Abstract
Important contributions to evolutionary developmental biology have been made using the comparative organismal approach. As examples I describe insights obtained from studies of Molgula ascidians and Astyanax cavefish.
Keywords: comparative organismal approach, evolutionary developmental biology, ascidians, Molgula, cavefish, Astyanax
1. INTRODUCTION
During the past several decades, considerable progress has been made in understanding the processes and genes underlying the evolution of development using the comparative organismal approach. In this approach organisms are compared to explore the molecular, cellular, and morphological basis for similarities and differences that have evolved between them. One version of the comparative organismal approach employs distantly related species to understand the extent of conservation in developmental processes. This version revealed a common set of conserved genes and signaling pathways controlling animal development (Scott, 1994). In a few cases, genes from one species have been shown to control the development of similar phenotypes after their expression in a distantly related species. The most famous case is the formation of ectopic eyes on Drosophila legs after mis-expression of the squid, ascidian, frog, or mouse pax6 genes (Gehring and Ikeo, 1999). The discovery of conserved genes and mechanisms unified developmental biology and provided a background for most of the contemporary research in evolutionary developmental biology.
A second version of the comparative organismal approach employs closely related species with different modes of development or the same species exhibiting different morphs to probe the developmental basis of evolutionary divergence. The second version targets the heart of evolutionary developmental biology and is especially compelling when the species or morphs can be hybridized, permitting the use of genetic techniques to study the evolution of development. This is the case for the model systems highlighted in this essay: (1) closely related species of molgulid ascidians with different modes of development (Fig. 1 A-E) and (2) conspecific surface- and cave-dwelling morphs of the teleost Astyanax mexicanus (Fig. 1 F-K). Using these models as examples, I discuss how they have revealed some of the key processes underlying the evolution of developmental diversity.
Figure 1.
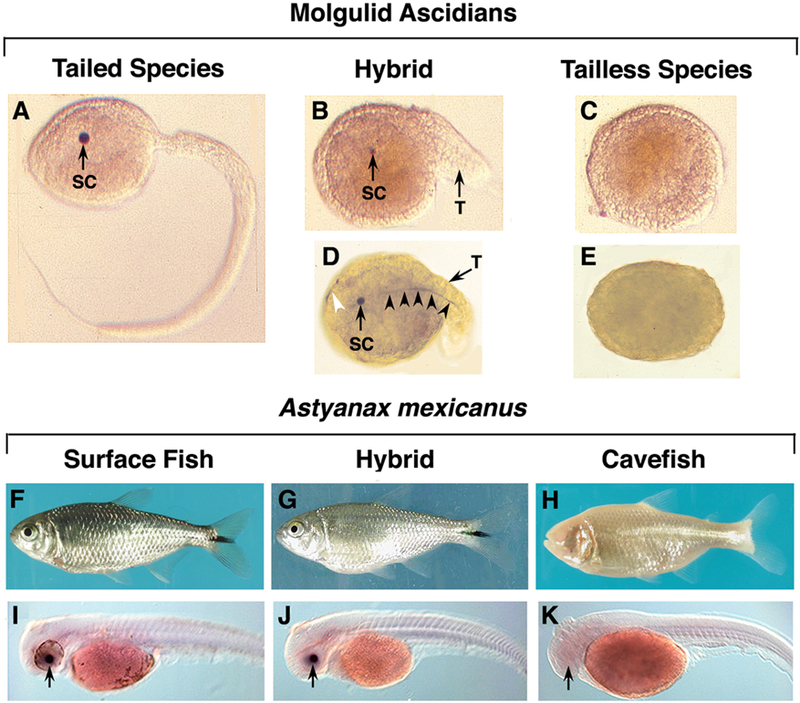
The Molgulid ascidian and Astyanax mexicanus models. Top. Molgulid ascidians. A. Tailed urodele larva of Molgula oculata (the tailed species). B. Short tailed urodele larva of a cross between Molgula occulta (the tailless species) eggs and Molgula oculata (the tailed species) sperm. C. Tailless anural larva of the Molgula occulta (the tailless species). D, E. Alpha acetylated-tubulin antibody staining showing the restoration of anterior and tail nerves, which are absent in the tailless species larva (E), in a tailless x tailed species hybrid (D). SC: brain sensory cells. White arrowhead: part of a restored anterior nerve. Black arrowheads: restored tail nerve. [A-B. From Swalla and Jeffery, 1996]. [C, D. Y. Yamamoto and W. R. Jeffery, unpublished]. Bottom. A. mexicanus. F. Adult surface fish with eyes and pigment. G. Adult surface fish x cavefish hybrid with eyes and pigment. H. Adult cavefish lacking eyes and pigment. I. Larval surface fish showing alphaA-crystallin gene expression in the lens. J. Larval surface fish x cavefish hybrid showing alphaA-crystallin gene expression in the lens. K. Larval cavefish lacking alphaA-crystallin gene expression in the lens. Arrows: lens. [I-K. From Ma et al., 2014].
2. MOLGULID ASCIDIANS: EVOLVING DIFFERENT MODES OF DEVELOPMENT
Ascidians are tunicates: invertebrate chordates inferred to be the closest living relatives of vertebrates (Bourlat et al., 2006; Delsuc et al., 2006). They have two stages in their life cycle, a non-feeding larval stage, the product of embryonic development, and an adult stage, produced by modification and reorganization of the larva during metamorphosis. With the exception of branchial gill slits, adult ascidians have no resemblances to vertebrates; their sac-like body is specialized for filter feeding and producing gametes. However, the motile larval stage, a tadpole-like organism consisting of a trunk (or head) and tail, has definitive chordate features: a dorsal central nervous system, a notochord of exactly 40 cells, and a post-anal tail equipped with striated muscle cells (reviewed by Satoh, 1994).
The majority of ascidian species form tadpole-like swimming larvae prior to metamorphosis, which is called urodele (tailed) development (Fig. 1A). In contrast, a small subset of ascidian species has lost the tailed larva (Fig. 1C) (reviewed by Jeffery and Swalla, 1990). The tailless (or anural) ascidian species, which are mostly found in the Family Molgulidae, develop more or less directly into the adult. The anural developers lack a melanin pigmented sensory cell in their brain, an extended notochord, differentiated tail muscle cells, and nerves leading from the brain into the head and tail (Fig. 1 C, E), which are present in the tadpole larvae of all urodele developers. The anural mode of development has evolved multiple times from ancestors with conventional urodele development (Hadfield et al., 1995). The anural ascidian Molgula occulta is closely related to the urodele ascidian Molgula oculata. To avoid confusion due to the similarity in names, we will refer to them as the tailed (urodele M. oculata) and the tailless (anural M. occulta) species. The tailed and tailless species are co-distributed on sub-tidal sand flats along the northwest coast of Europe, where they may have evolved recently from a common urodele ancestor. They are indeed so closely related that unlike most other ascidians can produce hybrids when crossed (Fig. 1 B, D) (Swalla and Jeffery, 1991). Because of radically different modes of development and capacity for inter-specific hybridization the Molgula species are an excellent comparative model in evolutionary developmental biology.
Ascidian embryonic development is controlled by maternal factors localized in the egg and segregated into stereotypical cell lineages during cleavage, as well as zygotic processes, such as induction (reviewed by Satoh, 1994). Do maternal processes, zygotic processes, or both control the evolution of different modes of ascidian development? Hybridization between the two Molgula species has addressed this question (Swalla and Jeffery, 1991). Remarkably, when eggs of the tailless species are fertilized with sperm of the tailed species some of the lost urodele features are restored in the hybrid embryos (tailless x tailed species hybrids) (Fig. 1B, D). The restored urodele features include a melanin-containing brain sensory cell, a short tail containing an extended notochord, and nerves emanating from the brain into the head and tail (Fig. 1B-E). The rescue of urodele features in inter-specific hybrids shows that anural development is controlled in large part by changes in zygotic processes. However, maternal processes may also be involved, as evidenced by the inability of the tailed species genome to restore the entire program of terminal cell divisions in the notochord and muscle lineages (Swalla and Jeffery, 1991) and by changes in cytoplasmic localization in eggs of the tailless species (Swalla et al., 1991).
The Molgula model also illustrates the importance of heterochrony (relative changes in the timing of developmental events) and cell death (apoptosis) and in the evolution of development. In urodele developers, the notochord, tail muscle cells, and parts of the brain that function in larval development undergo apoptosis during metamorphosis. In the tailless species, however, the precursors of larval brain and tail tissues undergo apoptosis prematurely during the embryonic period (Jeffery, 2002; 2006). In tailless x tailed hybrids, the temporal relationship between apoptosis and metamorphosis typical of urodele developers is restored, suggesting that zygotic processes also regulate heterochrony between the two Molgula species (Jeffery, 2002). Some of the genes involved in anural development through effects on cell survival have been identified. The Manx (Swalla and Jeffery, 1996) and foxA5 (Olsen et al., 1999) genes are expressed in the brain, notochord, and muscle cells of the tailed species but are downregulated or show abbreviated expression respectively in the tailless species, and their urodele expression patterns are restored in tailless x tailed hybrids. Functional studies have shown that these genes protect the urodele-related brain and tail tissues from premature apoptosis (Jeffery, 2002). Many other zygotic genes are also likely to be involved in the urodele to anural developmental transition, which are currently being investigated by transcriptome and genomic analysis.
Does anural development involve changes at the beginning or the end of gene regulatory cascades? Ascidian tail muscle cell development is controlled by a chain of events beginning with Macho-1, a transcription factor encoded by localized maternal mRNA (reviewed by Nishida, 2010), and ending with the synthesis of muscle structural proteins in the tail muscle cell lineages. The ability of a tailed species muscle actin gene construct to be expressed in the undifferentiated muscle cells of the tailless species suggests that all upstream regulatory factors in the muscle pathway are functional in the tailless species (Kusakabe et al., 1996). Although there is no published information about macho-1 mRNA in the tailless species, maternal macho-1 transcripts are present and segregated to the muscle lineage in another anural ascidian species, Molgula tectiformis (Gyoja, 2006). Furthermore, Tbx6, a Macho-1 target gene, is expressed in the muscle lineage of the tailless species (Takada et al., 2002a). Accordingly, instead of changing upstream regulatory genes, their downstream targets in the muscle differentiation pathway are affected; the muscle actin genes of the tailless species contain stop codons rendering them non-functional (Kusakabe et al., 1996). The conversion of larval muscle actin genes into pseudogenes has also occurred in two other anural species Molgula bleizi and M. tectiformis (Jeffery et al., 1999; Gioja et al., 2007), suggesting that the muscle structural gene decay may be a common theme in anural development. Thus, the evolutionary change(s) responsible for the absence of muscle cell differentiation in anural developers is controlled by changes at the end of the gene cascade. A similar situation may occur for the notochord gene cascade, as the transcription factor Brachyury, the initiator of the ascidian notochord developmental pathway (Yasuo and Satoh, 1993), is also expressed normally in the notochord cell lineage of the tailless species (Takada et al., 2002b).
Anural development is relatively common in the Molgulidae but it is encountered only rarely in other ascidian families (reviewed by Jeffery and Swalla, 1990). The repeated alteration of muscle development in anural Molgula species via the decay of muscle structural genes may be relevant one of the reasons why molgluid ascidians are so readily subject to this evolutionary change. T. Kusakabe (2001) has proposed an intriguing hypothesis to explain this paradox based on low muscle actin gene copy number and pseudogene formation. Most urodele ascidians have many copies of larval muscle actin genes (Kusakabe et al., 1997), which are probably needed to produce large amounts of structural protein during rapid larval myogenesis. However, molgluid ascidians are proposed to have a lower number of redundant muscle actin genes, making muscle cell differentiation more susceptible to evolutionary change via pseudogene formation. Because there are specific sets of larval and adult muscle structural genes in ascidians, the decay of the larval genes in anural developers would not have adverse effects in adults, possibly “pre-adapting” molgluid ascidians for anural development.
3. CAVEFISH: EVOLUTION IN THE DARK
The teleost Astyanax mexicanus is a single species with an eyed and a pigmented surface dwelling morph (surface fish) and many different eyeless and de-pigmented cave dwelling (cavefish) morphs (Fig. 1F, H) (see reviews in Keene et al., 2015). The cavefish morphs spend their entire life cycle in perpetual darkness. As a consequence of relaxed selection over many generations, traits essential for survival in lighted habitats, such as eyes and pigmentation, have been reduced or lost (Fig. 1 F, H). Constructive differences have also evolved between the surface fish and cavefish morphs. For instance, cavefish show substantially modified craniofacial morphology, have larger jaws, increased numbers of taste buds, and specialized behaviors, such as an attraction to water vibrations (Yoshizawa et al., 2010). Different cavefish populations have evolved multiple times from surface fish in an extensive karst region in northeastern Mexico (Gross, 2013). The time required for the separation of cavefish from surface fish ancestors is estimated to be 5 million to less than a million years (Mitchell et al., 1977; Porter et al., 2007).
The Astyanax conspecific morphs can be hybridized to produce viable F1 progeny (Fig. 1 F-H). As in the tailed and tailless ascidian species, hybridization has shown that zygotic processes are changed in cavefish. For example, surface fish x cavefish F1 hybrids develop eyes and pigmentation (Wilkens, 1988) and express genes that are downregulated during cavefish development, such as lens alphaA-crystallin (Fig. 1 I-K) (Ma et al., 2014). Intercrossing between the progeny of surface fish and cavefish has allowed the cavefish phenotypes to be mapped by genetic analysis (Protas et al., 2008; O’Quin et al., 2013; Kowalko et al., 2013). The genetic mapping studies revealed multiple genes controlling most of the cavefish phenotypes. An exception is albinism, the loss of melanin pigmentation, which evolved by mutations in the oca2 gene (Protas et al., 2006). Deletions in the oca2 coding regions are responsible for albinism in independently evolved cavefish morphs, implying that this gene is a frequent target of evolutionary change. The sequenced cavefish genome (McGaugh et al., 2014) will allow further progress in identifying and characterizing the genes underlying gained and lost traits during cavefish evolution. Below I provide examples using the eye and pigment phenotypes to illustrate how the Astyanax model has contributed to evolutionary developmental biology.
Although cavefish adults lack external eyes, eye primordia with an embryonic lens and optic cup are formed during embryogenesis. After larval hatching the cavefish lens undergoes massive apoptosis, and further lens development is arrested (Jeffery and Martasian, 1998; Soares et al., 2004). Lens dysfunction has widespread consequences on optic development, as demonstrated by lens transplantation experiments (Yamamoto and Jeffery, 2000). Transplantation of a surface fish embryonic lens into a cavefish optic cup rescues many aspects of eye development and growth, leading to the recovery of an external eye in adult cavefish. Reciprocally, transplantation of a cavefish embryonic lens into the surface fish optic cup triggers eye degeneration, resulting in the disappearance of an external eye in adult surface fish. Two specific consequences of the absence of a functional cavefish lens are worthy of further attention in the context of this essay. First, the cornea and iris, which are induced by the lens during normal eye development, fail to form in cavefish (Strickler et al., 2001). Second, the stem cell progeny that contribute differentiating cells to the growing retina also undergo apoptosis in the absence of a functional lens, retarding the growth of this optic tissue (Strickler et al., 2007). Both cornea induction and retinal growth can be rescued by transplanting a functional surface fish lens into the cavefish optic cup (Yamamoto and Jeffery, 2000), showing effects on inductive processes and cell survival during development are important in the evolution of cavefish eye degeneration.
During cavefish larval development, the small degenerating eye does not keep pace with growth of the body and is buried in the adult orbit as a tiny non-functional vestige. Why would the eye be formed during embryogenesis only to subsequently degenerate in the larva? The probable cause is a developmental constraint: the early steps of eye development are also required to control the development of other crucial parts of the embryo, such as the brain, and are thus indispensible. Another example of a developmental constraint is that retinal development is not caused by the loss of stem cells or by blocking their proliferation division, which would be the most energetically efficient mode of suppressing growth, but instead by inducing apoptosis after new cells are already produced by the retinal stem cells. The retina is actually a part of the brain. Thus, apoptosis of stem cell progeny may be more permissible than losing their function because they are likely important in regulating other parts of the growing brain. Accordingly, only developmental pathways or their parts that are not strongly intertwined with other essential pathways may be subject to change during evolution.
The cavefish model also illustrates the principle of developmental amplification: small changes in early development can be increased incrementally as development proceeds to result in major changes in adult morphology. Surface fish and cavefish have significant differences in head shape and craniofacial morphology. Some of these differences, namely the shape and number of circumorbital bones, are linked to eye loss (Yamamoto et al., 2003). Accordingly, transplantation of a surface fish lens into a cavefish optic cup can promote the formation of a surface fish-like craniofacial skeleton. The sequence of developmental amplification is: the functional surface fish lens promotes retinal growth, producing a large eye on the external surface of the head, and the eye controls the surrounding craniofacial morphology to develop the surface fish phenotype. In cavefish, loss of lens function (probably along with other factors) promotes the arrest of retinal growth, and subsequent eye degeneration leads to development of the cavefish-type craniofacial morphology.
At the molecular level, the Astyanax model has highlighted the importance of antagonistic pleiotropy (Williams, 1957) in the evolution of development. The Sonic Hedgehog (Shh) midline-signaling pathway has critical roles in cavefish development and evolution. The cavefish shh genes are overexpressed along the anterior embryonic midline relative to surface fish counterparts (Yamamoto et al., 2004), and this expansion of Shh signaling impacts the expression of downstream developmental targets. For example, negative regulation of pax6 through expanded Shh signaling causes size reduction in the cavefish retina (Menuet et al., 2007) and shh overexpression triggers lens apoptosis and eye degeneration in surface fish embryos, producing a phenocopy of the cavefish eye (Yamamoto et al., 2004). Cavefish larvae differ from those of surface fish in having a larger mouth with a wider jaw span and more taste buds. Shh signaling is also involved in the enhancement of these oral features. In a direct demonstration of shh gene pleiotropy, experimental shh overexpression causes enhancement of jaws and taste buds at the expense of eyes in the same surface fish larvae (Yamamoto et al., 2009). The tradeoff between eye and oral development may have adaptive significance due to benefits associated with increased feeding efficiency in the dark cave environment.
A metabolic tradeoff involving the melanin and catecholamine (CAT) synthesis pathways could be important in the evolution of cavefish albinism. In the melanin synthesis pathway, L-tyrosine is converted to L-DOPA, and then in several steps to melanin pigment. Melanin is deposited the eye and body pigment cells of surface fish, but is absent or decreased in cavefish. In the CAT pathway, L-tyrosine is converted to L-DOPA, which is then metabolized to dopamine, epinephrine, and norepineprhine. Dopamine and norepinephrine are increased in albino cavefish compared to pigmented surface fish (Bilandžija et al., 2013), possibly to support CAT-related neurological and behavioral changes. The cavefish melanin synthesis pathway is blocked at the conversion of L-tyrosine to L-DOPA (McCauley et al., 2004), making excess L-tyrosine available for utilization in the CAT synthesis pathway. The close relationship between these two pathways has been confirmed by oca2 knockdown in surface fish, which abolishes melanin synthesis while simultaneously increasing L-tyrosine and dopamine (Bilandzija et al., 2013). Thus, a potential benefit of cavefish albinism may be the use of an L-tyrosine stockpile for purposes that are more suitable in the dark cave environment.
4. CONCLUSIONS
Insights from the comparative organismal approach indicate that many aspects of development are flexible and subject to potential modification during evolution. I have described some examples in ascidians and cavefish, including changes in cytoplasmic localization, zygotic control processes, the complete development of cell lineages, embryonic inductive events, the balance between cell survival and death, and heterochrony of developmental events. Moreover, the cavefish model demonstrates that developmental processes are subject to tradeoffs through the function of pleiotropic genes, which may be important factors in the evolution of development. As appreciated early in the history of genetic research most genes have pleiotropic functions (Stearns, 2010). Thus, further studies of multifunctional genes may be helpful in understanding the rapid evolution of morphological traits. The ascidian and cavefish models also highlight developmental processes that are not readily subject to change, defining inflexible developmental constraints that limit and channel the possibilities for evolution.
The comparative organismal approach in ascidian, cavefish, and other model systems (see for example Goldstein, 2001) has provided a foundation for continued progress to understand how development is changed during evolution. However, little is known about the environmental cues and the evolutionary forces leading to these changes. Therefore, one of the future challenges is to determine not only “how” but also “why” development is changed during evolution. The comparative organismal approach will be an important tool in solving the next generation of questions in evolutionary developmental biology.
REFERENCES
- Bilandžija H, Ma L, Parkhurst A, and Jeffery WR (2013). A potential benefit of albinism in Astyanax cavefish: Downregulation of the oca2 gene increases L-tyrosine and catecholamine levels as an alternative to melanin synthesis. PLoS ONE 8, e80823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourlat SJ, Juliusdottir T, Lowe CJ, Freeman R, Aronowicz J, Kirschner M, Lander ES, Thorndyke M, Nakano M, Kohn AB, Heyland A Moroz LL, Copley RR, and Telford MJ (2006). Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature 444, 85–88. [DOI] [PubMed] [Google Scholar]
- Delsuc F, Brinkmann H, Chourrout D, and Phillipe H (2006). Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439, 965–968. [DOI] [PubMed] [Google Scholar]
- Gehring W,J, and Ikei K (1999). Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet 15, 371–377. [DOI] [PubMed] [Google Scholar]
- Goldstein B (2010). On the evolution of early development in the Nematoda. Philos. Trans. T. Soc. Lond., B Biol. Sci 356, 1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JB (2012). The complex origin of Astyanax cavefish. BMC Evol. Biol 12, 105 doi: 10.1186/1471-2148-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyoja F (2006). Expression of a muscle determination gene, macho 1, in the anural ascidian Molgula tectiformis. Dev. Genes Evol 216, 285–289. [DOI] [PubMed] [Google Scholar]
- Gyoja F, Satou Y, Shin-I T, Kohara Y, Swalla BJ, and Satoh N (2007). Analysis of large scale expression sequenced tags (ESTs) from the ascidian, Molgula tectiformis. Dev. Biol 307, 460–482. [DOI] [PubMed] [Google Scholar]
- Hadfield KA, Swalla BJ, and Jeffery WR (1995). Multiple origins of anural development in ascidians inferred from rDNA sequences. J. Mol. Evol 40, 413–427. [DOI] [PubMed] [Google Scholar]
- Jeffery WR, and Martasian DP (1998). Evolution of eye regression in the cavefish Astyanax: apoptosis and the Pax-6 gene. Amer. Zool 38, 685–696. [Google Scholar]
- Jeffery WR (2002). Programmed cell death in the ascidian embryo: modulation by Fox2A and Manx and roles in the evolution of larval development. Mech. Dev 118, 118–124. [DOI] [PubMed] [Google Scholar]
- Jeffery WR (2006). Evolution and development of brain sensory organs in molgulid ascidians. Evol. Dev 6, 170–179. [DOI] [PubMed] [Google Scholar]
- Jeffery WR, and Swalla BJ (1990). Anural development in ascidians: evolutionary modification and elimination of the tadpole larva. Sem. Dev. Biol 96, 125–143. [Google Scholar]
- Jeffery WR, Swalla BJ, Ewing N, and Kusakabe T (1999). Evolution of the ascidian anural larva: Evidence from embryos and molecules. Mol. Biol. Evol 16, 646–654. [DOI] [PubMed] [Google Scholar]
- Keene A, Yoshizawa M, and McGaugh S (2015). “Biology and Evolution of the Mexican Cavefish”. Elsevier, New York: (in press). [Google Scholar]
- Kowalko JE, Rohner N, Rompani SB, Peterson BK, Linden T, Yoshizawa M, Kay EH, Hoekstra HE, Jeffery WR, Borowsky R, and Tabin CJ (2013). Genetic analysis of the loss of schooling behavior in cavefish reveals both sight-dependent and independent mechanisms. Curr. Biol 23, 1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe T (2001). Evolution of anural development in ascidians: role of muscle specific differentiation genes In: “Biology of Ascidians”. Sawada H, Yokosawa H, and Lambert CC eds. Springer, Tokyo: Pp. 225–229. [Google Scholar]
- Kusakabe T, Swalla BJ, Satoh N, and Jeffery WR (1996). Mechanism of an evolutionary change in muscle differentiation in ascidians with different modes of development. Dev. Biol 174, 379–392. [DOI] [PubMed] [Google Scholar]
- Kusakabe T, Araki I. Satoh N, and Jeffery WR (1997). Evolution of chordate actin genes: evidence from genomic organization and amino acid sequences. J. Mol. Evol 44, 289–298. [DOI] [PubMed] [Google Scholar]
- Ma L, Parkhurst A, and Jeffery WR (2014). The role of a lens survival pathway including sox2 and αA-crystallin in the evolution of cavefish eye degeneration. EvoDevo 5, 28 doi: 10.1186/2041-9139-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RW, Russell WH, and Elliott WR (1977). Mexican eyeless Characin fishes, genus Astyanax: environment, distribution, and evolution. Spec. Publ. Mus. Texas Tech. Univ 12, 1–89. [Google Scholar]
- McCauley DW, Hixon E, and Jeffery WR (2004). Evolution of pigment cell regression in the cavefish Astyanax: A late step in melanogenesis. Evol. Dev 6, 209–218. [DOI] [PubMed] [Google Scholar]
- McGaugh SE, Gross JB, Aken B, Blin M, Borowsky R, Chalopin D, Hinaux H, Jeffery WR, Keene A, Ma L, Minx P, Murphy D, O’Quin KE, Rétaux S, Rohner N, Searle SMJ, Stahl B, Tabin C, Volff JN, Yoshizawa M, and Warren W (2014). The cavefish genome reveals candidate genes for eye loss. Nature Commun 5, doi. 10.1038/natcommun630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuet A, Alunni A, Joly J-S, Jeffery WR, and Rétaux S 2007. Shh overexpression in Astyanax cavefish: multiple consequences on forebrain development and evolution. Development 134, 845–855. [DOI] [PubMed] [Google Scholar]
- Nishida H (2012). The maternal muscle determinant in the ascidian egg. Wiley Interdisp. Rev. Dev. Biol 3, 425–433. [DOI] [PubMed] [Google Scholar]
- Olsen CL, Natzle JE, and Jeffery WR (1999). The forkhead gene FH1 is involved in evolutionary modification of the ascidian tadpole larva. Mech. Dev 85, 49–58. [DOI] [PubMed] [Google Scholar]
- O’ Quin KE, Yoshizawa M, Doshi P, and Jeffery WR (2013). Quantitative genetic analysis of retinal degeneration in the blind cavefish. PLoS ONE 8(2), e57281. doi: 10.1371/journal.pone.0057281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ML, Dittmar K, and Pérez-Losada M (2007). How long does evolution of the troglomorphic form take? Estimating divergence times in Astyanax mexicanus. Acta. Carsolog 36, 173–182. [Google Scholar]
- Protas ME, Hersey C, Kochanek D, Zhou Y, Wilkens H, Jeffery WR, Zon LI, Borowsky R, and Tabin CJ (2006). Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nature Genet 38, 107–111. [DOI] [PubMed] [Google Scholar]
- Protas ME, Tabansky I, Conrad M, Gross JB, Vidal O, Tabin CJ, and Borowsky R (2008). Multi-trait evolution in a cave fish, Astyanax mexicanus. Evol. Dev 10, 196–209. [DOI] [PubMed] [Google Scholar]
- Satoh N (1994). “Developmental Biology of Ascidians”. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Scott MP (1994). Intimations of a creature. Cell 79, 1121–1124. [DOI] [PubMed] [Google Scholar]
- Soares D, Yamamoto Y, Strickler AG, and Jeffery WR (2004). The lens has a specific influence on optic nerve and tectum development in the blind cavefish Astyanax. Dev. Neurosci 26, 308–319. [DOI] [PubMed] [Google Scholar]
- Stearns FW (2010). One hundred years of pleiotropy: A retrospective. Genetics 186, 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler AG, Yamamoto Y, and Jeffery WR (2001). Early and late changes in Pax 6 expression accompany eye degeneration during cavefish development. Dev. Genes Evol 211, 138–144. [DOI] [PubMed] [Google Scholar]
- Strickler AG, Yamamoto Y, and Jeffery WR (2007). The lens controls cell survival in the retina: evidence from the blind cavefish Astyanax. Dev. Biol 311, 512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalla BJ, and Jeffery WR (1991). Interspecific hybridization between an anural and urodele ascidian: differential expression of urodele features suggests multiple mechanisms control anural development. Dev. Biol 143, 319–334. [DOI] [PubMed] [Google Scholar]
- Swalla BJ, Badgett MR, and Jeffery WR (1991). Identification of a cytoskeletal protein localized in the myoplasm of ascidian eggs: Localization is modified during anural development. Development 111, 425–436 [DOI] [PubMed] [Google Scholar]
- Swalla BJ, and Jeffery WR (1996). Requirement of the Manx gene for expression of chordate features in a tailless ascidian larva. Science 274, 1205–1208. [DOI] [PubMed] [Google Scholar]
- Swalla BJ, Makabe KW, Satoh N, and Jeffery WR (1993). Novel genes expressed differentially in ascidians with alternate modes of development. Development 119, 307–318. [DOI] [PubMed] [Google Scholar]
- Takada N, Satoh N, and Swalla BJ (2002). Expression of Tbx6, a muscle lineage T-box gene, in the anural ascidian Molgula tectiformis. Dev. Genes Evol 212, 354–356. [DOI] [PubMed] [Google Scholar]
- Takada N, York J, Davis JM, Schumpert B, Yasuo H, Satoh N, and Swalla BJ (2002). Brachury expression in tailless Molgulid ascidian embryos. Evol. Dev 4, 205–211. [DOI] [PubMed] [Google Scholar]
- Wilkens H (1988). Evolution and genetics of epigean and cave Astyanax fasciatus (Characidae, Pisces)-support for the neutral mutation hypothesis. Evol. Biol 23, 271–367. [Google Scholar]
- Williams GC (1957). Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411. [Google Scholar]
- Yamamoto Y, and Jeffery WR (2000). Central role for the lens in cave fish eye degeneration. Science 289, 631–633. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Stock DW, and Jeffery WR (2004). Hedgehog signalling controls eye degeneration in blind cavefish. Nature 431, 844–847. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Espinasa L, Stock DW, and Jeffery WR (2003). Development and evolution of craniofacial patterning is mediated by eye-dependent and –independent processes in the cavefish Astyanax. Evol. Dev 5, 435–446. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Byerly MS, Jackman WR, and Jeffery WR (2009). Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cavefish evolution. Dev. Biol 330, 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuo H, and Satoh N (1994). An ascidian homolog of the mouse Brachyury (T) gene is expressed exclusively in notochord cells at the fate restricted stage. Dev. Growth Differ 36, 9–18. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Gorički Š, Soares D, and Jeffery WR (2010). Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Curr. Biol 20, 1631–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]


