Abstract
The aim of the current study is to investigate programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) expressions and to analyze the relationship between the expression of PD-L1 and PD-1 proteins and the molecular type, clinicopathological factors, and prognosis of invasive ductal carcinoma. We enrolled 136 patients with invasive ductal carcinoma of the breast. The expression of PD-L1 in tumor cells and that of PD-1 on paratumor-infiltrating immune cells was detected by immunohistochemistry, and the data were analyzed using SPSS software. The positive expression rates of PD-L1 and PD-1 in triple-negative breast cancer (TNBC) were 47.8 and 43.5%, which were higher than those of other subtypes (P<0.05). The expression of PD-L1 in tumor cells was correlated with the expression of estrogen receptor, progesterone receptor, and Ki-67 (P<0.05). The expression of PD-1 in the tumor-infiltrating immune cells was correlated with the expression of estrogen receptor, progesterone receptor, and Ki-67 and the histological grade (P<0.05). The expression of PD-L1 in tumor cells was correlated with the expression of PD-1 in paratumor-infiltrating immune cells (P<0.001). The expression of PD-L1 in tumor cells was found to be an independent prognostic risk factor with the progression-free survival rate for breast invasive ductal carcinoma (P=0.003). These results indicate that PD-L1 and PD-1 were highly expressed in TNBC which suggests that patients with TNBC may benefit from targeted immune therapies to a greater degree than patients with other subtypes. PD-L1 expression is an independent risk factor for breast invasive ductal carcinoma and expression of PD-L1 is expected to be a prognostic factor for breast cancer.
Keywords: programmed death-1, programmed death ligand-1, targeted immune therapy, triple-negative breast cancer
Introduction
Breast cancer is the most common female malignant tumor in the clinic. In China, the incidence of breast cancer is the highest among female malignant tumors in urban areas, showing an upward trend year by year, and the age of onset tends to be younger 1. At present, the treatment of breast cancer is divided into surgical treatment, chemotherapy, radiation therapy, targeted therapy, and endocrine therapy. However, for some special breast cancer subtypes such as triple-negative breast cancer (TNBC), it is still the difficulty and focus of research worldwide because of high invasiveness, a high recurrence rate over 3 years, poor prognosis, and single treatment.
In recent years, studies that have investigated multiple types of tumors have confirmed that the tumor microenvironment provides a favorable condition for the occurrence and development of tumors 2. Programmed death ligand-1 (PD-L1) and programmed death-1 (PD-1) are highly expressed in the tumor microenvironment. PD-1 is a type I transmembrane glycoprotein of the immunoglobulin CD28 superfamily and is a very important immune molecule 3,4. PD-1 is mainly expressed on the surface of activated T cells, B cells, monocytes, NK cells, and dendritic cells (DCs) and is a surface receptor of activated T cells. The current study of PD-1 includes two ligands: PD-L1 (B7-H1) and PD-L2 (B7-DC). The expression of PD-L2 is relatively limited. It is mainly expressed on the membrane of activated macrophages, DCs, and a few tumors 5. Studies have shown that the correlation with the clinicopathological parameters of the tumor is not significant 6. PD-L1 is expressed on activated T cells, B cells, macrophages, DCs, and various tumor cells.
PD-L1 and PD-1 interact to inhibit the proliferation and killing effect of T lymphocytes and to induce the apoptosis of T cells. PD-L1 and PD-1 interact with each other and function through the PD-1/PD-L1 pathway to inhibit the killing of tumor cells by the immune system. The discovery of the PD-1/PD-L1 pathway has made tumor immunotherapy to be applied clinically. Anti-PD-1/PD-L1 pathway immunogenic agents have led to new hope for the treatment of various solid tumors. PD-1/PD-L1 inhibitors have shown significant therapeutic effects in the treatment of various solid tumors.
PD-1/PD-L1 inhibitors have been approved by the FDA for the treatment of advanced melanoma, non-small-cell lung cancer, renal cell carcinoma, bladder cancer, and Hodgkin’s lymphoma. The efficacy of PD-1/PD-L1 immunoagents in the treatment of breast cancer has been confirmed in a detailed study on PD-1/PD-L1 and the immune escape of breast cancer cells. In 2015, at the San Antonio Breast Cancer Symposium, the first early data of the PD-1/PD-L1 immunosuppressive agent Keytruda for the treatment of metastatic TNBC were reported. Among patients with TNBC, patients with PD-L1 positive are using PD-1/PD. After L1 inhibitors, the overall remission rate reached 18.5%. PD-1/PD-L1 inhibitors show excellent antitumor activity 7.
Materials and methods
Methods
We enrolled 136 patients with breast invasive ductal carcinoma for whom paraffin tissue specimens had been deposited in the Department of Pathology at the First Affiliated Hospital of Dalian Medical University from 1 January 2013 to 30 September 2016, and who had complete clinical data. All specimens were confirmed by histopathology as breast invasive ductal carcinoma. All patients were women aged 30–84 years; the median age was 54 years. Among the patients recruited, 132 patients had undergone radical mastectomy. According to the status of sentinel lymph node biopsy during surgery, it was determined whether or not sexual axillary lymph node dissection was performed. Four patients underwent local puncture or lymph node puncture because of late clinical staging. None of the 136 patients received any form of chemotherapy, radiotherapy, endocrine therapy, or targeted therapy before surgery.
Immunohistochemical method to detect the expression of programmed death ligand-1 and programmed death-1 in tumor tissue
All breast tumor tissue specimens were fixed in methanol, embedded in paraffin, sectioned, deparaffinized, hydrated, and subjected to antigen retrieval. The immunohistochemical staining was performed using a two-step kit with the following steps: blocking, primary antibody incubation, PBS washing, secondary antibody incubation, horseradish peroxidase labeling, diaminobenzidine chromogenesis, hematoxylin staining, dehydration, and mounting in neutral resin. Rabbit anti-human PD-L1 monoclonal antibody (ab213524) was used by Abcam Technology Co. Ltd (Pudong, Shanghai, China), Mouse anti-human PD-1 monoclonal antibody (UMAB199), diaminobenzidine color reagent, and 0.01 mol/l pH 6.0 citrate buffer (2000 ml) were obtained from Beijing Zhongshan Jinqiao Biological Co. Ltd (Beijing, China).
Determination of pathological result
Estrogen receptor (ER) and progesterone receptor (PR) determination criterion was as follows: ER or PR expression greater than or equal to 1% of all tumor cell nuclei was identified as positive staining.
Human epidermal growth factor receptor 2 (HER-2) determination criteria were as follows: positive: cases with an immunohistochemical score of 3+, or with a score of 2+, but with a positive fluorescence in-situ hybridization test result; negative: those with an immunohistochemical score in the range of 0± to 1+, or a score of 2+, but with a verified negative fluorescence in-situ hybridization test result.
Ki-67 determination criteria were as follows: Ki-67 smaller than or equal to 20% was identified as low expression and Ki-67 greater than 20% was identified as high expression.
PD-L1 and PD-1 determination criteria were as follows: both PD-L1 and PD-1 were measured using a semiquantitative scoring method, and a histological analysis was carried out by combining the density of positively stained cells with the staining intensity 8,9. The entire field of view was observed under low magnification, and three high-magnification fields of view (×400) with more tumor cells were then selected from each section. The grading of the staining intensity was as follows: 0 for the absence of staining, 1 for light yellow staining, 2 for brownish yellow staining, and 3 for tawny staining. The positive cell density classification was as follows: 0 for less than 1% stained, 1 for 1–10%, 2 for 11–50%, and 3 for 51–100%. For the mean of the product of the positive cell density score and the staining intensity score, up to 1 was considered negative and more than 1 was considered positive.
Follow-up: progression-free survival (PFS) was determined by reviewing the patient’s original case data and by telephone follow-up. The deadline for follow-up was 15 February 2017. The shortest follow-up duration was 2 months and the median follow-up duration was 45.3 months.
Statistical methods
We used SPSS 23.0 (SPSS Inc., Chicago, Illinois, USA) for the statistical analysis. Numerical data were compared using the χ2-test. The expression of PD-L1 in tumor cells, that of PD-1 in paratumor-infiltrating immune cells, other clinicopathological parameters, and prognosis were analyzed by Kaplan–Meier analysis and a Cox regression analysis model. P value less than 0.05 indicated a statistically significant difference.
Results
Expression of programmed death ligand-1 in tumor cells and that of programmed death-1 in paratumor-infiltrating immune cells
PD-L1 is mainly expressed on the cell membrane and in the cytoplasm of tumor cells. In this study, the positive rate of PD-L1 expression in tumor cells was 33.1% (45/136) among the 136 patients with invasive ductal carcinoma of the breast. PD-1 is mainly expressed in paratumor-infiltrating immune cells. In this study, the positive rate of PD-1 in paratumor-infiltrating immune cells was 29.4% (40/136) among the 136 patients with invasive ductal carcinoma of the breast.
Relationships of the expression of programmed death ligand-1 in tumor cells and that of programmed death-1 in paratumor-infiltrating immune cells with the molecular tumor type
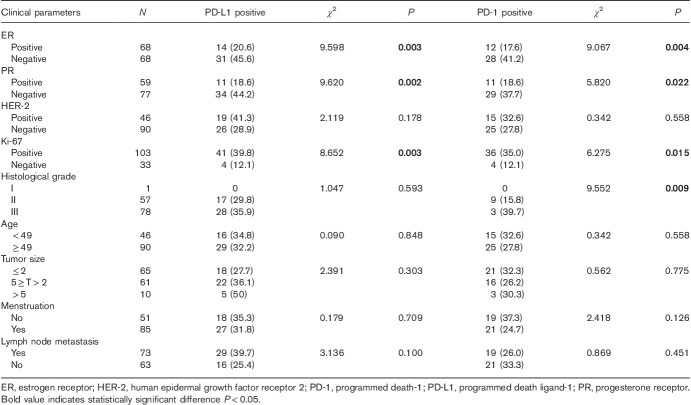
The expression of PD-L1 in tumor cells and that of PD-1 on paratumor-infiltrating immune cells differed among different molecular types of this cancer (Table 1). In TNBC, the expression rate of PD-L1 in tumor cells was 47.8% and that of PD-1 in paratumor-infiltrating immune cells was 43.5%, which were higher than the expression rates in the other subtypes; these differences were statistically significant (Fig. 1).
Table 1.
Expression of programmed death ligand-1 in tumor cells and programmed death-1 in paratumor-infiltrating immune cells in different molecular typing
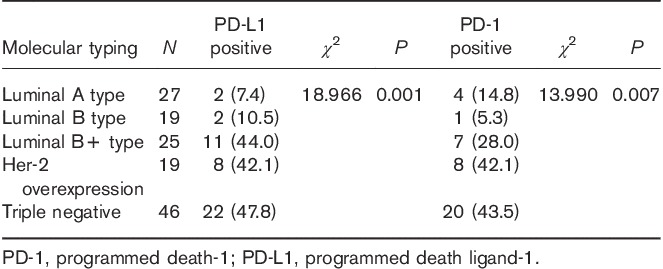
Fig. 1.
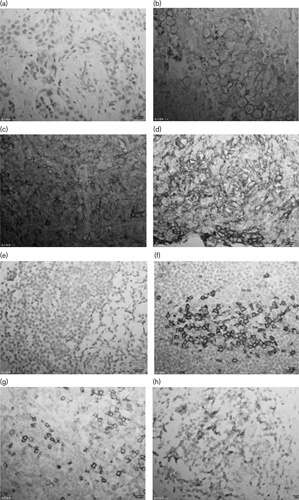
Expression of PD-L1 in in tumor cells and PD-1 in paratumor-infiltrating immune cells detected by immunohistochemical staining (magnification, ×400). (b–d) Positive expression of PD-L1 in tumor cells. (f–h) Positive expression of PD-1 in paratumor-infiltrating immune cells. (a) Negative expression of PD-L1 in tumor cells. (d) Negative expression of PD-1 in paratumor-infiltrating immune cells. PD-1, programmed death-1; PD-L1, programmed death ligand-1.
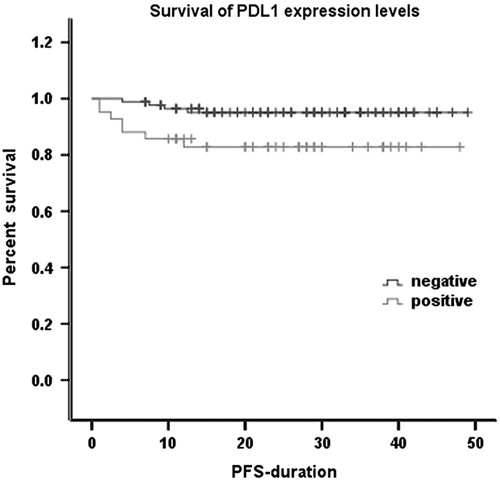
Expression of programmed death ligand-1 in tumor cells and programmed death-1 in paratumor-infiltrating immune cells and the correlation analysis of their expression and clinicopathological parameters
The expression of PD-L1 in tumor cells was correlated with the expression of ER, PR, and Ki-67 (P<0.05), but was not correlated with the expression of HER-2, histological grade, age, tumor size, menstrual status, or lymph node metastasis. The expression of PD-1 in the infiltrating immune cells around the tumor was correlated with the expression of ER, PR, Ki-67, and histological grade (P<0.05), but was not correlated with the expression of HER-2, age, tumor size, menstrual status, or lymph node metastasis (Table 2).
Table 2.
Programmed death ligand-1 in tumor cells, programmed death-1 in paratumor-infiltrating immune cells in the expression of the relationship between the clinicopathological parameters
Correlation between programmed death ligand-1 expression in tumor cells and programmed death-1 expression in paratumor-infiltrating immune cells
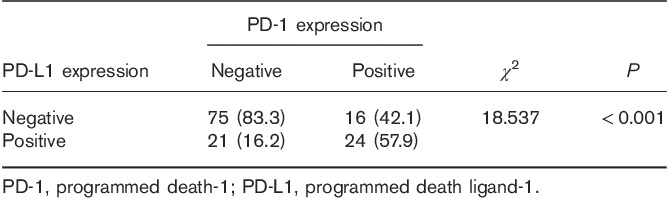
We carried out a χ2 correlation analysis to determine the correlation between PD-L1 expression on tumor cells and PD-1 expression on immune cells around the tumor. In this study, 24 (53.3%) of the 45 patients who expressed PD-L1 in tumor cells showed PD-1 expression in paratumor-infiltrating immune cells and 16 (17.6%) of the 91 patients who did not express PD-L1 in tumor cells showed PD-1 expression in paratumor-infiltrating immune cells. Patients with a positive PD-L1 expression in tumor cells had high PD-1 expression levels in the immune cells around the tumor (P<0.001; Table 3).
Table 3.
Correlation between programmed death ligand-1 expression in tumor cells and programmed death-1 expression in paratumor-infiltrating immune cells
Relationships among programmed death ligand-1 expression in tumor cells, programmed death-1 expression in paratumor-infiltrating immune cells, and clinical prognosis
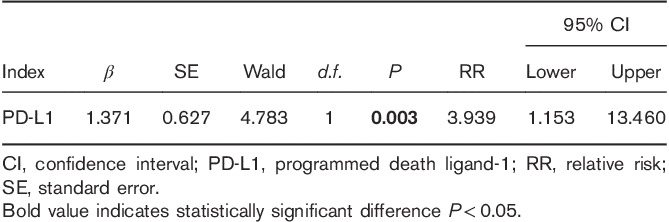
We used Kaplan–Meier analysis to carry out a single-factor survival analysis with the PFS for the 136 patients with invasive breast cancer who were enrolled. The results showed only one significant factor, PD-L1 (P=0.018; Table 4). The Kaplan–Meier survival curve of PD-L1 and PD-1 is shown in Fig. 2. Multivariate analysis using a Cox regression analysis model indicated that the expression of PD-L1 in tumor cells was found to be an independent prognostic risk factor with the PFS rate for breast invasive ductal carcinoma (P=0.003; Table 5).
Table 4.
Univariate Kaplan–Meier analysis affecting recurrence, metastasis, and death
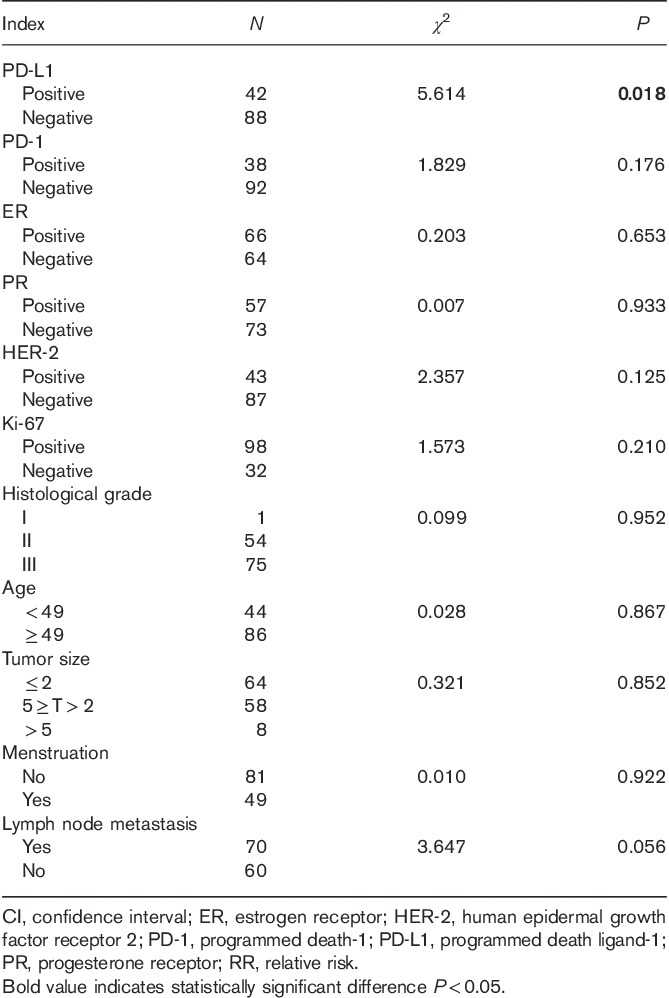
Fig. 2.
Kaplan–Meier estimates of the progression-free survival of patients with PD-L1 expression (N=130, median PFS did not reach the study endpoint, P=0.018). PD-L1, programmed death ligand-1; PFS, progression-free survival.
Table 5.
Multivariate Cox analysis affecting recurrence, metastasis, and death
Discussion
The PD-1/PD-L1 pathway is one of many inhibitory immunological checkpoints. PD-1 and PD-L1 bind to each other to express an inhibitory signal that prevents the antitumor killing activity of T cells, which is an important mechanism of tumor cell immune escape. Therefore, blocking PD-1/PD-L1 provides a new strategy for tumor immunotherapy. Studies have shown that PD-L1 expression is a predictor of the efficacy of immunotherapy. This hypothesis was supported by the American Society of Clinical Oncology in 2013. Among the 101 melanoma patients treated with nivolumab, PD-L1-positive patients showed a higher objective response rate, longer PFS, and better overall survival. Therefore, the expression of PD-L1 and its ligand PD-1 in breast cancer tissues and their significance were analyzed in this study.
This study first analyzed the expression of PD-L1 and PD-1 in breast cancer tissues. The results showed that the positive rate of PD-L1 in the tumor cells of breast cancer tissues was 33.1% and that the positive rate of PD-1 in lymphocytes around the tumor was 29.4%, of which the PD-L1-positive expression rate was similar to that of 34% found by Ghebeh et al. 10 in 44 cases of breast cancer. At present, increasingly more research on PD-L1 and PD-1 is being carried out, but mainly western women are the participants in these studies. The research data of oriental women is very rare. In this study, we included female patients in the Dalian area as participants and obtained the expression data of PD-1 and PD-L1 in breast cancer tissues, which were consistent with the current literature.
Although the current treatments have led to significant improvements in the survival time and treatment efficiency of breast cancer, some special subtypes still lack effective treatments. Three-negative breast cancer is a typical subtype lacking effective therapies. This study also found a correlation between PD-L1 expression on tumor cells and PD-1 expression on tumor-infiltrating immune cells and breast cancer molecular typing. The results showed that the positive rates of PD-1 and PD-L1 expression differed among different subtypes. However, the positive rates of expression of both were the highest in TNBC tissue, and the P values were both lower than 0.05, which indicates statistical significance, showing that the expression of PD-L1 and PD-1 in triple-negative breast cancer tissues increased. Whether this specific increase is related to the unique biological characteristics of TNBC is worth exploring.
The results showed that the PD-L1 and PD-1 expression levels were specifically increased in TNBC tissues, which suggests that immunosuppression may be related to the malignant biological behavior of TNBC. These data suggest that PD-L1 and PD-1 expression is an important part of the mechanisms of recurrence and metastasis of triple-negative breast cancer. PD-1/PD-L1 inhibitors can block the PD-1/PD-L1 negative regulatory pathway and inhibit the immune escape of tumor cells. Therefore, patients with TNBC may benefit from anti-PD-1/PD-L1-targeted immunotherapy.
This study showed that the expression of PD-1 in tumor cells and that of PD-L1 in paratumor-infiltrating immune cells were correlated with the expression of ER and PR. Whether the application of PD-1/PD-L1 inhibitors in patients with advanced breast cancer that is resistant to endocrine drugs can inhibit the progression of the disease and reverse the drug resistance is worthy of further investigation. The cell proliferation antigen Ki-67 is a nuclear antigen associated with the proliferation of cells. The expression of Ki-67 protein reflects the activity of tumor cells and is highly correlated with the development, metastasis, and prognosis of malignant tumors 11. This study found that PD-1 and PD-L1 expression was correlated with Ki-67 expression. This suggests that the expression of PD-L1 in breast cancer cells with strong proliferative activity may be upregulated by certain mechanisms, which would facilitate the immune escape of tumor cells. However, this mechanism should be investigated further.
Many studies have shown that the expression of PD-L1 and PD-1 is correlated negatively with prognosis in many solid tumor tissues and is one of the risk factors that affect the prognosis of cancer patients. In gastric cancer, Chen et al. 12 found that the higher the expression level of PD-L1 in gastric cancer tissues, the deeper the depth of infiltration of tumor tissues. Furthermore, the expression of PD-L1 in tumor cell lines was correlated positively with the invasiveness of tumor cell lines. Also in breast cancer, there is a direct correlation between the expression of PD-L1 in tumor tissue and tumor proliferation, which is consistent with the results of this study. In this study, we found that the expression of PD-L1 in tumor cells was correlated with the expression of PD-1 in paratumor-infiltrating immune cells. This result suggests a certain correlation between the expression of PD-L1 and that of PD-1 in the tumor microenvironment. In addition, these two proteins interacted with each other to generate a synergistic effect, which inhibited the attack on tumor cells by the immune system. By Kaplan–Meier analysis and Cox regression analysis, we showed that the expression of PD-L1 was an independent risk factor for the prognosis of breast cancer patients. Patients with PD-L1-positive breast invasive ductal carcinoma had an increased risk of recurrence and metastasis, which suggests that a reduction in PD-L1 expression or a reduction in the number of PD-L1-positive cells involved in the immune response could possibly be associated with a survival benefit in these patients.
Conclusion
Our results are consistent with the results of most previous studies. The expression of PD-L1 in tumor cells and that of PD-1 in tumor-infiltrating immune cells in breast invasive ductal carcinoma were closely correlated with the expression of Ki-67, as well as with the expression of ER and PR in tumors. These findings suggest that PD-L1 and PD-1 expression plays a very important role in the invasion, metastasis, immune escape, and other processes of breast invasive ductal carcinoma. Furthermore, patients with invasive ductal carcinoma with high expression of PD-L1 and PD-1 had a poor prognosis. However, because of the limited follow-up duration, this study only analyzed the progression-free survival as it related to PD-L1 and PD-1, but did not measure the total survival time. In addition, the total number of patients who experienced recurrence was 11 and the number of patients who achieved the endpoint was low; these findings as they related to PD-L1 and PD-1 expression were not statistically significant. Further follow-up observation should be performed in the future. In this study, we found that PD-L1 and PD-1 were expressed highly in TNBC. Future studies should enroll more TNBC cases to investigate the expression of PD-L1 and PD-1 in TNBC to determine its relationship with prognosis and to obtain more meaningful clinical data.
Acknowledgements
This work was supported by the First Affiliated Hospital Dalian Medical University, Department Oncology, (no. 193 United Road, Dalian City, Dalian 116000, China). This work was supported by the National Natural Science Foundation of China (No. 81341060).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol 2014; 15:279–289. [DOI] [PubMed] [Google Scholar]
- 2.Fridman WH. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298. [DOI] [PubMed] [Google Scholar]
- 3.Bachy E, Coiffier B. Anti-PD1 antibody:a new approach to treatment of lymphomas. Lancet Oncol 2014; 15:7. [DOI] [PubMed] [Google Scholar]
- 4.Gunturi A, Mcdermott DF. Potential of new therapies like anti-PD1 in kidney cancer. Curr Treat Options Oncol 2014; 15:137–146. [DOI] [PubMed] [Google Scholar]
- 5.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20:5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatam LJ, Devoti JA, Rosenthal DW, Lam F, Abramson AL, Steinberg BM. Immune suppression in premalignant respiratory papillomas: enriched functional CD4+Foxp3+ regulatory T cells and PD-1/PD-L1/L2 expression. Clin Cancer Res 2012; 18:1925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Hu Z, Wang Q, Ge Y, Bai L, Wang X. Generation and characterization of four novel monoclonal antibodies against human programmed death-1 molecule. Hybridoma 2010; 29:153–160. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine 2011; 56:231–238. [DOI] [PubMed] [Google Scholar]
- 9.Xie F, Wang Q, Chen Y, Gu Y, Mao H, Zeng W. Costimulatory molecule OX40/OX40L expression in ductal carcinoma in situ and invasive ductal carcinoma of breast. An immunohistochemistry-based pilot study. Pathology 2010; 206:735–739. [DOI] [PubMed] [Google Scholar]
- 10.Ghebeh H, Mohammed S, Alomair A, Qattan A, Lehe C, Alqudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 2006; 8:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez AF, Nunez MJ, Piqueras V, Lucas AR, Sanchez J, Tejerina A, Schneider J. Flow cytometry vs. Ki-67 labelling index in breast cancer: a prospective evaluation of 181 cases. Anticancer Res 2002; 22:295–298. [PubMed] [Google Scholar]
- 12.Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang J, Wu C. B7-H1 expression associates with tumor invasion and predicts patient’s survival in human esophageal cancer. Int J Clin Exp Pathol 2014; 7:6015–6023. [PMC free article] [PubMed] [Google Scholar]