Abstract
Disrupted white matter structure has been established in patients with diffuse axonal injury (DAI), but morphological changes in gray matter and local intrinsic activity in the short and midterm (before 6 months) have not been documented in DAI patients. We hypothesized that regionally selective atrophy observed in deep gray matter in the short-term and mid-term periods in patients with mild-to-moderate DAI, local atrophy, and/or dysfunction would be related to clinical characteristics. We evaluated the changes in regional density and synchronization in 18 DAI patients separately using Diffeomorphic Anatomical Registration through Exponentiated Lie algebra-enhanced voxel-based morphometry and regional homogeneity (ReHo). Compared with the controls, DAI patients showed a decreased density in the bilateral thalami and decreased ReHo values in the ventral anterior and ventral lateral nuclei of the bilateral thalami. Pearson’s correlation analysis showed that decreased density in the bilateral thalami was correlated negatively with time since injury and decreased ReHo values in the ventral anterior and ventral lateral nuclei of the bilateral thalami were associated with a worsened motor assessment scale. These findings suggest that mild-to-moderate traumatic DAI within the short and midterm could lead to thalamic atrophy and that dysfunction in the bilateral thalami is associated with declining motor function. This study could potentially provide complementary evidence as an important element in longitudinal studies.
Keywords: Diffeomorphic Anatomical Registration through Exponentiated Lie algebra-enhanced voxel-based morphometry, diffuse axonal injury, functional MRI, regional homogeneity, resting state
Introduction
As traditionally understood, diffuse axonal injury (DAI) is a severe and extensive type of traumatic brain damage that is the result of a traumatic shearing force causing the disruption of axons, accompanied by severe neurological impairment. However, in fact, more than half of mild-to-moderate DAI patients were misdiagnosed because of negative findings from computed tomography or conventional MRI. Advanced or state-of-the-art MRI sequences and imaging techniques could improve this dilemma; for example, detection of axonal injury or disconnection using diffusion tensor imaging (DTI) 1, detection of hemorrhagic shearing lesions using susceptibility-weighted imaging (SWI) 2,3, and detection of biochemistry metabolism using magnetic resonance spectroscopy could improve diagnoses 4. In general, SWI and diffusion-weighted imaging are sensitive for detecting lesions of microhemorrhage and edema that could confirm the DAI diagnosis by demonstrating lesions in white matter (WM) of the cerebral hemispheres (grade I), corpus callosum (grade II), and brainstem (grade III) 1,2.
Techniques such as voxel-based morphometry (VBM) can be used to detect changes in local tissue density caused by DAI. Warner et al. 5 found that, after 7.8 months of follow-up, significant and regionally selective post-traumatic atrophy was detected in the thalamus, putamen, and other cortical regions, rather than the global or the diffuse area. Other studies also found gray matter (GM) atrophy in patients with DAI after a long-term (≥1 year) follow-up 6,7. The reported that GM atrophy may have two potential mechanisms in long-term DAI patients, one of which could be cortical involvement, as the brain strikes the cranial vault in a coup–contrecoup manner 8. In addition, DAI can cause axonal damage and delayed neuronal cell death, leading to retrograde degeneration and neuronal somatic loss 9. However, in MR morphometry studies, the alterations in brain volume in the short and midterm after DAI trauma (trauma occurred no >6 months) remain unclear. For this reason, we hypothesized that previous regionally selective atrophy would be observed in the deep GM of mild-to-moderate DAI patients at short-term and mid-term periods after trauma. To improve anatomical precision and accuracy in locating morphologic alterations, a Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL)-enhanced VBM method was used to compare the relative density of brain parenchyma between the DAI and healthy controls (HCs) to investigate the possible alterations in the short-term and mid-term post-trauma periods.
Resting-state functional MRI (rfMRI) has also provided considerable information on disrupted functional homotopy 10, long-distance connectivity 11, and networks 12 after traumatic brain injury. Recently, a summary systemically reviewed the association between local morphometry and local intrinsic activity measures 13. Regional homogeneity (ReHo), providing an approach to investigate the local functional connectivity within neighbouring voxels in the resting state, shares the individual variability with a wide range of cortical morphologies 14. After investigating possible alterations in morphologies, we also investigated alterations in ReHo between groups, and whether local atrophy or dysfunction would relate to clinical characteristics. Studying the local atrophy and/or dysfunction in the short-term and mid-term periods after trauma in patients with mild-to-moderate DAI could provide complementary evidence as an important element in longitudinal studies or perhaps provide robust biomarkers of patient outcomes that longitudinal studies can focus on.
Participants and methods
Participants
Eighteen DAI patients were recruited from the Emergency and Trauma Center at the First Affiliated Hospital, Nanchang University. The following DAI criteria were used as in our previous study in which the homotopic functional connectivity results were reported 10. The following inclusion criteria were applied: (a) the patient had a closed-head TBI (involving acceleration-deceleration or high-velocity rotational forces); (b) the patient was hemodynamically stable (to ensure that transfer to the scanner was clinically safe); (c) the patient was 16–60 years of age; (d) the patient’s Glasgow coma scale (GCS) score within 24 h after trauma was more than 8 (mild-to-moderate traumatic DAI); and (e) the patient undergoes MRI scanning within 6 months after trauma occurring 1,15. Exclusion criteria were as follows: (a) any focal, mixed, or high-density lesion (including contusion, an extra-axial hematoma, and/or intra-parenchymal hemorrhages) more than 10 ml; (b) a midline shift more than 3 mm at the level of the septum pellucidum on admission computed tomography; (c) a pre-existing neurological disease, as assessed on the basis of a medical history or MRI; (d) bilaterally absent pupillary responses; and (e) any contraindication to MRI. For quality control of the rfMRI data, all patients were included if their head movement was less than 2 mm of translation along any axis and less than 2° of angular rotation along any axis during the rfMRI scan (see the ‘Regional homogeneity analysis’ section).
Eighteen age-matched and sex-matched HCs were recruited from the local community through advertisements as a control group. On the basis of a medical history and MRI, the control participants without any brain lesions were included. All participants self-reported as being right-handed. The study protocol was approved and carried out in accordance with the guidelines of the Institutional Review Board of the First Affiliated Hospital of Nanchang University. Written informed consent was obtained from each participant before the study. This study was carried out in accordance with the Declaration of Helsinki.
Clinical evaluation
In our study, each DAI patient was assessed by the GCS within 24 h after trauma, the mini-mental state examination (MMSE), and the motor assessment scale (MAS). Among these, the GCS is a neurological scale that assesses the level of consciousness after a head injury 16, the MMSE test is a 30-point questionnaire that is used to estimate the severity and progression of cognitive impairment 17, and the MAS is used extensively in clinical and evaluates a patient’s ability to move low muscle tone or whose movement synergistic or stereotypical upper motor neuron patterns turn actively into a normal movement pattern 18.
Data acquisition
All MRIs were obtained on a 3.0 Tesla MR scanner (Trio Tim, Siemens Medical Systems, Munich, Germany). Foam pads and earplugs were used to reduce head motion and scanner noise, respectively. Patients received a conventional MRI (including T2WI and SWI) for diagnosis and radiological evaluation. Three-dimensional high-resolution T1WI were acquired for a DARTEL-enhanced VBM analysis using the following sequence: TR/TE=1900/2.26 ms; NEX=1; matrix=240×256; FOV=215×230 mm; slices=176; slice thickness=1.0 mm; and orientation=sagittal. The rfMRI data were acquired for ReHo analysis using the following sequence: TR/TE=2000/30 ms, flip angle=90°, FOV=200×200 mm, matrix=64×64, 30 interleaved axial slices with 4-mm thickness with an interslice gap of 1.2 mm, number of time points=240. During the rfMRI scanning, participants were instructed to keep their eyes closed, to not systemically think about anything, and to not fall asleep.
Diffeomorphic Anatomical Registration through Exponentiated Lie algebra-enhanced voxel-based morphometry analysis
The DARTEL procedure is a relatively recent alternative to previous spatial normalisation methods and provides improved anatomical precision 19,20. The preprocessing included the following steps: (a) High-resolution T1WI were realigned and reconstructed to axial datasets and then reoriented manually so that the anterior commissure was positioned at coordinate [0, 0, 0]. (b) Nonbrain tissue voxels were removed from the reoriented T1WI, and brain tissues were then segmented into GM, WM, and cerebrospinal fluid in native space; this was performed using the statistical parametric mapping (SPM) pipeline. (c) To adjust for head size, GM and WM were normalized by individual intracranial volume as the GM fraction and WM fraction for group comparison, respectively, to create a set of group-specific templates. (d) Each participant’s output images were still in the average brain space. These images were transformed from each native space into the Montreal Neurological Institute space. (e) Finally, the resulting normalized images were smoothed with a 6-mm full-width–half-maximum Gaussian kernel. After spatial preprocessing, the normalized and smoothed GM and WM data sets were subjected to statistical analysis.
One of the limitations of DARTEL (and nonrigid registration in general) is the difficulty in obtaining an accurate alignment for various anatomical structures, for instance brains with severe injury. In our study, the 18 patients with DAI did not show cerebral deformation after trauma; thus, it ensures that this limitation of DARTEL would not affect the accurate alignment.
Regional homogeneity analysis
All rfMRI data were processed using Data Processing Assistant for Resting-State fMRI Advanced Edition V2.2. For each participant, the first ten volumes were discarded to avoid the possible effects of scanner instability and the adaptation of participants to the scanner environment. The next preprocessing steps included slice timing to correct within-scan acquisition time differences between slices, realignment to the first volume to correct head-motion (a six-parameter spatial transformation), spatial normalization to the Montreal Neurological Institute template using a six-parameter spatial transformation, and resampling images into a spatial resolution of 3×3×3 mm3. We then performed signal linear detrending and voxelwise temporal bandpass filtering (0.01–0.08 Hz), and subsequently regressed out the nuisance covariates, including eight covariates (i.e. signals from WM and cerebrospinal fluid as well as six head motion parameters).
The rfMRI data without spatial smoothing were used for ReHo analysis with Data Processing Assistant for Resting-State fMRI Advanced Edition V2.2 21. Kendall’s coefficient of concordance was calculated to measure the local synchronization of the 27 nearest-neighbouring voxels using the formula (a) given below, 22 and the ReHo value was assigned to the central voxel. Then, a voxelwise ReHo map was obtained. Thus, each individual ReHo map was generated. A standardized ReHo map was created by dividing every individual ReHo map by each participant’s global mean Kendall’s coefficient of concordance value within the brain mask. Finally, the standardized ReHo maps were smoothed spatially using a Gaussian kernel of 6 mm.
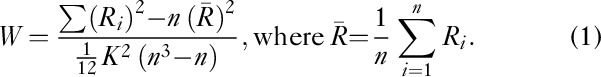 |
Statistical analysis
Statistical analysis was carried out using the software SPSS, version 17.0 (SPSS Inc., Chicago, Illinois, USA) for demographic and clinical data. An independent two-sample t-test was performed on the standardized DARTEL-enhanced VBM and ReHo maps using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). DARTEL-enhanced VBM and ReHo were reported with a two-tailed voxelwise significance level threshold of P value less than 0.01 and cluster level P value less than 0.05 with a Gaussian Random Field (GRF) correction. We used a Pearson’s correlation analysis to assess the relationship between the regional density (or amount) values, ReHo values indicating group differences and the clinical presentation of the participants, with age and sex as covariates of no interest. The statistical significance level was set at P value less than 0.05.
Results
Demographic and clinical data
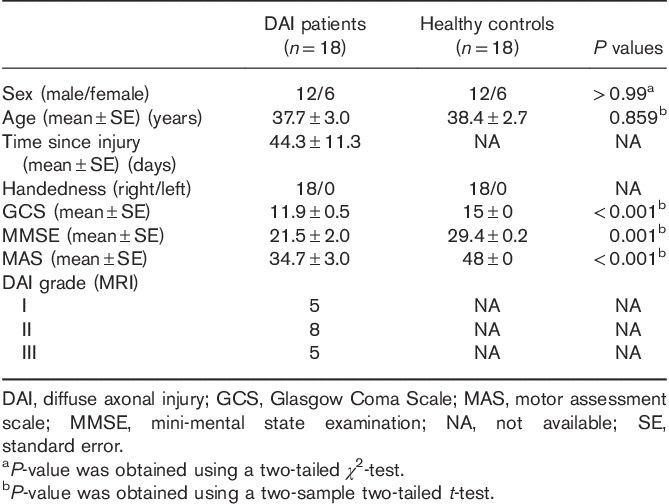
In total, 18 DAI patients and 18 well-matched HCs were enrolled in this study. Table 1 presents their demographic information and shows the clinical features of the DAI patients. The two groups showed no significant differences in age, sex, or handedness. Significantly lower values were observed in the DAI group for the evaluated clinical markers, including GCS, MMSE, and MAS, which measured the responses in the disturbance of consciousness, cognitive impairment, and movement disorder, respectively. By using SWI and diffusion-weighted imaging, DAI grade could be confirmed with lesions involving EM of the cerebral hemispheres (grade I), the corpus callosum (grade II), and the brainstem (grade III).
Table 1.
Demographics and clinical characteristics of the diffuse axonal injury patients and healthy controls
Decreased gray matter density in diffuse axonal injury patients
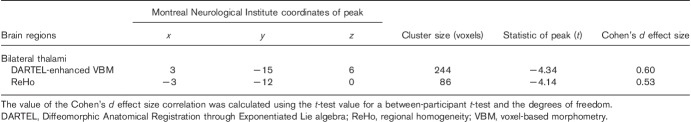
DARTEL-enhanced VBM analysis indicated that the DAI group showed a decrease in GM density in the bilateral thalami (Fig. 1a and Table 2). No significant difference in WM was present through DARTEL-enhanced VBM analysis. (voxel level P<0.01, GRF-corrected cluster level P<0.05)
Fig. 1.
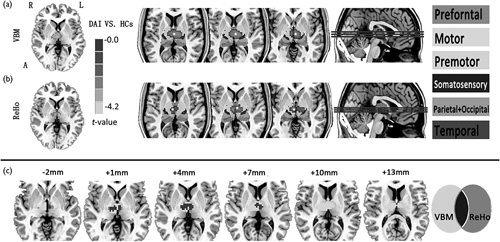
Group differences in DARTEL-enhanced VBM and ReHo between DAI patients and HCs. (a) Shows decreased grey matter density in the bilateral thalami in the DAI patients and decreased thalamic ReHo values are also observed in (b). The leftimage shows a probabilistic atlas of the subthalamic regions, segmented and coloured according to their white-matter connectivity to cortical areas 23. This template is derived from FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). Different regions (star) were overlaid with this template. (c) Shows regions of overlap between decreased DARTEL-enhanced VBM and ReHo analysis.
Table 2.
Brain regions showing a significant difference in voxel-based morphometry and the regional homogeneity map between diffuse axonal injury patients and healthy controls
Decreased thalamic regional homogeneity values in diffuse axonal injury patients
An ReHo comparative analysis was carried out on the basis of the GM mask by VBM segmentation. Decreased ReHo of the bilateral thalami, mainly located in the ventral anterior and the ventral lateral (VA and VL) nuclei, was also detected in the DAI patients compared with HCs (Fig. 1b; voxel-level P<0.01, GRF corrected cluster-level P<0.05). In addition, the abnormal region of ReHo analysis, ∼81.4% of the total voxels, was found to overlap with the bilateral thalami in the VBM analysis (Fig. 1c and Table 2). ReHo abnormalities were not found in other GM except for the thalamus.
Correlations between altered voxel-based morphometry and Regional homogeneity with clinical variables
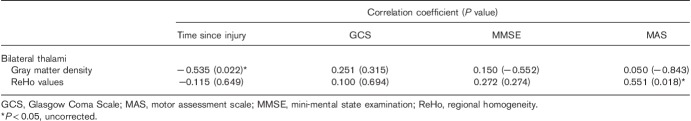
The decreased GM density in the bilateral thalami was correlated negatively with time since injury (P=0.022, uncorrected; Table 3). We also found that decreased ReHo values in the VA and VL nuclei of the thalami were correlated positively with the MAS score (P=0.018, uncorrected; Table 3).
Table 3.
Correlations between altered voxel-based morphometry, regional homogeneity, and clinical variables
Discussion
Combining morphometry and ReHo analysis, the current study shows that (a) thalamic atrophy and dysfunction occur in mild-to-moderate traumatic DAI patients within a short and midterm after trauma, and (b) time since injury is related to decreased thalamic density, and motor (MAS) is related to decreased thalamic ReHo. This evidence of structural and local functional homogeneity could potentially provide objective measures and contribute toward our understanding of post-traumatic symptoms.
In our study, decreased thalamic density was observed in the DAI group compared with HCs. The injured regions of traumatic shearing forces mostly occurred in areas mainly including the brainstem, the corpus callosum, and the junctions between white and GM. Although it is not a commonly affected area, several histopathologic studies have confirmed that injury and neuronal loss involve the thalamic nuclei 24,25. In-vivo morphometry studies and regional volume loss were also observed in the thalamus, basal ganglia, deep hemispheric nuclei, and the subcortical WM in longitudinal post-traumatic studies 5,6. The longitudinal investigations suggested that dynamic changes in cerebral areas are time dependent after trauma 26. In our study, we also found that the decreased GM density in the bilateral thalami is correlated negatively with time intervals of injury to MRI scanning. On the basis of previous studies, we speculated that the thalamus might be a kind of brain structure susceptible to atrophy.
Another finding is that DAI patients showed decreased ReHo in the VA and VL nuclei of the bilateral thalami, and the region of decreased ReHo was found to markedly overlap with the atrophic thalami in VBM analysis. The current results are consistent with previous results of functional abnormalities in the short and midterm following trauma, involving abnormal thalamic resting-state networks 27 and functional homotopy 10. The volume loss of the thalamus might alter thalamic GABAergic quantity, acting as an intermediary that can act within thalamic nuclei, 28,29 affecting the normal thalamic resting-state function. Alternatively, subtle injury-involving thalamic neurons might directly alter low-frequency fluctuations of rfMRI to reflect local functional connectivity 27. In addition, Zhang et al. 30,31 and Fair et al. 32 found that the VA and VL nuclei of the thalamus had strong structural and functional connections with the motor and the premotor cortex. In our study, Pearson’s correlation analysis showed that decreased ReHo values in the VA and VL nuclei of the thalamus were associated with worsened MAS scores. We suggest that the decreased ReHo of thalamic nuclei underlies the subtle injury, resulting in abnormal thalamocortical connectivity, and then affects motor function in patients with DAI.
Several limitations should be noted in the present study. First, our sample size was small, which precluded our ability to carry out analyses on additional patient differences, for example, whether different degrees of brain injury may cause different brain regions with abnormal densities. In addition, it is not clear to what extent WM lesions (i.e. traumatic microbleeds) may have affected GM density and function. Finally, the P-value uncorrected for the correlations analysis was a limitation. These results should be interpreted with caution.
Conclusion
These findings suggest that time since injury is related to thalamic atrophy, and thalamic local dysfunction underlies declining motor function in the short-term and mid-term periods for patients with mild-to-moderate DAI. In the future, non-invasive longitudinal studies that integrate the thalamic anatomical, functional, and diffusion MRI data are needed for a better understanding of the post-traumatic symptoms.
Acknowledgements
This study was supported by the National Science Foundation of China (grant nos 81260217, 81460263, 81560284) and the Science Foundation for Young Scientists of Jiangxi, China (grant no. 20171BAB215048).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Lin Wu and Fuqing Zhou contributed equally to the writing of this article.
References
- 1.Wang JY, Bakhadirov K, Abdi H, Devous MD, Sr, Marquez de la Plata CD, Moore C, et al. Longitudinal changes of structural connectivity in traumatic axonal injury. Neurology 2011; 77:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JH, Park SW, Kang SH, Nam TK, Min BK, Hwang SN. Detection of traumatic cerebral microbleeds by susceptibility-weighted image of MRI. J Korean Neurosurg Soc 2009; 46:365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson RR, Gattu R, Sewick B, Kou Z, Zakariah N, Cavanaugh JM, et al. Detection of hemorrhagic and axonal pathology in mild traumatic brain injury using advanced MRI: implications for neurorehabilitation. NeuroRehabilitation 2012; 31:261–279. [DOI] [PubMed] [Google Scholar]
- 4.Bruce ED, Konda S, Dean DD, Wang EW, Huang JH, Little DM. Neuroimaging and traumatic brain injury: state of the field and voids in translational knowledge. Mol Cell Neurosci 2015; 66:103–113. [DOI] [PubMed] [Google Scholar]
- 5.Warner MA, Youn TS, Davis T, Chandra A, Marquez de la Plata C, Moore C, et al. Regionally selective atrophy after traumatic axonal injury. Arch Neurol 2010; 67:1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidaros A, Skimminge A, Liptrot MG, Sidaros K, Engberg AW, Herning M, et al. Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. Neuroimage 2009; 44:1–8. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi MA, Ward MA, Hess TM, Gale SD, Dempsey RJ, Rowley HA, et al. Longitudinal changes in global brain volume between 79 and 409 days after traumatic brain injury: relationship with duration of coma. J Neurotrauma 2007; 24:766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill CS, Coleman MP, Menon DK. Traumatic axonal injury: mechanisms and translational opportunities. Trends Neurosci 2016; 39:311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil 2005; 20:76–94. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Gao L, Xie K, Zhan J, Luo X, Wang H, et al. Detection of Functional Homotopy in Traumatic Axonal Injury. Eur Radiol 2017; 27:325–335. [DOI] [PubMed] [Google Scholar]
- 11.Marquez de la Plata CD, Garces J, Shokri Kojori E, Grinnan J, Krishnan K, Pidikiti R, et al. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Arch Neurol 2011; 68:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharp DJ, Scott G, Leech R. Network dysfunction after traumatic brain injury. Nat Rev Neurol 2014; 10:156–166. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L, Zuo XN. Regional homogeneity: a multimodal, multiscale neuroimaging marker of the human connectome. Neuroscientist 2016; 22:486–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang L, Xu T, He Y, Hou XH, Wang J, Cao XY, et al. Toward neurobiological characterization of functional homogeneity in the human cortex: regional variation, morphological association and functional covariance network organization. Brain Struct Funct 2015; 220:2485–2507. [DOI] [PubMed] [Google Scholar]
- 15.Weiss N, Galanaud D, Carpentier A, Naccache L, Puybasset L. Clinical review: Prognostic value of magnetic resonance imaging in acute brain injury and coma. Crit Care 2007; 11:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iankova A. The Glasgow Coma Scale: clinical application in emergency departments. Emerg Nurse 2006; 14:30–35. [DOI] [PubMed] [Google Scholar]
- 17.Pangman VC, Sloan J, Guse L. An examination of psychometric properties of the mini-mental state examination and the standardized mini-mental state examination: implications for clinical practice. Appl Nurs Res 2000; 13:209–213. [DOI] [PubMed] [Google Scholar]
- 18.Aamodt G, Kjendahl A, Jahnsen R. Dimensionality and scalability of the Motor Assessment Scale (MAS). Disabil Rehabil 2006; 28:1007–1013. [DOI] [PubMed] [Google Scholar]
- 19.Goto M, Abe O, Aoki S, Hayashi N, Miyati T, Takao H, et al. Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra provides reduced effect of scanner for cortex volumetry with atlas-based method in healthy subjects. Neuroradiology 2013; 55:869–875. [DOI] [PubMed] [Google Scholar]
- 20.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 2009; 46:786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for ‘Pipeline’ Data Analysis of Resting-State fMRI. Front Syst Neurosci 2010; 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004; 22:394–400. [DOI] [PubMed] [Google Scholar]
- 23.Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003; 6:750–757. [DOI] [PubMed] [Google Scholar]
- 24.Ross DT, Graham DI, Adams JH. Selective loss of neurons from the thalamic reticular nucleus following severe human head injury. J Neurotrauma 1993; 10:151–165. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell WL, Pennington K, MacKinnon MA, Smith DH, McIntosh TK, Wilson JT, et al. Differential responses in three thalamic nuclei in moderately disabled, severely disabled and vegetative patients after blunt head injury. Brain 2004; 127:2470–2478. [DOI] [PubMed] [Google Scholar]
- 26.Gale SD, Baxter L, Roundy N, Johnson SC. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J Neurol Neurosurg Psychiatry 2005; 76:984–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang L, Ge Y, Sodickson DK, Miles L, Zhou Y, Reaume J, et al. Thalamic resting-state functional networks disruptionin patients with mild traumatic brain injury. Radiology 2011; 260:831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aron AR, Schlaghecken F, Fletcher PC, Bullmore ET, Eimer M, Barker R, et al. Inhibition of subliminally primed responses is mediated by the caudate and thalamus: evidence from functional MRI and Huntington’s disease. Brain 2003; 126:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steriade M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci 2005; 28:317–324. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Snyder AZ, Fox MD, Bullmore ET, Eimer M, Barker R, et al. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol 2008; 100:1740–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex 2010; 20:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, Zhang D, et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci 2010; 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]