Abstract
Introduction:
Latent HIV reservoirs are rapidly established in lymphoid tissues during acute HIV infection (AHI). Sampling these tissues provides important information about HIV pathogenesis. This period is associated with viral replication and immune activation that may affect procedure-related adverse events (AEs). We examined the safety and tolerability of inguinal lymph node (LN) biopsy in research participants with AHI in Bangkok, Thailand.
Methods:
Between 2013 and 2016, 67 AHI participants in the RV254/SEARCH010 study underwent at least one optional inguinal LN biopsy during AHI at the baseline visit and/or after antiretroviral therapy (median 48 weeks after antiretroviral therapy). Biopsy-related AEs were graded according to NIH Division of AIDS guidelines. Poisson regression was used to calculate incidence rate ratios and 95% confidence intervals to evaluate associations of demographic and HIV characteristics, procedure timing, and repetition with AE incidence.
Results:
Of the 67 participants, 97% were male with a median age of 26. Among 78 LN biopsies (39 at baseline and 39 at follow-up), 10 (12.8%) AEs were reported: 6 (7.7%) grade 1 and 4 (5.1%) grade 2. The AEs were biopsy-site discomfort (n = 8, 10.2%) and hematoma (n = 2, 2.6%). No factors were significantly associated with AE incidence. All biopsy-related AEs were transient and self-limited.
Conclusions:
Inguinal LN biopsies were safe and well tolerated in mostly Thai men with AHI. As LN biopsies become an integral part of HIV research, this study provides information to participants, researchers, and institutional review boards that these samples can be safely obtained.
Key Words: research risk, acute HIV infection, lymph node biopsy
INTRODUCTION
Despite the success of antiretroviral therapy (ART) in preventing HIV disease progression, ART does not eradicate the virus. Integrated viral DNA persists in reservoirs of latently infected resting CD4+ T cells and in lymphoid tissues, which develop soon after HIV acquisition during acute HIV infection (AHI).1–4 Serial sampling of these tissues can provide important information about HIV reservoirs and immune responses, advancing our understanding of HIV pathogenesis and informing HIV cure efforts.
Lymph node (LN) biopsies are safe in both chronically HIV-infected and HIV-uninfected populations.5–7 Complications of LN biopsy have included surgical site infection, seroma, hematoma, lymphedema, lymphocele, dorsal vein thrombosis, and epididymitis.5–7 However, only one study has examined the safety of LN biopsy in an HIV-infected population outside the United States.7 No studies have been conducted on AHI.
Unlike the steady-state viremia and immune dysfunction in untreated chronic HIV infection, AHI is associated with a surge of viral replication and immune activation, particularly in lymphoid tissues.8,9 It is unknown whether complications from tissue biopsies may be more common during this period. It is critical for researchers to understand the safety of invasive procedures performed in a variety of settings and at various stages of infection to optimally use these procedures during clinical research programs and to adequately inform research participants about the risks of study participation. Here, we examine the safety of inguinal LN biopsies in a prospective cohort of participants with AHI in Bangkok, Thailand.
METHODS
Study Population
The RV254/SEARCH010 study prospectively screened and enrolled participants with AHI from the Thai Red Cross Anonymous Clinic in Bangkok, Thailand (clinicaltrials.gov identification number NCT00796146). The study was approved by the institutional review boards of the Chulalongkorn University in Thailand and the Walter Reed Army Institute of Research in the United States. Informed consent was obtained separately for the main study and for optional procedures, which included inguinal LN biopsy. Participants in RV254/SEARCH010 who underwent LN biopsy are included in these analyses.
Participant Identification and Staging
The RV254/SEARCH010 study used pooled nucleic acid testing, fourth generation (4thG) HIV antigen/antibody immunoassay, and sequential less sensitive HIV antibody immunoassay to identify and enroll acutely HIV-infected participants attending a major voluntary counseling and testing site in Thailand, as previously described.10,11 Staging of AHI was performed using Fiebig staging.12 The number of days since HIV exposure was estimated using an algorithm that considers sexual encounters of varying risk levels over the preceding 30–60 days, as previously described.13 Participants in RV254/SEARCH010 were offered ART during AHI through a separate protocol (clinicaltrials.gov NCT00796263). They were followed at days 0, 3, 7; weeks 2, 4, 8, 12; and then every 12 weeks.
Inguinal LN Biopsy Procedure
Inguinal biopsies were offered at enrollment and after 24, 48, 96, 144, and 240 weeks in the study, with a maximum of 4 LN biopsies per participant allowed. LNs were identified by palpation. The biopsies were performed in the inguinal region by 2 experienced surgeons using local anesthesia and standard sterile technique. A single LN was removed through a 1–2-cm skin incision that was closed with sutures.
Repeated biopsies were performed on the side of the groin opposite to the previous biopsy. Participants were instructed to avoid strenuous activities for 24 hours after each procedure and advised to contact the study team in the event of any sign or symptom of postoperative complications. At the next scheduled study visit after the procedure, study physicians asked participants for feedback and adverse events (AEs) related to the biopsy. Participants were given the option to undergo photography of the biopsy wound immediately after the procedure.
Participants were excluded from the procedure if they had clinical bleeding abnormalities; platelet count below 80,000/mL or INR > 1.2; or had taken aspirin, nonsteroidal anti-inflammatory drugs, or anticoagulant medications within the previous 7 days.
AE Monitoring and Grading
Participants were asked to contact the study team after the procedure if any abnormal symptoms developed, and AEs were solicited and graded at every study visit by study physicians. Physicians recorded the start and end dates of the AE and association to ART or procedures. Complications of LN biopsies were evaluated retrospectively using the study database and medical record review.
AEs were graded according to the Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0.14 The DAIDS table was selected because it is a standardized and sequential scale for classifying complications. Grade 1 is characterized as “mild symptoms causing no or minimal interference with usual social and functional activities with intervention not indicated,” grade 2 as “moderate symptoms causing greater-than-minimal interference with usual social and functional activities with intervention indicated,” grade 3 as severe, and grade 4 as AEs that are potentially life-threatening.14
Statistical Methods
Data were summarized by median with interquartile range (IQR) for continuous variables and number with percentage for categorical variables. Incidence rate ratios (IRRs) of AE and 95% confidence interval (CI) were calculated using Poisson distribution. Longitudinal Poisson regression models were used to assess whether age, plasma HIV RNA, CD4 cell count, and sexually transmitted infections (including chlamydia, gonorrhea, and syphilis) at the time of biopsy were associated with AE incidence. Separate analyses evaluated whether baseline procedures were associated with the number of AEs and whether repeating a biopsy procedure more than once increased AE risk. Factors with a P-value <0.05 were considered significant. All analyses were performed using Stata Statistical Software Release 13 (StataCorp, College Station, TX).
RESULTS
Study Population Characteristics
Between June 2013 and May 2016, 67 acutely HIV-infected participants in the RV254/SEARCH 010 study (97% male, median age 26 years) consented to and underwent LN biopsy as an optional procedure. All biopsies that were attempted during this period were successful in removing LNs. Isolated LNs were a median of 13 mm (IQR 9–17 mm) at AHI and 12 mm (IQR 9–15 mm) at follow-up. Fifty-six participants underwent one LN biopsy and 11 participants underwent 2 procedures, although up to 4 biopsies were allowed per study protocol. A total of 78 LN biopsies were completed during the study period, 39 procedures during AHI at baseline and 39 at a follow-up visit, 24–240 weeks after ART initiation. Clinical characteristics of participants are summarized in Table 1.
TABLE 1.
Characteristics of Participants With Completed Lymph Node Biopsy

Safety of Optional Inguinal LN Biopsy
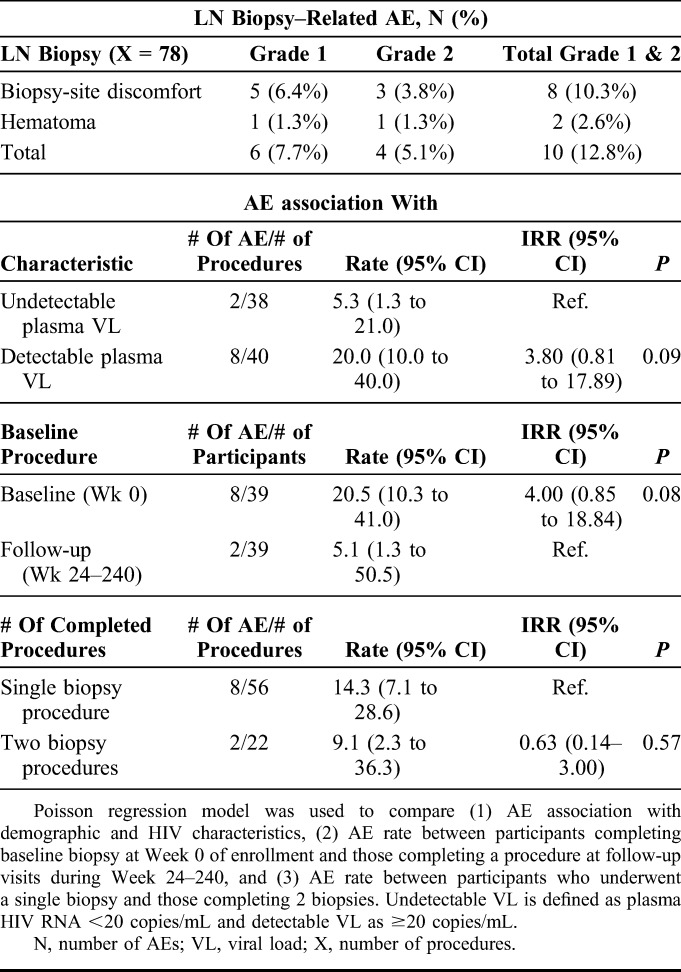
Among the 78 inguinal LN biopsies performed, there were a total of 10 procedure-related AEs (Table 2) for an overall AE rate of 12.8%, including 6 that were mild (grade 1, 7.7%) in severity and 4 that were moderate (grade 2, 5.1%). No participant encountered more than one AE associated with LN biopsy. The grade 1 AEs all resolved without any medical intervention. Prolonged biopsy-site discomfort was the most common grade 1 AE and persisted for a range of 10 days to 1 month.
TABLE 2.
Complications Associated With Biopsy and Association Factors
Two out of 3 of the grade 2 biopsy site discomfort AEs presented swelling, which resolved without treatment. In one participant with grade 2 biopsy-site discomfort, the wound was painful when sitting, and swelling was approximately 8 × 4 cm at 2 weeks after procedure when the study physician and surgeon inspected the biopsy site. No treatment was necessary and the wound healed without complications after 1.5 months. The second case presented discomfort when walking and swelling, which resolved 20 days after the procedure. The final case of grade 2 biopsy site discomfort was managed by 1000 mg of paracetamol daily and resolved within 9 days. A single complication of grade 2 hematoma healed within 10 days of the procedure. The participant was also treated with 1000 mg of paracetamol daily and received amoxicillin as a prophylactic antibiotic after the biopsy.
Factors Associated With LN Biopsy–Related AEs
Eight of the reported AEs were associated with biopsies during AHI, and the remaining 2 occurred after procedures performed at later study visits (Table 2). Although numerically more AEs occurred during AHI than during later study visits, the difference in event rates did not achieve statistical significance [IRR 4.00 (95% CI: 0.85–18.84), P = 0.08]. The rate of AEs was also higher in participants with detectable plasma HIV RNA, although not statistically significant [IRR 3.80 (95% CI: 0.81–17.89), P = 0.09]. Age, CD4 cell count, and sexually transmitted infections were not associated with AE incidence. No participant who completed an LN biopsy during AHI had viral load below 50 copies per milliliter, whereas 38 of 39 participants who completed biopsy at a later follow-up visit were fully suppressed (Table 1). Undergoing 2 LN procedures did not significantly increase AE risk.
DISCUSSION
Inguinal LN biopsies for research purposes were safe and well tolerated in this cohort of mostly young Thai men with AHI. Although AEs were reported in 12.8% of procedures, most required little or no intervention and did not substantially interfere with daily functions. All AEs resolved without sequelae.
For LN biopsy–associated complications, the grade 1 AE rate in the RV254/SEARCH010 study was 7.7%, which was slightly lower than the rate for minor complications reported by an HIV-infected cohort study in the United States.6 The grade 1 AEs in our study, which did not require medical intervention, were similar to the “minor complications” in the U.S. study. While that study reported 6 seromas, 1 lymphedema, 1 hematoma, and 1 reaction to adhesive tape in 95 procedures, our grade 1 AEs seemed milder in comparison: 5 cases of biopsy-site discomfort and 1 hematoma.6 Biopsy-site discomfort was not reported in previous studies most likely because it was considered expected and relatively minor from the surgical perspective.
The majority of complications reported in previous reports seem to commensurate with grade 2 AEs by the DAIDS grading table.14 Our grade 2 LN-associated AE rate was 5.1%, comparable with the complication rate of 5%–10% reported in other HIV cohorts.5,6 Rothenberger et al7 reported a 2.4% (1/41 procedures) complication rate in HIV-infected participants but used a different methodology for defining AEs. Our complication rate is also similar to the reported rates of LN-related AEs in HIV-uninfected populations in Netherlands and Germany.15,16
Although not statistically significant, more AEs were reported at baseline during AHI than at follow-up and in study participants with detectable plasma HIV RNA, defined as ≥20 copies per milliliter. All participants with AEs during AHI had viral loads above 50 copies per milliliter, whereas all participants with AEs at follow-up were below (HIV RNA < 50 copies/mL). Viremia and immune activation during AHI could theoretically increase susceptibility to biopsy-related complications.8 The more frequent follow-up shortly after baseline (6 visits within the first 4 weeks) as compared with later follow-up visits (every 12 weeks) may have contributed to more AEs being documented for biopsies during AHI.
The DAIDS AE grading table used in our study offered a standardized method of rating complications and is specific to HIV research, which likely increased sensitivity in detecting AEs as compared with previous studies of LN biopsy–related AEs. Unlike surgical grading table, this system does not include common surgical complications such as seromas and wound infections.17 Comparability between studies is therefore limited—our analysis in participants with AHI included mild, grade 1 AEs that did not compromise the safety of the procedure and most likely were not reported by other studies. We did not encounter rare or serious AEs. Findings from young Thai males may not be generalizable to other populations.
CONCLUSIONS
Inguinal LN biopsies are safe and well tolerated by participants with AHI in Thailand. Biopsy-related AEs occurred in 12.8% of participants and were mild and self-limited. Biopsy-site discomfort was the most common AE. As LN biopsies become an integral part of HIV research, this study provides information to participants, researchers, and institutional review boards that these samples can be safely obtained.
ACKNOWLEDGMENTS
The authors thank their study participants and staff from the Thai Red Cross AIDS Research Centre, Chulalongkorn University, and AFRIMS for their valuable contributions to this study. They are grateful to the Thai Government Pharmaceutical Organization, ViiV Healthcare, Gilead, and Merck for providing the antiretrovirals for this study. The RV254/SEARCH 010 Study Group includes: from SEARCH/TRCARC/HIV-NAT: Praphan Phanuphak, Nipat Teeratakulpisarn, Mark de Souza, James Fletcher, Ponpen Tantivitayakul, Sasiwimol Ubolyam, Pacharin Eamyoung, Jintana Intasan, Duanghathai Sutthichom, Peeriya Prueksakaew, Somprartthana Rattanamanee, Suwanna Puttamaswin, Somporn Tipsuk, Khunthalee Benjapornpong, Nisakorn Ratnaratorn, Chutharat Munkong, Kamonkan, and Tanjnareel; from AFRIMS: Robert J O'Connell, Siriwat Akapirat, Rapee Trichavaroj, Bessara Nuntapinit; from MHRP: Merlin Robb, Madelaine Ouellette, and Oratai Butterworth.
Footnotes
Supported by cooperative agreements (W81XWH-07-2-0067, W81XWH-11-2-0174) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the US Department of the Army and by an intramural grant from the Thai Red Cross AIDS Research Center. The US Army Medical Research Acquisition Activity (820 Chandler Street, Fort Detrick, MD 21702-5014) is the awarding and administering acquisition office for the cooperative agreement. It has also been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. This research was supported in part by awards from the National Institute of Allergy and Infectious Diseases (R01AI125127) and The National Institute of Neurologic Disorders and Stroke (R01NS084911). Antiretroviral therapy was supported by the Thai Government Pharmaceutical Organization, Gilead, Merck, and ViiV Healthcare.
M.C. interned at Royalty Pharma. T.A.C. has received a speaker fee from Gilead Sciences. S.S.S. codirects a clinical research study that receives donated study medications from ViiV Healthcare. J.A. has received honorarium for participating in advisory meetings for ViiV Healthcare, Merck, AbbVie, and Roche. The remaining authors have no conflicts of interest to disclose.
Clinical Trial Number: NCT00796146.
The views expressed are those of the authors and should not be construed to represent the positions of the participating institutions, the U.S. Army, the Department of Defense, or the Department of Health and Human Services. The investigators have adhered to the policies for protection of human subjects as prescribed in AR-70.
REFERENCES
- 1.Siliciano JD, Siliciano RF. The latent reservoir for HIV-1 in resting CD4+ T cells: a barrier to cure. Curr Opin HIV AIDS. 2006;1:121–128. [DOI] [PubMed] [Google Scholar]
- 2.Pace MJ, Agosto L, Graf EH, et al. HIV reservoirs and latency models. Virology. 2011;411:344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Churchill MJ, Deeks SG, Margolis DM, et al. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol. 2016;14:55–60. [DOI] [PubMed] [Google Scholar]
- 4.Pierson T, McArthur J, Siliciano RF. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu Rev Immunol. 2000;18:665–708. [DOI] [PubMed] [Google Scholar]
- 5.Skarda DE, Taylor JH, Chipman JG, et al. Inguinal lymph node biopsy in patients infected with the human immunodeficiency virus is safe. Surg Infect. 2007;8:173–177. [DOI] [PubMed] [Google Scholar]
- 6.Meditz AL, Connick E, McCarter M. Safety of excisional inguinal lymph node biopsies performed for research purposes in HIV-1-infected women and men. Surg Infect. 2014;15:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothenberger MK, Mutuluuza CK, Ssali F, et al. Inguinal lymph node and anorectal mucosal biopsies for human immunodeficiency virus research protocols in an emerging nation: patient outcomes and lessons learned. Surg Infect. 2015;16:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robb ML, Eller LA, Kibuuka H, et al. Prospective study of acute HIV-1 infection in Adults in East Africa and Thailand. N Engl J Med. 2016;374:2120–21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowell TA, Colby DJ, Pinyakorn S, et al. Acute retroviral syndrome is associated with high viral burden, CD4 depletion, and immune activation in systemic and tissue compartments. Clin Infect Dis. 2017;66:1540–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De souza MS, Phanuphak N, Pinyakorn S, et al. Impact of nucleic acid testing relative to antigen/antibody combination immunoassay on the detection of acute HIV infection. AIDS. 2015;29:793–800. [DOI] [PubMed] [Google Scholar]
- 11.Phanuphak N, Teeratakulpisarn N, van Griensven F, et al. Anogenital HIV RNA in Thai men who have sex with men in Bangkok during acute HIV infection and after randomization to standard vs. intensified antiretroviral regimens. J Int AIDS Soc. 2015;18:19470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. [DOI] [PubMed] [Google Scholar]
- 13.Crowell TA, Fletcher JLK, Sereti I, et al. Initiation of antiretroviral therapy before colonic infiltration by HIV reduces viral reservoirs, inflammation and immune activation. J Int AIDS Soc. 2016;19:21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institute of Health. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. Version 2.0. Bethesda, MD: US Department of Health and Health Services; 2014. [Google Scholar]
- 15.Kroon BK, Lont AP, Valdés Olmos RA, et al. Morbidity of dynamic sentinel node biopsy in penile carcinoma. J Urol. 2005;173:813–815. [DOI] [PubMed] [Google Scholar]
- 16.Kretschmer L, Thoms KM, Peeters S, et al. Postoperative morbidity of lymph node excision for cutaneous melanoma-sentinel lymphonodectomy versus complete regional lymph node dissection. Melanoma Res. 2008;18:16–21. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]


