Abstract
Decades of alcohol research have established the health risks and pharmacodynamic profile of oral alcohol consumption. Despite isolated periods of public health concern, comparatively less research has evaluated exposure to alcohol vapor. Inhaled alcohol initially bypasses first-pass metabolism and rapidly reaches the arterial circulation and the brain, suggesting that this route of administration may be associated with pharmacological effects that increase the risk of addiction. However, detailed reviews assessing the possible effects of inhaled alcohol in humans are lacking. A comprehensive, systematic literature review was conducted using Google Scholar and PubMed to examine manuscripts studying exposure to inhaled alcohol and measurement of biomarkers (biochemical or functional) associated with alcohol consumption in human participants. Twenty-one publications reported on alcohol inhalation. Fourteen studies examined inhalation of alcohol vapor associated with occupational exposure (e.g., hand sanitizer) in a variety of settings (e.g., naturalistic, laboratory). Six publications measured inhalation of alcohol in a controlled laboratory chamber, and 1 evaluated direct inhalation of an e-cigarette with ethanol-containing “e-liquid.” Some studies have reported that inhalation of alcohol vapor results in measurable biomarkers of acute alcohol exposure, most notably ethyl glucuronide. Despite the lack of significantly elevated blood alcohol concentrations, the behavioral consequences and subjective effects associated with repeated use of devices capable of delivering alcohol vapor are yet to be determined. No studies have focused on vulnerable populations, such as adolescents or individuals with alcohol use disorder, who may be most at risk of problems associated with alcohol inhalation.
Keywords: Ethanol Vapor, Alcohol Inhalation, Alcohol Without Liquid, Vaportini, Vaping
ALCOHOL USE IS a significant health problem that often co-occurs with other addictive disorders and mental health diagnoses (Bradizza et al., 2006; Falk et al., 2006; Grant et al., 2004; RachBeisel et al., 1999). While scores of human and animal studies have exhaustively characterized the behavioral and neurocognitive effects resulting from oral ingestion over a wide range of doses, the evaluation of effects from alternative routes of alcohol absorption has received comparatively little attention. Notably, alcohol inhalation in humans has been documented within contexts associated with incidental exposure (e.g., occupational or environmental) as well as intentional, or inadvertent, exposure while using devices that deliver alcohol vapor.
Concerns about the negative health impact of exposure to inhaled alcohol have been present for decades (e.g., Lester and Greenberg, 1951). The majority of research on incidental exposure to alcohol vapor has largely focused on occupational exposure, namely healthcare professionals that frequently use commercial alcohol-containing products such as hand sanitizer; but emerging research suggests that another source of exposure to inhaled alcohol is from the use of e-cigarettes that contain ethanol (EtOH) in the “e-liquid.” E-cigarettes are battery-operated devices that use an electrical current to heat small metal coils that then generate aerosols from an e-liquid reservoir (i.e., tank or saturated wicking material). E-liquids typically contain a “base mixture” of glycerol and propylene glycol to which various flavoring ingredients and nicotine are added. In addition, EtOH is a variable, but frequent constituent of e-liquids (Cai and Kendall, 2009; Ellicott, 2009; Herrington and Myers, 2015; Tygat, 2007; Valance and Ellicott, 2008; Varlet et al., 2015) and alcohol has been detected in the aerosols produced from them (Herrington et al., 2015; Laugesen, 2008). Furthermore, some Internet-based e-cigarette forums include recipes that recommend alcohol as an ingredient in self-made e-liquids. Consequently, the fact many e-cigarette users are repeatedly inhaling variable levels of alcohol during routine e-cigarette use has potential health implications.
In addition, “alcohol vaporizers” that use heat or physical agitation to generate alcohol vapor or aerosols in an enclosed system that are then inhaled have periodically been publicized as a novel mode of recreational alcohol use (Le Foll and Loheswaran, 2014). For example, one such method known as “alcohol without liquid” used a nebulizer to mix alcohol and oxygen to create a mist (Lovell, 2004). Although the specter of a public health menace from inhaled alcohol use emerged with these early reports, widespread recreational use of inhaled alcohol or of other routes of administration failed to materialize and these alternative forms of alcohol use have largely been relegated to Internet-based curiosities (Stogner et al., 2014). Collectively, the absence of sustained, identifiable public health risks resulting from recreational or coincidental exposure to inhaled alcohol, combined with the absence of evidence for acute safety risks within extant literature on studies simulating occupational exposure, has resulted in an implicit presumption that inhaled alcohol poses negligible risks as compared to the well-established consequences of ingested alcohol. With that said, the scientific assessment of harm from inhaled alcohol is incomplete because it has been based upon comparisons to the effects of ingested alcohol and has relied on methods and assumptions that do not address more subtle behavioral, cognitive, and physical manifestations that may result from the possibly dissimilar pharmacodynamics of inhaled alcohol (Fig. 1).
Fig. 1.
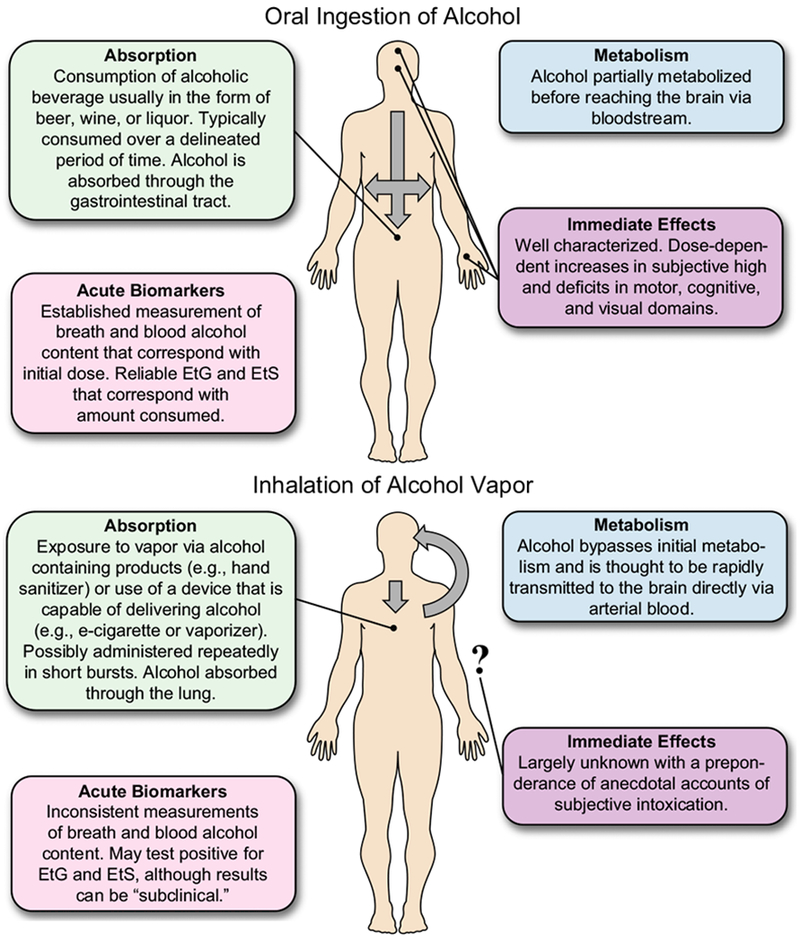
Comparison of oral and inhaled alcohol absorption, metabolism, immediate effects, and acute biomarkers.
Further exploration of the behavioral and pharmacological profile of inhaled alcohol use is particularly relevant for drugs of abuse, because the rate of delivery to brain receptor sites is positively correlated with their abuse potential and addiction risk (Allain et al., 2015). In well-controlled human laboratory studies, faster delivery of fixed doses of opioids, benzodiazepines, cocaine, and stimulants produce greater positive subjective drug effects (Marsch et al., 2001; de Wit et al., 1992). Preclinical studies suggest that greater psychomotor sensitization and immediate early gene expression are potential mechanisms by which rate of delivery impacts the behavioral effects for drugs of abuse (Samaha et al., 2005). As with smoked drugs such as nicotine and cocaine, inhaled alcohol bypasses first-pass metabolism and, compared with other routes of administration, should rapidly reach arterial circulation in the brain. Consequently, inhaled alcohol may be associated with enhanced behavioral effects including increased risk of addiction. Indeed, in rodents, chronic exposure to alcohol vapor was found to be the most effective mechanism for inducing alcohol dependence (Gilpin et al., 2008). If similar effects are present in humans, inhaled alcohol may produce a “priming” effect for alcohol consumption when small doses of alcohol are rapidly delivered. To determine the influence of inhaled alcohol, it is helpful to first evaluate whether exposure to alcohol vapor can be detected via established alcohol biomarkers.
The purpose of this focused review is to (i) summarize the translational foundation of alcohol inhalation and evaluate possible effects of exposure in humans, (ii) establish the objective evidence for systemic alcohol absorption after human inhalation, and (iii) discuss the most common contexts of alcohol inhalation and their differential risk profiles.
ANIMAL MODELS OF EXPOSURE TO ALCOHOL VAPOR
Preclinical studies provide the best evidence to date for characterizing possible risks associated with inhalation of alcohol vapor. Compared with oral administration, EtOH vapor provides a faster, more reliable method of inducing alcohol dependence in animal models (e.g., Heilig and Koob, 2007; Koob et al., 2009; Martin et al., 2012; Sommer and Spanagel, 2013; for review, see Vendruscolo and Roberts, 2014). The use of alcohol vapor inhalation for the induction of dependence has several advantages over other methods of forced alcohol administration (e.g., oral gavage, intragastric intubation) such as precise control of the dose, duration, and pattern of exposure that correspond to key features of alcohol dependence including withdrawal severity and tolerance (Gilpin et al., 2008; Goldstein and Pal, 1971; Rimondini et al., 2003). In a seminal study, Rogers and colleagues (1979) compared alcohol exposure via an inhalation chamber, intubation, and liquid diet in rats. Of these, alcohol inhalation was the only method to safely induce tolerance and dependence by producing sustained blood alcohol levels above 100 mg/dl while permitting rats to move normally and match nutritional needs between the control and experimental groups (Rogers et al., 1979). In subsequent operant conditioning paradigms, rats permitted to self-administer EtOH vapor achieved sustained blood alcohol levels between 100 and 150 mg/dl that significantly reduced the severity and presence of withdrawal criteria (Roberts et al., 1996). Withdrawal-associated increases in operant self-administration of EtOH vapor in alcohol-dependent rats has also been shown to persist for 4 to 8 weeks after induction of dependence (Roberts et al., 2000). Another study comparing liquid diet and vapor inhalation in rats concluded both routes of administration consistently produced alcohol withdrawal; however, vapor inhalation, relative to the liquid diet, was associated with a greater peak and average severity of withdrawal symptoms and a higher average blood alcohol level at the time of withdrawal (217.8 mg/dl vs. 94.3 mg/dl, respectively) (Macey et al., 1996). Finally, self-administration of an alternative reinforcer (i.e., saccharin) in rodents exposed to intermittent vapor was not significantly different than no alcohol controls, suggesting that the motivational mechanisms driving self-administration are likely specific to alcohol (Becker and Lopez, 2004; O’Dell et al., 2004).
Animal models using alcohol vapor have also contributed to our understanding of the impact of EtOH on the brain and other vital organs as well as in evaluating the mechanisms and efficacy of possible pharmacological interventions for chronic alcohol use. Alcohol vapor exposure in rodents results in alcohol-induced alterations in neural pathways that have been associated with alcohol use in humans such as dopamine (Budygin et al., 2007; Hirth et al., 2016), GABA (Roberto et al., 2004), glutamate (Rimondini et al., 2002; Roberto et al., 2006), and corticotrophin-releasing factors (Sommer et al., 2008; for review, see Heilig and Koob, 2007). Preclinical research using alcohol vapor to induce repeated cycles of intoxication and withdrawal has elucidated mechanisms critical to the development and evaluation of pharmacological intervention (Czachowski and Delory, 2009; Gewiss et al., 1991; Rimondini et al., 2002; for review, see Meinhardt and Sommer, 2015). For example, the administration of acamprosate blocks exposure-induced, but not basal, EtOH vapor intake and the resultant changes in gene expression mirror those in human models of alcohol dependence (Rimondini et al., 2002). Chronic intermittent EtOH vapor exposure in rats also produces widespread significant tissue injury including hepatic, pulmonary, and cardiovascular changes (Mouton et al., 2016). In sum, the use of alcohol vapor to induce dependence in preclinical models is an effective approach for evaluating motivational, biological, and pharmacological factors associated with chronic alcohol use and intoxication.
INHALED ALCOHOL IN HUMANS: POSSIBLE REINFORCING EFFECT
Although alcohol vapor is arguably the most effective method of inducing a state of dependence in animal models, there is a paucity of research on the behavioral and pharmacological profile of inhaled alcohol in humans. A cursory Internet search typically yields multiple news articles, instructional blogs, and user-uploaded videos of consumer and commercial devices that are designed to deliver alcohol vapor for recreational use. Most appear to be targeted to young adults and often include anecdotal evidence of a rapid “high” that quickly subsides. Additionally, patterns of use closely resemble binge drinking where the device is used frequently for a short period of time to achieve subjective levels of intoxication. Despite the digital presence of alcohol vaping devices and media reports of increasing use, no studies have directly compared behavioral and pharmacological profiles of oral and inhaled routes of alcohol administration in humans. As such, the relative reinforcing strength of vaporized alcohol transmitted to the brain via arterial uptake is largely unknown. By analogy, arterial levels of inhaled nicotine are reported to be as much as 10-fold higher than concurrent venous concentrations in blood (Benowitz, 2008; Hukkanen et al., 2005). Thus, the immediate impact of inhaled alcohol on brain sites of action may be out of proportion to those predicted from blood alcohol content (BAC) measurements.
Although oral and inhaled alcohol use likely entail differences in pharmacodynamics, human neuroimaging studies using oral and/or inhaled alcohol as conditioned reinforcers reveal meaningful similarities in cue reactivity in response to small doses of alcohol. Oral administration of just 1 ml of preferred brand of alcohol to humans during an fMRI scan is associated with increased craving and activation in reward-related regions of the brain, including the nucleus accumbens, amygdala, precuneus, dorsal striatum, and insula (Claus et al., 2011; Filbey et al., 2008; Ray et al., 2014). Furthermore, activation of these regions is positively associated with alcohol use disorder severity (Claus et al., 2011). Alcohol odors have also been used as conditioned reinforcers in human fMRI studies by forcing air into a closed container of alcohol that volatilizes alcohol into a nasal cannula attached to the participant (Lowen and Lukas, 2006; Lukas et al., 2013; Schneider et al., 2001). Exposure to inhaled alcohol, relative to neutral odors, has been associated with brain activation in the nucleus accumbens, amygdala, and ventral tegmental area (Kareken et al., 2004; Schneider et al., 2001). Thus, exposure to even a small amount of alcohol or inhalation of vaporized alcohol has analogous and potentially additive effects on brain regions known to be involved in the development and maintenance of addiction.
ACUTE BIOCHEMICAL AND FUNCTIONAL BIOMARKERS FOR ALCOHOL EXPOSURE
A biomarker is an objectively measured characteristic that is evaluated as a therapeutic indicator of a pharmacologic response to intervention or a diagnostic indicator to assess the risk or presence of a disease (Biomarkers Definitions Work Group, 2001; Gutman and Kessler, 2006). As inhaled alcohol is likely associated with low levels of alcohol exposure and not necessarily associated with chronic alcohol consumption, the current review will focus on the biomarkers of acute alcohol exposure (see Ingall, 2012).
Acute Biochemical Biomarkers
Two direct biochemical markers that have been used to study the bioavailability of inhaled alcohol are breath alcohol and the urinary alcohol metabolites, ethyl glucuronide (β-D-6-glucuronide or EtG) and ethyl sulfate (EtS).
Breath/Blood Alcohol Content
In both forensic and clinical settings, breath alcohol content (BrAC) is accepted as a valid estimation of BAC (Jones, 1993; Martin et al., 1984). The pharmacodynamic profile of ingested alcohol is well established. The principle effects (i.e., subjective, motor, cognitive) of acute alcohol exposure are most evident in the rising BAC curve (Friel et al., 1995; Wilkinson, 1980); moreover, individuals are typically more responsive to changes in BAC than to the absolute level of BAC (Martin et al., 2006). Although the pharmacodynamics of inhaled alcohol have not been established, it is possible that inhalation of alcohol vapor may correspond to low levels of total exposure that share features of the rising curve after acute oral alcohol exposure. Alterations in alcohol-specific motivational and attention processes are also evident at low BAC levels, including increasing craving and attention to alcohol-related stimuli (Schoenmakers et al., 2008). Collectively, these studies highlight the clinical importance of even a low dose of oral alcohol, particularly in the rising BAC curve, and possible shifts in motivation that are evident after exposure to relatively small amount of alcohol.
Ethyl Glucuronide and Ethyl Sulfate
Two additional direct biochemical biomarkers are EtG and EtS (Jatlow and O’Malley, 2010; Wurst et al., 2015). While they are only minor metabolites of EtOH, accounting for less than 0.1% of total EtOH dispersion (Helander et al., 2009), both EtG and EtS can detect alcohol a few hours after exposure (Wurst et al., 2006; Zimmer et al., 2002) and remain detectable for days depending upon the dose (Helander and Beck, 2005; Jatlow et al., 2014; Wurst et al., 2015). Particularly relevant to alcohol inhalation, EtG and EtS are effective at confirming that alcohol has been absorbed even in the absence of a positive BAC.
Acute Functional Biomarkers
The primary action of ingested alcohol is dose-dependent central nervous system depression exemplified by functional changes in multiple cognitive and motor domains (Little, 1991). Much of the existing literature on functional biomarkers has focused on deficits at or above the legal limit for intoxication (i.e., 80 mg/dl); but deficits in reaction time, response inhibition, working memory, and visuo-motor control are observable at BAC under the traditional cutoff for intoxication (for review, see Brick and Erickson, 2009; Chamberlain and Solomon, 2002). A review of over 100 studies on low-dose alcohol exposure suggested that functional impairment of skills related to driving begin with any departure from a BAC of zero (Moskowitz and Fiorentino, 2000). All of the above studies are based on oral ingestion and subsequent peripheral (venous) measurement or estimation of BAC, which may not be the most effective for quantifying alcohol after inhalation. Although the impairments associated with low-level alcohol exposure may not be sufficient to endanger personal safety (e.g., driving under the influence), subjective effects and functional impairments associated with alcohol consumption could trigger alcohol-specific expectancies that may increase reactivity to alcohol cues and motivate drinking behavior.
MATERIALS AND METHODS
Literature searches were performed in PubMed and Google Scholar using the following search terms: “alcohol OR ethanol AND inhalation OR vapor,” without any restrictions on date of manuscript publication. Potential studies were limited to those with human participants that included both exposure to inhaled alcohol and measurement of biomarkers (biochemical or functional) associated with alcohol consumption. Upon completion of literature search, the reference sections of identified articles and reviews (e.g., Stogner et al., 2014) were used to locate additional studies that met inclusion criteria.
RESULTS
A total of 21 publications met criteria to be included in the review. A summary of findings can be found in Table 1. Fourteen studies examined inhalation of alcohol vapor associated with occupational exposure (e.g., hand sanitizer) in a variety of settings (e.g., naturalistic, laboratory). Six publications measured alcohol inhalation of alcohol in a controlled laboratory chamber, and 1 evaluated direct inhalation of an e-cigarette with EtOH-containing e-liquid.
Table 1.
Summary of Literature Review of Inhaled Alcohol
| Article | Type of alcohol | Source of alcohol | Method of exposure | Study design | Biomarkers for alcohol exposure | Main finding |
|---|---|---|---|---|---|---|
| Ahmed-Lecheheb and colleagues (2012) | 70% EtOH | Hand-sanitizing gel | Sanitizer: 3 ml several times over 4-hour shift | Naturalistic | BrAC; urine and plasma levels of EtOH, acetaldehyde, and acetate | ↑ BrAC in 33% of participants |
| Ali and colleagues (2013) | 62% EtOH | Hand-sanitizing gel | Sanitizer: 3 groups with varying amounts and drying procedures | Between subjects; 3 conditions: 1.5 ml with hand rubbing, 1.5 ml no rubbing, 3.0 ml no rubbing | BrAC | ↑ BrAC (median, dose dependent) |
| Arndt and colleagues (2012) | 30% propan-1-ol; 45% 2-propanol | Hand-sanitizing gel | Sanitizer: 5 individuals applying every 15 minutes for 8 hours | Within subjects; 2 conditions: 2 individuals present in room but did not apply sanitizer (inhaled only) | EtG | ↑ EtG in 6 of 7 including both participants in inhaled only condition |
| Arndt and colleagues (2014) | 96% EtOH | Hand-sanitizing gel | Sanitizer: 3 ml 4 times per hour for 8 hours | Between subjects; dermal and inhalation versus inhalation only | EtG | ↑ EtG 6 hours after exposure |
| Below and colleagues (2012) | 70% propan-1-ol; 63.14% propan-2-ol; 45% propan-2-ol with 30% propan-1-ol | Hand-sanitizing gel | Sanitizer: 10 surgical hand rubs (4 ml repeated 5 times) over 80 minutes | Between subjects; dermal and inhalation of 3 sanitizers | Plasma propanol levels | ↑ In median propanol from dermal and inhalation |
| Brown and colleagues (2007) | 70% EtOH or 70% isopropanol | Hand-sanitizing gel | Sanitizer: 1 squirt (1.2 to 1.5 ml) every 2 minutes | Naturalistic | BrAC, serum | ↑ BrAC in 30% of participants within 2 minutes of exposure |
| Brugnone and colleagues (1983) | Occupational exposure to isopropanol | Isopropanol concentration in air | Air sample before shift, 30 minutes later, and hourly for next 7 hours | Naturalistic | Isopropanol in alveolar air, BAC, and urine | ↑ Alveolar concentration that correlated with environmental air; not detected in blood or urine |
| Campbell and Wilson (1986) | 1,000 ppm of EtOH air concentration | Inhaled alcohol | Inhaled alcohol for 3 hours | Case study, time series; blood assessed throughout 3 hours | BAC | No increases in EtOH blood content |
| Dumas-Campagna and colleagues (2014) | 125 to 1,000 ppm of EtOH air concentration | Inhaled alcohol | Inhaled alcohol for 4 hours in each condition | Within subjects; exposed to 5 concentrations over 6 days (excluding exercise condition) | BAC | Detectable BAC in all conditions with highest in 1,000 ppm after 4 hours (0.30 mg/dl) |
| Ernstgard and colleagues (2003) | 142 ppm of 2-propanol air concentration | Inhaled alcohol | Inhaled alcohol over 2 hours during light physical exercise | Within subjects; propanol and clean air exposures | BAC, urine, BrAC | ↑ 2-Propanol in BAC, urine, and blood up to 6 hours after exposure |
| Ernstgard and colleagues (2005) | 0,100,200 ppm of methanol | Inhaled alcohol | Inhaled alcohol over 2 hours in each condition with light physical exercise | Within subjects; exposed to 3 concentrations | BAC, urine, BrAC | Dose-dependent increase in methanol in BAC, urine, and BrAC |
| Hautemaniere and colleagues (2013a) | 70% EtOH | Hand-sanitizing gel | Sanitizer: used ad libitum over the course of a 4-hour shift | Naturalistic | BrAC, urine, and plasma levels of EtOH, acetaldehyde, and acetate | ↑ BrAC within 2 minutes of exposure |
| Jones and colleagues (2006) | 62% EtOH | Hand-sanitizing gel | Sanitizer: 0.5 g once per hour for 8 hours | Time series; urine assessed throughout 8 hours and next morning (18 hours) | EtG, EtS | ↑ EtG and EtS |
| Kramer and colleagues (2007) | 95, 85, and 55% EtOH | Hand-sanitizing gel | Sanitizer: 4 ml applied for 10 seconds repeated 20 times or 20 ml for 3 minutes repeated 10 times | Within subjects; 3 conditions: 55, 85, and 95% EtOH with 2 exposure durations | Blood levels of EtOH and acetaldehyde | Dose-dependent increase in median blood EtOH concentration |
| Miller and colleagues (2006) | 62% EtOH | Hand-sanitizing gel | Sanitizer: 5 ml applied 50 times over 4 hours | Within subjects; pre–post, repeated application | Plasma levels of EtOH | No increases in plasma EtOH levels |
| Nadeau and colleagues (2003) | 0 to 1,000 ppm of EtOH air concentration | Inhaled EtOH | Inhaled EtOH for 6 hours at each level | Within subjects; exposed to 4 concentrations (in ppm) over 4 days | Plasma levels of EtOH | Increases in plasma EtOH to 0.443 mg/dl at highest concentration (1,000 ppm) |
| Reisfield and colleagues (2011) | 62% EtOH | Hand-sanitizing gel | Sanitizer: 1 pump (~1 ml) every 5 minutes for 10 hours | Repeated measures; sanitizer procedure repeated for 3 days | EtG, EtS | ↑ EtG in all but 1 participant |
| Rohrig and colleagues (2006) | 62% EtOH | Hand-sanitizing gel | Sanitizer: applied 15 minutes for 4 hours | Between groups; sanitizer applied at various frequencies | EtG | ↑ EtG in 1 participant in the most frequent application group |
| Rosano and Lin (2008) | 61% EtOH | Hand-sanitizing gel | Sanitizer: 1 ml applied 20 times during 8 to 12 hours | Within subjects; comparing EtG after hand sanitizer and oral EtOH challenge | EtG | ↑ EtG in next day urine in 90% of sample |
| Skipper and colleagues (2009) | 62% EtOH | Hand-sanitizing gel | EthGel applied 2 squirts on hands every 4 minutes for 1 hour | Between groups; 4 conditions: control, skin exposure, vapor exposure or both | BrAC, EtG | ↑EtG with gel exposure, especially vapor |
| Valentine and colleagues (2016) | 23.5% EtOH | Electronic cigarette “liquid” | E-cigarette: Two 20-minute ad lib smoking sessions | Within subjects; pre–post smoking e-cigarette in high and low EtOH content | BrAC, EtG, EtS | No elevated BrAC, 38% of participants positive for EtG after high EtOH exposure |
BrAC, breath alcohol content; BAC, blood alcohol content; EtG, ethyl glucuronide; EtS, ethyl sulfate; EtOH, ethanol; ppm, parts per million.
Occupational Exposure via Hand Sanitizers
One possible complicating factor when studying hand sanitizer use is the ability to isolate inhalation of alcohol vapor, and not dermal resorption, of alcohol. To address this, a double-blind, randomized, 3 times crossover study included either dermal application of 74.1% EtOH, 10% 2-propanol, or a combination of both, to the participant’s back (thus reducing opportunity to inhale alcohol vapor) (Kirschner et al., 2009). All 3 conditions resulted in undetectable BAC suggesting that increases in alcohol biomarkers are unlikely to result from transdermal resorption. To determine whether use of hand sanitizer presents an opportunity for inhaled alcohol exposure, 1 study used a specialized apparatus that measures alcohol vapor in the breathing zone (150 cm) above individuals exposed to 3 ml of hand sanitizer (Bessonneau and Thomas, 2012). Results suggest that alcohol levels within the breathing zone peaked around 1.3 to 1.4 mg/dl after 30 seconds of exposure and 1.8 to 2.0 mg/dl after 40 seconds of exposure. Assuming an average breathing frequency, the authors calculated that after 30 seconds and 90 seconds of hand disinfection, the total inhaled dose of EtOH were 74.9 mg and 328.9 mg, respectively (Bessonneau and Thomas, 2012). Therefore, although transdermal alcohol absorption remains a possibility, it is more likely that the positive tests for alcohol biomarkers after hand sanitizer use reviewed below are due to inhalation of alcohol vapor and not dermal resorption of alcohol.
Healthcare workers have been the focus of many studies because infection control plans encourage the repeated use of alcohol-based hand sanitizers. To evaluate whether a naturalistic exposure to alcohol-containing hand sanitizers results in an increase in alcohol biomarkers, Hautemaniere and colleagues (2013a) repeatedly measured alcohol concentrations present in blood, breath, and urine at the beginning of a work shift and 4 hours later. Participants were asked to maintain their typical frequency of hand sanitizer use during the course of the study. Four hours into a shift, EtOH was not detectable in blood or urine; however, mean alcohol in inhaled air was 46.2 mg/m3 and expelled air contained 0.003 mg/dl of alcohol up to 2 minutes after exposure (Hautemaniere et al., 2013a). In a similar study with a larger sample of 86 healthcare workers tested before and after a 4-hour shift, alcohol was not detected in blood and urine, but approximately one-third of healthcare workers demonstrated an elevated mean expired EtOH level of 0.008 mg/dl within 2 minutes of exposure (Ahmed-Lecheheb et al., 2012). Furthermore, another study found a significant positive correlation between the amount of hand sanitizer used and EtOH concentration in inhaled air, suggesting that increased sanitizer use will increase inhaled alcohol exposure (Hautemaniere et al., 2013b).
Another set of experimental studies have evaluated direct biomarkers for alcohol exposure after replicating various recommended hand-sanitizing procedures in the laboratory. With notable exceptions (Miller et al., 2006), multiple studies have reported increases in BAC after exposure to alcohol vapor during hand-sanitizing protocols modeled from clinical settings. For example, Kramer and colleagues (2007) evaluated 3 hand rubs (95, 85, and 55% EtOH) in hygienic (4 ml applied 20 times) and surgical (20 ml applied 10 times) hand disinfection. The highest median blood levels of EtOH 30 minutes postexposure in the hygienic (2.1 mg/dl) and surgical (3.0 mg/dl) conditions were low, but still demonstrate a small dose-dependent increase in blood EtOH levels after exposure to alcohol vapor from hand sanitizer (Kramer et al., 2007). Additional studies using other hand-sanitizing protocols that mimic healthcare settings have reported consistent increases in BAC (Below et al., 2012; Brown et al., 2007) and BrAC (Ali et al., 2013; Brown et al., 2007) with the median BrAC at times exceeding the legal limit (119 mg/ dl after 3.0 ml) (Ali et al., 2013). Taken together, these results suggest that incidental exposure to alcohol vapor from hand sanitizer corresponds to inconsistent or extremely small increases in BrAC and BAC biomarkers.
In contrast, a number of studies have evaluated EtG and EtS levels after exposure to alcohol vapor. For example, in a simulation of a typical clinical shift, individuals used 3 ml of 96% EtOH sanitizer 4 times an hour for 8 hours and then provided repeated urine samples over 24 hours (Arndt et al., 2014). Use of sanitizer was associated with elevated EtG levels up to 2,100 ng/ml and individuals who were exposed only to alcohol vapor demonstrated concentrations between 600 and 800 ng/ml (Arndt et al., 2014). Notably, application of hand sanitizer under an exhaust fan (i.e., no vapor) resulted in undetectable EtG, further supporting the likelihood that positive biomarkers are a result of alcohol inhalation and not dermal resorption. In another study, daily pre- and posturinary analyses revealed that use of 1 ml of 62% EtOH hand sanitizer every 5 minutes for a 10-hour period on 3 consecutive days resulted in positive EtG levels in 90% of participants with a mean EtG of 278 ng/ml (range = 0 to 2,001 ng/ml). Positive urine EtS was present in 72% of the sample with a mean value of 9 ng/ml (range = 0 to 84 ng/ml) (Reisfield et al., 2011). Several other studies report detectable EtG and EtS after exposure to alcohol vapor from hand sanitizer (Arndt et al., 2012; Jones et al., 2006; Rohrig et al., 2006; Rosano and Lin, 2008) with reported average peak concentrations sometimes exceeding clinical thresholds commonly used to indicate alcohol consumption (Skipper et al., 2009). Therefore, in contrast to BAC, urine EtG may at least for a short period time document systemic exposure to alcohol after incidental exposure to EtOH vapor from hand sanitizer.
Other Occupational Exposure to Alcohol Vapor and Laboratory Alcohol Vapor Chambers
Compared with the numerous studies on biochemical biomarkers associated with exposure to alcohol vapors from hand sanitizer, we identified only 6 studies that explored possible functional biomarkers associated with direct exposure to inhaled alcohol. One study measured environmental air concentration of isopropanol in a printing works while measuring corresponding isopropanol concentration in alveolar air, blood, and urine of workers (Brugnone et al., 1983). This occupational exposure resulted in detectable levels in alveolar air (range 4 and 437 mg/m3), but not in blood or urine. While 1 study reported no detectable increases in blood EtOH content after exposure to vaporized EtOH (1,000 parts per million [ppm]) (Campbell and Wilson, 1986), 2 other studies combined alcohol vapor exposure with light exercise and reported increases in blood, urine, and BrAC after exposure to 142 ppm of 2-propanol vapor (Ernstgard et al., 2003) and a dose-dependent increase after exposure to 3 concentrations (0, 100, 200 ppm) of methanol (Ernstgard et al., 2005). Another study reported that exposure to 6 EtOH concentrations (125 to 1,000 ppm) in an inhalation chamber resulted in peak BAC of 0.3 (men) and 0.27 (women) mg/dl after 4 hours in the highest concentration (Dumas-Campagna et al., 2014). Finally, associations between BrAC and performance on neuromotor tasks, including reaction time, body sway, postural tremor, and velocity of hand movements, were evaluated during 6 hours of EtOH inhalation at 4 concentrations: 0, 250, 500, and 1,000 ppm (Nadeau et al., 2003). Results demonstrated negative BrAC in all conditions except for a negligible increase after 3 and 6 hours at the highest concentration and neuromotor effects were largely nonsignificant at all concentrations. This study was limited in that the total sample consisted of only 5 males and neuromotor tests were associated with high variability (Nadeau et al., 2003). Assessment of BAC/BrAC has been established after oral ingestion of alcohol, but arterial or brain levels may prove more relevant. If true, even a modest BAC or detectable EtG in a larger sample could potentially reflect functional impairment.
Alcohol Inhalation via E-Cigarette
A recent study measured motor performance and urine EtG after inhaling from an e-cigarette filled with e-liquids that contained high or low alcohol concentrations (Valentine et al., 2016). The e-liquids used in an e-cigarette contain numerous chemicals which have as-yet-unknown toxicities. Ethyl alcohol (alcohol) is one such constituent, but has received little scientific interest in this context (Hutzler et al., 2014; Varlet et al., 2015). The study evaluated the acute effects of puffing from commercially available e-liquids containing 23% or trace (<0.4%) alcohol in young adult social drinkers who also smoke cigarettes. While no differences in subjective drug effects were observed between alcohol conditions, puffing from an e-liquid with 23% alcohol was associated with diminished performance on the Purdue Pegboard Dexterity Test, a test known to be sensitive to alcohol exposure (Breckenridge and Berger, 1990; Buddenberg and Davis, 2000; Marczinski et al., 2012; Tarter et al., 1971). Further, in 3 of the 8 subjects with undetectable baseline urine EtG levels, just 1 test session of puffing from the e-cigarette with 23% alcohol resulted in positive EtG levels (average 371 ng/ml) verifying systemic exposure (Valentine et al., 2016). Importantly, the results may underestimate the intensity of exposure that could result after repeated, heavy e-cigarette use with alcohol-containing e-liquid.
DISCUSSION
The current review of the literature highlights that biomarkers of alcohol exposure in humans are measurable after inhalation of alcohol vapor, but at levels that are generally considered subthreshold for legal intoxication based on oral ingestion of alcohol. Thus, it is not surprising that inhalation of alcohol vapor is commonly perceived as innocuous; however, the acute pharmacodynamics of inhaled alcohol are presently unknown. Guided by preclinical and addiction literature, it is possible that inhaled alcohol is especially reinforcing due to immediate and rapid transmission to the brain. As such, the usual methods of quantifying alcohol exposure (i.e., BAC and BrAC) may be largely irrelevant to the behavioral and pharmacological impact of inhaled alcohol. That is, compared with oral consumption of alcohol, a “hit” of alcohol to brain is not likely to produce analogous BAC levels that are associated with well-characterized impairments in cognitive and motor performance. The most sensitive biomarkers for alcohol inhalation seem to be EtG (and/or EtS), and the presence of EtG confirms that some amount of alcohol has been absorbed and metabolized. With that said, the literature reviewed above generally reflect very low levels of exposure (e.g., alcohol vapor from hand sanitizer) that may not reflect the levels or exposure resulting from the recreational inhalation of alcohol.
Recreational use of alcohol vapor is most likely to resemble a binge-like pattern where inhalation occurs repeatedly in short bursts (e.g., e-cigarette inhalation, recreational alcohol vaping). Based on animal literature highlighting that intermittent, relative to continuous, EtOH vapor exposure results in rapid increases in self-administration and greater overall intake (O’Dell et al., 2004; Rimondini et al., 2002), the reinforcing effect of taking “hits” of alcohol vapor may be greater than, or serve to enhance, oral ingestion. Furthermore, akin to oral alcohol use, the volume of alcohol inhaled may be a critical factor in determining the clinical effect of repeated exposure over the course of day. Therefore, positive biomarkers found in the above studies, especially EtG, may significantly underestimate the clinical significance of recreational alcohol vapor exposure. It may also be possible that home-made vaporizers, relative to commercial products, may deliver higher volumes of alcohol vapor, potentially resulting in greater levels of alcohol exposure. Additional research is needed to characterize the consequences of different methods of alcohol inhalation (e.g., deliberate vaping, inadvertent exposure) and, perhaps, the likely pharmacokinetics of brain exposure as reflected by arterial levels. The behavioral consequences of alcohol inhalation also need to be better defined and measured after repeated intentional exposures that simulate recreational binge patterns of use.
Overall, the immediate safety concerns for inhaled alcohol may be relatively minor. However, there may be specific contexts of use that are more likely to result in acute behavioral effects such as while using high-powered electronic cigarette designs in combination with high-alcohol-content e-liquids. The psychomotor impact of repeated intentional inhalation of alcohol vapor has not been comprehensively studied in a controlled laboratory setting. Such studies are needed to determine whether the amount or frequency of inhaled alcohol poses similar risks to complex tasks, such as driving. Safety concerns aside, the clinical significance of inhaled alcohol is likely to dependent on the specific populations or individuals exposed. For example, subtle changes in interoceptive states resulting from inhaled alcohol may serve as conditioned reinforcers for alcohol use, regardless of whether they are consciously perceived or attributed to the inhalation of alcohol. Therefore, it is noteworthy that the existing studies on inhaled alcohol deliberately exclude vulnerable populations such as those with alcohol use disorder.
Prior research in clinical populations has suggested that reactivity to drug cues can be consciously perceived via controlled processing or subject to automatic processing that is unavailable to introspection (Ryan, 2002). Drug expectancy plays a critical role in the generation of craving such that an individual can experience craving after becoming aware of drug-related stimuli or interpreting a state (e.g., physiological arousal) as representing a need for drug use. Exposure to the odor of a preferred alcoholic beverage is an example of a consciously perceived drug cue that can readily generate craving and motivate drinking behavior. Exposure to “preattentive,” or subliminal, drug cues, is also associated with increased craving, motivation for drug seeking behavior, and activation of reward-related limbic brain regions (Childress et al., 2008; Franken et al., 2000; Wetherill et al., 2014; Young et al., 2014). Compared with similarly masked control images, alcohol cues that were presented for 30 ms were associated with deceleration of heart rate in heavy drinkers that is thought to be indicative of an orienting index for the automatic analysis of a stimulus (Ingjaldsson et al., 2003b), and this effect was more pronounced within “high cravers” (Ingjaldsson et al., 2003a). Additionally, exposure to subliminal alcohol-related, but not nonalcoholic, words resulted in activation of alcohol expectancy-consistent aggressive behavior (Friedman et al., 2007; Subra et al., 2010) and cognitive impairment (van Koningsbruggen and Stroebe, 2011). Thus, it may not be important that incidental or inadvertent (e.g., e-cigarettes) exposure to alcohol vapor results in biomarkers above or even near to legal limits; instead, the level of absorption after inhaling alcohol may only need to be sufficient to produce interoceptive or subjective states that have previously been associated with oral alcohol intoxication. Therefore, exposure to even small amounts of alcohol vapor by individuals in recovery from alcohol use disorders may facilitate relapse via reinforcement of alcohol-related expectancies that increase craving and/or attention to alcohol cues.
An alternative scenario is that exposure to alcohol vapor may influence the susceptibility to problematic or habitual oral alcohol use. The etiology of alcohol use disorders is complex and believed to be associated with a host of individual factors such as positive family history of alcohol problems, age of alcohol use onset, and co-occurring mental health and substance use disorders (for review, see Moss et al., 2007; de Wit and Phillips, 2012). In general, subjective positive effects resulting from initial drug use are believed to play a key role in escalation of problematic use and the development of drug use disorders (de Wit and Griffiths, 1991) and measures of drug liking have been posited to be both sensitive and reliable indicators of likelihood of abuse (Carter and Griffiths, 2009; Griffiths et al., 2003; McColl and Sellers, 2006). For example, evidence suggests that positive effects of nicotine are associated with abuse liability and increased smoking behavior while a sensitivity to negative or aversive subjective effects of nicotine may prevent both the initiation of tobacco product use as well as the amount of nicotine intake in established tobacco users (Carter et al., 2009; Hu et al., 2011; Jensen et al.,2015; Sartor et al.,2010). The neuropharmacological and behavioral effects of oral alcohol intoxication in heavy drinkers are believed to be multifaceted and include concurrent dimensions of reinforcement and punishment (Ray et al., 2009). Models that describe the development of alcohol use disorders are equally complicated with some highlighting increased experience of reinforcing effects of alcohol (e.g., Newlin and Thomson, 1990) and others emphasizing a reduced sensitivity to unpleasant effects of alcohol (e.g., Schuckit, 1994). Consistent with anecdotal and proposed behavioral and pharmacological consequences of inhaled alcohol, successive “hits” of alcohol may result in more reinforcing positive effects, and absent or attenuated aversive effects, when compared to oral alcohol consumption. As such, similar to the proposed etiology of nicotine addiction (Fowler and Kenny, 2014; Laviolette and van der Kooy, 2003; Riley, 2011), the potential for inhalation of alcohol vapor to influence susceptibility to the development of habitual alcohol use may be a function of the relative balance between inhaled alcohol’s positive and negative/aversive effects and is likely moderated by established risk factors for problematic drinking (e.g., positive family history).
Exposure to subthreshold doses of inhaled alcohol may also maintain or facilitate development of addiction to other substances, most notably nicotine. The frequent covariation of alcohol use and cigarette smoking is well documented with about 20% of those dependent on tobacco also dependent on alcohol, and with half of those who are alcohol dependent also being dependent on nicotine (Falk et al., 2006; Grant et al., 2004). Use of alcohol and nicotine together may lead to a greater reinforcement than either substance alone. Indeed, co-administration of subthreshold doses of nicotine and alcohol induces dopamine release in the nucleus accumbens, a region implicated in the reinforcing effects of drugs of abuse (Tizabi et al., 2007). In human laboratory studies, acute alcohol administration increases urges to smoke, smoking behavior, and satisfaction from smoking in both heavy and light smokers (Kahler et al., 2014; King et al., 2009; McKee et al., 2006; Sayette et al., 2005). Collectively, numerous studies across varying levels of analysis indicate that the development of nicotine addiction may be facilitated by the use of combustible tobacco products or e-cigarettes that also contain alcohol. Considering the rapid rise in e-cigarette use, particularly among adolescents (Bunnell et al., 2015), more research on the prevalence and patterns of alcohol absorption from e-cigarette use, and on the biological effects of co-administered nicotine and alcohol, is needed to understand the influence of inhaled alcohol vapors from e-cigarette use on the development of nicotine addiction, especially in specific populations with increased vulnerability.
CONCLUSIONS AND FUTURE RESEARCH
This review of the current literature evaluating inhaled alcohol use has revealed a predominate focus on the inhalation of alcohol vapor from hand sanitizer use, a ubiquitous behavior in the healthcare worker population. When using standards established by decades of research on oral ingestion, exposure to alcohol vapor is often assumed to pose a negligible risk. However, many studies have reported that inhalation of alcohol vapor can result in measurable biomarkers of alcohol exposure, most notably EtG and/or EtS documenting systemic alcohol exposure by the inhalation route. Importantly, the acute behavioral consequences and subjective effects of repeated exposure using devices capable of delivering alcohol vapor await further characterization. Finally, inhalation exposure may also be especially important in individuals with an alcohol use disorder, or those at risk of developing alcohol use disorder for whom priming doses of alcohol, as might occur with inadvertent inhalation via e-cigarettes or from other sources, may lead to greater craving and further alcohol use.
Acknowledgments
FUNDING SOURCES
Research reported in this publication was supported by the VA New England Mental Illness Research, Education, and Clinical Center (MIRECC) and by the P50DA036151 (Yale TCORS) from the National Institute on Drug Abuse of the National Institutes of Health and the U.S. Food and Drug Administration Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or of the U.S. Food and Drug Administration. Drs. MacLean, Valentine, Jatlow, and Sofuoglu declare they have no conflicts of interest.
REFERENCES
- Ahmed-Lecheheb D, Cunat L, Hartemann P, Hautemaniere A (2012) Der-mal and pulmonary absorption of ethanol from alcohol-based hand rub. J Hosp Infect 81:31–35. [DOI] [PubMed] [Google Scholar]
- Ali SS, Wilson MP, Castillo EM, Witucki P, Simmons TT, Vilke GM (2013) Common hand sanitizer may distort readings of breathalyzer tests in the absence of acute intoxication. Acad Emerg Med 20:212–215. [DOI] [PubMed] [Google Scholar]
- Allain F, Minogianis EA, Roberts DC, Samaha AN (2015) How fast and how often: the pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev 56:166–179. [DOI] [PubMed] [Google Scholar]
- Arndt T, Gruner J, Schrofel S, Stemmerich K (2012) False-positive ethyl glu-curonide immunoassay screening caused by a propyl alcohol-based hand sanitizer. Forensic Sci Int 223:359–363. [DOI] [PubMed] [Google Scholar]
- Arndt T, Schrofel S, Gussregen B, Stemmerich K (2014) Inhalation but not transdermal resorption of hand sanitizer ethanol causes positive ethyl glucuronide findings in urine. Forensic Sci Int 237:126–130. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF (2004) Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 28:1829–1838. [DOI] [PubMed] [Google Scholar]
- Below H, Partecke I, Huebner NO, Bieber N, Nicolai T, Usche A, Assadian O, Below E, Kampf G, Parzefall W, Heidecke CD, Zuba D, Bessonneau V, Kohlmann T, Kramer A (2012) Dermal and pulmonary absorption of propan-1-ol and propan-2-ol from hand rubs. Am J Infect Control 40:250–257. [DOI] [PubMed] [Google Scholar]
- Benowitz NL (2008) Clinical pharmacology of nicotine: Implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther 84:531–541. [DOI] [PubMed] [Google Scholar]
- Bessonneau V, Thomas O (2012) Assessment of exposure to alcohol vapor from alcohol-based hand rubs. Int J Environ Res Public Health 9:868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biomarkers Definitions Work Group (2001) Biomarkers and surrogate end-points: preferred definitions and conceptual framework. Clin Pharmacol Ther 69:89–95. [DOI] [PubMed] [Google Scholar]
- Bradizza CM, Stasiewicz PR, Paas ND (2006) Relapse to alcohol and drug use among individuals diagnosed with co-occurring mental health and sub-stance use disorders: a review. Clin Psychol Rev 26:162–178. [DOI] [PubMed] [Google Scholar]
- Breckenridge RL, Berger RS (1990) Locus of control and perceived alcohol ingestion in performance of a fine motor skill. Psychol Rep 66:179–185. [DOI] [PubMed] [Google Scholar]
- Brick J, Erickson CK (2009) Intoxication is not always visible: an unrecognized prevention challenge. Alcohol Clin Exp Res 33:1489–1507. [DOI] [PubMed] [Google Scholar]
- Brown TL, Gamon S, Tester P, Martin R, Hosking K, Bowkett GC, Gerostamoulos D, Grayson ML (2007) Can alcohol-based hand-rub solutions cause you to lose your driver’s license? Comparative cutaneous absorption of vari-ous alcohols. Antimicrob Agents Chemother 51:1107–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnone F, Perbellini L, Apostoli P, Bellomi M, Caretta D (1983) Iso-propanol exposure: environmental and biological monitoring in a printing works. Br J Ind Med 40:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddenberg LA, Davis C (2000) Test-retest reliability of the Purdue Pegboard Test. Am J Occup Ther 54:555–558. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Mathews TA, Lack AK, Diaz MR, McCool BA, Jones SR (2007) Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology 193:495–501. [DOI] [PubMed] [Google Scholar]
- Bunnell RE, Agaku IT, Arrazola RA, Apelberg BJ, Caraballo RS, Corey CG, Coleman BN, Dube SR, King BA (2015) Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011–2013. Nicotine Tob Res 17:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Kendall MW (2009) Gas Chromatography Mass Spectrometry (GC-MS) Analysis Report. Evans Analytical Group, Sunnyvale, CA. [Google Scholar]
- Campbell L, Wilson HK (1986) Blood alcohol concentrations following the inhalation of ethanol vapour under controlled conditions. J Forensic Sci Soc 26:129–135. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR (2009) Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend 105(Suppl 1):S14–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Stitzer ML, Henningfield JE, O’Connor RJ, Cummings KM, Hatsukami DK (2009) Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol Biomarkers Prev 18:3241–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain E, Solomon R (2002) The case for a 0.05% criminal law blood alcohol concentration limit for driving. Inj Prev 8 (Suppl 3):iii1–iii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP (2008) Prelude to passion: limbic activation by “unseen” drug and sex-ual cues. PLoS One 3:e1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE (2011) Iden-tifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology 36:2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Delory MJ (2009) Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology 204:335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas-Campagna J, Tardif R, Charest-Tardif G, Haddad S (2014) Ethanol toxicokinetics resulting from inhalation exposure in human volunteers and toxicokinetic modeling. Inhal Toxicol 26:59–69. [DOI] [PubMed] [Google Scholar]
- Ellicott M (2009) Analysis of Components from “e-Juice XX High 36 mg/ml Rated Nicotine Solution” ref S 55434. Report Number: E249A. LPD Lab Services BMS, Ltd, Lancashire, UK. [Google Scholar]
- Ernstgard L, Shibata E, Johanson G (2005) Uptake and disposition of inhaled methanol vapor in humans. Toxicol Sci 88:30–38. [DOI] [PubMed] [Google Scholar]
- Ernstgard L, Sjogren B, Warholm M, Johanson G (2003) Sex differences in the toxicokinetics of inhaled solvent vapors in humans 2. 2-propanol. Tox-icol Appl Pharmacol 193:158–167. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S (2006) An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health 29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE (2008) Exposure to the taste of alcohol elicits activetion of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology 33:1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ (2014) Nicotine aversion: neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology 76:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, Kroon LY, Wiers RW, Jansen A (2000) Selective cognitive processing of drug cues in heroin dependence. J Psychopharmacol 14:395–400. [DOI] [PubMed] [Google Scholar]
- Friedman RS, McCarthy DM, Bartholow BD, Hicks JA (2007) Interactive effects of alcohol outcome expectancies and alcohol cues on nonconsumptive behavior. Exp Clin Psychopharmacol 15:102–114. [DOI] [PubMed] [Google Scholar]
- Friel PN, Baer JS, Logan BK (1995) Variability of ethanol absorption and breath concentrations during a large-scale alcohol administration study. Alcohol Clin Exp Res 19:1055–1060. [DOI] [PubMed] [Google Scholar]
- Gewiss M, Heidbreder C, Opsomer L, Durbin P, De Witte P (1991) Acamprosate and diazepam differentially modulate alcohol-induced behavioural and cortical alterations in rats following chronic inhalation of ethanol vapour. Alcohol Alcohol 26:129–137. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF (2008) Vapor inhalation of alcohol in rats. Curr Protoc Neurosci Chapter 9:Unit 9.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Pal N (1971) Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science 172:288–290. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K (2004) Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 61:807–816. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA (2003) Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend 70(3 Suppl):S41–S54. [DOI] [PubMed] [Google Scholar]
- Gutman S, Kessler LG (2006) The US Food and Drug Administration perspective on cancer biomarker development. Nat Rev Cancer 6:565–571. [DOI] [PubMed] [Google Scholar]
- Hautemaniere A, Ahmed-Lecheheb D, Cunat L, Hartemann P (2013a) Assessment of transpulmonary absorption of ethanol from alcohol-based hand rub. Am J Infect Control 41:e15–e19. [DOI] [PubMed] [Google Scholar]
- Hautemaniere A, Cunat L, Ahmed-Lecheheb D, Hajjard F, Gerardin F, Morele Y, Hartemann P (2013b) Assessment of exposure to ethanol vapors released during use of alcohol-based hand rubs by healthcare workers. J Infect Public Health 6:16–26. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF (2007) A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci 30:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander A, Beck O (2005) Ethyl sulfate: a metabolite of ethanol in humans and a potential biomarker of acute alcohol intake. J Anal Toxicol 29:270–274. [DOI] [PubMed] [Google Scholar]
- Helander A, Bottcher M, Fehr C, Dahmen N, Beck O (2009) Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol 44:55–61. [DOI] [PubMed] [Google Scholar]
- Herrington JS, Myers C (2015) Electronic cigarette solutions and resultant aerosol profiles. J Chromatogr A 1418:192–199. [DOI] [PubMed] [Google Scholar]
- JS Herrington, C Myers, A Rigdon (2015) Analysis of nicotine and impurities in electronic cigarette solutions and vapor. Restek ChromatoGraphy Technical Resource Document, Bellefonte, PA: Available at: http://www.restek.com/pdfs/FFAN2127-UNV.pdf. Accessed December 15, 2016. [Google Scholar]
- Hirth N, Meinhardt MW, Noori HR, Salgado H, Torres-Ramirez O, Uhrig S, Broccoli L, Vengeliene V, Rossmanith M, Perreau-Lenz S, Kohr G, Sommer WH, Spanagel R, Hansson AC (2016) Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proc Natl Acad Sci USA 113:3024–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Griesler P, Schaffran C, Kandel D (2011) Risk and protective factors for nicotine dependence in adolescence. J Child Psychol Psychiatry 52:1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, Benowitz NL (2005) Metabolism and disposition kinetics of nicotine. Pharmacol Rev 57:79–115. [DOI] [PubMed] [Google Scholar]
- Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, Luch A (2014) Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol 88:1295–1308. [DOI] [PubMed] [Google Scholar]
- Ingall GB (2012) Alcohol biomarkers. Clin Lab Med 32:391–406. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Thayer JF, Laberg JC (2003a) Craving for alcohol and pre-attentive processing of alcohol stimuli. Int J Psychophysiol 49:29–39. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Thayer JF, Laberg JC (2003b) Preattentive processing of alcohol stimuli. Scand J Psychol 44:161–165. [DOI] [PubMed] [Google Scholar]
- Jatlow PI, Agro A, Wu R, Nadim H, Toll BA, Ralevski E, Nogueira C, Shi J, Dziura JD, Petrakis IL, O’Malley SS (2014) Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: results of a dose ranging alcohol challenge study and 2 clinical trials. Alcohol Clin Exp Res 38:2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatlow P, O’Malley SS (2010) Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin Exp Res 34:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, DeVito EE, Herman AI, Valentine GW, Gelernter J, Sofuoglu M (2015) A CHRNA5 smoking risk variant decreases the aversive effects of nicotine in humans. Neuropsychopharmacology 40:2813–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW (1993) Pharmacokinetics of ethanol in saliva: comparison with blood and breath alcohol profiles, subjective feelings of intoxication, and diminished performance. Clin Chem 39:1837–1844. [PubMed] [Google Scholar]
- Jones JT, Jones MR, Plate CA, Lewis D (2006) Ethyl glucuronide and ethyl sulfate concentrations following use of ethanol containing hand sanitizer. USDTL Res Monograph 2. Available at: http://www.usdtl.com/assets/whitepapers_etghandsanitizer_fd_010710.pdf. Accessed December 1, 2016. [Google Scholar]
- Kahler CW, Metrik J, Spillane NS, Day A, Leventhal AM, McKee SA, Tidey JW, McGeary JE, Knopik VS, Rohsenow DJ (2014) Acute effects of low and high dose alcohol on smoking lapse behavior in a laboratory ana-logue task. Psychopharmacology 231:4649–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AE, Radnovich AJ, Hector D, Ramchandani VA, O’Connor SJ, Lowe M, Li TK (2004) Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcohol Clin Exp Res 28:550–557. [DOI] [PubMed] [Google Scholar]
- King A, McNamara P, Conrad M, Cao D (2009) Alcohol-induced increases in smoking behavior for nicotinized and denicotinized cigarettes in men and women. Psychopharmacology 207:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner MH, Lang RA, Breuer B, Breuer M, Gronover CS, Zwingers T, Bottrich JG, Arndt A, Brauer U, Hintzpeter M, Burmeister MA, Fauteck JD (2009) Transdermal resorption of an ethanol- and 2-propanol-containing skin disinfectant. Langenbecks Arch Surg 394:151–157. [DOI] [PubMed] [Google Scholar]
- van Koningsbruggen GM, Stroebe W (2011) Lasting effects of alcohol: sub-liminal alcohol cues, impairment expectancies, and math performance. Eur J Soc Psychol 41: 807–811. [Google Scholar]
- Koob GF, Kenneth Lloyd G, Mason BJ (2009) Development of pharmacotherapies for drug addiction: a Rosetta stone approach. Nat Rev Drug Discov 8:500–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A, Below H, Bieber N, Kampf G, Toma CD, Huebner NO, Assadian O (2007) Quantity of ethanol absorption after excessive hand disinfecttion using three commercially available hand rubs is minimal and below toxic levels for humans. BMC Infect Dis 7:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugesen M (2008) Safety Report on the Ruyan e-Cigarette Cartridge and Inhaled Aerosol. Health New Zealand, Christchurch, New Zealand. [Google Scholar]
- Laviolette SR, van der Kooy D (2003) The motivational valence of nicotine in the rat ventral tegmental area is switched from rewarding to aversive following blockade of the alpha7-subunit-containing nicotinic acetylcholine receptor. Psychopharmacology 166:306–313. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Loheswaran G (2014) Alcohol inhalation. CMAJ 186:E399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester D, Greenberg L (1951) The inhalation of ethyl alcohol by man. I. Industrial hygiene and medicolegal aspects. II. Individuals treated with tetraethylthiuram disulfide. Q J Stud Alcohol 12:168–178. [PubMed] [Google Scholar]
- Little HJ (1991) Mechanisms that may underlie the behavioural effects of ethanol. Prog Neurobiol 36:171–194. [DOI] [PubMed] [Google Scholar]
- Lovell J (2004) Vaporized, oxygenated cocktail. The New York Times. Available at: http://www.nytimes.com/2004/12/12/magazine/vaporized-oxygenated-cocktail-the.html. Accessed December 15,2016. [Google Scholar]
- Lowen SB, Lukas SE (2006) A low-cost, MR-compatible olfactometer. Behav Res Methods 38:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, Mallya G, Palmer C, Penetar DM (2013) Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. NeuroImage 78:176–185. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF (1996) Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol 13:163–170. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT, Henges AL, Ramsey MA, Young CR (2012) Effects of energy drinks mixed with alcohol on information processing, motor coordination and subjective reports of intoxication. Exp Clin Psychopharmacol 20:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Rathmell JP, Swedberg MD, Jonzon B, Norsten-Hoog C (2001) Effects of infusion rate of intravenously adminis-tered morphine on physiological, psychomotor, and self-reported mea-sures in humans. J Pharmacol Exp Ther 299:1056–1065. [PubMed] [Google Scholar]
- Martin CS, Balaban CD, McBurney DH (2006) Tonic and phasic processes in the acute effects of alcohol. Exp Clin Psychopharmacol 14:209–218. [DOI] [PubMed] [Google Scholar]
- Martin E, Moll W, Schmid P, Dettli L (1984) The pharmacokinetics of alcohol in human breath, venous and arterial blood after oral ingestion. Eur J Clin Pharmacol 26:619–626. [DOI] [PubMed] [Google Scholar]
- Martin SA, McLanahan ED, El-Masri H, LeFew WR, Bushnell PJ, Boyes WK, Choi K, Clewell HJ 3rd, Campbell JL Jr (2012) Development of multi-route physiologically-based pharmacokinetic models for ethanol in the adult, pregnant, and neonatal rat. Inhal Toxicol 24:698–722. [DOI] [PubMed] [Google Scholar]
- McColl S, Sellers EM (2006) Research design strategies to evaluate the impact of formulations on abuse liability. Drug Alcohol Depend 83(Suppl 1):S52–S62. [DOI] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS (2006) Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology 189:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Sommer WH (2015) Postdependent state in rats as a model for medication development in alcoholism. Addict Biol 20:1–21. [DOI] [PubMed] [Google Scholar]
- Miller MA, Rosin A, Levsky ME, Patel MM, Gregory TJ, Crystal CS (2006) Does the clinical use of ethanol-based hand sanitizer elevate blood alcohol levels? A prospective study. Am J Emerg Med 24:815–817. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, Fiorentino D (2000) A Review of the Literature on the Effects of Low Doses of Alcohol on Driving-Related Skills. National Highway and Traffic Safety Administration, Washington, DC. [Google Scholar]
- Moss HB, Chen CM, Yi HY (2007) Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend 91:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton AJ, Maxi JK, Souza-Smith F, Bagby GJ, Gilpin NW, Molina PE, Gardner JD (2016) Alcohol vapor inhalation as a model of alcohol-induced organ disease. Alcohol Clin Exp Res 40:1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau V, Lamoureux D, Beuter A, Charbonneau M, Tardif R (2003) Neuromotor effects of acute ethanol inhalation exposure in humans: a prelim-nary study. J Occup Health 45:215–222. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB (1990) Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull 108:383–402. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF (2004) Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor expo-sure. Alcohol Clin Exp Res 28:1676–1682. [DOI] [PubMed] [Google Scholar]
- RachBeisel J, Scott J, Dixon L (1999) Co-occurring severe mental illness and substance use disorders: a review of recent research. Psychiatr Serv 50:1427–1434. [DOI] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Hutchison KE, Mackillop J, Galvan A, Ghahremani DG (2014) Initial evidence that OPRM1 genotype moderates ventral and dorsal striatum functional connectivity during alcohol cues. Alcohol Clin Exp Res 38:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, Leventhal A, Hutchison KE (2009) Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res 33:2154–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfield GM, Goldberger BA, Crews BO, Pesce AJ, Wilson GR, Teitelbaum SA, Bertholf RL (2011) Ethyl glucuronide, ethyl sulfate, and ethanol in urine after sustained exposure to an ethanol-based hand sanitizer. J Anal Toxicol 35:85–91. [DOI] [PubMed] [Google Scholar]
- Riley AL (2011) The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav 103:69–78. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M (2002) Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J 16:27–35. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M (2003) A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol 64:445–449. [DOI] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR (2006) Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology 31:988–996. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR (2004) Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci 24:10159–10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF (1996) Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res 20:1289–1298. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF (2000) Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology 22:581–594. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE (1979) Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol 27:466–486. [DOI] [PubMed] [Google Scholar]
- Rohrig TP, Huber C, Goodson L, Ross W (2006) Detection of ethylglucuronide in urine following the application of Germ-X. J Anal Toxicol 30:703–704. [DOI] [PubMed] [Google Scholar]
- Rosano TG, Lin J (2008) Ethyl glucuronide excretion in humans following oral administration of and dermal exposure to ethanol. J Anal Toxicol 32:594–600. [DOI] [PubMed] [Google Scholar]
- Ryan F (2002) Detected, selected, and sometimes neglected: cognitive processing of cues in addiction. Exp Clin Psychopharmacol 10:67–76. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Yau WY, Yang P, Robinson TE (2005) Rapid delivery of nicotine promotes behavioral sensitization and alters its neurobiological impact. Biol Psychiatry 57:351–360. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lessov-Schlaggar CN, Scherrer JF, Bucholz KK, Madden PA, Pergadia ML, Grant JD, Jacob T, Xian H (2010) Initial response to cigarettes predicts rate of progression to regular smoking: findings from an off-spring-of-twins design. Addict Behav 35:771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Perrott MA, Peters AR (2005) The effects of alcohol on cigarette craving in heavy smokers and tobacco chippers. Psychol Addict Behav 19:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K (2001) Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychia-try 158:1075–1083. [DOI] [PubMed] [Google Scholar]
- Schoenmakers T, Wiers RW, Field M (2008) Effects of a low dose of alcohol on cognitive biases and craving in heavy drinkers. Psychopharmacology 197:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA (1994) Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 151:184–189. [DOI] [PubMed] [Google Scholar]
- Skipper GE, Wurst FM, Weinmann W, Liepman M (2009) Ethanol-based hand sanitizing gel vapor causes positive alcohol marker ethylglucuronide and positive breathalyzer. J Addict Med 3:1–5.21768996 [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA (2008) Upregulation of voluntary alcohol intake, behaveioral sensitivity to stress, and amygdala crhr1 expression following a his-tory of dependence. Biol Psychiatry 63:139–145. [DOI] [PubMed] [Google Scholar]
- Sommer W, Spanagel R (2013) Behavioral neurobiology of alcohol addiction. Preface. Curr Top Behav Neurosci 13:v–vii. [DOI] [PubMed] [Google Scholar]
- Stogner JM, Eassey JM, Baldwin JM, Miller BL (2014) Innovative alcohol use: assessing the prevalence of alcohol without liquid and other non-oral routes of alcohol administration. Drug Alcohol Depend 142:74–78. [DOI] [PubMed] [Google Scholar]
- Subra B, Muller D, Begue L, Bushman BJ, Delmas F (2010) Automatic effects of alcohol and aggressive cues on aggressive thoughts and behaveiors. Pers Soc Psychol Bull 36:1052–1057. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Jones BM, Simpson CD, Vega A (1971) Effects of task complexity and practice on performance during acute alcohol intoxication. Percept Mot Skills 33:307–318. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL Jr, Taylor RE (2007) Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol 42:413–416. [DOI] [PubMed] [Google Scholar]
- Tygat J (2007) “Super Smoker” Expert Report, Final Report. Toxicology Laboratory, Catholic University, Leuven, Belgium. [Google Scholar]
- Valance C, Ellicott M (2008) Analysis of Chemical Components from High, med & Low Nicotine Cartridges. LPD Lab Services BMS, Ltd., Lancashire, UK. [Google Scholar]
- Valentine GW, Jatlow PI, Coffman M, Nadim H, Gueorguieva R, Sofuoglu M (2016) The effects of alcohol-containing e-cigarettes on young adult smokers. Drug Alcohol Depend 159:272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlet V, Farsalinos K, Augsburger M, Thomas A, Etter JF (2015) Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health 12:4796–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ (2014) Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol 48:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Childress AR, Jagannathan K, Bender J, Young KA, Suh JJ, O’Brien CP, Franklin TR (2014) Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacology 231:1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson PK (1980) Pharmacokinetics of ethanol: a review. Alcohol Clin Exp Res 4:6–21. [DOI] [PubMed] [Google Scholar]
- de Wit H, Bodker B, Ambre J (1992) Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology 107:352–358. [DOI] [PubMed] [Google Scholar]
- de Wit H, Griffiths RR (1991) Testing the abuse liability of anxiolytic and hypnotic drugs in humans. Drug Alcohol Depend 28:83–111. [DOI] [PubMed] [Google Scholar]
- de Wit H, Phillips TJ (2012) Do initial responses to drugs predict future use or abuse? Neurosci Biobehav Rev 36:1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst FM, Dresen S, Allen JP, Wiesbeck G, Graf M, Weinmann W (2006) Ethyl sulphate: a direct ethanol metabolite reflecting recent alcohol con-sumption. Addiction 101:204–211. [DOI] [PubMed] [Google Scholar]
- Wurst FM, Thon N, Yegles M, Schruck A, Preuss UW, Weinmann W (2015) Ethanol metabolites: their role in the assessment of alcohol intake. Alcohol Clin Exp Res 39:2060–2072. [DOI] [PubMed] [Google Scholar]
- Young KA, Franklin TR, Roberts DC, Jagannathan K, Suh JJ, Wetherill RR, Wang Z, Kampman KM, O’Brien CP, Childress AR (2014) Nipping cue reactivity in the bud: baclofen prevents limbic activation elicited by subliminal drug cues. J Neurosci 34:5038–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer H, Schmitt G, Aderjan R (2002) Preliminary immunochemical test for the determination of ethyl glucuronide in serum and urine: comparison of screening method results with gas chromatography-mass spectrometry. J Anal Toxicol 26:11–16. [DOI] [PubMed] [Google Scholar]


