Abstract
PI3K/AKT signalling is commonly disrupted in human cancers, with AKT being a central component of the pathway, influencing multiple processes that are directly involved in tumourigenesis. Targeting AKT is therefore a highly attractive anti-cancer strategy with multiple AKT inhibitors now in various stages of clinical development. In this review, we summarise the role and regulation of AKT signalling in normal cellular physiology. We highlight the mechanisms by which AKT signalling can be hyperactivated in cancers and discuss the past, present and future clinical strategies for AKT inhibition in oncology.
Keywords: AKT, PKB, PI3K/AKT signalling, AKT inhibitors, cancer
1. Introduction
The AGC kinases, named after the protein A, G and C kinases, are an evolutionarily conserved group of proteins that share a hydrophobic motif at the c-terminus of their catalytic core. This motif, composed of F-X-X-F/Y-S/T-Y/F, is known as the PIF-pocket and regulates catalytic activity (Arencibia et al., 2013; Manning and Cantley, 2007). The AGC kinase family comprises 14 family members, of which AKT (also known as PKB; protein kinase B) is a key member.
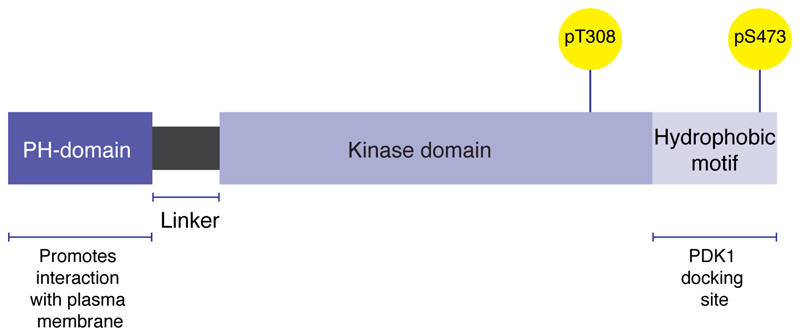
There are three AKT isoforms, transcribed from separate genes, which share three highly conserved domains: a central catalytic domain and two regulatory domains: a lipid-binding N-terminal PH (plektrin homology) domain, and the hydrophobic motif (Figure 1). The PH domain contains a lipid-binding module that promotes the interaction of AKT with the plasma membrane, an important step in AKT activation. The hydrophobic motif contains an important docking site for the activating kinase PDK1 (3-phosphoinositide-dependent kinase-1) and also provides allosteric regulation of catalytic activity (Scheid & Woodgett, 2003; Figure 1). There is approximately 80% sequence homology between the isoforms with most variability occurring in the linker region between the PH and catalytic domains (Brodbeck et al., 1999; Cheng et al., 1992; Hanada et al., 2004; Jones et al., 1991).
Figure 1.
Structural domains of AKT.
2. AKT activation
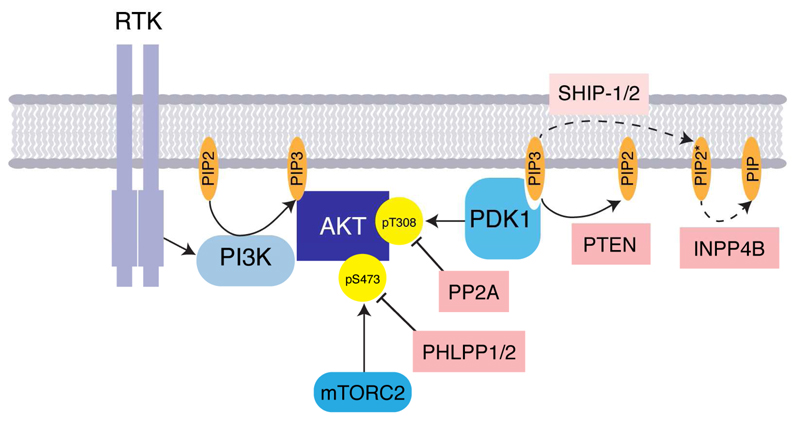
Mechanisms of AKT activation have been reviewed previously (Liao and Hung, 2010; Scheid and Woodgett, 2003), but essentially, AKT activity is regulated downstream of receptor tyrosine kinases (RTKs), such as those within the EGF (epidermal growth factor), Insulin, PDGF (platelet derived growth factor), FGF (fibroblast growth factor) and VEGF (vascular endothelial growth factor) families. RTKs activate class I phosphatidylinositol 3-kinases (PI3K), either directly, or in conjunction with adaptor proteins such as IRS-1/2 (insulin receptor substrate -1/2; Figure 2). The PI3Ks phosphorylate phosphatidylinositol-4,5-bisphosphate (PIP2) to generate phosphatidylinositol-3,4,5-trisphosphate (PIP3). AKT binding to PIP3 at the plasma membrane induces a conformational change that results in phosphorylation of AKT, predominantly on two highly conserved residues, Thr308 and Ser473 leading to AKT activation (Figure 1). Phosphorylation of Thr308 in the activation T-loop of the catalytic domain by PDK1, results in a conformational change that enhances substrate affinity and promotes AKT kinase activity (Alessi et al., 1997). Phosphorylation of Ser473 within the PIF pocket of AKT by mTORC2 (mammalian target of rapamycin complex 2) is thought to promote AKT activity by increasing the affinity of AKT to PDK1 (Sarbassov et al., 2005). In fact, multiple different kinases for Ser473 have been described in the literature and it’s likely that mechanisms determining full activation of AKT are context dependent. It has been accepted, for example, that following DNA damage, the PI3K-like kinase (PIKK) DNA-PK (DNA-dependent protein kinase) is responsible for AKT Ser473 phosphorylation and that AKT activation prevents apoptosis following ionizing radiation (Bozulic et al., 2008). Multiple other phosphorylation sites on AKT have been described, although the physiological importance of these is not yet fully understood (Risso et al., 2015) and mechanisms of constitutative activation of AKT signalling in cancer are discussed further below.
Figure 2.
Activation and negative regulation of AKT. See text for details.
Important negative regulators of the PI3K/AKT signalling pathway include the tumour suppressor genes and phosphatases PTEN (phosphatase and tensin homolog), PP2A (protein phosphatase 2A) and PHLPP (PH domain and leucine rich repeat protein phosphatase 1; Gao et al. 2005), which dephosphorylate PIP3, AKT pThr308 and AKT pSer473 respectively (Toker & Marmiroli, 2014; Figure 2). PTEN hydrolyses the 3′- phosphate on PIP3 to terminate PI3K signalling. The SH2 domain-containing inositol phosphatases (SHIP-1/2) are able to hydrolyse the 5′-phosphate on PIP3 to generate PI(3,4)P2, the function of which is not clear, although some studies suggest that like PIP3, PI(3,4)P2 is able to facilitate PDK1 activation of AKT (Gewinner et al., 2009). Recently, a fourth putative tumour suppressor of the PI3K/AKT pathway has been described namely, polyphosphate 4-phosphatase type II (INPP4B; Gewinner et al. 2009). This is able to dephosphorylate PI(3,4)P2 to PI(3)P in vitro, resulting in attenuation of AKT signalling (Gewinner et al., 2009).
Of all the tumour suppressors, PHLPP in particular is a key regulator of AKT activity and it’s ability to fine-tune the PI3K/AKT pathway is discussed in greater detail below.
3. Downstream signalling of AKT
A consensus phosphorylation motif has been described for AKT substrates: R-X-R-X-X-S/T-B where X represents any amino acid and B represents bulky hydrophobic residues (Alessi et al., 1996). Numerous AKT substrates have been published in the literature, with several being extensively validated that impact on multiple cellular processes including: cell growth, proliferation and survival, cellular metabolism, glucose uptake and angiogenesis (Table 1; Manning & Cantley 2007). Importantly there is great overlap and crosstalk between substrate functions, with many substrates being regulated directly by AKT phosphorylation, as well as indirectly, through AKT-mediated phosphorylation of transcription factors. AKT signalling therefore plays a central role in multiple pathways involved in tumourigenesis and hyperactivation of the PI3K/AKT pathway is a common occurrence in cancer (Liu et al., 2009).
Table 1. The effect of AKT phosphorylation on well-described substrates.
(Manning and Cantley, 2007). BAD (BCL2 Associated Agonist Of Cell Death); FOXO (Forkhead Box); MDM2 (MDM2 oncogene); TSC2 (Tuberous Sclerosis 2); PRAS40 (Proline Rich Akt substrate); CDKN (Cyclin-Dependent Kinase Inhibitor); GSK3β (Glycogen Synthase Kinase Beta).
| Cellular function | Substrate | Effect of AKT phosphorylation |
|---|---|---|
| Cell survival | BAD | Release of pro-survival Bcl-2 proteins from BAD. |
| FOXO1 FOXO3a FOXO4 |
14-4-4 mediated export of FOXO transcription factors from the nucleus, blocking transcription of pro-apoptotic proteins such as BIM and FasL. | |
| MDM2 | Nuclear translocation of MDM2 resulting in P53 ubiquitin-mediated degredation. | |
| Cell growth | TSC2 | Inhibits TSC2 function, resulting in mTORC1 activation. |
| PRAS40 | Phosphorylation of PRAS40 by AKT results in 14-3-3 mediated sequestration of PRAS40 and mTORC1 activation. | |
| Cell proliferation | CDKN1B/p27 CDKN1A/p21 |
Prevents nuclear localisation of cyclin dependent kinases, blocking cell-cycle inhibitory effects and resulting in loss of the G1-S checkpoint. |
| Glucose homeostasis | Unknown | Results in translocation of glucose transporter 4 (GLUT4) to the plasma membrane. |
| GSK3β | Inhibits glycogen synthase 3 (GSK3), thereby inhibiting glycogen synthesis. | |
| Angiogenesis | eNOS | Activates endothelial nitric oxide synthase (eNOS), resulting in vasodilatation, vascular remodelling and angiogenesis |
4. AKT in normal physiology
As well as playing a central role in cancer, AKT signalling is also essential for normal cellular physiology. In adults, AKT1 expression is ubiquitous, expression of AKT2 is elevated in insulin-responsive tissues and expression of AKT3, whilst found at low levels in all tissues, is more highly expressed in the brain and endocrine tissues (http://www.proteinatlas.org/; Uhlen et al. 2010; Uhlén et al. 2015).
AKT plays an essential role in glucose homeostasis and a deleterious effect of pharmacological inhibition of AKT is hyperglycaemia and hyperinsulinaemia which correlates with peak plasma level drug concentrations (Crouthamel et al., 2009). The AKT substrate GSK3β (glycogen synthase kinase beta) phosphorylates and inhibits glycogen synthase, preventing glycogen synthesis. AKT activation results in phosphorylation and inhibition of GSK3β relieving the inhibitory effect on glycogen synthase, resulting in glycogen synthesis. As well as stimulating glycogen synthesis, AKT also promotes cellular uptake of glucose by prompting translocation of the glucose transporter GLUT4 to the plasma membrane. The AKT substrate directly involved in this process has not been well-defined and several candidate proteins are described in the literature (Manning and Cantley, 2007).
AKT function (especially AKT1 and AKT2) is also important for regulating cardiac growth, contractile function and coronary angiogenesis. It is essential for maintaining normal endothelial function and endothelial nitric oxide synthesis, with increased atherosclerosis and peripheral vascular disease demonstrated in Akt1 knockout mice (Hers et al., 2011). AKT also has a pro-thrombotic effect, promoting platelet activation and aggregation, highlighting the need for tight control of AKT signalling (Hers et al., 2011). As well as its role in the cardiovascular and endocrine systems, AKT has also been shown to be neuro-protective and plays a role in synaptic transmission between neurons (Hers et al., 2011). Taken together therefore, given the wide-ranging effects of AKT signalling in normal human physiology, there is vast scope for significant on-target as well as off-target effects of AKT inhibitors in the clinic.
5. Selectivity within the AKT pathway
The phenotypes of Akt null mice have suggested non-redundant roles for the three isoforms, with Akt1 knockout being embryonic lethal, Akt2 null mice developing insulin-resistant diabetes and Akt3 knock-out resulting in microcephaly (Toker and Marmiroli, 2014). In keeping with this, findings from pre-clinical studies, are consistent with AKT isoforms having partially overlapping, but distinct functions in cancer cells (Clark and Toker, 2014; Toker and Marmiroli, 2014). Phosphoproteomic screens have demonstrated unique and common substrates for each of the AKT isoforms and functional studies have started to highlight the physiological relevance of these findings, for example during RNA processing in lung cancer (Sanidas et al., 2014). Isoform-mediated specificity has been best studied in breast cancer models however, where differential functions of AKT1, -2 and -3 in both inhibiting and promoting cell migration and proliferation have been demonstrated (Clark and Toker, 2014).
AKT1 has been shown to be important for G1-S checkpoint transition and proliferation, whereas AKT2 regulates cell-cycle exit through its interaction with p21 (Héron-Milhavet et al., 2006). In a recent study in triple negative breast cancer, AKT3, rather than AKT1 activity was most important for cellular proliferation (Chin et al., 2014a), suggesting a degree of context specificity for these findings. Interestingly, it has been observed that although activating mutations in AKT1 result in accelerated tumourigenesis, metastatic dissemination is decreased due to inhibitory effects of AKT1 on tumour cell invasion and migration (Chin and Toker, 2010; Hutchinson et al., 2004). By contrast, AKT2 activity promotes tumour invasion and metastatic dissemination in mouse breast cancer models (Dillon et al., 2009; Maroulakou et al., 2007). In addition, it has recently been shown that INPP4B inhibition of AKT2 is important for metastatic spread of follicular thyroid cancer (Chew et al., 2015). A number of different AKT1/2 substrates have been described that may account for the differential phenotype of cellular migration, which play a role in epithelial-mesenchymal transition (EMT), including the NFAT transcription factor (Yoeli-Lerner et al., 2005), palladin (Chin and Toker, 2010), the integrin β subunit (Arboleda et al., 2003) and the miR-200 family of micro-RNAs (Iliopoulos et al., 2009). There are likely to be many more (Clark and Toker, 2014) and the relative importance of each of these is likely to depend on tumour subtype, as well as perhaps tumour stage and genetic background. An example of this is that PTEN deficient tumour cells have recently been shown to be dependent, specifically on AKT2 for survival (Chin et al., 2014b). This was shown to be true for prostate cancer, breast cancer and glioblastoma PTEN deficient cells lines and was at least in part, mediated through AKT2 dependent up regulation p21 (Chin et al., 2014b).
Several lines of evidence suggest that AKT does not need to be fully activated in order to phosphorylate all substrates. This suggests that different AKT substrates and therefore different cellular functions of AKT depend on varying levels of AKT activation. For example AKT Ser473 phosphorylation appears to be dispensable for phosphorylation of the AKT targets TSC2 and GSK3, as well as the TORC1 effectors, S6K and 4E-BP1, but not for FOXO1/3a (Jacinto et al., 2006). Interestingly, in patient non-small cell lung cancer samples, AKT Thr308 correlates with phosphorylation of AKT substrates PRAS40, TSC2 and TBC1D4, whereas AKT Ser473 phosphorylation does not (Vincent et al., 2011). This raises an important point clinically, when trying to determine whether or not a tumour might be dependent on AKT signalling for survival, suggesting the AKT Thr308 is a more suitable biomarker of AKT activation than AKT Ser473.
Relative levels of the negative regulators of the PI3K/AKT pathway will also ultimately affect amplitude of AKT activation. For example, the PHLPP isoforms -1 and -2 have been shown to preferentially hydrolyse Thr308 on AKT2 and AKT3 respectively (Brognard et al., 2007). Cellular location and post-translational modification of AKT isoforms also contribute to specificity within the pathway (Clark and Toker, 2014) and untangling all of these factors within the context of cancer is proving to be a considerable challenge.
6. Feedback loops within the PI3K/AKT signalling pathway
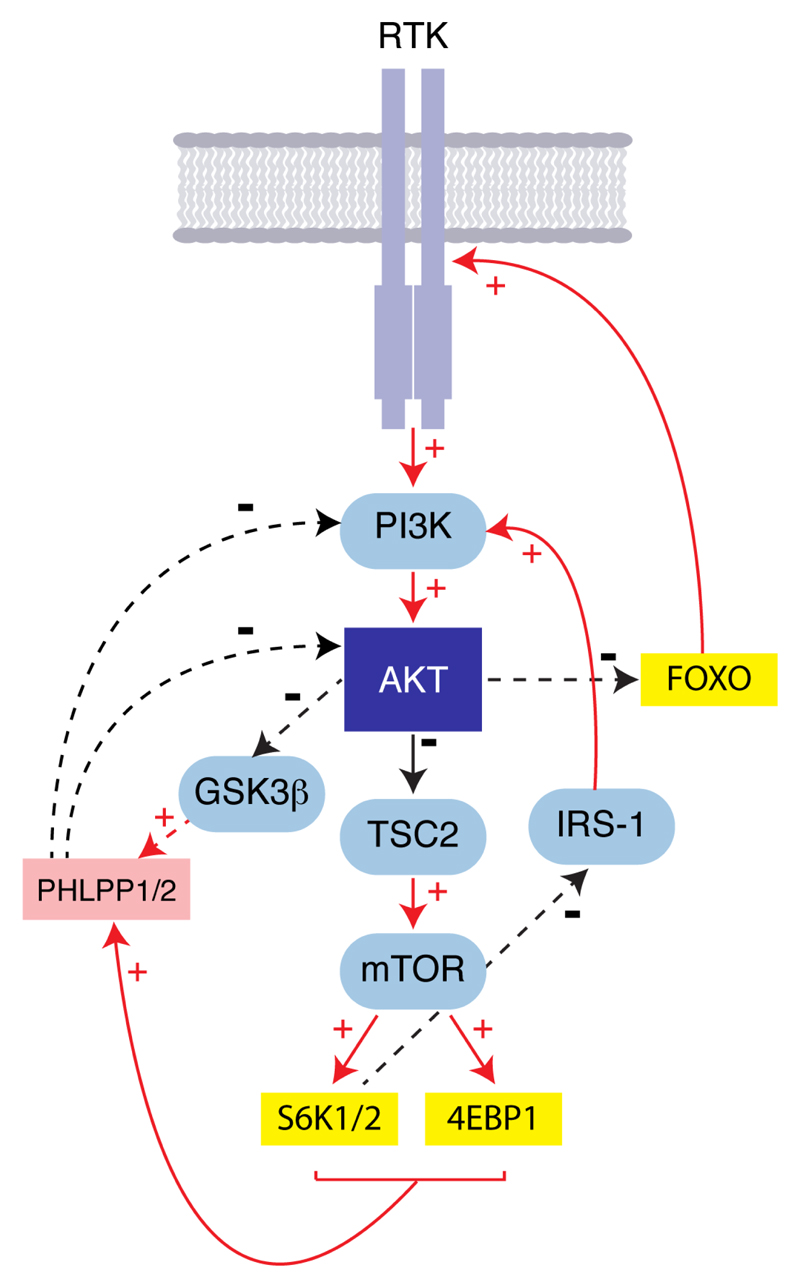
The PI3K/AKT signalling pathway is far from linear with multiple negative feedback loops fine-tuning overall activation levels (Carracedo and Pandolfi, 2008). The activity of the S6 kinases (S6K1/2), which are phosphorylation targets of mTORC1 play a pivotal role in negatively regulating AKT activity. IRS-1 (insulin receptor substrate-1) is an adaptor protein required for binding of the class IA PI3Ks with insulin and insulin growth factor (IGF) receptors. Phosphorylation of IRS-1 by S6K1 inhibits its interaction with insulin and IGF receptors and also promotes proteasomal degredation of IRS-1 (Harrington et al., 2005), thereby dampening PI3K signalling and reducing AKT activity. In addition to its effects on AKT activity via upstream inhibition of PI3K signalling, the mTORC1 complex also inhibits AKT by up-regulating PHLPP1 translation (Liu et al., 2011), the phosphatase responsible for dephosphorylating AKT pSer473 (Figure 3).
Figure 3.
Diagram illustrating feedback inhibition and activation within AKT signalling.
AKT activity is also self-limiting through its substrate GSK3β ; the kinase activity of which is negatively regulated by AKT phosphorylation (Figure 3; Li et al. 2009). GSK3β activation results in phosphorylation and subsequent ubiquitin mediated degredation of PHLPP (Li et al., 2009). AKT activation therefore results in GSK3β ???????????? and high levels of PHLPP, which in turn, puts the brakes on the AKT signalling pathway.
Opposing the above mechanisms that limit the activity of the PI3K/AKT pathway, AKT inhibition results in up-regulation of RTKs, in part through loss of mTORC1 signalling, but also due to FOXO-dependent transcriptional increases in RTK expression (Chandarlapaty et al., 2011). This up-regulation of upstream pathway components in part will compensate for pharmacological inhibition of more downstream players and has been shown to contribute to resistance to AKT inhibitors (Garrett et al., 2011b).
In the context of normal growth factor signalling, it’s easy to appreciate why it’s advantageous that in ‘times of plenty’, chronic activation of mTORC1, leads to attenuation of AKT signalling (Manning, 2004). As discussed below, multiple components of the PI3K pathway are mutated or deleted in cancer cells and the level of the aberration within the pathway will have profoundly different effects on AKT activity depending on whether or not the negative regulatory feedback loops are intact. Equally, chronic vs acute inhibition of the pathway may result in very different signalling patterns. This makes targeting the pathway a significant challenge in oncology and also has implications for dosing schedules of AKT inhibitors.
7. AKT activation in cancer
The PI3K/AKT pathway is the most commonly disrupted signalling pathway in human cancers (Millis et al., 2016). PI3K/AKT pathway aberrations have been identified in up to 40% of all tumour types, with PTEN loss by immunohistochemistry occurring most frequently (30%), followed by mutations in PIK3CA (13%), PTEN (6%) and AKT (1%; Millis et al. 2016). Whilst activating AKT mutations are relatively uncommon, AKT activation can arise as a result of several other genomic aberrations.
AKT amplification
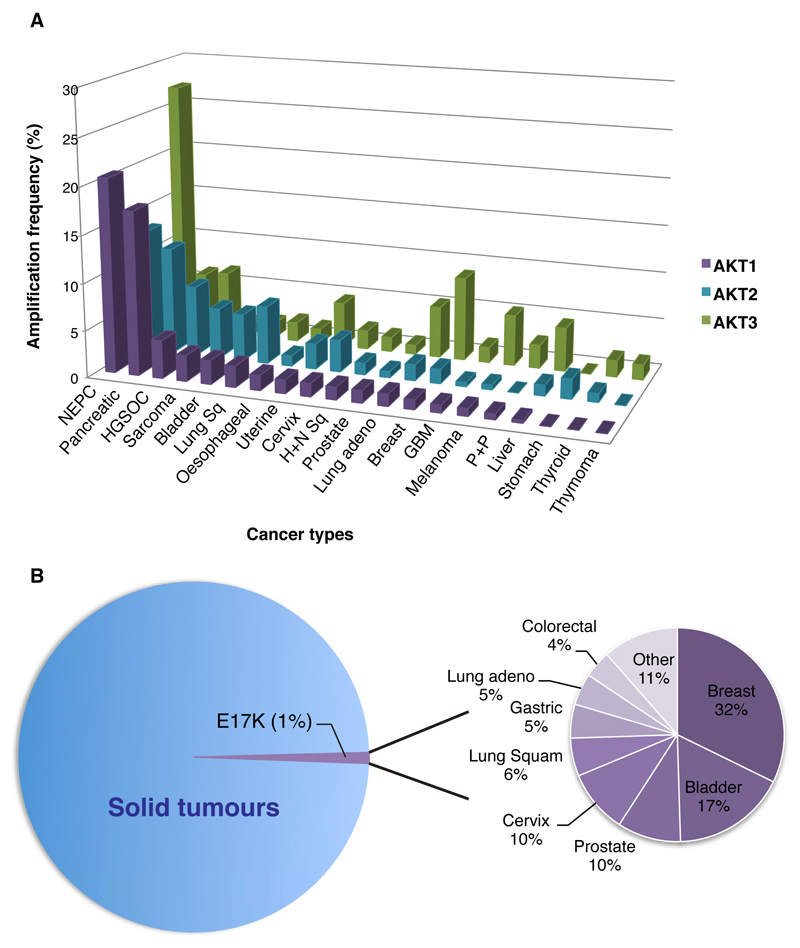
In keeping with its role in promoting EMT transition and promoting metastatic disease, AKT2 is frequently amplified in cancer. Analysis of the cBioPortal database for cancer genomics demonstrates that AKT2 amplification is found in up to 16% of pancreatic cancers, 16% of uterine cancers, 13% of breast cancers and 5-10% of ovarian, lung and bladder cancers (Figure 4A). Early work also demonstrated that siRNA depletion of AKT2 resulted in reduced cell growth and invasiveness only in cells overexpressing AKT2, consolidating its role in tumourigenesis (Cheng et al., 1996). By contrast, AKT1 amplification is found less frequently across tumour types, occurring in 20% of neuroendocrine prostate cancers, 10% of pancreatic cancers and 3-5% of breast and serous ovarian cancers. To date, high levels of AKT3 amplification have been identified most often in breast cancers and as discussed, AKT3 may be the predominant isoform responsible for growth and proliferation of triple negative breast cancers (Chin et al., 2014a). Interestingly, high levels of AKT3 expression have also been demonstrated in over 25% of neuroendocrine prostate cancers (Figure 4A).
Figure 4.
(A) Frequency of AKT1-3 amplification across a range of tumour types.. (B) AKT mutations are found in approximately 1% of all cancers. The frequency of AKT mutations vary between cancer types as shown. Data gathered from cbioportal.org database (Cerami et al. 2012; Gao et al. 2013). Only studies with n>100 included in analysis.
AKT mutations
In contrast to amplifications, mutations in AKT2 are found much less frequently and to our knowledge, no commonly occurring functionally activating mutations in AKT2 have been described. AKT1 is the most frequently mutated isoform in tumours and this occurs at low frequency (approximately 1% of all cancers; Figure 4B; www.cbioportal.org; Cerami et al. 2012; Gao et al. 2013). Several functionally transforming mutations of AKT1 have been described however (Yi et al., 2013), the predominant mutation is AKT1E17K (Carpten et al., 2007). This glutamic acid to lysine substitution within the lipid-binding pocket of AKT1 results in pathological localisation of AKT1E17K to the plasma membrane and subsequent constitutative activation of AKT1 signalling (Carpten et al., 2007). In contrast to physiological AKT1 activation that occurs upon binding of AKT1 to PIP3 at the plasma membrane, the E17K mutation in AKT1 alters PIP specificity, resulting in high affinity binding to highly abundant PIP2 within the plasma membrane (Landgraf et al., 2008). AKT1E17K therefore utilises both PIP3 as well as PIP2 for its activation and as a result, remains constitutively active at the cell membrane (Landgraf et al., 2008).
Both allosteric and catalytic inhibitors of AKT are capable of suppressing AKT1E17K activity and subsequently inhibiting growth of tumours harbouring AKT1E17K in breast cancer models (Davies et al., 2015). AKT1E17K mutations are found most frequently in breast, bladder, cervix and prostate cancers (Figure 4B). Inhibition of AKT1E17K tumours in a clinical setting has also now been demonstrated and will be discussed in more detail below.
Alterations of other proteins of the PI3K/AKT signalling pathway
Over-expression or activating mutations of proteins upstream of AKT in the PI3K/AKT pathway result in AKT activation, as do loss of the tumour suppressor proteins: PTEN, PHLPP, PP2A and INPP4B (Liu et al., 2009). Germline mutations in PTEN result in PTEN hamartoma syndromes, of which Cowden’s syndrome is the best described; characterised by the development of multiple hamartomas and with increased risk of carcinomas of the breast, thyroid, endometrium and kidney. Somatic PTEN loss is most commonly identified in endometrial carcinoma, prostate carcinoma and glioblastoma and can arise due to mutations, insertions and deletions throughout the PTEN gene (Hollander et al., 2011).
8. The clinical development of AKT inhibitors
Inhibiting PI3K/AKT signalling has long been an attractive therapeutic approach in oncology. Numerous compounds that inhibit the pathway at all levels are now in clinical development, including those targeting AKT (Yap et al., 2008). AKT inhibitors fall predominantly into two separate classes. Allosteric inhibitors of the AKT PH-domain prevent localisation of AKT to the plasma membrane, thereby blocking AKT phosphorylation and activation. The second class of inhibitors comprise ATP- competitive inhibitors of AKT, of which there are several in the early stages of clinical development (Table 2).
Table 2. Table showing trials of AKT inhibitors in clinical development.
Trials that are currently recruiting or remain open are marked in red. Allosteric inhibitors are shaded blue. Competitive ATP inhibitors are shaded purple. Dex = dexamethasone. CRPC (castrate resistant prostate cancer). TNBC (triple negative breast cancer).
| AFURESERTIB (GSK2110183) | ||
| Monotherapy | Phase I: | Multiple myeloma (NCT02177682); Haematological malignancies (NCT00881946) (Spencer et al., 2014); Healthy volunteers (NCT01827644, NCT02040480) |
| Phase I/II: | Solid tumours (NCT01531894) (Yan et al., 2011) | |
| Chemotherapy | Carboplatin + Paclitaxel | Platinum resistant ovarian cancer (NCT01653912) (Blagden et al., 2016) |
| Paclitaxel | Gastric cancer (NCT02240212) | |
| MAPK | Trametinib | Solid tumours (NCT01476137) (Tolcher et al., 2015b) |
| Other | Bortezomib + Dex | Multiple myeloma (NCT01428492) |
| Ofatumumab | CLL (NCT01532700) | |
| ARQ 751 and ARQ 092 | ||
| Monotherapy | Phase I: | Solid tumours (NCT01473095); Solid tumours with PIK3CA or AKT1/2/3 activating mutations (NCT02761694,); Proteus syndrome (non-oncological indication; NCT02594215) |
| Combinations | Carboplatin +/- paclitaxel or anastrazole | Ovarian, endometrial, cervical and TNBC (NCT02476955) |
| AZD5363 | ||
| Monotherapy |
Phase I: Solid tumours (NCT01226316, NCT01353781, NCT01895946) (Banerji et al., 2015; Dean et al., 2015; Tamura et al., 2016); mCRPC (NCT01692262). Expansion cohorts in PIK3CA and AKT mutant breast and gynaecological cancers. Phase II: Solid tumours with molecular match (NCT02465060); ER+ve breast cancer vs placebo (NCT02077569); NSCLC (NCT02664935, NCT02117167); Lymphoma (NCT02465060); metastatic breast cancer (NCT02299999) |
|
| Chemotherapy/Radiotherapy | Paclitaxel | TNBC (NCT02423603); ER+ breast cancer (NCT01625286); Gastric cancer (NCT02449655, NCT02451956) |
| Docetaxel + Prednisolone | CRPC (NCT02121639) (Crabb et al., 2016) | |
| Targeted therapy/hormonal therapy | Olaparib | Solid tumours (NCT02338622; NCT02576444) (Michalarea et al., 2016) |
| Olaparib + AZD2014 | Endometrial and ovarian cancers (NCT02208375) | |
| Enzalutamide | CRPC (NCT02525068) | |
| Fulvestrant | ER+ breast cancer (NCT01992952) | |
| BAY1125976 | ||
| Monotherapy | Phase I: | Solid tumours (NCT01915576) |
| GSK2141795 | ||
| Monotherapy | Phase I: | Solid tumours (NCT00920257); Ovarian cancer (NCT01266954) |
| MAPK | Trametinib | Solid tumours (NCT01138085, NCT01138085); AML (NCT01907815); Multiple Myeloma (NCT01989598); Endometrial cancer (NCT01935973, NCT02093546); TNBC (NCT01964924); Uveal melanoma (NCT01979523) (Shoushtari et al., 2016); BRAF WT melanoma (NCT01941927) (Algazi et al., 2015); Cervical cancer (NCT01958112) |
| Trametinib/Dabrafenib | Solid tumours (NCT01902173) | |
| GSK690693 | ||
| Monotherapy | Phase I: | Solid tumours (NCT00493818) |
| IPATASERTIB (GDC-0068) | ||
| Monotherapy | Phase I: | Solid tumours (NCT01090960); Healthy subjects (NCT02536391, NCT02063581, NCT02390492) |
| Phase II: | GBM (NCT02430363) | |
| Chemotherapy | Paclitaxel | TNBC (NCT02301988, NCT02162719); solid tumours (NCT01362374) (Isakoff et al., 2014) |
| mFOLFOX6 | Gastro-oesophageal cancer (NCT01896531); solid tumours (NCT01362374) | |
| Docetaxel | Solid tumours (NCT01362374) | |
| MAPK | Cobimetinib | Any cancer (NCT01562275) (Bendell et al., 2014) |
| Hormonal | Abiraterone + Pred | CRPC (NCT01485861) (De Bono et al., 2016) |
| Enzalutamide | Solid tumours (NCT01362374) | |
| LY2780301 | ||
| Monotherapy | Phase I: | Single agent (NCT01115751) (Azaro et al., 2015) |
| Combinations | Paclitaxel | Breast cancer (NCT01980277) |
| Gemcitabine | Solid tumours (NCT02018874) | |
| TRICIRIBINE (TCN-PM; VD-0002) | ||
| Monotherapy | Phase I: | Solid tumours (NCT00363454) (Garrett et al., 2011a);Haematological malignancies(NCT00642031) |
| Combinations | Carboplatin | Ovarian cancer (NCT01690468) |
| MK2206 | ||
| Monotherapy |
Phase I: Solid tumours (NCT00670488, NCT01071018) (Doi et al., 2015; Yap et al., 2011, 2014); Paediatric tumours (NCT01231919) (Fouladi et al., 2014) Phase II: Solid tumours: Adenoid cystic carcinoma (NCT01604772) (Ho et al., 2015); Breast cancer (NCT01277757); Early breast cancer (NCT01319539); Biliary cancer (NCT01425879) (Ahn et al., 2015); Colorectal (NCT01802320) (Dasari et al., 2016); KRAS WT PIK3CA mutant CRC (NCT01186705); Endometrial cancer (NCT01312753, NCT01307631) (Konstantinopoulos et al., 2014); Gastro-oesophageal (NCT01260701) (Ramanathan et al., 2015); Head and neck cancer (NCT01349933) (Ma et al., 2015); Liver cancer (NCT01239355); Nasopharyngeal carcinoma (NCT01370070); Neuroendocrine tumours (NCT01169649); Ovarian/primary peritoneal/fallopian tube cancers with PI3K/AKT mutations or PTEN loss (NCT01283035) Haematological malignancies: AML (NCT01253447) (Konopleva et al., 2014); Lymphoma (NCT01258998) (Oki et al., 2015); Diffuse large B-cell lymphoma (NCT01466868, NCT01481129) |
|
| Chemotherapy | Paclitaxel | Solid tumours + breast cancer (NCT01263145) (Gonzalez-Angulo et al., 2015) |
| Paclitaxel + Trastuzumab | HER2+ve tumours (NCT01235897) (Chien et al., 2016) | |
| Carbo-taxol or Docetaxel | Solid tumours (NCT00848718) (Molife et al., 2014) | |
| HER2-directed | Lapatinib | HER2+ve breast cancer (NCT01281163, NCT01245205) (Wisinski et al., 2016) |
| Trastuzumab +/- Lapatinib | HER2+ve breast cancer (NCT01042379); HER2+ve solid tumours (NCT00963547, NCT01705340)(Hudis et al., 2013; Tripathy et al., 2015) | |
| MAPK | Selumetinib | Solid tumours (NCT01021748) (Tolcher et al., 2015a); Colorectal (NCT01333475) (Do et al., 2015); Melanoma (NCT01519427); NSCLC (NCT01248247) (Papadimitrakopoulou et al., 2014) |
| Selumetinib + mFOLFOX | Pancreatic cancer (NCT01658943) (Chung et al., 2015) | |
| PI3K/mTOR | Gefitinib | NSCLC (NCT01147211) (Lin et al., 2014) |
| Erlotinib | NSCLC (NCT01248247) (Papadimitrakopoulou et al., 2014); Erlotinib resistant NSCLC (NCT01294306) (Lara et al., 2015); Solid tumours (NCT00848718) (Molife et al., 2014) | |
| Dalotuzumab | Any cancer (NCT01243762) (Brana et al., 2014) | |
| Everolimus | Renal cancer (NCT01239342) | |
| Ridaforolimus (MK8669) | Any cancer (NCT01295632) (Gupta et al., 2015) | |
| Hormonal | Anastrozole +/or Fulvestrant | ER +ve breast cancer (NCT01344031) (Ma et al., 2016) |
| Anastrozole +/- Goserelin | ER +ve HER2-ve breast cancer (NCT01776008) | |
| Bicalutamide | Prostate cancer (NCT01251861) | |
| Other | Dinaciclib | Pancreatic carcinoma (NCT01783171) |
| Bendamustine Hydrochloride, and Rituximab | CLL or small lymphocytic leukaemia (NCT01369849) | |
| Hydroxychloroquine | Solid tumours (NCT01480154) | |
| PERIFOSINE | ||
| Monotherapy |
Phase I: All solid tumours (Crul et al., 2002; Figg et al., 2014; Van Ummersen et al., 2004; Unger et al., 2010) (NCT00019656, NCT00062387, NCT00005794; NCT00005794); Phase II: Breast cancer (Leighl et al., 2008)(NCT00054145); NSCLC (NCT00399789); Malignant glioma (NCT00590954); Head and neck (Argiris et al., 2006)(NCT00062387); Melanoma (Ernst et al., 2005) (NCT00053781); Pancreatic cancer (Marsh et al., 2007) (NCT00059982, NCT00053924); Castrate resistant prostate cancer (Posadas et al., 2005)(NCT00060437); Biochemically recurrent prostate cancer (Chee et al., 2007) (NCT00058214); Renal carcinoma (Cho et al., 2012) (NCT00448721, NCT00498966); Soft tissue sarcoma (Bailey et al., 2006; Knowling et al., 2006) (NCT00053794, NCT00064324); Chemo resistant sarcoma (NCT00401388); Waldenstrom's macroglobulinemia (NCT00422656, NCT00398710); Leukaemia (NCT00391560). |
|
| Chemotherapy/Radiotherapy | Docetaxel | Epithelial ovarian cancer (Fu et al., 2012)(NCT00431054) |
| Radiotherapy | Solid tumours (Vink et al., 2006) | |
| Capecitabine | Colorectal cancer (Bendell et al., 2011, 2012; Eng et al., 2014) (NCT01097018; NCT00398879) | |
| Targeted therapy | Temsirolimus | Paediatric solid tumours (NCT01049841);Malignant Gliomas (NCT01051557,NCT02238496) |
| Imatinib | GIST (NCT00455559) | |
| Bortezomib +Dex | Multiple myeloma (Richardson et al., 2011)(NCT00401011, NCT01002248) | |
| Lenalidomide +Dex | Multiple myeloma (Jakubowiak et al., 2012) (NCT00415064) | |
| Sorafenib | Lymphoprolierative diseases (Guidetti et al., 2014) | |
| UCN-01 | Acute leukaemia, CML, high risk MDS (Gojo et al., 2013)(NCT00301938) | |
Monotherapy with AKT inhibitors
The AKT inhibitors that have entered clinical evaluation have been discussed in alphabetical order.
Afuresertib (GSK2110183) and GSK2141795
Afuresertib (GSK2110183) and GSK2141795 (GlaxoSmithKline) are both orally bioavailable, ATP-competitive inhibitors of AKT1-3 which have anti-tumour effects in vitro and in vivo as monotherapy and in combination with the MEK inhibitor trametinib (GSK1120212; Dumble et al. 2014). In a phase I trial of afuresertib in patients with haematological malignancies, DLTs consisted of deranged liver function tests and the MTD was established as 125mg daily. The most frequent (>10% of patients) treatment-related adverse events (AEs) were nausea (23.3%), diarrhoea (20.5%), dyspepsia (19.2%), fatigue (16.4%), gastrointestinal reflux disease (15.1%), and anorexia (13.7%). Promising anti-tumour activity was demonstrated in patients with multiple myeloma (Spencer et al., 2014). Preliminary data from a phase I study of GSK2141795 demonstrated an MTD of 75mg daily with DLTs of G3 hyperglycaemia, G4 hypoglycaemia, G3 hypoglycaemia and G2 stomatitis with promising tumour responses seen in patients with an activated PI3K/AKT pathway (Burris et al., 2011).
ARQ 092 and ARQ 751
ARQ 092 and ARQ 751 (ArQule) are both highly potent allosteric inhibitors of wild type and AKTE17K, with ARQ 751 having enhanced PK properties and potency (Table 2; Lapierre et al., 2016; Yu et al., 2015). Structural studies have shown that allosteric inhibitors bind to a region between the PH-domain and kinase domain on AKT (Wu et al., 2010). This stabilises the PH-domain in a ‘closed’ conformation which blocks ATP binding to the kinase site, obstructing the phospholipid binding site, and thereby inhibiting AKT recruitment to the plasma membrane (Wu et al., 2010).
Preliminary data from a phase Ia dose escalation trial of ARQ 092 has demonstrated common drug-related adverse events (≥10%) of hyperglycaemia (30%), macular-papular rash (28%), nausea (20%), diarrhoea (19%), pruritis (16%), fatigue (15%_ and decreased appetite (14%; Tolcher et al., 2016). Early evidence of anti-tumour activity has been shown with two durable partial responses in patients with activating PIK3CA mutations (Tolcher et al., 2016) and promising clinical activity in patients with AKTE17K mutations has also now been demonstrated in the phase Ib expansion cohort (Abbadessa et al., 2015).
Interestingly, AKT inhibition using ARQ 092 is also being explored for the treatment of Proteus syndrome (Lindhurst et al., 2015). Rarely a somatic AKT1E17K mutation can arise during development that results in Proteus syndrome; a progressive, mosaic, segmental overgrowth syndrome that can affect any part of the body (Biesecker, 2001). The severity and extent of tissue involvement is unique to each patient, with bone, fat, skin and connective tissue being most commonly involved (Biesecker, 2001). Affected patients have an increased risk of malignancy, including mesothelioma and breast cancer, and are at high risk of life-threatening venous thromboembolism (Biesecker, 2001). In preclinical studies, ARQ 092 has been shown to suppress AKT signalling in cell lines and tissues from patients with Proteus syndrome (Lindhurst et al., 2015). In contrast to the treatment of AKT-mutated cancers, the aim of such treatments is to restore physiological levels of AKT signalling in cells rather cytotoxicity. A phase I trial of ARQ 092 is currently recruiting children and adults with Proteus syndrome (NCT02594215) and preliminary data suggest that doses of ARQ 092 that are significantly lower than the MTD for cancer will be sufficient to treat patients with Proteus syndrome (Lindhurst et al., 2015).
AZD5363
AZD5363 (Astrazeneca) is a potent competitive kinase domain inhibitor of AKT1-3, which in pre-clinical studies has been shown to inhibit phosphorylation of AKT substrates (PRAS40, GSK3β and S6) and tumour growth in xenograft models (Davies et al., 2012). The presence of an activating PIK3CA mutation, AKT1E17K mutation or PTEN loss, in the absence of a significant KRAS (or other activating mutation in an alternative pro-survival pathway) significantly correlated with sensitivity to AZD5363 across a range of tumour cell lines (Davies et al., 2012, 2015). Interestingly, continuous low doses of AZD5363 had a predominantly anti-proliferative effect on cells when given as monotherapy and significant levels of apoptosis were only observed using an intermittent high dose schedule, suggesting that intermittent high dose schedules might be a more effective clinical strategy for the treatment of AKT dependent tumours (Davies et al., 2012).
A phase I trial of AZD5363 monotherapy in patients with solid tumours has investigated the toxicity, pharmacokinetics (PK) and PD of intermittent vs continuous dosing (Banerji et al., 2015). Three schedules were explored: continuous dosing (7/7), four days a week (4/7) and two days a week (2/7) of which the MTDs were 320mg BID, 480mg BID and 640mg BID respectively. The DLTs were rash and diarrhoea for 7/7, and hyperglycaemia for 2/7 with no DLTs identified for 4/7. As expected following AKT inhibition, the most common causally related adverse events ≥ CTC Grade 3 were hyperglycaemia (20%), diarrhoea (10%), rash (10%), nausea (3%) and fatigue (1%). PK profiles at 480mg BID (4/7) resulted in higher steady state levels of AZD5363 compared to continuous dosing at lower drug concentrations and were consistent with exposures that resulted in tumour regression in preclinical models. Pre- and post-treatment biopsies confirmed target engagement in tumour tissue, with an increase in pAKT and reductions in pGSK3β and pPRAS40. Based on PK, PD and toxicity data, 480mg BID (4/7) was determined as the RP2D of AZD5363. Early data from an expansion cohort at this dose in patients with breast or gynaecological tumours harbouring PIK3CA mutations demonstrated anti-tumour activity in approximately 50% of patients with PIK3CA mutations although the magnitude of response in most patients was not deemed sufficient to go forward with monotherapy in a PIK3CA mutant population. Preliminary expansion data at the RP2D in patients with AKT1 E17K mutations (without concomitant RAS/RAF mutations) has shown very promising anti-tumour activity. At the time of reporting, 13/17 (76%) ER+ve breast, 8/10 (80%) gynaecological and 10/12 (83%) other solid tumour patients demonstrated target lesion responses with 4 (24%), 2 (20%) and 3 (25%) partial responses (PRs) respectively (Hyman et al., 2015). A phase I trial of AZD5363 monotherapy in Japanese patients with solid tumours has also determined 480mg BID (4/7) to be the RP2D and demonstrated 2 PRs in patients with AKT1E17K mutations (Tamura et al., 2016). AKT1E17K is therefore a promising predictive biomarker for response to AZD5363 monotherapy across a range of tumour types and the full results of this trial are awaited with interest.
Ipatasertib (GDC-0068)
Optimisation of ATP-competitive AKT inhibitors using structure based design led to the discovery of ipatasertib (GDC-0068; Genentech), a potent and highly selective inhibitor of AKT1-3 that causes dose-dependent AKT signalling inhibition in cancer cells and xenograft tumour models (Blake et al., 2012; Lin et al., 2013). In pre-clinical models, markers of hyperactive AKT signalling correlated with sensitivity to ipatasertib, including high basal phospho-AKT levels, PTEN loss and PIK3CA mutations (Lin et al., 2013). Preliminary data from a phase I study established the MTD as 600mg QD of ipatasertib, with two DLTs of fatigue at higher dose levels and a low incidence of clinically significant hyperglycaemia (Yan et al., 2011). A dose- and time-dependent PD response was demonstrated through inhibition of pGSKβ in platelet rich plasma in doses above 200mg (Yan et al., 2011, 2013). As with AZD5363, the clinical focus for ipatasertib is now on combination studies (see below).
MK-2206
MK-2206 (Merck; MSD) is the most clinically advanced allosteric inhibitor of AKT, although other allosteric inhibitors of AKT include ARQ092 (ArQule; Lapierre et al., 2016), ARQ751 (ArQule; Yu et al., 2015) and BAY1125976 (Bayer; Politz et al., 2016; Table 2).
MK-2206 is a predominantly AKT1/2 inhibitor with reduced potency against AKT3 (Yan, 2009) and has been shown to have single agent activity against a range of cell lines harbouring RTK activation, PTEN loss/mutation and/or AKT2 amplification (Lu et al., 2009). A phase I trial of MK-2206 monotherapy in patients with solid tumours demonstrated a MTD of 60mg on alternate days with DLTs of skin rash and stomatitis (Yap et al., 2011). Drug related AEs included rash (51.5%), nausea (36.4%), pruritus (24.2%), hyperglycaemia (21.2%), and diarrhoea (21.2%). MK-2206 had a long half life of 40-100 hrs and plasma concentrations declined in a biphasic manner (Yap et al., 2011). On-target PD tumour effects were also demonstrated (decrease in AKT pSer473). Similarly to other AKT inhibitors, intermittent dosing schedules seem to be better tolerated and equally, if not more effective. Compared to the alternate day schedule, once weekly dosing of MK-2206 was equally as well tolerated up to a recommended phase II dose (RP2D) of 200mg with pulsatile inhibition of AKT demonstrated in tumour biopsies (Yap et al., 2014). Given the limited anti-tumour activity seen with MK-2206 monotherapy in phase II trials across a range of tumour types, attention has focused on combination trials (Table 2 and see below).
Perifosine
The first AKT inhibitor to enter clinical development was in fact an alkylphospholipid, perifosine (D-21266; KRX0401; Aeterna Zentaris). Alkylphospholipids accumulate in the plasma membrane and inhibit AKT activation by disrupting the interaction between AKT and the phospholipids (Kondapaka et al., 2003). As such, these compounds are relatively non-selective (Hideshima et al., 2006). In clinical studies, doses of oral perifosine are limited by gastro-intestinal (GI) toxicity when administered daily and several studies have tested different loading and maintenance schedules in order to achieve administration of biologically active doses without intolerable toxicity (Crul et al., 2002; Figg et al., 2014; Van Ummersen et al., 2004; Unger et al., 2010). Multiple Phase II trials have been conducted using a loading dose/maintenance dose schedule (Table 2) and of those reported, perifosine monotherapy has shown modest anti-tumour activity in soft tissue sarcomas and renal cell carcinomas (Bailey et al., 2006; Cho et al., 2012; Knowling et al., 2006).
Combination strategies with AKT inhibitors
Activation of the PI3K/AKT pathway has been implicated in resistance to a number of anti-cancer agents, providing a strong rationale for the clinical exploration of AKT inhibitor combinations.
Chemotherapy
AKT activation has been linked to both chemotherapy and radiation resistance. DNA-PK is one of the apical kinases involved in DNA double strand break (DSB) repair and is essential for classical non-homologous end-joining, the predominant repair pathway of DSBs in human cells (Jette and Lees-Miller, 2015). Following the generation of DNA DSBs, AKT is activated in a DNA-PK – dependent manner and promotes survival through anti-apoptotic mechanisms that are not well described (Bozulic et al., 2008; Brown et al., 2015; Wendel et al., 2004). In pre-clinical studies, AKT inhibition has been shown to hypersensitise cells to a number of chemotherapeutic agents (Davies et al., 2012; Hirai et al., 2010; VanderWeele et al., 2004).
Continuous low dose perifosine has been combined safely with a number of chemotherapies (Table 2). A randomised phase II trial of capecitabine +/- perifosine as second or third line treatment for metastatic colorectal cancer showed a significant OS benefit of the combination (17.7 v 7.6 months; P = .0052; Bendell et al. 2011), however these results were not validated in the phase III setting (Bendell et al., 2012). Interestingly, the presence of a PIK3CA mutation or PTEN loss did not predict for response to the capecitabine/perifosine combination which may in part be due to the high levels of KRAS mutations in colorectal cancers (Eng et al., 2014).
AZD5363 significantly enhances the activity of docetaxel in xenograft studies (Davies et al., 2012). Preliminary data from a phase I trial of AZD5363 in combination with docetaxel in patients with metastatic castrate resistant prostate cancer (mCRPC) has determined a RP2D of 320 mg BID 4 days on/3 days off in combination with full dose docetaxel (75mg/m2 q3w) and prednisolone (5mg BID D1-21; Crabb et al. 2016). DLTs were G3 rash and G3 diarrhoea. The randomised phase II trial is currently recruiting (ProCAID, NCT02121639). Early results from a phase Ib study determined a RP2D of 400mg ipatasertib QD days 1-21 q4w in combination with weekly paclitaxel 90mg/m2 with grade 3 AEs of diarrhoea, fatigue and hyperglycaemia at higher doses (Bendell et al., 2014). Promising anti-tumour activity was demonstrated in breast cancer patients with 6 PRs (22%) including 4 patients who had previously progressed on paclitaxel (Bendell et al., 2014). Similarly, phase I/II trials of AZD5363 in combination with weekly paclitaxel are on going in breast and gastric cancer populations with results awaited (Table 2).
Activation of AKT has been associated with platinum resistance in pre-clinical studies of ovarian cancer (Stronach et al., 2011). In a phase I/II study of afuresertib in combination with carboplatin (iv AUC5 q3w) and paclitaxel (iv 175mg/m2 q3w), the MTD of afuresertib was 150mg daily with DLTs of G3 rash, G2 febrile neutropenia and G2 lip swelling (Blagden et al., 2016). Promising anti-tumour activity of the combination was demonstrated and at the time of reporting, overall response rate (ORR) for platinum-resistant patients was 32.1% (95% CI: 15.9–52.4) and median progression free survival (PFS) was 7.1 months (95% CI: 6.3–9.0).
PARP Inhibitors
Pre-clinical studies have demonstrated synergy between PI3K inhibition and PARP inhibition in BRCA1/2 mutant mouse models (Juvekar et al., 2012). The mechanism of this synergy has not yet been fully explained. Published data suggests that PI3K inhibition impairs DSB repair by homologous recombination through reduced expression of BRCA1 and BRCA2 (Ibrahim et al., 2012). It is also likely however, that as with chemotherapy, the anti-apoptotic/pro-survival effects of PI3K inhibition are important in this setting. Preliminary data have shown promising anti-tumour activity of AZD5363 in combination with olaparib across a range of tumour types (Michalarea et al., 2016). At the time of reporting, out of 37 evaluable patients, there were 10 RECIST partial or complete responses, including 3 patients with BRCA wild-type tumours and 1 patient who had previously been treated with a PARP inhibitor. The recommended phase II schedules of this combination are 300mg BID olaparib + 400mg BID 4/7 AZD5363 or 300mg BID olaparib + 640mg BID 2/7 AZD5363. Common G1-2 toxicities were nausea, fatigue, anaemia, diarrhoea, anorexia, mucositis and vomiting on both schedules.
RTK/PI3K/AKT/mTOR Inhibitors
Given the multiple feedback loops within the PI3K-AKT signalling pathway, dual inhibition at different levels seems like a sensible strategy, although over-lapping toxicities limits dosing.
Pre-clinical studies have demonstrated synergy between EGFR and AKT inhibition in EGFR-wild type and mutant NSCLC cell lines (Puglisi et al., 2014) and a number of early phase studies of gefitinib or erlotinib in combination with MK-2206 have been completed (Table 2). The most common grade 3 AEs in a phase II trial of erlotinib 150mg orally OD with MK-2206 200mg weekly, in patients with erlotinib-resistant NSCLC, were rash, diarrhoea, fatigue and mucositis (Lara et al., 2015). Although partial response rates were low in both EGFR wild-type and -mutant tumours (9% and 3% respectively) a disease control rate of 43% in EGFR-wild type tumours met pre-specified levels of clinical activity to warrant further investigation. A phase I trial has also shown tolerability of gefitinib in combination with MK-2206 with a RP2D of 250mg gefitinib daily and 200mg MK-2206 weekly with early signs of clinical activity in EGFR resistant patients with NSCLC (Lin et al., 2014). A phase II trial exploring erlotinib in combination with MK-2206 as part of the BATTLE-2 program (NCT01248247) is on going.
PTEN loss and activating PIK3CA mutations have been associated with relative resistance to trastuzumab both in clinical and pre-clinical studies (Berns et al., 2007; Junttila et al., 2009; Nagata et al., 2004). Inhibition of HER2/ErbB2 with trastuzumab has been shown to activate PTEN and attenuate PI3K/AKT signalling (Junttila et al., 2009; Nagata et al., 2004). Trastuzumab-induced growth inhibition has also been shown to correlate with inhibition of AKT signalling in HER2 +ve cells and combined inhibition of HER2 and AKT signalling is synergistic (Junttila et al., 2009). MK-2206 has in particular, been shown to be well-tolerated in combination with trastuzumab and paclitaxel/trastuzumab in phase I trials, demonstrating promising anti-tumour activity in HER2+ and paclitaxel refractory tumours (Chien et al., 2016; Hudis et al., 2013).
Results of a phase I trial of the mTOR inhibitor ridaforolimus in combination with MK-2206 demonstrated an MTD of 10mg ridaforolimus OD for 5/7 and 90mg MK-2206 weekly with 1/17 patients experiencing a DLT (G3 rash) at this dose (Gupta et al., 2015). Other common AEs included skin rash, stomatitis, diarrhoea and anorexia. Promising anti-tumour activity was demonstrated in a number of heavily pre-treated breast cancer patients in an expansion cohort comprising patients with a low ‘RAS pathway signature score’, a 147-gene signature that has been shown to correlate with tumour dependence on RAS signalling and resistance to PI3K pathway inhibition (Loboda et al., 2010).
MAPK Pathway Inhibitors
There are now several lines of evidence showing that tumours harbouring activating mutations in both the PI3K/AKT and MAPK signalling networks, require inhibition of both to negatively affect tumour growth and survival, due to significant crosstalk and redundancy between the pathways (Davies et al., 2012; She et al., 2010). Doses of MK-2206 in combination with the MEK inhibitor selumetinib in a phase I trial of patients with solid tumours were limited predominantly by skin rash, diarrhoea and stomatitis (Tolcher et al., 2015a). The RP2D was defined as MK-2206 135mg weekly and selumetinib 100mg daily, with dose reductions of both drugs required due to overlapping toxicity. Significant tumour responses were seen in patients with KRAS-mutant NSCLC and low-grade ovarian cancer, but not in KRAS-mutant colorectal cancer, again suggesting differences in biology based on tumour context. Preliminary data from a phase II trial of MK-2206 in combination with selumetinib in patients with pre-treated KRAS mutant NSCLC has shown a disease control rate of 61% (Papadimitrakopoulou et al., 2014) and the full results of this study are awaited with interest.
Preliminary results from a phase Ib study of ipatasertib in combination with the MEK inhibitor cobimetinib has shown early evidence of anti-tumour activity, but tolerability has been challenging (Bendell et al., 2014). Afuresertib has also been tested in combination with a MEK inhibitor, trametinib with only intermittent dosing being tolerable (Tolcher et al., 2015b). Continuous dosing schedules of GSK214795 in combination with trametinib have been achieved (Algazi et al., 2015; Shoushtari et al., 2016) and a number of trials exploring GSK2141795 in combination with trametinib are ongoing (Table 2). Preclinical data has clearly demonstrated that sub-maximal inhibition of the combination of MEK and AKT does not significantly increase growth inhibition of BRAF or PIK3CA mutant cells, compared to maximally inhibiting MEK or AKT alone (Stewart et al., 2015). This has significant implications for the clinical development of such combinations and requires intelligent phase I trial design that tests optimal schedules as well as doses (Lopez and Banerji, 2016).
Hormonal Treatments
The development of castrate resistant prostate cancer (CRPC) is associated with reactivation of the androgen receptor axis as well as up-regulation of other pro-survival pathways including the PI3K pathway (Morgan et al., 2009). The PI3K/AKT pathway is activated in 30-50% of prostate cancers, most commonly through loss of PTEN, however inhibition of this pathway alone results in up-regulation of AR signalling leading to resistance (Carver et al., 2011). In pre-clinical studies, synergistic inhibition of AKT and androgen receptor signalling using either bicalutamide or enzalutamide results in sustained tumour growth inhibition and PSA stabilisation in mouse models of CRPC (Thomas et al., 2013; Toren et al., 2015). Preliminary data from a phase I/II study of ipatasertib in combination with abiraterone, has shown promising clinical benefit in patients with CRPC (De Bono et al., 2016). Patients with mCRPC, previously treated with docetaxel, were randomized 1:1:1 to three arms: ipatasertib 400mg, ipatasertib 200mg, or placebo, all in combination with abiraterone 1000 mg and prednisone 10 mg daily. Compared to placebo, ipatasertib 400mg showed increased radiological PFS (median 8.2 vs 6.4 m; HR = 0.75; p= 0.17) and increased OS for ipatasertib 400mg vs placebo (median 18.9m vs 15.6m; HR = 0.72; p= 0.22) (De Bono et al., 2016). Patients with PTEN loss had a superior PFS benefit (De Bono et al., 2016). Full results of this trial are awaited with interest. A randomised phase II trial of enzalutamide +/- AZD5363 in patients with mCRPC (RE-AKT, NCT02525068) is currently recruiting. Similarly, in ER +ve breast cancer, hormone resistance is also associated with an increased dependence on PI3K/AKT signalling (Miller et al., 2010) and treatment of breast cancer cell lines and xenografts with AZD5363 in combination with fulvestrant is superior to AZD5363 alone (Fox et al., 2013; Ribas et al., 2015). FAKTION (NCT01992952), a randomised phase II trial of fulvestrant +/- AZD5363 is ongoing.
9. Future directions and conclusions
AKT remains a central player in the PI3K-AKT signalling network and promising data from a number of phase II combination trials look set to pave the way for phase III studies and hopefully, clinical registration of some these compounds. Patient selection remains key, with AKT1E17K looking like a particularly strong biomarker predictive of response to AZD5363 monotherapy. A basket trial approach to clinical trial design is becoming increasingly popular and suits collections of low frequency, but high potency mutations such as AKT1E17K. Although potentially slow to complete, the clinical impact for patients harbouring such mutations should not deter licensing of compounds for such niche tumour populations.
The presence of AKT1E17K and other genetic aberrations (such as activating PIK3CA mutations and PTEN loss) needs to be put in molecular context however as co-activation of other pro-survival pathways is likely to require combination treatment strategies as demonstrated with AKT and MEK inhibition. Other potential biomarkers such as the RAS pathway signature score and proteomic signatures predictive of response to AKT inhibitors attempt to look beyond the PI3K/AKT pathway itself and should be explored further (Cheraghchi-Bashi et al., 2015; Loboda et al., 2010).
Interestingly, intermittent high doses of AKT inhibitors have been shown to be a more effective strategy both clinically and pre-clinically. High doses appear to be required for induction of apoptosis and intermittent schedules overcome the low therapeutic index of these compounds. This is particularly important in combination studies where toxicity is often limiting (Shimizu et al., 2012). Current preclinical data suggests intermittent dosing that inhibits AKT signalling to a greater extent is more efficacious than chronically inhibiting AKT to a lesser degree as a clinical strategy (Lopez and Banerji, 2016; Stewart et al., 2015). Interestingly due to feedback inhibition within the PI3K/AKT pathway, intermittent dosing may also delay treatment resistance. Despite the challenges, there remains intense enthusiasm in establishing AKT inhibitors within the future collection of personalised treatments for cancer and numerous clinical trials of AKT inhibitor combinations are actively recruiting.
The potential applications of AKT inhibitors are numerous. The scientific community has multiple drugs in the clinic with exciting potential to influence treatment paradigms in multiple cancers. Carefully thought out clinical trials with tolerable clinical schedules, appropriate combinations and patient selection biomarkers will be needed to maximally exploit the full potential of this class of agents.
Acknowledgements
The Drug Development Unit of the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research is supported in part by a programme grant from Cancer Research UK. Support is also provided by the Experimental Cancer Medicine Centre (programme grant) (to The ICR) and the National Institute for Health Research Biomedical Research Centre (jointly to the RMH NHS Foundation Trust and The ICR).
Abbreviations
- AE
adverse event
- CRPC
castrate resistant prostate cancer
- DLT
dose limiting toxicity
- DNA-PK
DNA-dependent protein kinase
- DSB
double strand break
- GSK3β
glycogen synthase kinase beta
- INPP4B
polyphosphate 4-phosphatase type II;
- MTD
maximum tolerated dose
- OS
overall survival
- PD
pharmacodynamics
- PDK1
3-phosphoinositide-dependent kinase-1
- PFS
progression free survival
- PH domain
plektrin homology domain
- PHLPP
PH domain and leucine rich repeat protein phosphatase 1
- PI3K
phosphatidylinositol 3-kinases
- PIP3
phosphatidylinositol-3,4,5-trisphosphate
- PK
pharmacokinetics
- PP2A
protein phosphatase 2A
- PR
partial response
- PTEN
phosphatase and tensin homolog
- RP2D
recommended phase II dose
- RTKs
receptor tyrosine kinases
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr Udai Banerji is employed by The Institute of Cancer Research. The Institute of Cancer Research was involved in the discovery of the AKT inhibitor AZD536 and has commercial interests related to the drug. The author is not named on the patent and does not receive payment as part of rewards to inventors scheme related to AZD5363.
Bibliography
- Abbadessa G, Eathiraj S, Meade J, Wick M, Tolcher A, Papadopoulos K, Saleh M, Sachdev J, Chai F, Schwartz B. Association of AKT1E17K and PIK3CAH1047R mutations with efficacy of ARQ 092 in vitro, in vivo and in patients. Molecular Cancer Therapeutics. 2015;14(12 Supplement 2):C134. doi: 10.1158/1535-7163.TARG-15-C134. [DOI] [Google Scholar]
- Ahn DH, Li J, Wei L, Doyle A, Marshall JL, Schaaf LJ, Phelps MA, Villalona-Calero MA, Bekaii-Saab T. Results of an abbreviated phase-II study with the Akt Inhibitor MK-2206 in Patients with Advanced Biliary Cancer. Sci Rep. 2015;5:12122. doi: 10.1038/srep12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Algazi AP, O’Brien K, Lencioni A, Tsai KK, Kadafour M, Chapman PB, Daud A. Phase II trial of trametinib in combination with the AKT inhibitor GSK 2141795 in BRAF wild-type melanoma. 2015 [Google Scholar]
- Arboleda M, Lyons J, Kabbinavar F, Bray M, Snow B, Ayala R, Danino M, Karlan B, Slamon D. Overexpression of AKT2/protein kinase B beta leads to up-regulation of beta 1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196–206. [PubMed] [Google Scholar]
- Arencibia JM, Pastor-Flores D, Bauer AF, Schulze JO, Biondi RM. AGC protein kinases: from structural mechanism of regulation to allosteric drug development for the treatment of human diseases. Biochim Biophys Acta. 2013;1834:1302–1321. doi: 10.1016/j.bbapap.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Argiris A, Cohen E, Karrison T, Esparaz B, Mauer A, Ansari R, Wong S, Lu Y, Pins M, Dancey J, et al. A phase II trial of perifosine, an oral alkylphospholipid, in recurrent or metastatic head and neck cancer. Cancer Biol Ther. 2006;5:766–770. doi: 10.4161/cbt.5.7.2874. [DOI] [PubMed] [Google Scholar]
- Azaro A, Rodon J, Calles A, Braña I, Hidalgo M, Lopez-Casas PP, Munoz M, Westwood P, Miller J, Moser BA, et al. A first-in-human phase I trial of LY2780301, a dual p70 S6 kinase and Akt Inhibitor, in patients with advanced or metastatic cancer. Invest New Drugs. 2015;33:710–719. doi: 10.1007/s10637-015-0241-7. [DOI] [PubMed] [Google Scholar]
- Bailey HH, Mahoney MR, Ettinger DS, Maples WJ, Fracasso PM, Traynor AM, Erlichman C, Okuno SH. Phase II study of daily oral perifosine in patients with advanced soft tissue sarcoma. Cancer. 2006;107:2462–2467. doi: 10.1002/cncr.22308. [DOI] [PubMed] [Google Scholar]
- Banerji U, Dean EJ, Perez-Fidalgo J, Batist G, Bedard P, You B, Westin S, Kabos P, Davies B, Elvin P, et al. A pharmacokinetically (PK) and pharmacodynamically (PD) driven phase I trial of the pan-AKT inhibitor AZD5363 with expansion cohorts in PIK3CA mutant breast and gynecological cancers. J Clin Oncol. 2015;33 2015 (suppl; abstr 2500) [Google Scholar]
- Bendell JC, Nemunaitis J, Vukelja SJ, Hagenstad C, Campos LT, Hermann RC, Sportelli P, Gardner L, Richards DA. Randomized Placebo-Controlled Phase II Trial of Perifosine Plus Capecitabine As Second- or Third-Line Therapy in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2011;29:4394–4400. doi: 10.1200/JCO.2011.36.1980. [DOI] [PubMed] [Google Scholar]
- Bendell JC, Ervin TJ, Senzer NN, Richards DA, Firdaus I, Lockhart C, Cohn AL, Saleh MN, Gardner LR, Sportelli P, et al. Results of the X-PECT study: A phase III randomized double-blind, placebo-controlled study of perifosine plus capecitabine (P-CAP) versus placebo plus capecitabine (CAP) in patients (pts) with refractory metastatic colorectal cancer (mCRC) J Clin Oncol. 2012;30 2012 (Suppl; Abstr LBA3501) [Google Scholar]
- Bendell JC, LoRusso P, Cho DC, Musib L, Yan Y, Chang I, Patel P, Chang IT, Meng RD, Shapiro GI. Clinical results of a phase Ib dose-escalation study of the Mek inhibitor cobimetinib (GDC-0973) and the Akt inhibitor ipatasertib (GDC-0068) in patients (pts) with solid tumors. Cancer Res. 2014;74(19 Suppl) Abstract Nr CT328. [Google Scholar]
- Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Biesecker LG. The multifaceted challenges of Proteus syndrome. JAMA. 2001;285:2240–2243. doi: 10.1001/jama.285.17.2240. [DOI] [PubMed] [Google Scholar]
- Blagden SP, Gabra H, Hamilton AL, Wong SS, Michael A, Mileshkin LR, Hall M, Goh J, Sergeevua L, DeSilvio M, et al. Phase I/II dose-escalation and expansion study of afuresertib + carboplatin and paclitaxel in recurrent ovarian cancer. J Clin Oncol. 2016;34 doi: 10.1158/1078-0432.CCR-18-2277. 2016 (suppl; abstr 2551) [DOI] [PubMed] [Google Scholar]
- Blake JF, Xu R, Bencsik JR, Xiao D, Kallan NC, Schlachter S, Mitchell IS, Spencer KL, Banka AL, Wallace EM, et al. Discovery and preclinical pharmacology of a selective ATP-competitive Akt inhibitor (GDC-0068) for the treatment of human tumors. J Med Chem. 2012;55:8110–8127. doi: 10.1021/jm301024w. [DOI] [PubMed] [Google Scholar]
- De Bono JS, Giorgi U De, Massard C, Bracarda S, Kocak I, Font A, Arija JAA, Shih KC, Radovoi GD, Yu W, et al. Randomized phase II study of AKT blockade with ipatasertib (GDC-0068) and abiraterone (Abi) vs. abi alone in patients with metastatic castration-resistant prostate cancer (mCRPC) after docetaxel chemotherapy (A. MARTIN Study) J Clin Oncol. 2016;34 2016 (suppl; abstr 5017) [Google Scholar]
- Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 Acts Downstream of DNA-PK in the DNA Double-Strand Break Response and Promotes Survival. Mol Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Brana I, Berger R, Golan T, Haluska P, Edenfield J, Fiorica J, Stephenson J, Martin LP, Westin S, Hanjani P, et al. A parallel-arm phase I trial of the humanised anti-IGF-1R antibody dalotuzumab in combination with the AKT inhibitor MK-2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK-0752, in patients with advanced solid tumours. Br J Cancer. 2014;111:1–13. doi: 10.1038/bjc.2014.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck D, Cron P, Hemmings BA. A human protein kinase Bgamma with regulatory phosphorylation sites in the activation loop and in the C-terminal hydrophobic domain. J Biol Chem. 1999;274:9133–9136. doi: 10.1074/jbc.274.14.9133. [DOI] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a Second Isoform, PHLPP2, Differentially Attenuate the Amplitude of Akt Signaling by Regulating Distinct Akt Isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Brown KK, Montaser-Kouhsari L, Beck AH, Correspondence AT, Toker A. MERIT40 Is an Akt Substrate that Promotes Resolution of DNA Damage Induced by Chemotherapy. Cell Rep. 2015;11:1–9. doi: 10.1016/j.celrep.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HA, Siu LL, Infante JR, Wheler JJ, Kurkjian C, Opalinska J, Smith DA, Antal JM, Gauvin JL, Gonzalez T, et al. Safety, pharmacokinetics (PK), pharmacodynamics (PD), clinical activity of the oral AKT inhibitor GSK2141795 (GSK795) in a phase I first-in-human study. J Clin Oncol. 2011;29 2011 (suppl; abstr 3003) [Google Scholar]
- Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, et al. Reciprocal Feedback Regulation of PI3K and Androgen Receptor Signaling in PTEN-Deficient Prostate Cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee KG, Longmate J, Quinn DI, Chatta G, Pinski J, Twardowski P, Pan C-X, Cambio A, Evans CP, Gandara DR, et al. The AKT Inhibitor Perifosine in Biochemically Recurrent Prostate Cancer: A Phase II California/Pittsburgh Cancer Consortium Trial. Clin Genitourin Cancer December. 2007;5:433–437. doi: 10.3816/CGC.2007.n.031. [Article] [DOI] [PubMed] [Google Scholar]
- Cheng JQ, Godwin aK, Bellacosa a, Taguchi T, Franke TF, Hamilton TC, Tsichlis PN, Testa JR. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U S A. 1992;89:9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheraghchi-Bashi A, Parker CA, Curry E, Salazar J-F, Gungor H, Saleem A, Cunnea P, Rama N, Salinas C, Mills GB, et al. A putative biomarker signature for clinically effective AKT inhibition: correlation of in vitro, in vivo and clinical data identifies the importance of modulation of the mTORC1 pathway. Oncotarget. 2015;6:41736–41749. doi: 10.18632/oncotarget.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew CL, Lunardi A, Gulluni F, Ruan DT, Chen M, Salmena L, Nishino M, Papa A, Ng C, Fung J, et al. In vivo role of INPP4B in tumor and metastasis suppression through regulation of PI3K/AKT signaling at endosomes. Cancer Discov. 2015:740–752. doi: 10.1158/2159-8290.CD-14-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Cockerill A, Fancourt C, Schmidt E, Moasser MM, Rugo HS, Melisko ME, Ko AH, Kelley RK, Korn WM, et al. A phase 1b study of the Akt-inhibitor MK-2206 in combination with weekly paclitaxel and trastuzumab in patients with advanced HER2-amplified solid tumor malignancies. Breast Cancer Res Treat. 2016;155:521–530. doi: 10.1007/s10549-016-3701-7. [DOI] [PubMed] [Google Scholar]
- Chin YR, Toker A. The Actin-Bundling Protein Palladin Is an Akt1-Specific Substrate that Regulates Breast Cancer Cell Migration. Mol Cell. 2010;38:333–344. doi: 10.1016/j.molcel.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YR, Yoshida T, Marusyk A, Beck AH, Polyak K, Toker A. Targeting Akt3 signaling in triple-negative breast cancer. Cancer Res. 2014a;74:964–973. doi: 10.1158/0008-5472.CAN-13-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YR, Yuan X, Balk SP, Toker A. PTEN-deficient tumors depend on AKT2 for maintenance and survival. Cancer Discov. 2014b;4:945–955. doi: 10.1158/2159-8290.CD-13-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DC, Hutson TE, Samlowski W, Sportelli P, Somer B, Richards P, Sosman JA, Puzanov I, Michaelson MD, Flaherty KT, et al. Two phase 2 trials of the novel Akt inhibitor perifosine in patients with advanced renal cell carcinoma after progression on vascular endothelial growth factor-targeted therapy. Cancer. 2012;118:6055–6062. doi: 10.1002/cncr.27668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung VM, McDonough SL, Philip PA, Cardin DB, Wang-Gilliam A, Hui L, Lowy AM, Guthrie K, Blanke CD, Hochster HS. SWOG S1115: Randomized phase II trial of selumetinib (AZD6244; ARRY 142886) hydrogen sulfate (NSC-748727) and MK-2206 (NSC-749607) vs. mFOLFOX in pretreated patients (Pts) with metastatic pancreatic cancer. J Clin Oncol. 2015;33 2015 (suppl; abstr 4119) [Google Scholar]
- Clark AR, Toker A. Signalling specificity in the Akt pathway in breast cancer. Biochem Soc Trans. 2014;42:1349–1355. doi: 10.1042/BST20140160. [DOI] [PubMed] [Google Scholar]
- Crabb SJ, Birtle AJ, Martin K, Downs N, Bowers M, Ratcliffe I, Ellis M, Griffiths G, Thompson S, Khoo V, et al. ProCAID: A phase I clinical trial to combine the AKT inhibitor AZD5363 with docetaxel and prednisolone (DP) chemotherapy for metastatic castration resistant prostate cancer (mCRPC) J Clin Oncol. 2016;34(suppl 2S) doi: 10.1007/s10637-017-0433-4. 2016 abstr 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouthamel MC, Kahana JA, Korenchuk S, Zhang SY, Sundaresan G, Eberwein DJ, Brown KK, Kumar R. Mechanism and management of AKT inhibitor-induced hyperglycemia. Clin Cancer Res. 2009;15:217–225. doi: 10.1158/1078-0432.CCR-08-1253. [DOI] [PubMed] [Google Scholar]
- Crul M, Rosing H, de Klerk GJ, Dubbelman R, Traiser M, Reichert S, Knebel NG, Schellens JHM, Beijnen JH, ten Bokkel Huinink WW. Phase I and pharmacological study of daily oral administration of perifosine (D-21266) in patients with advanced solid tumours. Eur J Cancer. 2002;38:1615–1621. doi: 10.1016/s0959-8049(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Dasari A, Overman MJ, Fogelman DR, Kee BK, Menter D, Raghav KPS, Morris VK, Oh J, Wu J, Jiang Z, et al. A phase II and co-clinical study of an AKT inhibitor in patients (pts) with biomarker-enriched, previously treated metastatic colorectal cancer (mCRC) J Clin Oncol. 2016;34 2016 (suppl; abstr 3563) [Google Scholar]
- Davies BR, Greenwood H, Dudley P, Crafter C, Yu D-H, Zhang J, Li J, Gao B, Ji Q, Maynard J, et al. Preclinical Pharmacology of AZD5363, an Inhibitor of AKT: Pharmacodynamics, Antitumor Activity, and Correlation of Monotherapy Activity with Genetic Background. Mol Cancer Ther. 2012;11:873–887. doi: 10.1158/1535-7163.MCT-11-0824-T. [DOI] [PubMed] [Google Scholar]
- Davies BR, Guan N, Logie A, Crafter C, Hanson L, Jacobs V, James N, Dudley P, Jacques K, Ladd B, et al. Tumors with AKT1E17K Mutations Are Rational Targets for Single Agent or Combination Therapy with AKT Inhibitors. Mol Cancer Ther. 2015;14:2441–2451. doi: 10.1158/1535-7163.MCT-15-0230. [DOI] [PubMed] [Google Scholar]
- Dean E, Banerji U, Schellens J, Krebs M, Jimenez B, van der Noll R, Foxley A, Yates J, Taylor N, Evens S, et al. Results of OAK: A phase 1, open-label, multicentre study to compare two dosage forms of AZD5363 and to explore the effect of food on the pharmacokinetic (PK) exposure, safety, and tolerability of AZD5363 in patients with advanced solid malignancies. J Clin Oncol. 2015;33 doi: 10.1007/s00280-018-3558-z. 2015(suppl; abstr 2577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon RL, Marcotte R, Hennessy BT, Woodgett JR, Mills GB, Muller WJ. Akt1 and Akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res. 2009;69:5057–5064. doi: 10.1158/0008-5472.CAN-08-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do K, Speranza G, Bishop R, Khin S, Rubinstein L, Kinders RJ, Datiles M, Eugeni M, Lam MH, Doyle LA, et al. Biomarker-driven phase 2 study of MK-2206 and selumetinib (AZD6244, ARRY-142886) in patients with colorectal cancer. Invest New Drugs. 2015;33:720–728. doi: 10.1007/s10637-015-0212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Tamura K, Tanabe Y, Yonemori K, Yoshino T, Fuse N, Kodaira M, Bando H, Noguchi K, Shimamoto T, et al. Phase 1 pharmacokinetic study of the oral pan-AKT inhibitor MK-2206 in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2015;76:409–416. doi: 10.1007/s00280-015-2810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumble M, Crouthamel MC, Zhang SY, Schaber M, Levy D, Robell K, Liu Q, Figueroa DJ, Minthorn EA, Seefeld MA, et al. Discovery of novel AKT inhibitors with enhanced anti-tumor effects in combination with the MEK inhibitor. PLoS One. 2014;9:1–11. doi: 10.1371/journal.pone.0100880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C, Bendell JC, Kopetz S, Tarco E, Xiao L, Wang X, Philips J, Sportelli P, Meric-Bernstam F. Impact of PI3K aberrations on efficacy of perifosine (P), x-PECT: A phase III randomized study of P plus capecitabine (PC) versus placebo plus capecitabine (C) in refractory metastatic colorectal cancer (mCRC) patients. J Clin Oncol. 2014;32(5s) 2014 (suppl; abstr 3606) [Google Scholar]
- Ernst DS, Eisenhauer E, Wainman N, Davis M, Lohmann R, Baetz T, Belanger K, Smylie M. Phase II study of perifosine in previously untreated patients with metastatic melanoma. Invest New Drugs. 2005;23:569–575. doi: 10.1007/s10637-005-1157-4. [DOI] [PubMed] [Google Scholar]
- Figg WD, Monga M, Headlee D, Shah A, Chau CH, Peer C, Messman R, Elsayed YA, Murgo AJ, Melillo G, et al. A phase I and pharmacokinetic study of oral perifosine with different loading schedules in patients with refractory neoplasms. Cancer Chemother Pharmacol. 2014;74:955–967. doi: 10.1007/s00280-014-2569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouladi M, Perentesis JP, Philips CL, Leary S, Reid JM, McGovern RM, Ingle AM, Ahern CH, Ames MM, Houghton P, et al. A Phase I Trial of MK-2206 in Children with Refractory Malignancies: A Children’s Oncology Group Study Maryam. Pediatr Blood Cancer. 2014;61:1246–1251. doi: 10.1002/pbc.25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EM, Kuba MG, Miller TW, Davies BR, Arteaga CL. Autocrine IGF-I/insulin receptor axis compensates for inhibition of AKT in ER-positive breast cancer cells with resistance to estrogen deprivation. Breast Cancer Res. 2013;15:R55. doi: 10.1186/bcr3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Hennessy BT, Ng CS, Ju Z, Coombes KR, Wolf JK, Sood AK, Levenback CF, Coleman RL, Kavanagh JJ, et al. Perifosine plus docetaxel in patients with platinum and taxane resistant or refractory high-grade epithelial ovarian cancer. Gynecol Oncol. 2012;126:47–53. doi: 10.1016/j.ygyno.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: A phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Garrett CR, Coppola D, Wenham RM, Cubitt CL, Neuger AM, Frost TJ, Lush RM, Sullivan DM, Cheng JQ, Sebti SM. Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a smallmolecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT. Invest New Drugs. 2011a;29:1381–1389. doi: 10.1007/s10637-010-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sánchez V, Chakrabarty A, Dave B, Cook RS, Pao W, McKinely E, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 2011b;108:5021–5026. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, Barretina J, Lin WM, Rameh L, Salmena L, et al. Evidence that Inositol Polyphosphate 4-Phosphatase Type II Is a Tumor Suppressor that Inhibits PI3K Signaling. Cancer Cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojo I, Perl A, Luger S, Baer MR, Norsworthy KJ, Bauer KS, Tidwell M, Fleckinger S, Carroll M, Sausville EA. Phase I study of UCN-01 and perifosine in patients with relapsed and refractory acute leukemias and high-risk myelodysplastic syndrome. Invest New Drugs. 2013;31:1217–1227. doi: 10.1007/s10637-013-9937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Krop I, Akcakanat A, Chen H, Liu S, Li Y, Culotta KS, Tarco E, Piha-Paul S, Moulder-Thompson S, et al. SU2C phase Ib study of paclitaxel and MK-2206 in advanced solid tumors and metastatic breast cancer. J Natl Cancer Inst. 2015;107:1–9. doi: 10.1093/jnci/dju493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti A, Carlo-Stella C, Locatelli SL, Malorni W, Mortarini R, Viviani S, Russo D, Marchianò A, Sorasio R, Dodero A, et al. Phase II study of perifosine and sorafenib dual-targeted therapy in patients with relapsed or refractory lymphoproliferative diseases. Clin Cancer Res. 2014;20:5641–5651. doi: 10.1158/1078-0432.CCR-14-0770. [DOI] [PubMed] [Google Scholar]
- Gupta S, Argilés G, Munster PN, Hollebecque A, Dajani O, Cheng JD, Wang R, Swift A, Tosolini A, Piha-Paul SA. A phase I trial of combined ridaforolimus and MK-2206 in patients with advanced malignancies. Clin Cancer Res. 2015;21:5235–5244. doi: 10.1158/1078-0432.CCR-15-0180. [DOI] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT - A major therapeutic target. Biochim Biophys Acta-Proteins Proteomics. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Héron-Milhavet L, Franckhauser C, Rana V, Berthenet C, Fisher D, Hemmings Ba, Fernandez A, Lamb NJC. Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Mol Cell Biol. 2006;26:8267–8280. doi: 10.1128/MCB.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, Podar K, Munshi NC, Chauhan D, Richardson PG, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- Ho AL, Foster NR, Meyers JP, Vasudeva SD, Katabi N, Antonescu CR, Pfister DG, Horvath LE, Erlichman C, Schwartz GK. Alliance A091104: A phase II trial of MK-2206 in patients (pts) with progressive, recurrent/metastatic adenoid cystic carcinoma. J Clin Oncol. 2015;33 2015 (suppl; abstr 6039) [Google Scholar]
- Hollander MC, Blumenthal GM, Dennis Pa. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudis C, Swanton C, Janjigian YY, Lee R, Sutherland S, Lehman R, Chandarlapaty S, Hamilton N, Gajria D, Knowles J, et al. A phase 1 study evaluating the combination of an allosteric AKT inhibitor (MK-2206) and trastuzumab in patients with HER2-positive solid tumors. Breast Cancer Res. 2013;15:R110. doi: 10.1186/bcr3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt-1 ( PKB- α ) Can Accelerate ErbB-2-Mediated Mammary Tumorigenesis but Suppresses Tumor Invasion. Cancer Research. 2004;64:3171–3178. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- Hyman DM, Smyth L, Bedard PL, Oza A, Dean E, Armstrong A, Lima J, Bando H, Kabos P, Perez-Fidalgo JA, et al. Abstract B109: AZD5363, a catalytic pan-Akt inhibitor, in Akt1 E17K mutation positive advanced solid tumors. Mol Cancer Ther. 2015;14:B109–B109. [Google Scholar]
- Ibrahim YH, García-garcía C, Serra V, He L, Torres-lockhart K, Prat A, Anton P, Cozar P, Guzmán M, Grueso J, et al. PI3K Inhibition Impairs and Sensitizes Triple-Negative Breast Cancer to PARP Inhibition. Cancer Discov. 2012;2:1036–1048. doi: 10.1158/2159-8290.CD-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Polytarchou C, Hatziapostolou M, Kottakis F, Maroulakou IG, Struhl K, Tsichlis PN. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2:ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakoff SJ, Infante JR, Juric D, Chan WY, Jia S, Musib L, Zhu J, Meng R, Patel PH, Bendell J. Phase Ib dose-escalation study of the Akt inhibitor ipatasertib (Ipat) with paclitaxel (P) in patients (pts) with advanced solid tumors. Annals of Oncology. 2014;25(suppl_4):iv146–iv164. doi: 10.1093/annonc/mdu331. [DOI] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 Maintains rictor-mTOR Complex Integrity and Regulates Akt Phosphorylation and Substrate Specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Jakubowiak AJ, Richardson PG, Zimmerman T, Alsina M, Kaufman JL, Kandarpa M, Kraftson S, Ross CW, Harvey C, Hideshima T, et al. Perifosine plus lenalidomide and dexamethasone in relapsed and relapsed/refractory multiple myeloma: A Phase I Multiple Myeloma Research Consortium study. Br J Haematol. 2012;158:472–480. doi: 10.1111/j.1365-2141.2012.09173.x. [DOI] [PubMed] [Google Scholar]
- Jette N, Lees-Miller SP. The DNA-dependent protein kinase: A multifunctional protein kinase with roles in DNA double strand break repair and mitosis. Prog Biophys Mol Biol. 2015;117:194–205. doi: 10.1016/j.pbiomolbio.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings Ba. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci U S A. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-Independent HER2/HER3/PI3K Complex Is Disrupted by Trastuzumab and Is Effectively Inhibited by the PI3K Inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Juvekar A, Burga LN, Hu H. Combining a PI3K Inhibitor with a PARP Inhibitor Provides an Effective Therapy for BRCA1-Related Breast Cancer. Cancer Discov. 2012;2:1048–1063. doi: 10.1158/2159-8290.CD-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowling M, Blackstein M, Tozer R, Bramwell V, Dancey J, Dore N, Matthews S, Eisenhauer E. A phase II study of perifosine (D-21226) in patients with previously untreated metastatic or locally advanced soft tissue sarcoma: A National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2006;24:435–439. doi: 10.1007/s10637-006-6406-7. [DOI] [PubMed] [Google Scholar]
- Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- Konopleva MY, Walter RB, Faderl SH, Jabbour EJ, Zeng Z, Borthakur G, Huang X, Kadia TM, Ruvolo PP, Feliu JB, et al. Preclinical and early clinical evaluation of the oral AKT inhibitor, MK-2206, for the treatment of acute myelogenous leukemia. Clin Cancer Res. 2014;20:2226–2235. doi: 10.1158/1078-0432.CCR-13-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinopoulos P, Makker V, Barry WT, Liu J, Horowitz NS, Birrer MJ, Doyle A, Berlin ST, Whalen C, Van Hummelen P, et al. Phase II, single stage, cohort expansion study of MK-2206 in recurrent endometrial serous cancer. J Clin Oncol. 2014;32:5s. 2014 (suppl; abstr 5515) [Google Scholar]
- Landgraf KE, Pilling C, Falke JJ. Molecular mechanism of an oncogenic mutation that alters membrane targeting: Glu17Lys modifies the PIP lipid specificity of the AKT1 PH domain. Biochemistry. 2008;47:12260–12269. doi: 10.1021/bi801683k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre J-M, Eathiraj S, Vensel D, Liu Y, Bull CO, Cornell-Kennon S, Iimura S, Kelleher EW, Kizer DE, Koerner S, et al. Discovery of 3-(3-(4-(1-Aminocyclobutyl)phenyl)-5-phenyl-3 H -imidazo[4,5- b ]pyridin-2-yl)pyridin-2-amine (ARQ 092)An Orally Bioavailable, Selective, and Potent Allosteric AKT Inhibitor. J Med Chem. 2016;59:6455–6469. doi: 10.1021/acs.jmedchem.6b00619. [DOI] [PubMed] [Google Scholar]
- Lara PN, Longmate J, Mack PC, Kelly K, Socinski MA, Salgia R, Gitlitz B, Li T, Koczywas M, Reckamp KL, et al. Phase II study of the AKT inhibitor MK-2206 plus erlotinib in patients with advanced non-small cell lung cancer who previously progressed on erlotinib. Clin Cancer Res. 2015;21:4321–4326. doi: 10.1158/1078-0432.CCR-14-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighl NB, Dent S, Clemons M, Vandenberg TA, Tozer R, Warr DG, Crump RM, Hedley D, Pond GR, Dancey JE, et al. A Phase 2 study of perifosine in advanced or metastatic breast cancer. Breast Cancer Res Treat. 2008;108:87–92. doi: 10.1007/s10549-007-9584-x. [DOI] [PubMed] [Google Scholar]
- Li X, Liu J, Gao T. beta-TrCP-mediated ubiquitination and degradation of PHLPP1 are negatively regulated by Akt. Mol Cell Biol. 2009;29:6192–6205. doi: 10.1128/MCB.00681-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19–42. [PMC free article] [PubMed] [Google Scholar]
- Lin C-C, Yu C-J, Ho C-C, Chen K-Y, Shih J-Y, Lin Z-Z, Lin Y, Liao W, Tsai S-H, Yan L, et al. A phase I dose-defining study for MK-2206 combined with gefitinib in NSCLC population enriched with EGFR mutation. J Clin Oncol. 2014;32 2014 (suppl; abstr e19013) [Google Scholar]
- Lin J, Sampath D, Nannini MA, Lee BB, Degtyarev M, Oeh J, Savage H, Guan Z, Hong R, Kassees R, et al. Targeting activated Akt with GDC-0068, a novel selective Akt inhibitor that is efficacious in multiple tumor models. Clin Cancer Res. 2013;19:1760–1772. doi: 10.1158/1078-0432.CCR-12-3072. [DOI] [PubMed] [Google Scholar]
- Lindhurst MJ, Yourick MR, Yu Y, Savage RE, Ferrari D, Biesecker LG. Repression of AKT signaling by ARQ 092 in cells and tissues from patients with Proteus syndrome. Sci Rep. 2015;5:17162. doi: 10.1038/srep17162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Stevens PD, Gao T. mTOR-dependent regulation of PHLPP expression controls the rapamycin sensitivity in cancer cells. J Biol Chem. 2011;286:6510–6520. doi: 10.1074/jbc.M110.183087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loboda A, Nebozhyn M, Klinghoffer R, Frazier J, Chastain M, Arthur W, Roberts B, Zhang T, Chenard M, Haines B, et al. A gene expression signature of RAS pathway dependence predicts response to PI3K and RAS pathway inhibitors and expands the population of RAS pathway activated tumors. BMC Med Genomics. 2010;3:26. doi: 10.1186/1755-8794-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JS, Banerji U. Combine and conquer: challenges for targeted therapy combinations in early phase trials. Nat Rev Clin Oncol. 2016:1–10. doi: 10.1038/nrclinonc.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Defeo-Jones D, Davis L, Hang G, Tammam J, Hatch H, Lutterbach B, Ware C, Haskell K, Drakas B, et al. Abstract #3714: In vitro and in vivo antitumor activities of MK-2206, a new allosteric Akt inhibitor. Cancer Res. 2009;69:3714–3714. [Google Scholar]
- Ma BBY, Goh BC, Lim WT, Hui EP, Tan EH, Lopes G, de L, Lo KW, Li L, Loong H, Foster NR, et al. Multicenter phase II study of the AKT inhibitor MK-2206 in recurrent or metastatic nasopharyngeal carcinoma from patients in the mayo phase II consortium and the cancer therapeutics research group (MC1079) Invest New Drugs. 2015;33:985–991. doi: 10.1007/s10637-015-0264-0. [DOI] [PubMed] [Google Scholar]
- Ma CX, Sanchez C, Gao F, Crowder R, Naughton M, Pluard T, Creekmore A, Guo Z, Hoog J, Lockhart AC, et al. A Phase I Study of the AKT Inhibitor MK-2206 in Combination with Hormonal Therapy in Postmenopausal Women with Estrogen Receptor Positive Metastatic Breast Cancer. Clin Cancer Res. 2016;22:2650–2658. doi: 10.1158/1078-0432.CCR-15-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD. Balancing Akt with S6K: Implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/Neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67:167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- Marsh Rdew, Rocha Lima CM, Levy DE, Mitchell EP, Rowland KM, Benson AB. A phase II trial of perifosine in locally advanced, unresectable, or metastatic pancreatic adenocarcinoma. Am J Clin Oncol. 2007;30:26–31. doi: 10.1097/01.coc.0000251235.46149.43. [DOI] [PubMed] [Google Scholar]
- Michalarea V, Roda D, Drew Y, Carreira S, O’Carrigan B, Shaw H, Roux R, Kumar S, Ward S, Parmar M, et al. Phase I trial combining the PARP inhibitor olaparib (Ola) and AKT inhibitor AZD5363 (AZD) in germline (g)BRCA and non- BRCA mutant (m) advanced cancer patients (pts) incorporating noninvasive monitoring of cancer mutations. AACR; Cancer Res. 2016 2016 Abstract Nr CT010. [Google Scholar]
- Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, Higham C, Garcia-Echeverria C, Shyr Y, Arteaga CL. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis SZ, Ikeda S, Reddy S, Gatalica Z, Kurzrock R. Landscape of Phosphatidylinositol-3-Kinase Pathway Alterations Across 19 784 Diverse Solid Tumors. JAMA Oncol. 2016;2:489–501. doi: 10.1001/jamaoncol.2016.0891. [DOI] [PubMed] [Google Scholar]
- Molife LR, Yan L, Vitfell-Rasmussen J, Zernhelt AM, Sullivan DM, Cassier PA, Chen E, Biondo A, Tetteh E, Siu L, et al. Phase 1 trial of the oral AKT inhibitor MK-2206 plus carboplatin/ paclitaxel, docetaxel, or erlotinib in patients with advanced solid tumors. J Hematol Oncol. 2014;7 doi: 10.1186/1756-8722-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 2009;9:237–249. doi: 10.2174/156800909787580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Oki Y, Fanale M, Romaguera J, Fayad L, Fowler N, Copeland A, Samaniego F, Kwak LW, Neelapu S, Wang M, et al. Phase II study of an AKT inhibitor MK2206 in patients with relapsed or refractory lymphoma. Br J Haematol. 2015;171:463–470. doi: 10.1111/bjh.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitrakopoulou V, Lee JJ, Wistuba II, Tsao AS, Fossella FV, Heymach J, Kaihor N, Gupta S, Gettinger SN, Byers LA, et al. BATLLE-2: KRAS mutation and outcome in a biomarker-integrated study in previously treated patients (pts) with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2014;32:5s. doi: 10.1200/JCO.2015.66.0084. 2014 (suppl; abstr 8042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politz O, Siegel F, Bärfacker L, Bömer U, Hägebarth A, Scott WJ, Michels M, Ince S, Neuhaus R, Meyer K, et al. BAY 1125976, a selective allosteric AKT1/2 inhibitor, exhibits high efficacy on AKT signaling-dependent tumor growth in mouse models. Int J Cancer. 2016 doi: 10.1002/ijc.30457. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Posadas EM, Gulley J, Arlen PM, Trout A, Parnes HL, Wright J, Lee M-J, Chung EJ, Trepel JB, Sparreboom A, et al. A phase II study of perifosine in androgen independent prostate cancer. Cancer Biol Ther. 2005;4:1133–1137. doi: 10.4161/cbt.4.10.2064. [DOI] [PubMed] [Google Scholar]
- Puglisi M, Thavasu P, Stewart A, de Bono JS, O’Brien MER, Popat S, Bhosle J, Banerji U. AKT inhibition synergistically enhances growth-inhibitory effects of gefitinib and increases apoptosis in non-small cell lung cancer cell lines. Lung Cancer. 2014;85:141–146. doi: 10.1016/j.lungcan.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Ramanathan RK, McDonough SL, Kennecke HF, Iqbal S, Baranda JC, Seery TE, Lim HJ, Hezel AF, Vaccaro GM, Blanke CD. Phase 2 study of MK-2206, an allosteric inhibitor of AKT, as second-line therapy for advanced gastric and gastroesophageal junction cancer: A SWOG cooperative group trial (S1005) Cancer. 2015;121:2193–2197. doi: 10.1002/cncr.29363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas R, Pancholi S, Guest SK, Marangoni E, Gao Q, Thuleau A, Simigdala N, Polanska UM, Campbell H, Rani A, et al. AKT Antagonist AZD5363 Influences Estrogen Receptor Function in Endocrine-Resistant Breast Cancer and Synergizes with Fulvestrant (ICI182780) In Vivo. Mol Cancer Ther. 2015:2035–2049. doi: 10.1158/1535-7163.MCT-15-0143. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Wolf J, Jakubowiak A, Zonder J, Lonial S, Irwin D, Densmore J, Krishnan A, Raje N, Bar M, et al. Perifosine plus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma previously treated with bortezomib: Results of a multicenter phase I/II trial. J Clin Oncol. 2011;29:4243–4249. doi: 10.1200/JCO.2010.33.9788. [DOI] [PubMed] [Google Scholar]
- Risso G, Blaustein M, Pozzi B, Mammi P, Srebrow A. Akt/PKB: one kinase, many modifications. Biochem J. 2015;468:203–214. doi: 10.1042/BJ20150041. [DOI] [PubMed] [Google Scholar]
- Sanidas I, Polytarchou C, Hatziapostolou M, Ezell SA, Kottakis F, Hu L, Guo A, Xie J, Comb MJ, Iliopoulos D, et al. Phosphoproteomics Screen Reveals Akt Isoform-Specific Signals Linking RNA Processing to Lung Cancer. Mol Cell. 2014;53:577–590. doi: 10.1016/j.molcel.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin Da, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Woodgett JR. Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett. 2003;546:108–112. doi: 10.1016/s0014-5793(03)00562-3. [DOI] [PubMed] [Google Scholar]
- She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, Solit DB, Rosen N. 4E-BP1 Is a Key Effector of the Oncogenic Activation of the AKT and ERK Signaling Pathways that Integrates Their Function in Tumors. Cancer Cell. 2010;18:39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, Smith LS, Gunn S, Smetzer L, Mays TA, Kaiser B, et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin Cancer Res. 2012;18:2316–2325. doi: 10.1158/1078-0432.CCR-11-2381. [DOI] [PubMed] [Google Scholar]
- Shoushtari AN, Kudchadkar RR, Panageas K, Murthy RK, Jung M, Shah R, O’Donnell B, Khawaja YS, Prempeh-Keteku NA, Ambrosini G, et al. A randomized phase 2 study of trametinib with or without GSK2141795 in patients with advanced uveal melanoma. J Clin Oncol. 2016;34 2016 (suppl; abstr 9511) [Google Scholar]
- Spencer A, Yoon SS, Harrison SJ, Morris SR, Smith DA, Brigandi RA, Gauvin J, Kumar R, Opalinska JB, Chen C. The novel AKT inhibitor afuresertib shows favorable safety, pharmacokinetics, and clinical activity in multiple myeloma. Blood. 2014;124:2190–2195. doi: 10.1182/blood-2014-03-559963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Thavasu P, De Bono JS, Banerji U. Titration of signalling output: Insights into clinical combinations of MEK and AKT inhibitors. Ann Oncol. 2015;26:1504–1510. doi: 10.1093/annonc/mdv188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronach EA, Chen M, Maginn EN, Agarwal R, Mills GB, Wasan H, Gabra H. DNA-PK mediates AKT activation and apoptosis inhibition in clinically acquired platinum resistance. Neoplasia. 2011;13:1069–1080. doi: 10.1593/neo.111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Hashimoto J, Tanabe Y, Kodaira M, Yonemori K, Seto T, Hirai F, Arita S, Toyokawa G, Chen L, et al. Safety and tolerability of AZD5363 in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016;77:787–795. doi: 10.1007/s00280-016-2987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Lamoureux F, Crafter C, Davies BR, Beraldi E, Fazli L, Kim S, Thaper D, Gleave ME, Zoubeidi A. Synergistic targeting of PI3K/AKT pathway and androgen receptor axis significantly delays castration-resistant prostate cancer progression in vivo. Mol Cancer Ther. 2013;12:2342–2355. doi: 10.1158/1535-7163.MCT-13-0032. [DOI] [PubMed] [Google Scholar]
- Toker A, Marmiroli S. Signaling specificity in the Akt pathway in biology and disease. Adv Biol Regul. 2014;55:28–38. doi: 10.1016/j.jbior.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolcher A, Harb W, Sachdev J, Papadopoulos K, Bordoni R, Chai F, Larmar M, Savage R, Abbadessa G, Saleh M. 338 Results from a phase 1 study of ARQ 092, a novel pan AKT-inhibitor, in subjects with advanced solid tumors or recurrent malignant lymphoma. Eur J Cancer. 2016;51:S66. [Google Scholar]
- Tolcher AW, Khan K, Ong M, Banerji U, Papadimitrakopoulou V, Gandara DR, Patnaik A, Baird RD, Olmos D, Garrett CR, et al. Antitumor activity in ras-driven tumors by blocking akt and mek. Clin Cancer Res. 2015a;21:739–748. doi: 10.1158/1078-0432.CCR-14-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolcher AW, Patnaik A, Papadopoulos KP, Rasco DW, Becerra CR, Allred AJ, Orford K, Aktan G, Ferron-Brady G, Ibrahim N, et al. Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma. Cancer Chemother Pharmacol. 2015b;75:183–189. doi: 10.1007/s00280-014-2615-5. [DOI] [PubMed] [Google Scholar]
- Toren P, Kim S, Cordonnier T, Crafter C, Davies BR, Fazli L, Gleave ME, Zoubeidi A. Combination AZD5363 with enzalutamide significantly delays enzalutamide-resistant prostate cancer in preclinical models. Eur Urol. 2015;67:986–990. doi: 10.1016/j.eururo.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Tripathy D, Chien AJ, Hylton N, Buxton MB, Ewing CA, Wallace AM, Forero A, Kaplan HG, Nanda R, Albain KS, et al. Adaptively randomized trial of neoadjuvant chemotherapy with or without the Akt inhibitor MK-2206: Graduation results from the I-SPY 2 Trial. J Clin Oncol. 2015;33 2015 (suppl; abstr 524) [Google Scholar]
- Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- Van Ummersen L, Binger K, Volkman J, Marnocha R, Tutsch K, Kolesar J, Arzoomanian R, Alberti D, Wilding G. A phase I trial of perifosine (NSC 639966) on a loading dose/maintenance dose schedule in patients with advanced cancer. Clin Cancer Res. 2004;10:7450–7456. doi: 10.1158/1078-0432.CCR-03-0406. [DOI] [PubMed] [Google Scholar]
- Unger C, Berdel W, Hanauske AR, Sindermann H, Engel J, Mross K. First-time-in-man and pharmacokinetic study of weekly oral perifosine in patients with solid tumours. Eur J Cancer. 2010;46:920–925. doi: 10.1016/j.ejca.2009.12.028. [DOI] [PubMed] [Google Scholar]
- VanderWeele DJ, Zhou R, Rudin CM. Akt up-regulation increases resistance to microtubule-directed chemotherapeutic agents through mammalian target of rapamycin. Mol Cancer Ther. 2004;3:1605–1613. [PubMed] [Google Scholar]
- Vincent EE, Elder DJE, Thomas EC, Phillips L, Morgan C, Pawade J, Sohail M, May MT, Hetzel MR, Tavaré JM. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br J Cancer. 2011;104:1755–1761. doi: 10.1038/bjc.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink SR, Schellens JHM, Beijnen JH, Sindermann H, Engel J, Dubbelman R, Moppi G, Hillebrand MJX, Bartelink H, Verheij M. Phase I and pharmacokinetic study of combined treatment with perifosine and radiation in patients with advanced solid tumours. Radiother Oncol. 2006;80:207–213. doi: 10.1016/j.radonc.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- Wisinski KB, Tevaarwerk AJ, Burkard ME, Rampurwala M, Eickhoff J, Bell MC, Kolesar JM, Flynn C, Liu G. Phase I Study of an AKT Inhibitor (MK-2206) Combined with Lapatinib in Adult Solid Tumors Followed by Dose Expansion in Advanced HER2+ Breast Cancer. Clin Cancer Res. 2016;22:2659–2667. doi: 10.1158/1078-0432.CCR-15-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WI, Voegtli WC, Sturgis HL, Dizon FP, Vigers GPA, Brandhuber BJ. Crystal structure of human AKT1 with an allosteric inhibitor reveals a new mode of kinase inhibition. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L. Abstract #DDT01-1: MK-2206: A potent oral allosteric AKT inhibitor. Cancer Res. 2009;69:DDT01–1. [Google Scholar]
- Yan Y, Wagle MC, Punnoose E, L M, Budha N, Nannini M, Lin K, Liederer BM, Murli S, Ramakrishnan V, et al. A first-in-human trial of GDC-0068: A novel, oral, ATP-competitive Akt inhibitor, demonstrates robust suppression of the Akt pathway in surrogate and tumor tissues. AACR; Mol Cancer Ther. 2011;10(11 Suppl) 2011; Abstract Nr B154. [Google Scholar]
- Yan Y, Serra V, Prudkin L, Scaltriti M, Murli S, Rodríguez O, Guzman M, Sampath D, Nannini M, Xiao Y, et al. Evaluation and clinical analyses of downstream targets of the akt inhibitor GDC-0068. Clin Cancer Res. 2013;19:6976–6986. doi: 10.1158/1078-0432.CCR-13-0978. [DOI] [PubMed] [Google Scholar]
- Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, Baird RD, Delgado L, Taylor A, Lupinacci L, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- Yap TA, Yan L, Patnaik A, Tunariu N, Biondo A, Fearen I, Papadopoulos KP, Olmos D, Baird R, Delgado L, et al. Interrogating two schedules of the AKT inhibitor MK-2206 in patients with advanced solid tumors incorporating novel pharmacodynamic and functional imaging biomarkers. Clin Cancer Res. 2014;20:5672–5685. doi: 10.1158/1078-0432.CCR-14-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KH, Axtmayer J, Gustin JP, Rajpurohit A, Lauring J. Functional analysis of non-hotspot AKT1 mutants found in human breast cancers identifies novel driver mutations: implications for personalized medicine. Oncotarget. 2013;4:29–34. doi: 10.18632/oncotarget.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20:539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Yu Y, Savage RE, Eathiraj S, Meade J, Wick MJ, Hall T, Abbadessa G, Schwartz B. Targeting AKT1-E17K and the PI3K/AKT pathway with an allosteric AKT inhibitor, ARQ 092. PLoS One. 2015;10:1–26. doi: 10.1371/journal.pone.0140479. [DOI] [PMC free article] [PubMed] [Google Scholar]