Abstract
We investigated sex as a potential modifier of influenza vaccine effectiveness (VE) between 2010–2011 and 2016–2017 in Canada. Overall VE was 49% (95% confidence interval [CI], 43% to 55%) for females and 38% (95% CI, 28% to 46%) for males (absolute difference [AD], 11%; P = .03). Sex differences were greatest for influenza A(H3N2) (AD, 17%; P = .07) and B(Victoria) (AD, 20%; P = .08) compared with A(H1N1)pdm09 (AD, 10%; P = .19) or B(Yamagata) (AD, –3%; P = .68). They were also more pronounced in older adults ≥50 years (AD, 19%; P = .03) compared with those <20 years (AD, 4%; P = .74) or 20–49 years (AD, –1%; P = .90) but with variation by subtype/lineage. More definitive investigations of VE by sex and age are warranted to elucidate these potential interactions.
Keywords: effect modification, gender, influenza vaccine, influenza virus, sex, vaccine effectiveness
Sex is a potential confounder in the evaluation of influenza vaccine effectiveness (VE) due to its plausible association both with the likelihood of receiving influenza vaccine and with having an influenza exposure [1]. Females tend to have higher influenza vaccination coverage rates than males (at least among young adults in Canada and the United States [2, 3]), likely related to their greater propensity for seeking health care. Women also tend to have greater opportunities for exposure to influenza associated with their traditional gender roles as primary caregivers for children and the elderly or their greater propensity to work in health care or other occupations that may increase the likelihood of exposure [1]. Conversely, morbidity and mortality due to influenza are generally thought to be higher among males (the so-called “man flu” phenomenon [4]). This association may have a biological basis, with greater sex differences in influenza risk reported in the youngest and oldest age groups [5–9].
Multivariable VE analyses are typically adjusted for sex to account for this potential source of bias. Although there may be true biological differences in response to influenza vaccine between males and females, sex is rarely considered a potential effect modifier of influenza VE. Here we investigate the interaction between sex and influenza vaccination for VE against medically attended, laboratory-confirmed influenza illness across 7 seasons (2010–2011 to 2016–2017) in Canada. We further explore whether sex differences in VE vary by influenza subtype/lineage, age, or season.
METHODS
The current analysis utilized historical databases of the Canadian Sentinel Practitioner Surveillance Network (SPSN) from 2010–2011 to 2016–2017 according to methods previously described [10–16]. Briefly, respiratory specimens were collected from outpatients 1 or more years old presenting to sentinel practitioners within 7 days of onset of influenza-like illness using a standardized case definition. Specimens were tested for influenza viruses by real-time reverse transcription polymerase chain reaction at public health reference laboratories in each participating province (Alberta, British Columbia, Ontario, and Quebec). Patients testing positive for influenza were considered cases; those testing negative for influenza were considered controls.
Patient data, including sex and vaccination status, were recorded on the laboratory requisition form by the ordering physician at the time of specimen collection before influenza diagnosis. Vaccination status was based on patient self-report but may have also been documented in the physician’s records. Patients were considered vaccinated if they received seasonal influenza vaccine ≥2 weeks before symptom onset; patients who received influenza vaccine <2 weeks before symptom onset were excluded. All patients or their parent/guardian provided verbal consent. Institutional review boards in each province provided approval for this study.
Differences between male and female patients were compared by chi-square test for categorical variables or the nonparametric Wilcoxon rank-sum test for continuous variables. Odds ratios (ORs) for influenza test positivity (by influenza A subtype or influenza B lineage) comparing vaccinated with unvaccinated patients were derived using logistic regression according to a test-negative study design for all seasons combined. Seasons for which there was minimal circulation of a given subtype/lineage were excluded from the pooled analysis for that outcome. Covariates included in the interaction models were vaccination status, sex, age group, comorbidity, province, specimen collection interval (days from symptom onset to specimen collection), calendar time (based on week of specimen collection, modeled as a cubic B-spline function with 3 equal knots), season, and an interaction term for vaccine*sex. VE was derived as (1−OR)×100%, where OR = exp[βvac] for females (sex = 0) and OR = exp[βvac + βvac*sex] for males (sex = 1). To verify the results and ensure that our interaction models were adequately specified, we also conducted separate analyses in male and female strata; sex-stratified VE estimates generally differed by ≤5% from the interaction models (data not shown). All analyses were performed in SAS, version 9.4 (SAS Inc., Cary, NC).
RESULTS
Females were over-represented among SPSN participants (60% vs 40%), both among influenza cases (58% vs 42%) and test-negative controls (61% vs 39%; P < .01). The age distribution varied by sex, with greater over-representation of females among adults age 20–49 years (62% vs 38%) and older adults age ≥50 years (63% vs 37%) than children and adolescents younger than age 20 years (51% vs 49%; P < .01). Overall, females were slightly less likely to test positive for influenza compared with males (40% vs 43%; P < .01), mostly driven by detection of A(H3N2) (17% vs 19%; P < .01), and less so by A(H1N1)pdm09 (10% vs 10%; P = .71), B(Yamagata) (6% vs 7%; P = .18), or B(Victoria) (4% vs 5%; P = .04). Females also had higher vaccination coverage than males overall (29% vs 23%; P < .01) and among negative controls (34% vs 27%; P < .01) but not among influenza cases (21% vs 19%; P = .10) (Supplementary Tables 1 and 2).
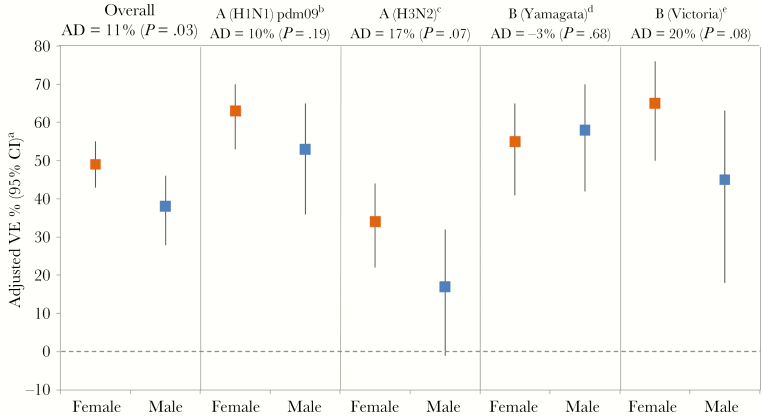
In general, adjusted VE was higher among females than males, although this varied by influenza subtype/lineage, age group, and season. Overall for any influenza, adjusted VE was 49% (95% confidence interval [CI], 43% to 55%) for females vs 38% (95% CI, 28% to 46%) for males (absolute difference [AD], 11%; P value for interaction term = .03) (Figure 1). The greatest absolute differences between females and males were for A(H3N2) and B(Victoria). For A(H3N2), adjusted VE in all seasons combined was 34% (95% CI, 22% to 44%) for females vs 17% (95% CI, –1% to 32%) for males (AD, 17%; P = .07). Excluding the 2014–2015 season, for which overall VE against A(H3N2) was negligible [14], adjusted VE was 49% (95% CI, 37% to 58%) and 29% (95% CI, 9% to 44%) for females and males, respectively (AD, 20%; P = .03). For B(Victoria), adjusted VE was 65% (95% CI, 50% to 76%) and 45% (95% CI, 18% to 63%) for females and males, respectively (AD, 20%; P = .08). The same pattern was observed for A(H1N1)pdm09 but with a smaller absolute difference in VE among females (63%; 95% CI, 53% to 70%) vs males (53%; 95% CI, 36% to 65%; AD, 10%; P = .19). No sex differences were seen for B(Yamagata), with a VE of 55% (95% CI, 41% to 65%) for females vs 58% (95% CI, 42% to 70%) for males (AD, –3%; P = .68). When restricted to vaccinated participants, the adjusted odds of influenza diagnosis was significantly higher among males than females overall (OR, 1.31; 95% CI, 1.12 to 1.54) and for A(H3N2) (OR, 1.45; 95% CI, 1.17 to 1.81) and B(Victoria) (OR, 1.83; 95% CI, 1.13 to 2.94; not adjusted for calendar time due to sample size limitations), but not A(H1N1)pdm09 (OR, 1.29; 95% CI, 0.92 to 1.80) or B(Yamagata) (OR, 1.03; 95% CI, 0.71 to 1.50). Conversely, this same effect was not observed among unvaccinated participants overall (OR, 1.06; 95% CI, 0.97 to 1.16) or for any subtype/lineage (data not shown).
Figure 1.
Vaccine effectiveness estimates by sex for influenza A subtype and influenza B lineage. P values indicate significance of vaccine*sex interaction. aCovariates included in the interaction model were vaccination status (no, yes), sex (female, male), age group (1–8, 9–19, 20–49, 50–64, ≥65 years), comorbidity (no, yes), province (AB, BC, ON, QC), collection interval (≤4, 5–7 days), calendar time (week of specimen collection based on cubic B-spline with 3 equal knots), season, and vaccine*sex. bA(H1N1)pdm09 analysis excludes 2014–2015 and 2016–2017 due to low A(H1N1)pdm09 circulation those seasons. cA(H3N2) analysis excludes 2013–2014 due to low A(H3N2) circulation that season. Excluding both the 2013–2014 and 2014–2015 seasons, VE for A(H3N2) was 49% (95% CI, 37% to 58%) among females and 29% (95% CI, 9% to 44%) among males (AD, 20%; P = .03). dB(Yamagata) analysis excludes 2010–2011 due to low B(Yamagata) circulation that season. eB(Victoria) analysis excludes 2013–2014, 2014–2015, and 2016–2017 due to low B(Victoria) circulation those seasons. Abbreviations: AD, absolute difference (Δ female – male); CI, confidence interval; VE, vaccine effectiveness.
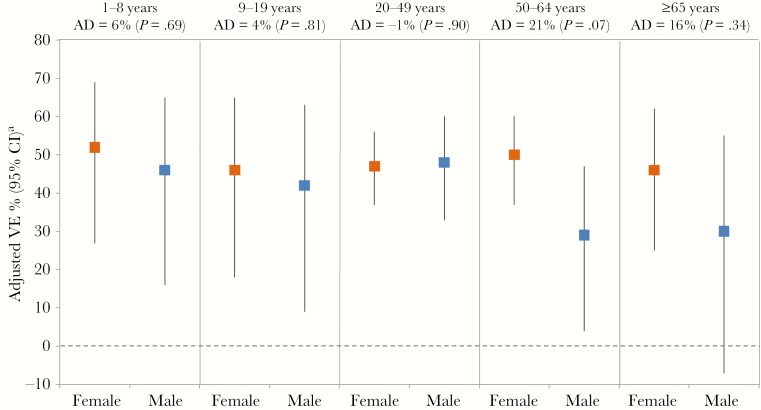
In the overall age-stratified analysis, the greatest absolute differences in VE between females and males were observed in older adults ≥50 years (Figure 2). Among adults ≥50 years, the adjusted VE was 48% (95% CI, 38% to 57%) vs 29% (95% CI, 10% to 44%) in females and males, respectively (AD, 19%, P = .03), whereas the VE was 49% (95% CI, 31% to 62%) vs 45% (95% CI, 24% to 59%; AD, 4%; P = .74) in those age <20 years and 47% (95% CI, 37% to 56%) vs 48% (95% CI, 33% to 60%; AD, –1%; P = .90) in those age 20–49 years, the latter age group comprising the majority of SPSN participants. The prevalence of comorbidities increased with age but did not significantly differ between females and males (20% vs 19%; P = .23), except in those age 50–64 years (27% vs 33%; P < .01). When an additional interaction term for comorbidity*sex was included in the fully adjusted VE model, including age adjustment, sex differences persisted: 48% (95% CI, 42% to 54%) in females vs 39% (95% CI, 29% to 48%) in males (AD, 9%; P = .08 for vaccine*sex and P = .21 for comorbidity*sex).
Figure 2.
Vaccine effectiveness estimates by sex for patient age groups. P values indicate significance of vaccine*sex interaction. aCovariates included in the interaction model were vaccination status (no, yes), sex (female, male), comorbidity (no, yes), province (AB, BC, ON, QC), collection interval (≤4, 5–7 days), calendar time (week of specimen collection based on cubic B-spline with 3 equal knots), season, and vaccine*sex. Abbreviations: AD, absolute difference (Δ female – male); CI, confidence interval; VE, vaccine effectiveness.
By subtype/lineage, larger absolute differences were seen among children and adolescents age <20 years for A(H1N1)pdm09 and B(Victoria) and older adults age ≥50 years for A(H3N2), A(H1N1)pdm09, and B(Victoria) (Supplementary Table 3). Smaller or negative absolute differences were seen across all subtypes/lineages for adults age 20–49 years. The addition of an interaction term for age*sex did not meaningfully change the pattern of higher VE in females (48%; 95% CI, 41% to 54%) vs males (40%; 95% CI, 30% to 49%) in the overall analysis (AD, 8%; P = .16 for vaccine*sex and P < .01 for age*sex). By subtype/lineage, the VE estimates were also similar when adjusted for the age*sex interaction, although the P value for the age*sex interaction term was only statistically significant for B(Yamagata) (Supplementary Table 4).
The finding of higher VE in females was generally consistent across seasons, although the opposite pattern of higher VE in males was observed in 2011–2012 for B(Victoria), 2012–2013 for A(H3N2) and A(H1N1)pdm09, and in 2014–2015 and 2015–2016 for B(Yamagata) (Supplementary Table 5). Few season-specific comparisons reached statistical significance, likely due to the smaller sample size.
DISCUSSION
Our analysis investigated sex as an effect modifier in the association between influenza vaccination and medically attended outpatient illness across 7 influenza seasons in Canada. Sex is often considered a potential confounder in the analysis of influenza VE [1], although it is not consistently included in adjusted VE estimation and the association will likely vary by study population and setting [17]. In our own SPSN VE analyses, sex was included as a covariate for some seasons where a bivariate association was seen for both vaccination status and influenza test positivity [14, 15] but was otherwise omitted.
Few studies have investigated the role of sex as an effect modifier for influenza-related outcomes. In a cohort study of community-dwelling elderly adults from 1990–2000, Nichol et al. found evidence of an interaction between vaccination and sex for all-cause mortality, with lower effectiveness found in males (P = .03) [18]. Vila-Córcoles et al. found a lower risk for all-cause mortality in females compared with males across all age groups in community-dwelling elderly adults in Spain from 2002–2005, but with the difference in mortality risk between females and males narrowing as age increased [19]. However, both of these studies were limited to a population of elderly adults age ≥65 years, nonspecific outcomes, and observational study designs; as such, they are likely not directly comparable to our findings and may suffer from other systematic biases.
In our own study using laboratory-confirmed outcomes and a test-negative design, we observed a pattern of higher VE in females, suggesting that females may respond better to influenza vaccine than their male counterparts. Our finding of increased odds of influenza among vaccinated but not unvaccinated male vs female participants for A(H3N2) and B(Victoria) reinforces our interpretation. A theoretical advantage of the test-negative design for influenza VE evaluation is that it minimizes biases associated with health care–seeking behavior as all participants presented to health care and met a standardized testing indication for influenza-like illness. Together, these findings suggest that biological sex differences in the response to vaccine, rather than gender differences in health care seeking or vaccination status reporting, likely explain the observed differences in influenza VE between males and females.
Although few studies have investigated sex differences in vaccine protection, females have been shown to have stronger innate and adaptive immune responses, including more pronounced antibody response to influenza vaccine, in association with higher rates of local and systemic adverse events following immunization [20–24]. The biological mechanisms underpinning these sex differences are not well understood. Some have attributed these differences to sex steroids that alter the function of immune cells by binding to specific receptors and influencing cell signaling pathways [20, 21]. At certain concentrations, estrogens, particularly estradiol, can function in a pro-inflammatory role, whereas testosterone and progesterone are considered immunosuppressive [20, 21, 25]. Hormone-mediating factors, such as pregnancy, can also modulate the immune response to influenza infection [1, 20, 21]. Although data on pregnancy were not available for this study, sex effects were not apparent among adults during their prime reproductive years, and pregnant women would have comprised only a small proportion of study participants. We observed the greatest absolute differences in VE among older adults (during or after the onset of menopause in females), and to a lesser extent in young children (before the onset of puberty) for certain subtypes/lineages. As such, our age-related findings are consistent with the epidemiological studies cited above but may be contrary to expected patterns based on sex hormone mechanisms alone [20, 21]. Mutations or polymorphisms in genes on the X chromosome that encode immunological proteins can affect activation of cytokine receptors and regulatory processes [20, 21]. Other sex- or gender-dependent factors should also be considered in the interaction between sex and influenza VE.
This study was limited by the available sample size despite pooling across multiple seasons. Although results were generally consistent across outcomes and seasons, the overall effects were small and may be due to chance. In the combined all-season analysis, the interaction term for vaccine*sex was marginally significant at the α < .10 level for A(H3N2) and B(Victoria) outcomes. The interaction terms for A(H1N1)pdm09 and B(Yamagata) were not statistically significant, despite higher VE in females for A(H1N1)pdm09. In our analysis, sex differences were greatest for influenza A(H3N2) and B(Victoria) in older age groups ≥50 years. Influenza A(H3N2) is associated with greater disease burden among elderly adults, whereas A(H1N1)pdm09 is notable for its impact in younger adults [26–27]. Both influenza B(Victoria) and B(Yamagata) disproportionately affect children, with B(Yamagata) exhibiting a bimodal age distribution also affecting older adults [28]. Other factors, such as the higher prevalence of comorbidities in older adults, may also contribute to these findings, although VE analyses were adjusted for age and comorbidity. Ultimately, however, it remains possible that our observational study design did not adequately account for other potential biases, residual confounders, or interactions.
In conclusion, we observed a modest effect of sex on influenza VE across most outcomes and seasons, with higher VE estimates generally seen among females. These sex effects were age dependent, with greater effects in older adults age ≥50 years compared with those age <20 years or 20–49 years, but with some variation by subtype/lineage. Overall, these effects likely represent a complex interplay between birth cohort (ie, immunological prime-boost) effects, hormonal influences, and other contributing agent–host–environment factors [1, 15, 20, 21]. The clinical implications are unclear, although some have argued for sex-based design of influenza vaccination strategies [21]. More definitive investigations of VE by sex and age are ultimately needed to elucidate these potential interactions.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We gratefully acknowledge the contribution of those at the sentinel sites whose regular submission of specimens and data provided the basis of our analyses. We acknowledge the coordination and technical support provided by epidemiologic and laboratory staff in all participating provinces. We thank the following for network coordination and data entry activities in each province: Lisan Kwindt for national database management and sentinel network coordination activities in British Columbia; Elaine Douglas, Kinza Rizvi, Sandra Berzins, and Kasim Qureshi for TARRANT in Alberta; Romy Olsha for Public Health Ontario; and Sophie Auger for the Institut National de Santé Publique du Québec. We thank those who provided laboratory support at the British Columbia Centre for Disease Control Public Health Laboratory, the Alberta Provincial Laboratory for Public Health (ProvLab), the Public Health Ontario Laboratory, and the Laboratoire de Santé Publique du Québec and the National Microbiology Laboratory in Manitoba. We would also like to thank Dr. Naveed Janjua of the BC Centre for Disease Control for his previous work with the SPSN.
Financial support. This work was supported by the Canadian Institutes of Health Research (grant number TPA-90193), the British Columbia Centre for Disease Control, Alberta Health and Wellness, Public Health Ontario, Ministère de la Santé et des Services Sociaux du Québec, l’Institut National de Santé Publique du Québec, and the Public Health Agency of Canada.
Potential conflicts of interest. D.M.S. is Principal Investigator on grants received from the Canadian Institutes of Health Research and the Public Health Agency of Canada in support of this work. G.D.S. has received grants for investigator-initiated studies unrelated to influenza vaccine from GSK and Pfizer and provided paid expert testimony for the Ontario Nurses Association, the Quebec Ministry of Justice and GSK. J.G. has received research grants from GlaxoSmithKline Inc. and Hoffman-La Roche Ltd. to study antiviral resistance in influenza and from Pfizer Inc. to conduct microbiological surveillance of Streptococcus pneumoniae. M.K. has received research grants from Roche, Siemens, and Hologic for unrelated studies. S.J.D. is assisting Johnston & Johnston (Janssen) Pharmaceuticals on a literature search for point of care testing of respiratory viruses. The other authors have no conflicts of interest to declare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Sex, Gender and Influenza. Geneva: World Health Organization; 2010. http://apps.who.int/iris/handle/10665/44401. Accessed 31 May 2018. [Google Scholar]
- 2. Buchan SA, Kwong JC. Trends in influenza vaccine coverage and vaccine hesitancy in Canada, 2006/07 to 2013/14: results from cross-sectional survey data. CMAJ Open 2016; 4:E455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Flu vaccination coverge, United States, 2016–17 influenza seasonhttps://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. Accessed 3 May 2018.
- 4. Sue K. The science behind “man flu.” BMJ 2017; 359:j5560. [DOI] [PubMed] [Google Scholar]
- 5. Crighton EJ, Elliott SJ, Moineddin R, et al. . An exploratory spatial analysis of pneumonia and influenza hospitalizations in Ontario by age and gender. Epidemiol Infect 2007; 135:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jensen-Fangel S, Mohey R, Johnsen SP, et al. . Gender differences in hospitalization rates for respiratory tract infections in Danish youth. Scand J Infect Dis 2004; 36:31–6. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen AM, Noymer A. Influenza mortality in the United States, 2009 pandemic: burden, timing and age distribution. PLoS One 2013; 8:e64198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quandelacy TM, Viboud C, Charu V, Lipsitch M, Goldstein E. Age- and sex-related risk factors for influenza-associated mortality in the United States between 1997–2007. Am J Epidemiol 2014; 179:156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang XL, Yang L, Chan KH, et al. . Age and sex differences in rates of influenza-associated hospitalizations in Hong Kong. Am J Epidemiol 2015; 182:335–44. [DOI] [PubMed] [Google Scholar]
- 10. Skowronski DM, Janjua NZ, De Serres G, et al. . A sentinel platform to evaluate influenza vaccine effectiveness and new variant circulation, Canada 2010–2011 season. Clin Infect Dis 2012; 55:332–42. [DOI] [PubMed] [Google Scholar]
- 11. Skowronski DM, Janjua NZ, Sabaiduc S, et al. . Influenza A/subtype and B/lineage effectiveness estimates for the 2011–2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014; 210:126–37. [DOI] [PubMed] [Google Scholar]
- 12. Skowronski DM, Janjua NZ, De Serres G, et al. . Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One 2014; 9:e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skowronski DM, Chambers C, Sabaiduc S, et al. . Integrated sentinel surveillance linking genetic, antigenic, and epidemiologic monitoring of influenza vaccine-virus relatedness and effectiveness during the 2013–2014 influenza season. J Infect Dis 2015; 212:726–39. [DOI] [PubMed] [Google Scholar]
- 14. Skowronski DM, Chambers C, Sabaiduc S, et al. . A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skowronski DM, Chambers C, Sabaiduc S, et al. . Beyond antigenic match: possible agent-host and immuno-epidemiological influences on influenza vaccine effectiveness during the 2015–2016 season in Canada. J Infect Dis 2017; 216:1487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Skowronski DM, Chambers C, Sabaiduc S, et al. . Interim estimates of 2016/17 vaccine effectiveness against influenza A(H3N2), Canada, January 2017. Euro Surveill. 2017; 22:pii=30460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. Evaluation of Infleunza Vaccine Effectiveness: A Guide to the Design and Interpretation of Observational Studies. Geneva: World Health Organization; 2017. http://apps.who.int/iris/handle/10665/255203. Accessed 31 May 2018. [Google Scholar]
- 18. Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. New Engl J Med 2007; 357:1373–81. [DOI] [PubMed] [Google Scholar]
- 19. Vila-Corcoles A, Rodriguez T, de Diego C, et al. . Effect of influenza vaccine status on winter mortality in Spanish community-dwelling elderly people during 2002-2005 influenza periods. Vaccine 2007; 25:6699–707. [DOI] [PubMed] [Google Scholar]
- 20. Gabriel G, Arck PC. Sex, immunity and influenza. J Infect Dis 2014; 209(Suppl 3):S93–9. [DOI] [PubMed] [Google Scholar]
- 21. Klein SL, Pekosz A. Sex-based biology and the rational design of influenza vaccination strategies. J Infect Dis 2014; 209(Suppl 3):S114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Engler RJ, Nelson MR, Klote MM, et al. . Half- vs full-dose trivalent inactivated influenza vaccine (2004-2005): age, dose, and sex effects on immune responses. Arch Intern Med 2008; 168:2405–14. [DOI] [PubMed] [Google Scholar]
- 23. Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–80. [DOI] [PubMed] [Google Scholar]
- 24. Khurana S, Verma N, Talaat KR, Karron RA, Golding H. Immune response following H1N1pdm09 vaccination: differences in antibody repertoire and avidity in young adults and elderly populations stratified by age and gender. J Infect Dis 2012; 205:610–20. [DOI] [PubMed] [Google Scholar]
- 25. Furman D, Hejblum BP, Simon N, et al. . Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A 2014; 111:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson WW, Shay DK, Weintraub E, et al. . Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–86. [DOI] [PubMed] [Google Scholar]
- 27. Skowronski DM, Chambers C, Sabaiduc S, et al. . Pre- and postpandemic estimates of 2009 pandemic influenza A(H1N1) seroprotection to inform surveillance-based incidence, by age, during the 2013-2014 epidemic in Canada. J Infect Dis 2015; 211:109–14. [DOI] [PubMed] [Google Scholar]
- 28. Skowronski DM, Chambers C, De Serres G, et al. . Age-related differences in influenza B infection by lineage in a community-based sentinel system, 2010-2011 to 2015-2016, Canada. J Infect Dis 2017; 216:697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.