Abstract
OBJECTIVE
To characterize pharmacokinetics of cyclophosphamide and 4-hydoxycyclophosphamide (4-OHCP) in the plasma of healthy cats after oral, intravenously, or intraperitoneal administration of cyclophosphamide.
ANIMALS
6 healthy adult cats.
PROCEDURES
Cats were randomly assigned to receive 200 mg/m2 of cyclophosphamide via each of 3 routes of administration (oral, IV, and IP); there was a 30-day washout period between successive treatments. Plasma samples were obtained at various time points for up to 8 hours after administration. Samples were treated with semicarbazide hydrochloride to trap the 4-OHCP in stable form, which allowed for cyclophosphamide and trapped 4-OHCP to be simultaneously measured by use of tandem mass spectrometry. Pharmacokinetic parameters were determined from drug concentration–versus-time data for both cyclophosphamide and 4-OHCP.
RESULTS
Cyclophosphamide was tolerated well regardless of route of administration. Pharmacokinetic parameters for 4-OHCP were similar after oral, IV, and IP administration. Area under the concentration-time curve for cyclophosphamide was lower after oral administration than after IV or IP administration.
CONCLUSIONS AND CLINICAL RELEVANCE
Cyclophosphamide can be administered interchangeably to cats as oral, IV, and IP, formulations, which should provide benefits with regard to cost and ease of administration to certain feline patients. (Am J Vet Res 2017;78:862–866)
Cyclophosphamide is an alkylating agent commonly used in veterinary oncology. It frequently is administered in combination with other chemotherapy drugs as part of multiagent protocols or orally in a low-dose continuous manner.1,2 Cyclophosphamide has been administered IV, orally, and IP to cats.4–7
Cyclophosphamide is a prodrug that is oxidized by hepatic P450 enzymes to form the metabolite 4-OHCP.3,7–10 Subsequently, 4-OHCP is in equilibrium with its open-ring tautomer aldophosphamide; 4-OHCP and aldophosphamide then move intracellularly. Aldophosphamide spontaneously decomposes to the active alkylating metabolite, phosphoramide mustard, and the less active by-product acrolein.1,3,8,10 Phosphoramide mustard has cytotoxic effects that are 4 to 10 times the cytoxic effects for 4-OHCP; however, phosphoramide mustard is highly reactive and intracellular, thereby making it unstable and difficult to quantify.9 Therefore, for bioavailability purposes, concentrations of 4-OCHP are often measured as a surrogate for the active concentrations of phosphoramide mustard.9–11 There is equal bioavailability for 4-OHCP in dogs with lymphoma receiving cyclophosphamide via oral or IV administration.1,12 This confirms that the 2 administration routes could be used interchangeably without substantial differences in 4-OHCP concentrations. Safety and efficacy of cyclophosphamide and vincristine administered IP to 26 cats with various forms of malignant lymphoma have been confirmed.7 No specific IP-related adverse effects were seen in any cats of that study,7 and cats had similar survival times after administration via that route, compared with survival times for other reports,4–6,13 which indicated probable efficacy. A pharmacokinetic analysis revealed equal bioavailability of the parent compound (cyclophosphamide) given IV or IP; however, the active metabolite 4-OHCP was not measured.7,8
Although the safety and efficacy for IV, IP, and oral administration of cyclophosphamide have been reported,7,8 bioavailability of the active metabolite 4-OHCP has not been evaluated in cats. The objective of the study reported here was to assess the bioavailability of cyclophosphamide and 4-OHCP after IV, IP, and oral administration of cyclophosphamide to cats.
Materials and Methods
Animals
Six apparently healthy adult cats (3 males and 3 females) were included in the study. Mean body weight of the cats was 4 kg (mean body surface area, 0.252 m2), and mean age was 1.4 years (range, 0.85 to 2.75 years). Cats were housed individually in cages beginning the night prior to drug administration until 1 week after drug administration. They were then moved to group housing for 1 month, until their next drug administration. The study was approved by the Institutional Animal Care and Use Committee at Colorado State University.
Treatment protocol
A randomized triple crossover prospective study was performed. Cats were randomly assigned to their initial dosing group (their names were written on pieces of paper, the papers were placed into a container, and an investigator blindly selected the names). Cats in each group were administered cyclophosphamide (200 mg/m2). Cats received cyclophosphamide solutiona IV and IP and cyclophosphamide capsulesb orally; orally administered doses were rounded down to the nearest 25-mg capsule. There was a 30-day washout period between subsequent treatments; cats were arbitrarily rotated to another administration group. Thus, each cat received cyclophosphamide once via each route of administration (total of 3 treatments).
A physical examination was performed daily for 1 week after each treatment. Adverse events were recorded in accordance with published criteria.9 A CBC and biochemical analysis were performed 1 to 2 days before each treatment, and a CBC was performed 1 week after each treatment.
Sample collection
The evening before each cyclophosphamide administration, cats were sedated with butorphanol tartrate (0.2 mg/kg, IV) and ketamine hydrochloride (20 mg, IV), and a catheter was inserted in a jugular vein. The following morning, cyclophosphamide was administered via the specified route, and 1 to 2 mL of blood was collected immediately before (time 0) and 15, 30, and 60 minutes and 2, 4, 6, 8, and 12 hours after administration. Food was not withheld from cats before cyclophosphamide administration. Preliminary results obtained after the first treatment of each group revealed undetectable amounts of cyclophosphamide and 4-OHCP in plasma at the 12-hour sample; therefore, this time point was eliminated for the second and third treatments.
Blood samples were collected into sodium heparin tubes, placed immediately on ice, and centrifuged (2,000 × g for 10 minutes at 4°C) within 15 minutes after sample collection. Plasma was harvested and combined with analytic-grade 2M SCZc (1 part SCZ to 10 parts plasma) to trap the 4-OHCP.11,12 Samples were mixed in a vortex device and stored frozen at −80°C until analysis. Standard dilutions of analytic-grade cyclophosphamide monohydrated and 4-OOHCPe (synthetic preactivated [preoxidized] precursor to 4-OHCP; purity, 98%) were prepared in water at concentrations of 10 and 1 mg/mL, respectively. The standard dilutions were immediately added to naïve feline plasma, which resulted in standard curves ranging from 1 to 7,500 ng/mL for cyclophosphamide and from 10 to 7,500 ng/mL for 4-OOHCP. When placed in aqueous solutions, 4-OOHCP spontaneously converts to 4-OHCP; therefore, 2M SCZ was added to trap the 4-OHCP. Samples were then mixed in a vortex device, separated into aliquots, and stored at −80°C until analysis.
Liquid chromatography–tandem mass spectrometry
Plasma samples were analyzed in accordance with methods reported previously.12 Briefly, protein precipitation was achieved by mixing an equal volume of acetonitrile (50 μL) with 2M SCZ–treated plasma (50 μL) and then adding 5 μL of analytic-grade hexamethylphosphoramided (internal standard]); the mixture was then centrifuged. An aliquot (50 μL) of the supernatant was mixed with 150 μL of 1mM ammonium hydroxided (pH, 10.1), and 5 μL of this mixture was evaluated on a chromatograph by use of a C18 columnf (5 μm; 2.1 × 150 mm) maintained at room temperature (23 ± 2°C). Gradient elution (flow rate, 440 μL/min) was performed as follows: initial elution with 80% aqueous phase (1mM ammonium hydroxide) and 20% organic phase (acetonitriled) for 1 minute, linear increase in acetonitrile to 50% for 2 minutes, maintain at 50% acetonitrile for 1.5 minutes, linear return to 20% acetonitrile for 0.5 minutes, and maintain at 20% acetonitrile for 1 minute (total run time, 6 minutes). A triple-quadrupole mass spectrometerg in positive ion electrospray ionization mode was used to acquire mass spectra for each analyte by multiple reaction monitoring of the ion transitions m/z 261.2 → 140.2 for cyclophosphamide, m/z 334.3 → 221.2 for the SCZ derivative of 4-OHCP, and m/z 180.0 → 135.0 for the internal standard. Settings were specified for the mass spectrometer as follows: turbo ion spray temperature, 600°C; ion spray voltage, 3,500 V; declustering potential, 40.1, 29.4, and 21.0 V for cyclophosphamide, the SCZ derivative of 4-OHCP, and the internal standard, respectively; entrance potential, 5.2, 3.9, and 4.0 V for cyclophosphamide, the SCZ derivative of 4-OHCP, and the internal standard, respectively; collision energy, 26, 22, and 10 V for cyclophosphamide, the SCZ derivative of 4-OHCP, and the internal standard, respectively; collision cell entrance potential, 55.0, 38.6, and 15.2 V for cyclophosphamide, the SCZ derivative of 4-OHCP, and the internal standard, respectively; collision cell exit potential, 1.8, 2.4, and 3.0 V for cyclophosphamide, the SCZ derivative of 4-OHCP, and the internal standard, respectively; curtain gas, 60 U of N2; and auxiliary gas, 60 U of N2.
Statistical analysis
The AUC0−∞ of cyclophosphamide and 4-OHCP was calculated by use of noncompartmental analysis.h Parameters calculated included Cmax, clearance, AUC0−∞, t1/2, and time to Cmax. All values were reported as mean ± SD. A 1-way repeated-measures ANOVAi was conducted to compare the effect of route of administration on AUC0−∞, Cmax, and clearance. Values were considered significantly different at P < 0.05.
Results
Only 1 cat had gastrointestinal toxicosis (grade 11 diarrhea) after cyclophosphamide administration; however, this cat had positive results when tested for giardiasis 2 weeks after the second cyclophosphamide administration. Diarrhea resolved after treatment with fenbendazole and metronidazole. This same cat developed soft feces 1 day after the third cyclophosphamide administration; however, this resolved without any additional treatment. No hematologic toxic events were detected after cyclophosphamide administration, and all hematologic and biochemical variables evaluated were within reference limits. One cat had a grade IV toxicosis; however, it was a consequence secondary to the procedural technique and was not a drug-related event. This cat developed cardiac outflow obstruction secondary to catheter placement in the jugular vein, and the cat recovered fully once the catheter was removed. Cyclophosphamide had been orally administered to this cat 4 hours before the cardiac outflow obstruction event; therefore, on that day of the study, data for this cat were eliminated from statistical analysis.
Each cat received 1 dose of cyclophosphamide (200 mg/m2) IV and IP as well as 1 dose of cyclophosphamide (median, 158 mg/m2; range, 99 to 180 mg/m2) orally. Mean concentration-versus-time curves for cyclophosphamide and 4-OHCP were plotted for each of the 3 routes of administration (Figure 1).
Figure 1.
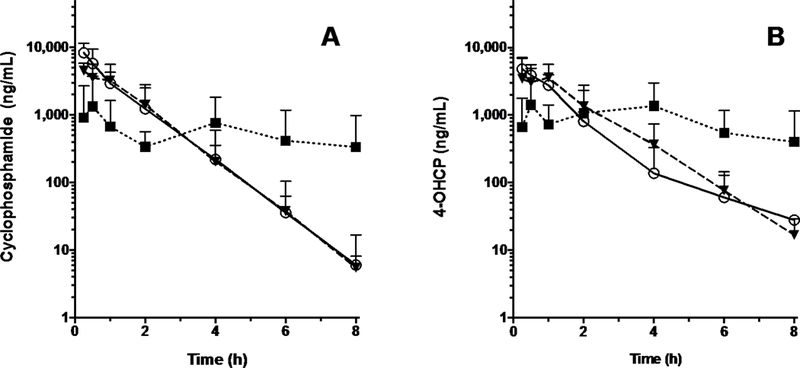
— Mean ± SD concentrations of cyclophosphamide (A) and 4-OHCP (B) in plasma of 6 healthy cats after oral (median, 158 mg/m2; range, 108 to 198 mg/m2; triangles and dashed line), IV (200 mg/m2; circles and solid line), and IP (200 mg/m2; squares and dotted line) administration. There was a 30-day washout period between successive treatments.
Mean ± SD AUC0−∞ for cyclophosphamide was similar after IV (8.96 ± 764 μg/h/mL) and IP (7.48 ± 307 μg/h/mL) administration, and those values did not differ significantly (P = 0.011) from the AUC0−∞ after oral administration (4.20 ± 3.29 μg/h/mL). Route of administration had a significant (P = 0.03) effect on Cmax of cyclophosphamide, which was highest for IV administration (9.18 ± 4.67 μg/mL), followed by IP administration (4.79 ± 1.41 μg/mL) and then oral administration (2.11 ± 2.11 μg/mL). Mean clearance did not differ significantly (P = 0.36) after oral (4.56 ± 3.63 mL/h), IV (3.24 ± 3.23 mL/h), and IP (2.21 ± 0.65 mL/h) administration. Mean t1/2 after IP and IV administration was 0.62 ± 0.13 hours and 0.59 ± 0.24 hours, respectively; t1/2 could not be determined after oral administration because the terminal elimination phase was not reached.
Mean ± SD AUC0−∞ for 4-OHCP did not differ significantly (P = 0.75) among the 3 routes of administration (6.36 ± 1.76 μg/h/mL, 7.62 ± 3.10 μg/h/mL, and 6.53 ± 4.13 μg/h/mL after IV, IP, and oral administration, respectively). Additionally, Cmax for 4-OHCP did not differ significantly (P = 0.20) among the 3 routes of administration (4.95 ± 2.25 μg/mL, 5.39 ± 2.77 μg/mL, and 3.20 ± 1.81 μg/mL after IV, IP, and oral administration, respectively). There was no difference in mean terminal t1/2 for 4-OHCP between IV and IP administration (0.78 ± 0.61 hours and 0.77 ± 0.27 hours, respectively). The t1/2 for 4-OHCP could not be determined after oral administration because the terminal elimination phase was not reached. Mean time to Cmax for 4-OHCP was 0.29 ± 0.10 hours after IV administration, 0.75 ± 0.39 hours after IP administration, and 2.17 ± 1.57 hours after oral administration.
Discussion
Similar to findings for humans15 and dogs,12 whereby oral and IV administration can be used interchangeably with resulting similar exposure to an active metabolite, there was no difference detected in the AUC0−∞ for 4-OHCP among the 3 routes of administration in the study reported here. The concentrations were similar among all routes of administration, despite the larger dose range for oral administration (range, 108 to 198 mg/m2), which suggested that the AUC0–∞ may be dose independent within this range for oral administration.
In contrast to results of a previous study7 in which cyclophosphamide exposure was greater, albeit not significantly different, after IV and IP administration, there was nearly equal bioavailability for the study reported here. Findings for the present study more closely resembled results of another study12 in which IV and IP administration resulted in equal bioavailability for both cyclophosphamide and 4-OHCP. One explanation for the difference in results for the former study7 may involve the fact that only cyclophosphamide was measured in that study, whereas cyclophosphamide and 4-OHCP were measured in the latter study12 and present study.
Additionally, similar to previous findings for dogs,12 oral administration of cyclophosphamide to cats in the present study resulted in an AUC0–∞ that was approximately half the AUC0–∞ after IV or IP administration. This can be explained by first-pass elimination in the liver because cyclophosphamide must undergo transformation to 4-OHCP via P450 enzymatic activation.10,16,17 Therefore, only a portion of a drug administered orally is detected systemically after first-pass metabolism.
The main intent of the present study was to detect drug exposure in healthy cats; thus, we decided to administer a dose of 200 mg/m2 instead of commonly used higher doses of 250 mg/m2. Despite the lower dose, all 3 routes of administration resulted in adequate exposure to 4-OHCP. The main variability in dosage was with the oral formulation because of the commercially available capsule size (25 mg). We elected to forego compounding of capsules for oral administration because of concerns about variability in quality and the ability to accurately reproduce capsule sizes at clinical practices. It is possible that the variability in the dose used for oral administration (median, 158 mg/m2; range, 108 to 198 mg/m2) may have influenced results of the present study. However, analysis indicated that despite the variability in the dose range, there was no difference in the AUC0–∞ for the active metabolite after oral administration of cyclophosphamide. This may suggest that exposure was a dose-independent phenomenon at the doses administered, which further supported the contention that oral administration can be interchangeable with IV and IP administration.
The present study was designed to include cats with little interanimal variability (all were of similar age and body weight, had a similar environment and diet, and did not receive prior chemotherapy) to properly establish baseline pharmacokinetic data. It is possible that exposure to drugs may be altered in cats with gastrointestinal lymphoma or liver dysfunction or that are receiving concurrent medications. Assessment of drug exposure in cats with gastrointestinal lymphoma would provide a more clinically relevant picture.
The present study provided information that can be of clinical benefit because the cost of oral formulations of cyclophosphamide is substantially less than that of formulations for IV or IP administration. In addition, IP administration may be easier to perform in fractious cats that are not amenable to catheter placement or oral administration of a drug. Furthermore, IP administration can potentially minimize exposure of personnel to chemotherapeutic agents, compared with exposure of personnel administering drugs orally.
Acknowledgments
Supported by the Angelo Fund for Feline Therapeutics, the University of Colorado Cancer Center Shared Resource Grant (No. P30CA046934), and the Flint Animal Cancer Center.
Presented in abstract form at the annual Veterinary Cancer Society Conference, Tyson’s Corner, Va, October 2015.
The authors thank Drs. Jessica Quimby and Kristen Weishaar for technical assistance.
ABBREVIATIONS
- 4-OHCP
4-hydroxycyclophosphamide
- 4-OOHCP
4-hydroperoxycyclophosphamide
- AUC0–∞
Area under the curve from time 0 extrapolated to infinity
- Cmax
Maximum concentration
- SCZ
Semicarbazide hydrochloride
- t1/2
Half-life
Footnotes
Footnotes
a. Baxter Healthcare Corp, Deerfield, Ill.
b. Roxane Laboratories, Columbus, Ohio.
c. Sigma-Aldrich Co, St Louis, Mo.
d. Sigma-Aldrich Co, St Louis, Mo.
e. IIT GmbH/NIOMECH, Bielefeld, Germany.
f. Waters Inc, Milford, Mass.
g. AB Sciex 3200 Q-TRAP, Applied Biosystems Inc, Foster City, Calif.
h. Phoenix WinNonlin, Certara, Princeton, NJ.
i. ANOVA, GraphPad Prism, version 7.00 for Windows, GraphPad Software Inc, La Jolla, Calif.
References
- 1.Chun R, Garrett L, Vail D. Cancer chemotherapy In: Withrow S, Vail D, eds. Withrow and MacEwen’s small animal clinical oncology. 4th ed London: Saunders, 2007; 163–193. [Crossref] [Google Scholar] [Google Scholar]
- 2.Hayes A Feline lymphoma; principles of diagnosis and management. In Pract 2006; 28: 516–524. [Crossref] [Google Scholar] [Google Scholar]
- 3.Gerson S, Friedman H. Alkylating agents (including methylating agents) In: Chabner B, Longo D, eds. Cancer chemotherapy and biotherapy. 5th ed Philadelphia: Lippincott, Williams & Wilkins, 2010; 267–309. [Google Scholar] [Google Scholar]
- 4.Collette SA, Allstadt SD, Chon EM, et al. Treatment of feline intermediate- to high-grade lymphoma with a modified university of Wisconsin–Madison protocol: 119 cases (2004–2012). Vet Comp Oncol 2016; 14: 136–146. [Crossref] [Medline] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon D, Eberle N, Laacke-Singer L, et al. Combination chemotherapy in feline lymphoma: treatment outcome, tolerability, and duration in 23 cats. J Vet Intern Med 2008; 22: 394–400. [Crossref] [Medline] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 6.Taylor SS, Goodfellow MR, Browne WJ, et al. Feline extra-nodal lymphoma: response to chemotherapy and survival in 110 cats. J Small Anim Pract 2009; 50: 584–592. [Crossref] [Medline] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 7.Teske E, van Lankveld AJ, Rutteman GR. Intraperitoneal antineoplastic drug delivery: experience with a cyclophosphamide, vincristine and prednisolone protocol in cats with malignant lymphoma. Vet Comp Oncol2014; 12: 37–46. [Crossref] [Medline] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 8.Voorhorst MJ, van Maarseveen EM, van Lankveld AJ, et al. Bioavailability of cyclophosphamide and vincristine after intraperitoneal administration in cats. Anticancer Drugs 2014; 25: 1211–1214. [Crossref] [Medline][Google Scholar] [DOI] [PubMed] [Google Scholar]
- 9.Huitema ADR, Tibben MM, Kerbusch T, et al. High-performance liquid chromatographic determination of the stabilized cyclophosphamide metabolite 4-hydroxycyclophosphamide in plasma and red blood cells. J Liq Chromatogr Relat Technol 2000; 23: 1725–1744. [Crossref] [Google Scholar] [Google Scholar]
- 10.Moore MJ. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 1991; 20: 194–208. [Crossref] [Medline] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 11.de Jonge ME, van Dam SM, Hillebrand MJ, et al. Simultaneous quantification of cyclophosphamide, 4-hydroxycyclophosphamide, N,N′,N″-triethylenethiophosphoramide (thiotepa) and N,N′,N″-triethylenephosphoramide (tepa) in human plasma by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J Mass Spectrom 2004; 39: 262–271. [Medline] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 12.Warry E, Hansen RJ, Gustafson DL, et al. Pharmacokinetics of cyclophosphamide after oral and intravenous administration to dogs with lymphoma. J Vet Intern Med 2011; 25: 903–908. [Crossref] [Medline] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teske E, van Straten G, van Noort R, et al. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (cop) in cats with malignant lymphoma: new results with an old protocol. J Vet Intern Med 2002; 16: 179–186. [Crossref] [Medline] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 14.Veterinary Co-operative Oncology G. Veterinary Co-operative Oncology Group–Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol 2004; 2: 195–213. [Crossref] [Medline] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 15.Struck RF, Alberts DS, Horne K, et al. Plasma pharmacokinetics of cyclophosphamide and its cytotoxic metabolites after intravenous versus oral administration in a randomized, crossover trial. Cancer Res 1987; 47: 2723–2726. [Medline] [Google Scholar] [PubMed] [Google Scholar]
- 16.Frye RF, Zgheib NK, Matzke GR, et al. Liver disease selectively modulates cytochrome P450–mediated metabolism. Clin Pharmacol Ther 2006; 80: 235–245. [Crossref] [Medline] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 17.Holford NHG. Pharmacokinetics and pharmacodynamics: rational dosing and time course of drug action In: Katzung B, Masters S, Trevor A, eds. Basic and clinical pharmacology. 11th ed New York: McGraw-Hill Medical Publishing Division, 2009; 37–66. [Google Scholar [Google Scholar]


