Abstract
Skin pigmentation is primarily dependent on melanogenesis, a physiological process that occurs in melanosomes of melanocytes. Solar radiation modulates pigmentation through variety of signaling pathways, but the mechanism of visible light-induced hyperpigmentation remains uncharacterized. Passeron’s group recently reported that visible light stimulates opsin3-regulated calcium-dependent microphthalmia-associated transcription factor activation that increases pigment gene expression and that it also causes the clustering of melanogenic enzymes. Together, these processes possibly contribute to long-lasting hyperpigmentation in the melanocompetent skins.
Human skin pigmentation (scaled as Fitzpatrick phototype I–VI) depends on intra- and extracellular factors, including genotype, and is highly regulated and maintained via multiple signaling networks operating in melanocytes (Steingrimsson et al., 2004). Melanocytes populate the choroid of the eye, the inner ear, and hair follicles in addition to skin epidermis and produce melanin pigments in membrane-bound organelles called melanosomes. The amount of pigment that is produced in melanosomes and the extent of transfer of melanosomes to neighboring keratinocytes further determine skin color, which is modulated by solar radiation. These pigments absorb UV rays and protect the skin from ionizing radiation. Solar radiation consists of UV, visible, and infrared light. UV-induced melanogenesis has been studied in detail (Passeron and Ortonne, 2016). Although visible light (390–700 nm) accounts for approximately 50% of solar spectrum, its effect on melanocyte pigmentation and skin color is largely unknown. Previous studies suggest that the action of visible light, such as 415 nm radiation (blue-violet light, referred to here as blue light), but not red light (630 nm) on the dark skin (types III–VI) of healthy volunteers led to longer-lasting hyperpigmentation than UVA/B (Duteil et al., 2014; Mahmoud et al., 2010). However, mechanisms involved in blue light-induced skin pigmentation are completely unknown.
A recent study from Thierry Passeron’s group attempted to identify the signaling pathways that were activated by blue light exposure and contributed to the skin hyperpigmentation (Regazzetti et al., 2017). The authors exposed primary human melanocytes to 415 nm radiation (≥50 J/cm2 for 2 days), which caused increased nuclear accumulation of phosphorylated microphthalmia-associated transcription factor (p-MITF), a transcription factor that is a master regulator of melanocyte pigmentation and differentiation. After exposure to blue light for 10 days, melanocytes increased their melanin content by several fold without proliferating, suggesting a direct effect of blue light on melanogenesis. In co-culture experiments, Passeron’s group excluded the involvement of signaling in keratinocytes and production of free radical species during this process. They hypothesized that “opsins,” G protein-coupled light sensors described in the eye, were involved. It has been reported that both keratinocytes and melanocytes express varying levels of five different opsins (OPN1-SW, OPN2, OPN3, OPN4, and OPN5) that are predicted to absorb shorter wavelengths of visible light (de Assis et al., 2016; Haltaufderhyde et al., 2015). Passeron’s group detected higher levels of opsin3 (also called encephalopsin or panopsin) relative to other opsins in both primary melanocytes and skin prototypes III to VI, suggesting a role for opsin3 in skin pigmentation. Interestingly, OPN3 transcript levels were unchanged after irradiation of melanocytes with blue light. Knockdown of opsin3 decreased MITF activation and its dependent melanogenesis gene expression, suggesting that opsin3 maintains levels of pigment gene expression in skin through blue light.
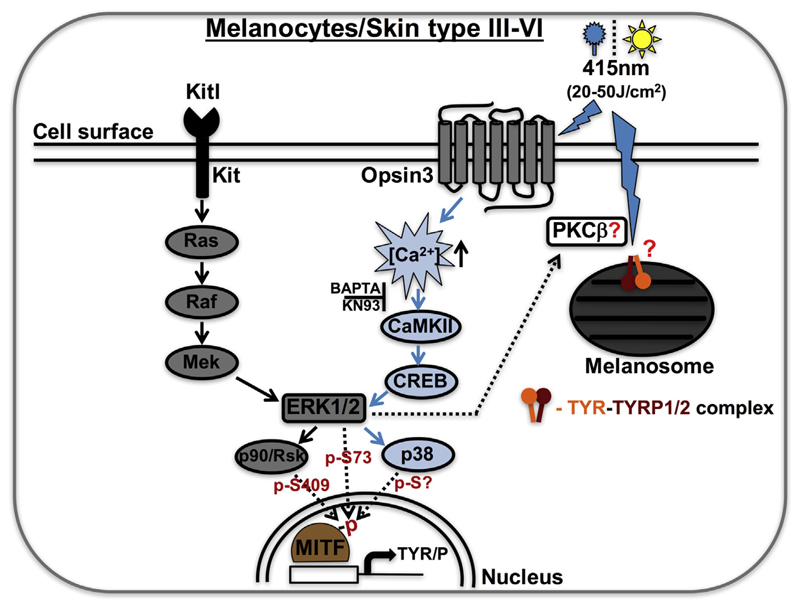
During melanocyte development, paracrine Kit/Kitl signaling activates intracellular signal transduction involving Ras, Raf, and Mek kinases, which further activates extracellular signal-regulated kinase 2 (ERK2; a mitogen-activated protein kinase) and p90/Rsk (serine-threonine kinase). Ultimately, MITF gets phosphorylated at Ser73 and Ser409 by ERK2 and p90/Rsk kinases, respectively, resulting in increased pigment gene expression (Figure 1) that maintains melanocyte pigmentation and skin color (Steingrimsson et al., 2004). In contrast, blue light-activated opsin3 induces a unique pathway involving CaMKII (calcium/calmodulin-dependent protein kinase II, activated via increased cytosolic calcium flux), which further amplifies the intracellular signaling cascade involving CRE-binding protein (CREB), ERK1/2, and p38 after phosphorylation of unknown serine residues on MITF (Figure 1). This pathway requires opsin-3-dependent calcium flux, because the treatment of melanocytes with small molecules such as BAPTA (a calcium chelator) or KN93 (a CaMKII inhibitor) or the knockdown of OPN3 completely abolishes the blue light-induced opsin3-dependent MITF activation (Regazzetti et al., 2017). It is interesting to note that the blue light-activated opsin3 pathway shares the downstream components with those of Kit/Kitl signaling, indicating that cross-talk between these pathways may maintain skin pigmentation (Figure 1).
Figure 1. Blue light-induced opsin3-mediated MITF activation in melanocytes or melanocompetent phototypes III to VI.
G protein-coupled receptor Opsin3 absorbs visible light of wavelength 415 nm (≥50 J/cm2) and increases cytosolic calcium flux followed by activation and phosphorylation of CaMKII, CREB, ERK1/2, p38, and MITF, resulting in the expression of pigment-specific genes. Note that small molecules such as BAPTA (calcium chelator) or KN93 (CaMKII inhibitor) block the visible light-dependent opsin3 signaling. In parallel, 415 nm (blue light) exposure results in the formation of multimeric tyrosinase (TYR) and tyrosinase-related protein-2/-1 (TYRP2/1) complexes. We hypothesized that activated ERK1/2 increases the activity of protein kinase C-β (PKC-β), which possibly phosphorylates the TYR and then pTYR forms the complex with TYRP1/2 on melanosomal membranes. The paracrine-based Kit/Kitl signaling pathway in melanocytes was shown separately. “?” indicates the role of these molecules or their formation/modification requires future investigation. CaMKII, calcium/calmodulin-dependent protein kinase II; CREB, CRE-binding protein; ERK, extracellular signal-regulated kinase; MITF, microphthalmia-associated transcription factor.
Unexpectedly, blue light exposure did not alter the expression levels of p-MITF/p-CaMKII/p-ERK/p-p38 or transcript levels of OPN3 in skin phototypes I to VI. In addition, only phototypes III to VI responded to blue light and increased pigmentation. These observations prompted Passeron’s group to investigate the differences in signaling between the phototypes. Interestingly, the authors identified higher molecular size protein complexes in melanocompetent skin type III to VI cells but not in type I or II cells after exposure to blue light (Regazzetti et al., 2017). Further characterization revealed that these complexes (TYR/P multimers) comprised tyrosinase (TYR) and tyrosinase-related protein-2 (TYRP2/DCT) and that they were observed only after blue light illumination. Increased levels of complexes including TYRP1 were not observed. Where and how these complexes are generated is unknown and their ability to modulate pigment synthesis is uncharacterized.
In vitro studies from Pawelek’s group reported the existence of these complexes and showed reduced TYR activity in complexes including TYRP2 or TYRP1 (Orlow et al., 1994). In different skin types, this process might have different dynamics due to increased stability, rate of formation, and number of TYR/P complexes. Consistently, Passeron’s group observed higher levels of these complexes in type III to VI cells that could result in long-lasting hyperpigmentation after blue light exposure. Multimeric TYR/P complexes were also observed in denaturing conditions, indicating possible covalent linkage induced by blue light (Figure 1). Interestingly, similar types of TYR/P complexes were observed after activation of protein kinase C(PKC)-β, where PKC phosphorylates TYR at Ser505 and Ser509 and then p-TYR associates with TYRP1 (Wu and Park, 2003). Additionally, ERK1/2 has been shown to modulate the activity of PKC during apoptosis induced by singlet oxygen (Zhuang et al., 1998). We predict that this might be one mechanism that promotes formation of TYR/P complexes, where ERK1/2 activates PKC after blue light irradiation leading to phosphorylation of TYR on melanosomal membranes and complex formation with TYRP1/2 (Figure 1). However, other possibilities should be explored in future.
In conclusion, nonretinal opsin3 in melanocytes senses blue light in the solar spectrum and activates calcium-dependent MITF activation/pigment gene expression and also increases the activity and/or stability of multimeric TYR/P protein complexes resulting in long-lasting skin hyperpigmentation. These studies also suggest that including physical shields such as iron oxide in combination with antioxidants (that protects against UVA/B) in sunscreens will protect skin from solar radiation-induced hyperpigmentation.
Visible light activates opsin3-mediated calcium-dependent MITF signaling and increases the formation of multimeric TYR/P protein complexes, which results in solar radiation-induced skin hyperpigmentation.
Acknowledgments
We acknowledge the financial support from Wellcome Trust-DBT India Alliance Senior Fellowship (500122/Z/09/Z) and Indo-French Centre for the Promotion of Advanced Research (CEFIPRA 4903-1).
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- de Assis LV, Moraes MN, da Silveira Cruz-Machado S, Castrucci AM. The effect of white light on normal and malignant murine melanocytes: a link between opsins, clock genes, and melanogenesis. Biochim Biophys Acta. 2016;1863:1119–33. doi: 10.1016/j.bbamcr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Duteil L, Cardot-Leccia N, Queille-Roussel C, Maubert Y, Harmelin Y, Boukari F, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822–6. doi: 10.1111/pcmr.12273. [DOI] [PubMed] [Google Scholar]
- Haltaufderhyde K, Ozdeslik RN, Wicks NL, Najera JA, Oancea E. Opsin expression in human epidermal skin. Photochem Photobiol. 2015;91:117–23. doi: 10.1111/php.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud BH, Ruvolo E, Hexsel CL, Liu Y, Owen MR, Kollias N, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092–7. doi: 10.1038/jid.2010.95. [DOI] [PubMed] [Google Scholar]
- Orlow SJ, Zhou BK, Chakraborty AK, Drucker M, Pifko-Hirst S, Pawelek JM. High-molecular-weight forms of tyrosinase and the tyrosinase-related proteins: evidence for a melanogenic complex. J Invest Dermatol. 1994;103:196–201. doi: 10.1111/1523-1747.ep12392743. [DOI] [PubMed] [Google Scholar]
- Passeron T, Ortonne J-P. Atlas of pigmentary disorders. Switzerland: Springer International Publishing Switzerland; 2016. [Google Scholar]
- Regazzetti C, Sormani L, Debayle D, Bernerd F, Tulic MK, De Donatis GM, et al. Melanocytes sense blue light and regulate pigmentation through the Opsin-3. J Invest Dermatol. 2018;138:171–8. doi: 10.1016/j.jid.2017.07.833. [DOI] [PubMed] [Google Scholar]
- Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- Wu H, Park HY. Protein kinase C-beta-mediated complex formation between tyrosinase and TRP-1. Biochem Biophys Res Commun. 2003;311:948–53. doi: 10.1016/j.bbrc.2003.10.092. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Lynch MC, Kochevar IE. Activation of protein kinase C is required for protection of cells against apoptosis induced by singlet oxygen. FEBS Lett. 1998;437:158–62. doi: 10.1016/s0014-5793(98)01222-8. [DOI] [PubMed] [Google Scholar]