.
Conclusions
Despite these limitations, an island-wide surveillance in Puerto Rico allowed for comprehensive data collection to characterize GBS associated with ZIKV infection. Indicated pathophysiologic mechanisms merit continued investigation, especially to identify risk factors for developing GBS after ZIKV infection and to appropriately prepare resources to improve patients’ long-term prognosis.
eFigure 1. Puerto Rico Department of Health Guillain-Barré Syndrome Case Report Form
eFigure 2. Case Identification Flow Diagram
eTable 1. Zika Virus Diagnostic Test Results Among Confirmed GBS Cases With Evidence of Zika Virus Infection (N = 71)
eTable 2. Detection of Zika Virus RNA and Anti-Zika Virus IgM Antibodies Among Guillain-Barré Syndrome Patients Body Fluids (Serum, Urine, and Cerebrospinal Fluid) by Day Prior to and Post Neurologic Illness Onset, Puerto Rico, 2016
References
- 1.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388(10045):717-727. [DOI] [PubMed] [Google Scholar]
- 2.Rees JH, Soudain SE, Gregson NA, Hughes RA. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med. 1995;333(21):1374-1379. [DOI] [PubMed] [Google Scholar]
- 3.McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide: a systematic literature review. Neuroepidemiology. 2009;32(2):150-163. [DOI] [PubMed] [Google Scholar]
- 4.Hund EF, Borel CO, Cornblath DR, Hanley DF, McKhann GM. Intensive management and treatment of severe Guillain-Barré syndrome. Crit Care Med. 1993;21(3):433-446. [DOI] [PubMed] [Google Scholar]
- 5.Salinas JL, Major CG, Pastula DM, et al. . Incidence and clinical characteristics of Guillain-Barré syndrome before the introduction of Zika virus in Puerto Rico. J Neurol Sci. 2017;377:102-106. [DOI] [PubMed] [Google Scholar]
- 6.Cao-Lormeau VM, Blake A, Mons S, et al. . Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parra B, Lizarazo J, Jiménez-Arango JA, et al. . Guillain-Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375(16):1513-1523. [DOI] [PubMed] [Google Scholar]
- 8.Dirlikov E, Medina NA, Major CG, et al. . Acute Zika virus infection as a risk factor for Guillain-Barré syndrome in Puerto Rico. JAMA. 2017;318(15):1498-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias A, Torres-Tobar L, Hernández G, et al. . Guillain-Barré syndrome in patients with a recent history of Zika in Cúcuta, Colombia: a descriptive case series of 19 patients from December 2015 to March 2016. J Crit Care. 2017;37:19-23. [DOI] [PubMed] [Google Scholar]
- 10.Uncini A, Shahrizaila N, Kuwabara S. Zika virus infection and Guillain-Barré syndrome: a review focused on clinical and electrophysiological subtypes. J Neurol Neurosurg Psychiatry. 2017;88(3):266-271. [DOI] [PubMed] [Google Scholar]
- 11.Dirlikov E, Torres JV, Martines RB, et al. . Postmortem findings in patient with Guillain-Barré syndrome and Zika virus infection. Emerg Infect Dis. 2018;24(1):114-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas DL, Sharp TM, Torres J, et al. . Local transmission of Zika virus: Puerto Rico, November 23, 2015–January 28, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(6):154-158. [DOI] [PubMed] [Google Scholar]
- 13.Dirlikov E, Major CG, Mayshack M, et al. . Guillain-Barré syndrome during ongoing Zika virus transmission: Puerto Rico, January 1–July 31, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(34):910-914. [DOI] [PubMed] [Google Scholar]
- 14.Major C, Dirlikov E, Medina N, et al. . Implementation and evaluation of Guillain-Barré syndrome surveillance in Puerto Rico during the 2016 Zika virus epidemic. P R Health Sci J. 2018;37. [PMC free article] [PubMed] [Google Scholar]
- 15.Puerto Rico Department of Health Administrative Order 358. 2016. http://www.salud.gov.pr/Sobre-tu-Salud/Documents/OA%20358%20-%20PARA%20ENMENDAR%20LA%20OA%20302.pdf. Accessed July 12, 2017.
- 16.U.S. Food and Drug Administration Emergency use authorizations: Zika virus emergency use authorization. https://www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161496.htm. Accessed July 12, 2017.
- 17.Lanciotti RS, Kosoy OL, Laven JJ, et al. . Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.InBios International Inc Anti–dengue virus immunoglobulin M (IgM) antibody-capture (MAC) enzyme-linked immunosorbent assay (ELISA). http://www.inbios.com/denvdetecttm-igm-elisa-kit-usa/. Accessed July 12, 2017.
- 19.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol. 2000;38(5):1823-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sejvar JJ, Kohl KS, Gidudu J, et al. ; Brighton Collaboration GBS Working Group . Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29(3):599-612. [DOI] [PubMed] [Google Scholar]
- 21.Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19(12):1497-1500. [DOI] [PubMed] [Google Scholar]
- 22.Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial prednisolone in acute polyneuropathy. Lancet. 1978;2(8093):750-753. [DOI] [PubMed] [Google Scholar]
- 23.Merkies IS, Schmitz PI, van der Meché FG, Samijn JP, van Doorn PA; Inflammatory Neuropathy Cause and Treatment (INCAT) Group . Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. J Neurol Neurosurg Psychiatry. 2002;72(5):596-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanSwearingen JM, Brach JS. The Facial Disability Index: reliability and validity of a disability assessment instrument for disorders of the facial neuromuscular system. Phys Ther. 1996;76(12):1288-1298. [DOI] [PubMed] [Google Scholar]
- 25.United States Census Bureau Puerto Rico Commonwealth Population Totals Tables: 2010-2016. 2017. https://www.census.gov/data/tables/2016/demo/popest/total-puerto-rico.html. Accessed June 14, 2017.
- 26.Dos Santos T, Rodriguez A, Almiron M, et al. . Zika virus and the Guillain-Barré syndrome: case series from seven countries. N Engl J Med. 2016;375(16):1598-1601. [DOI] [PubMed] [Google Scholar]
- 27.Dirlikov E, Kniss K, Major C, et al. . Guillain-Barré syndrome and healthcare needs during Zika virus transmission, Puerto Rico, 2016. Emerg Infect Dis. 2017;23(1):134-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lozier M, Adams L, Febo MF, et al. . Incidence of Zika virus disease by age and sex: Puerto Rico, November 1, 2015–October 20, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(44):1219-1223. [DOI] [PubMed] [Google Scholar]
- 29.Coelho FC, Durovni B, Saraceni V, et al. . Higher incidence of Zika in adult women than adult men in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. Int J Infect Dis. 2016;51:128-132. [DOI] [PubMed] [Google Scholar]
- 30.Duffy MR, Chen TH, Hancock WT, et al. . Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536-2543. [DOI] [PubMed] [Google Scholar]
- 31.Lozier MJ, Burke RM, Lopez J, et al. . Differences in prevalence of symptomatic Zika virus infection by age and sex: Puerto Rico, 2016 [published online December 6, 2017]. J Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brito Ferreira ML, Antunes de Brito CA, Moreira ÁJP, et al. . Guillain-Barré syndrome, acute disseminated encephalomyelitis and encephalitis associated with Zika virus infection in Brazil: detection of viral RNA and isolation of virus during late infection. Am J Trop Med Hyg. 2017;97(5):1405-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paz-Bailey G, Rosenberg ES, Doyle K, et al. . Persistence of Zika virus in body fluids: preliminary report. N Engl J Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552-1563. [DOI] [PubMed] [Google Scholar]
- 35.Muñoz LS, Parra B, Pardo CA; Neuroviruses Emerging in the Americas Study . Neurological implications of Zika virus infection in adults. J Infect Dis. 2017;216(suppl 10):S897-S905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langerak T, Yang H, Baptista M, et al. . Zika virus infection and Guillain-Barré syndrome in three patients from Suriname. Front Neurol. 2016;7:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassavetis P, Joseph JM, Francois R, Perloff MD, Berkowitz AL. Zika virus–associated Guillain-Barré syndrome variant in Haiti. Neurology. 2016;87(3):336-337. [DOI] [PubMed] [Google Scholar]
- 38.Adams L, Bello-Pagan M, Lozier M, et al. . Update: ongoing Zika virus transmission: Puerto Rico, November 1, 2015–July 7, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(30):774-779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Puerto Rico Department of Health Guillain-Barré Syndrome Case Report Form
eFigure 2. Case Identification Flow Diagram
eTable 1. Zika Virus Diagnostic Test Results Among Confirmed GBS Cases With Evidence of Zika Virus Infection (N = 71)
eTable 2. Detection of Zika Virus RNA and Anti-Zika Virus IgM Antibodies Among Guillain-Barré Syndrome Patients Body Fluids (Serum, Urine, and Cerebrospinal Fluid) by Day Prior to and Post Neurologic Illness Onset, Puerto Rico, 2016