Abstract
Outstanding results have been obtained in the treatment of chronic myeloid leukemia (CML) with first-line imatinib therapy. However, approximately 35% of patients will not obtain long-term benefit with this approach. Allogeneic hematopoietic stem cell transplantation (HCT) is a valuable second- and third-line therapy for appropriately selected patients. To identify useful prognostic indicators of transplantation outcome in postimatinib therapeutic interventions, we investigated the role of the HCT comorbidity index (HCT-CI) together with levels of C-reactive protein (CRP) before HCT in 271 patients who underwent myeloablative HCT for CML in first chronic phase. Multivariate analysis showed both an HCT-CI score higher than 0 and CRP levels higher than 9 mg/L independently predict inferior survival and increased nonrelapse mortality at 100 days after HCT. CML patients without comorbidities (HCT-CI score 0) with normal CRP levels (0-9 mg/L) may therefore be candidates for early allogeneic HCT after failing imatinib.
Introduction
The past decade has witnessed a dramatic change in the management of chronic myeloid leukemia (CML) such that the majority of patients initially receive therapy with imatinib rather than being offered allogeneic hematopoietic cell transplantation (HCT).1 However, approximately 35% of these patients will fail to respond and/or develop intolerance. The treatment choices then include second-generation tyrosine kinase inhibitors and HCT, and the current challenge is to optimize the relative timings of these alternatives.
The results of HCT in CML can be predicted using a prognostic score devised by the European Group for Blood and Marrow Transplantation (EBMT) based on 5 variables: donor type, disease phase, recipient age, donor/recipient sex combination, and interval from diagnosis to transplantation.2 As the variable of disease phase resulted in higher relative risks than other variables in the model,2 Passweg et al attempted to modify the score for patients in chronic phase for whom the decision of whether to undergo transplantation is most difficult. They found only one parameter with additional prognostic value, the Karnofsky performance score, and after inclusion the improvement over the original EBMT score was minimal.3
The hematopoietic cell transplantation comorbidity index (HCT-CI) was developed to analyze the impact of comorbidities on outcome of HCT.4 In several studies, the HCT-CI predicted for nonrelapse mortality (NRM) and overall survival (OS),5–8 but it has never been studied specifically in patients with CML. C-reactive protein (CRP) is a sensitive marker of inflammation and persons with elevated levels of this acute phase protein are known to have an increased risk of cardiovascular diseases and malignancies.9–10 We and others have previously shown the value of CRP levels shortly before HCT in predicting its outcomes.11–12 However, it was unclear whether the elevated CRP was a reflection of an underlying comorbidity. In this study, we investigated the prognostic value of HCT-CI together with preconditioning levels of CRP for patients with CML in first chronic phase. We demonstrate that both are independent predictors of NRM and OS.
Methods
Study design
We carried out a retrospective analysis of the outcomes of HCT in 312 consecutive patients who underwent myeloablative HCT between January 1991 and July 2008; 41 patients with incomplete data were omitted from the analysis. The median age of 271 analyzed patients was 34 years (range, 10-60 years). Fifteen (5.5%) patients received peripheral blood stem cells and 256 (94.5%) patients received bone marrow. Donors were HLA-identical siblings (130 patients, 48.0%), matched unrelated donors (134 patients, 49.4%), and mismatched unrelated donors (7 patients, 2.6%). Conditioning consisted of cyclophosphamide and total body irradiation. In addition, in vivo T-cell depletion with alemtuzumab was used for 2 sibling donors and 136 (96.5%) of 141 unrelated donor transplantations. CRP data were missing in 27 of the 271 patients so they were excluded from the multivariate analysis. The study was approved by the ethical review board of Imperial College.
EBMT scores were calculated for each patient as described previously.2 Comorbidities were assessed by comprehensive review of medical records and computer database by a single investigator (B.T.-R.), who was unaware of transplantation outcomes. They were defined and assigned different weights (1-3) by the HCT-CI.4 Preconditioning serum CRP levels were measured at a median of 16 days before stem cell infusion using a standard latex immunoassay (CRP Vario; Abbott Architect ci8200 analyzer; normal range, 0-9 mg/L). Two outcomes were considered: OS, calculated from the day of transplantation until death from any cause; and NRM, defined as any cause of death not related to relapse and measured at 100 days after transplantation. Probability curves were calculated using the Kaplan-Meier method for survival and the cumulative incidence procedure for NRM. Groups were compared using the log-rank test, whereas the Cox regression analysis was used in the multivariate setting. P values less than .05 were considered significant.
Results and discussion
The proportion of patients with CRP levels higher than 10 mg/L was equal among patients with or without comorbidities (11%), confirming the independence of these 2 factors. In multivariate analysis, the EBMT scores lacked association with NRM, but scores higher than 3 predicted for inferior OS (Table 1). Sibling transplant recipients had better overall (P = .04) and disease-free survival (P = .001) than unrelated (T cell–depleted) transplant recipients. Patients without comorbidities (HCT-CI score 0) had better NRM as well as OS compared with patients with comorbidities (HCT-CI score > 0; NRM, 5.3% and 18.5%, respectively; 5-year OS, 69.6% and 55.5%, respectively; Table 1, Figure 1A,C). Patients with HCT-CI scores of 1 to 2 and those with HCT-CI scores higher than 2 had similar NRM (18.1% and 19.8%, respectively; P = .82) and 5-year OS (56.2% and 53.0%, respectively; P = .91). Preconditioning CRP levels higher than 9 mg/L predicted for both inferior NRM and OS (Table 1, Figure 1B,D), but did not influence the relapse rate or disease-free survival. With CRP as a continuous variable, an increase of 1 mg/L gives a hazard ratio change of 1.019 (95% confidence interval, 1.003-1.035; P = .022). The prognostic value of high CRP levels was independent of the HCT-CI and its individual components and vice versa (data not shown). Specifically, there was no association between elevated preconditioning CRP levels and infection, either as a comorbidity or as a cause of death. Similarly, there was no relationship between CRP levels or comorbidities and grades of acute and chronic graft-versus-host disease.
Table 1.
Multivariate analyses describing NRM at 100 days and OS using the HCT comorbidity index, preconditioning CRP, and adjusted EBMT score
| Variable | No. (%) | Day 100 NRM |
OS |
||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| HCT-CI | |||||
| 0 | 146 (60) | 1.0 | 1.0 | ||
| 1 or more | 98 (40) | 4.29 (1.8-10.5) | .001 | 1.58 (1.04-2.4) | .03 |
| CRP | |||||
| 0-9 mg/L | 217 (89) | 1.0 | 1.0 | ||
| More than 9 mg/L | 27 (11) | 4.22 (1.6-11.2) | .004 | 2.33 (1.4-4.0) | .002 |
| Data missing | 27 | ||||
| EBMT score | |||||
| 0-1 | 45 (18) | 1.0 | 1.0 | ||
| 2 | 65 (27) | 1.2 (.3-4.9) | .78 | .96 (.5-2.1) | .91 |
| 3 | 87 (36) | 1.5 (.4-5.7) | .50 | 1.6 (.8-3.1) | .18 |
| More than 3 | 47 (19) | 1.1 (.3-4.8) | .89 | 2.1 (1.04-4.4) | .038 |
Twenty-seven patients were excluded from the analyses because of lack of CRP data.
NRM indicates nonrelapse mortality; OS, overall survival; HR, hazard ratio; CI, confidence interval; HCT-CI, hematopoietic cell transplantation comorbidity index; CRP, C-reactive protein; and EBMT, European Group for Blood and Marrow transplantation.
Figure 1.
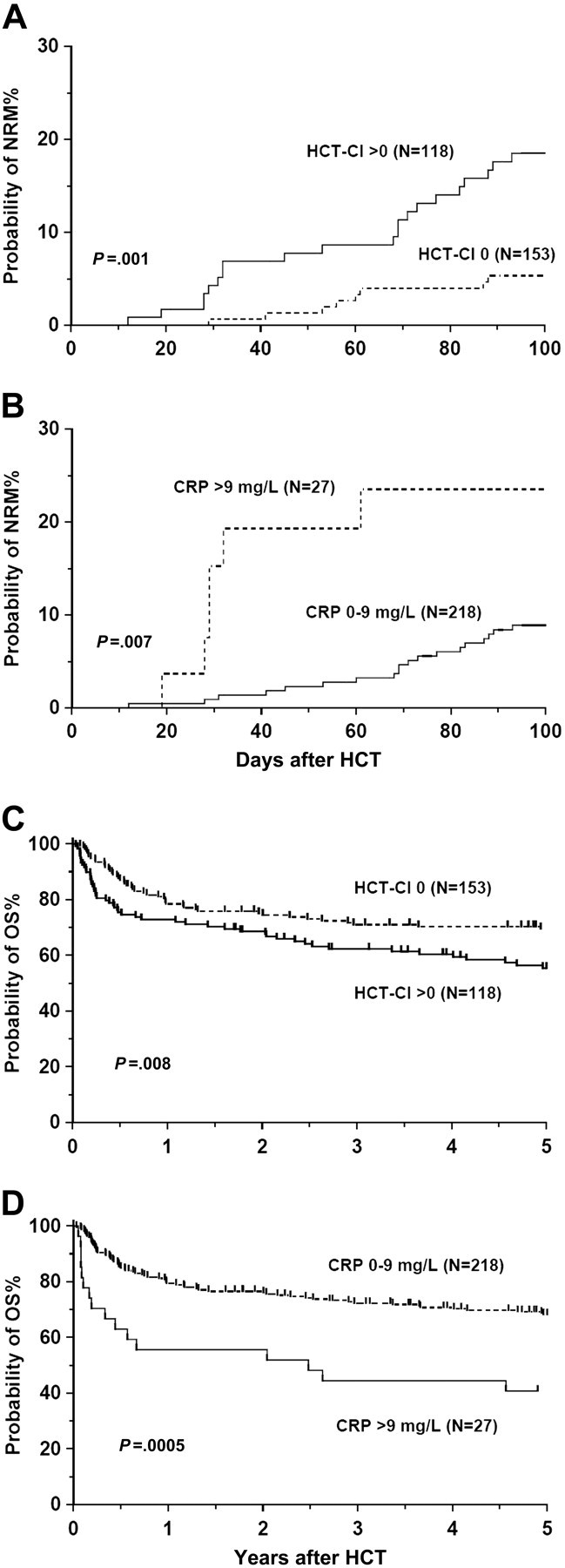
Nonrelapse mortality and survival. Probabilities of nonrelapse mortality (A-B) and overall survival (C-D) stratified by hematopoietic stem cell transplantation comorbidity index (HCT-CI; 0 vs > 0; A,C) and preconditioning C-reactive protein (CRP) levels (0-9 mg/L vs > 9 mg/L; B,D).
This study is the first to investigate the prognostic value of comorbidities on outcomes of HCT in CML. Previously patients who underwent transplantation for CML were contained within comorbidity studies that included patients with other diagnoses. In the study of Sorror et al, patients with CML accounted for 20% of the total cohort of 1055 patients.4 Subsequent studies supporting the prognostic value of HCT-CI included only few5 or no6–8 CML patients. However, other studies have not confirmed the prognostic role of HCT-CI.13–15 These include one study of 187 patients (21% with CML) in which HCT-CI did not correlate with NRM or OS.13
Increased levels of CRP were observed in newly diagnosed patients with CML,16 and serum interleukin-6 levels were found to correlate with monocyte, basophil, and blast cell counts in CML.17 Polymorphisms within the CRP gene promoter region were found to be associated with elevated CRP levels,18 and family and twin studies suggest that additive genetic factors account for as much as 40% of the variance in plasma CRP levels.19 Polymorphisms of several genes involved in host defense/inflammatory responses are associated with transplantation outcomes.20 It is possible that increased levels of CRP could reflect underlying inflammatory processes or gene polymorphisms that modify immune interactions of the host and graft leading to inferior outcomes of transplantation. We have, however, observed no association of CRP levels with incidence or severity of graft-versus-host disease (data not shown).
The probability of response to second-generation tyrosine kinase inhibitors in patients who fail imatinib can be predicted from their Sokal score at diagnosis, best cytogenetic response, and recurrent neutropenia on imatinib.21 The level of cytogenetic response at 3 or 6 months after starting dasatinib or nilotinib correlates with a major cytogenetic response at 1 year.22 A careful consideration of these factors taken in conjunction with the predicted outcome after HCT that takes account of CRP and comorbidity status could be valuable for deciding whether and when to offer HCT to a given patient with CML in first chronic phase who has a suitably matched donor after imatinib failure.
Acknowledgment
The authors are grateful for support from the National Institute for Health Research Biomedical Research Centre funding scheme.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.W.A., A.K.K., and B.T.-R. collected the data; J.F.A., J.M.G., J.P., and R.M.S. designed the study, analyzed the data, and wrote the paper; and E.J.K., D.H.M., D. Marin, D. Milojkovic, E.O., A.R., and K.R. provided assistance in data collection and analysis and paper preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jiří Pavlů, Department of Haematology, Hammersmith Hospital, Du Cane Rd, London, United Kingdom W12 0HS; e-mail: jiri.pavlu@imperial.nhs.uk.
References
- 1.Giralt SA, Arora M, Goldman JM, et al. Impact of imatinib therapy on the use of allogeneic haematopoietic progenitor cell transplantation for the treatment of chronic myeloid leukaemia. Br J Haematol. 2007;137(5):461–467. doi: 10.1111/j.1365-2141.2007.06582.x. [DOI] [PubMed] [Google Scholar]
- 2.Gratwohl A, Hermans J, Goldman JM, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation: chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352(9134):1087–1092. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 3.Passweg JR, Walker I, Sobocinski KA, Klein JP, Horowitz MM, Giralt SA. Validation and extension of the EBMT Risk Score for patients with chronic myeloid leukaemia (CML) receiving allogeneic haematopoietic stem cell transplants. Br J Haematol. 2004;125(5):613–620. doi: 10.1111/j.1365-2141.2004.04955.x. [DOI] [PubMed] [Google Scholar]
- 4.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112(9):1992–2001. doi: 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 6.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110(13):4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zipperer E, Pelz D, Nachtkamp K, et al. The hematopoietic stem cell transplantation comorbidity index is of prognostic relevance for patients with myelodysplastic syndrome. Haematologica. 2009;94(5):729–732. doi: 10.3324/haematol.2008.002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farina L, Bruno B, Patriarca F, et al. The hematopoietic cell transplantation comorbidity index (HCT-CI) predicts clinical outcomes in lymphoma and myeloma patients after reduced-intensity or non-myeloablative allogeneic stem cell transplantation. Leukemia. 2009;23(6):1131–1138. doi: 10.1038/leu.2009.1. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 10.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291(5):585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 11.Kew AK, Szydlo RM, Olavarria E, Goldman JM, Apperley JF. C-reactive protein on admission predicts transplant-related mortality in recipients of allogeneic stem cell transplant [abstract]. Blood. 2007;110(11) Abstract 3005. [Google Scholar]
- 12.Artz AS, Wickrema A, Dinner S, et al. Pretreatment C-reactive protein is a predictor for outcomes after reduced-intensity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(11):1209–1216. doi: 10.1016/j.bbmt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guilfoyle R, Demers A, Bredeson C, et al. Performance status, but not the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI), predicts mortality at a Canadian transplant center. Bone Marrow Transplant. 2009;43(2):133–139. doi: 10.1038/bmt.2008.300. [DOI] [PubMed] [Google Scholar]
- 14.Xhaard A, Porcher R, Chien JW, et al. Impact of comorbidity indexes on non-relapse mortality. Leukemia. 2008;22(11):2062–2069. doi: 10.1038/leu.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terwey TH, Hemmati PG, Martus P, et al. A modified EBMT risk score and the hematopoietic cell transplantation-specific comorbidity index for pre-transplant risk assessment in adult acute lymphoblastic leukemia. Haematologica. doi: 10.3324/haematol.2009.011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humlová Z, Klamová H, Janatková I, et al. Immunological profiles of patients with chronic myeloid leukaemia: I, state before the start of treatment. Folia Biol (Praha) 2006;52(3):47–58. [PubMed] [Google Scholar]
- 17.Anand M, Chodda SK, Parikh PM, Nadkarni JS. Abnormal levels of proinflammatory cytokines in serum and monocyte cultures from patients with chronic myeloid leukemia in different stages, and their role in prognosis. Hematol Oncol. 1998;16(4):143–154. doi: 10.1002/(sici)1099-1069(199812)16:4<143::aid-hon628>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 18.Carlson CS, Aldred SF, Lee PK, et al. Polymorphisms within the C-reactive protein (CRP) promoter region are associated with plasma CRP levels. Am J Hum Genet. 2005;77(1):64–77. doi: 10.1086/431366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pankow JS, Folsom AR, Cushman M, et al. Familial and genetic determinants of systemic markers of inflammation: the NHLBI family heart study. Atherosclerosis. 2001;154(3):681–689. doi: 10.1016/s0021-9150(00)00586-4. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson AM, Middleton PG, Rocha V, Gluckman E, Holler E. Genetic polymorphisms predicting the outcome of bone marrow transplants. Br J Haematol. 2004;127(5):479–490. doi: 10.1111/j.1365-2141.2004.05216.x. [DOI] [PubMed] [Google Scholar]
- 21.Milojkovic D, Nicholson E, Apperley JF, et al. Early prediction of success or failure using second generation tyrosine kinase inhibitors for chronic myeloid leukemia. Haematologica. 2010;95(2):224–231. doi: 10.3324/haematol.2009.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tam CS, Kantarjian H, Garcia-Manero G, et al. Failure to achieve a major cytogenetic response by 12 months defines inadequate response in patients receiving nilotinib or dasatinib as second or subsequent line therapy for chronic myeloid leukemia. Blood. 2008;112(5):516–518. doi: 10.1182/blood-2008-02-141580. [DOI] [PMC free article] [PubMed] [Google Scholar]


