Abstract
Objective
Age at onset of walking has been shown as an early predictor of physical activity in infants and children. However, little is known about whether age at onset of walking may predict sedentary behavior (SB). The aim of the present study was to examine the association between the timing of onset of walking and objectively measured SB, and whether this association is mediated by moderate-to-vigorous physical activity (MVPA) in children.
Methods
The subjects were 388 elementary school children aged 6–12 years. Current weight and height data were collected. Birth weight and the age in months the child first walked independently were reported based on the parents’ recall. Children’s SB and physical activity were objectively measured using a triaxial accelerometer (Active style Pro HJA-350IT, OMRON). The following summary outcome variables were derived from accelerometer data: Time (min/day) spent in SB (≤1.5 metabolic equivalents [METs]) and MVPA (≥3.0 METs).
Results
The mean ± SD time (min/day) spent in sedentary was 376 ± 62 and MVPA was 67.6 ± 20.8. Multiple linear regression analyses revealed that a later age at independent walking was associated with increased time spent in SB (β = 0.15, P < 0.001) and decreased time spent in MVPA (β = -0. 18, P < 0.001) after adjusting for gender, birth weight, current age, body weight, schools, and time spent wearing the accelerometer. When MVPA was introduced as a covariate in the model predicting SB, the association between the age at independent walking and time spent in SB was completely attenuated (β = 0.04, P = 0.215), while MVPA was significantly associated with SB (β = -0.61, P < 0.001).
Conclusions
Our results indicate that infants who walked at a later age spent more time in SB in childhood, and this association is mediated by MVPA. Appropriate interventions which focus on increasing MVPA and thereby reducing SB may be beneficial in infants who demonstrate a later age at onset of independent walking.
Introduction
Numerous epidemiological studies have identified consistent associations between physical inactivity, defined as “not performing sufficient amounts of moderate-to-vigorous physical activity (MVPA)”[1], and all-cause and cardiovascular disease mortality [2,3].
Sedentary behavior (SB), defined as “any waking activity characterized by an energy expenditure ≤1.5 metabolic equivalents and a sitting or reclining posture” [4], has also increasingly been recognized as an important risk factor associated with health outcomes [5]. Recent studies have shown that having a high level of SB negatively impacts health after considering the benefits of MVPA [6]. SB is an independent risk factor for diseases and is therefore worth considering in addition to MVPA. Sedentary lifestyles have become major health problem worldwide, and the identification of factors which determine SB is of great importance to public health.
Previous reviews have shown that SBs [7] and physical activities [8] track at moderate levels from childhood, suggesting that SB and physical activity (PA) during childhood may have a particularly important role over an individual’s life course. Thus, a better understanding of the determinants of children’s SB and PA is needed in order to effectively decrease SB and increase PA across the lifespan. Systematic reviews have mainly focused on environmental [9], psychological, social, and behavioral [10,11] factors as correlates of SB and PA during childhood and adolescence. Recent evidence also suggests that early-life factors make a significant contribution to SB [12] and PA [13] in young people, and a few studies have shown that indicators of motor development were an early predictor of PA in children [14–16].
A birth cohort study by Ridgway et al. (2009) reported that an earlier age at standing unaided and walking supported in infancy predicts higher levels of PA as indicated by an increased frequency of sports participation during adolescence [14]. Mattocks et al. (2008) reported motor coordination at 6 months, which was based on combined score from 12 questions, was positively associated with objectively measured PA (cpm) using accelerometry in children aged 11–12 years [15]. Associations between age at onset of walking and objectively measured PA patterns already exist in the first two years [17,18]. Hnatiuk et al. (2013) reported that 19-month-old toddlers who walked earlier had a higher total time spent in light-to-vigorous-intensity PA than those who walked later, regardless of how long the toddler had been walking [17]. A study by Prioreschi et al. (2017) reported diurnal distributions of mean vector magnitude by developmental stage and showed that in most cases walkers were more active during the day than crawlers, even after adjusting for age [18]. Therefore, these associations between age at onset of walking and PA during the first two years may carry over to childhood.
We also recently reported that a later age at onset of independent walking predicts lower levels of MVPA (min/day) measured by an accelerometer in 6- to 12-year-old children [16]. However, no studies have shown significant associations between indicators of motor development and SB. It’s important that increased SB and decreased MVPA often occur in combination, although these factors are mutually independent to some degree [19]. Therefore, it is plausible that a later age at onset of walking may also predict increased time spent in SB. However, interventions designed to increase PA or MVPA generally resulted in reductions in sedentary time [20,21]. These studies suggest that the differences in sedentary time may be partly caused by the amount of MVPA in an individual. Therefore, we hypothesized that MVPA might mediate a potential association between age at independent walking and SB.
In order to develop successful strategies to prevent prolonged sedentary time during childhood, it is essential to elucidate the substantial role of the timing of onset of walking in the determining levels of SB in later life, taking MVPA into consideration. The aim of the present study was to examine the association between the age at onset of independent walking and objectively measured SB, and whether this association is mediated by MVPA in children.
Materials and methods
Study subject
The subjects were Japanese primary school children, who were recruited from 14 primary schools in urban areas of Tokyo, Kanagawa, and Kyoto prefectures. The anthropometry, accelerometry, and questionnaire data were collected from June 2012 to January 2015 during the school year.
A total of 569 individuals participated in this study. A questionnaire filled out by parents was used to evaluate the children’s medical history, bedtime and wake-up time, age at onset of independent walking, and birth weight. Those who rescinded their consent (n = 8), had no questionnaire data (n = 16), or had a history of conditions affecting PA such as respiratory disease or heart disease (n = 28) were excluded. Additional subjects were excluded if the accelerometer data did not conform with the study criteria (see below) (n = 91). A final data set for 388 children was used for subsequent analyses after the exclusion of children with incomplete data on age at onset of independent walking (n = 24) including an outlier (≥25 months) [22], those born with a very low birth weight (<1.5 kg) (n = 2), and those who were multiples (n = 12).
The research project was approved by the Ethical Committee of Oberlin University (receipt number: 12023). The study procedures were explained in writing to all children and parents, and written informed consent was obtained from each participant and his/her parents.
Age at independent walking and birth weight
Information on independent walking and birth weight in children was retrospectively reported on a questionnaire according to the parents’ recall. The parents were asked to provide their children’s birth weight and the age in months when their children were first able to walk without assistance [23,24]. Birth weight was investigated as a factor potentially related to SB [25] and PA [13].
Sedentary behavior and physical activity
Daily SB and PA were objectively measured using a triaxial accelerometer (Active style Pro HJA-350IT, Omron Healthcare, Kyoto, Japan; dimensions 74 × 46 × 34 mm and weight 60 g including batteries) for seven consecutive days. The device is described in detail elsewhere [26]. The subjects wore the accelerometer on the left side of the waist and were requested to wear the device at all times except under special circumstances such as dressing, bathing, and swimming. An epoch of 10 seconds was used, and the data were converted using following conversion equations for primary school children based on the results of Hikihara et al. (2014), because the metabolic equivalent (MET) values recorded by the accelerometer are overestimated in primary school children [26].
Ambulatory activities (e.g., walking and running) and non-locomotive activities (e.g., playing games, cleaning, playing with blocks, tossing a ball, and aerobic dance) were discriminated based on the ratio of unfiltered synthetic acceleration to filtered synthetic acceleration [26]. Filtered synthetic acceleration was defined as the integrated acceleration ((X2+ Y2+ Z2)0.5) after the gravitational acceleration was removed from each dimensional acceleration (X, Y, Z) by passing it through a second-order Butterworth high-pass filter with a cut-off frequency of 0.7 Hz [27].
We analyzed data collected between 7:00 and 21:00 to exclude sleep. The average (± SD) bedtime and wake-up time of children assessed by a questionnaire were 21:27 ± 2:00 and 6:49 ± 0:27, respectively, in this study. Therefore, we determined the time window for the analyses as above (between 7:00 and 21:00). Awake data were partly excluded but data accumulated during sleep were not included in the analyses in many cases. It was difficult to discriminate the bedtime and wake-up time of each child day by day. If sleep periods were included in the time window (7:00 to 21:00), misclassifications of sleep into SB would increase, while excluding awake periods between 21:00 and 7:00 from the analyses would hardly affect duration of MVPA because little MVPA is observed during the time immediately after wake-up and before bedtime. Subjects with data obtained from wearing the accelerometer >10 hours on at least two weekdays and at least one weekend day [28,29] were included in the analysis. Periods with >60 min of consecutive zero counts (no signal) were classified as “no wearing time”.
Three variables were analyzed in this study: time (min/day) spent in SB (≤1.5 METs), light PA (LPA, 1.6–2.9 METs), and MVPA (≥3.0 METs). The mean weekly values were then calculated. The mean values were calculated by weighting for five weekdays and two weekend days (weighted data = ([mean for weekdays × 5] + [mean for weekend days × 2]) / 7).
Anthropometry
Body height and weight were measured without shoes, but with clothing to the nearest 0.1 cm and 0.1 kg, respectively. We used scales that were typically used in primary schools; otherwise, we brought a “Karada Scan HBF-370” (Omron Healthcare, Kyoto, Japan) scale into the schools and used it. Net body weight was calculated as the measured body weight minus the weight of the clothing. We used 0.5 kg as the weight of clothing in all children except for children who underwent measurements in physical exercise uniforms; their light clothing was regarded as weighing 0.35 kg. The weight used for clothing was determined by weighing typical children’s clothing.
Statistical analyses
The measured and calculated values are presented as means ± standard deviations (SD). Student’s t-test was used to investigate potential differences between boys and girls. Partial correlation analysis was used to test the relationships between study variables controlled for gender and months of age as covariates. Multiple linear regression analyses were performed to assess the associations between age at onset of independent walking and SB or PA. We first entered the age at independent walking (Model 1) and then added the birth weight and current weight as independent variables to examine how the association is altered by body weights (Model 2). In order to investigate whether MVPA acts as a mediator in the association between motor development and SB, we finally introduced MVPA as a covariate in the model for predicting SB according to the methods suggested by Baron and Kenny [30].
For illustrative purposes, the age at onset of walking was categorized into three groups according to tertiles: early (10.4 ± 0.7 months), middle (12.4 ± 0.5 months), and late (15.4 ± 2.0 months). Linear trends of SB, LPA, and MVPA were evaluated using an ordinal variable for the three groups [31]. All models were adjusted for gender, birth weight, current weight, months of age, interaction term (gender × months of age), schools, and accelerometer wearing time.
The statistical analyses were performed using IBM SPSS statistics 22.0 for Windows (IBM Japan Ltd., Tokyo, Japan). The statistical significance level was set at P < 0.05.
Results
The characteristics of the subjects are shown in Table 1. The average age of the subjects was 111.8 ± 19.4 months for boys and 112.0 ± 19.0 months for girls. No significant differences between boys and girls were observed in current age, height, weight, or the age at which they walked independently in infancy. Birth weight was slightly higher in boys than girls (95% CI: 7.5, 161.2). Boys showed higher MVPA values compared with girls (95% CI: 13.3, 20.9), whereas SB was significantly lower in boys than in girls (95% CI: -26.3, -1.6).
Table 1. Characteristics of the 388 children aged 6–12 years.
| Variables | All (n = 388) | Boys (n = 179) | Girls (n = 209) | P value† | |
|---|---|---|---|---|---|
| Months of age (mos.) | 111.9 ± 19.2 | (73–151) | 111.8 ± 19.4 | 112.0 ± 19.0 | 0.929 |
| Height (cm) | 132.5 ± 10.7 | (107.5–163.5) | 132.4 ± 10.0 | 132.6 ± 11.3 | 0.804 |
| Weight (kg) | 29.2 ± 7.6 | (16.4–57.2) | 29.7 ± 7.9 | 28.7 ± 7.2 | 0.178 |
| Birth weight (g) | 3045 ± 385 | (1678–4288) | 3090 ± 376 | 3006 ± 390 | 0.032 |
| Age at independent walking (mos.) | 12.8 ± 2.3 | (8–24) | 12.9 ± 2.4 | 12.8 ± 2.2 | 0.414 |
| 8 mos. (%) | 0.5 | 0.6 | 0.5 | ||
| 9 mos. (%) | 2.6 | 2.2 | 2.9 | ||
| 10 mos. (%) | 8.2 | 8.4 | 8.1 | ||
| 11 mos. (%) | 15.5 | 14.5 | 16.3 | ||
| 12 mos. (%) | 25.8 | 26.8 | 24.9 | ||
| 13 mos. (%) | 14.7 | 12.8 | 16.3 | ||
| 14 mos. (%) | 13.7 | 13.4 | 13.9 | ||
| 15 mos. (%) | 10.1 | 10.1 | 10.0 | ||
| 16 mos. (%) | 2.8 | 3.4 | 2.4 | ||
| 17 mos. (%) | 2.1 | 3.4 | 1.0 | ||
| 18 mos. (%) | 1.8 | 2.2 | 1.4 | ||
| ≥19 mos. (%) | 2.3 | 2.2 | 2.4 | ||
| SB (min/day) | 376 ± 62 | (204–550) | 368 ± 59 | 382 ± 64 | 0.027 |
| LPA (min/day) | 359 ± 50 | (219–516) | 354 ± 48 | 363 ± 51 | 0.060 |
| MVPA (min/day) | 67.6 ± 20.8 | (17.6–134.5) | 76.8 ± 20.0 | 59.7 ± 18.1 | <0.001 |
Data are means ± SD (range) or proportions.
SB, sedentary behavior; LPA, light physical activity; MVPA, moderate-to-vigorous physical activity.
† P values were calculated for gender difference by t-test.
Table 2 shows the partial correlation matrix between early life factors (birth weight and age at onset of independent walking) and current measures (height, weight, SB, LPA, and MVPA) controlled for gender and months of age as covariates. Birth weight was positively and weakly correlated with current height (P < 0.01) and weight (P < 0.05). The age at independent walking was weakly correlated positively with current weight (P < 0.05) and SB (P < 0.01) and inversely with MVPA (P < 0.001), but these associations were weak. Current height (P < 0.05) and weight (P < 0.01) were also weakly associated with SB and LPA. SB was strongly associated with LPA and moderately associated with MVPA (P < 0.001). LPA was weakly associated with MVPA (P < 0.001).
Table 2. Partial correlation matrix controlled for gender and months of age.
| Variables | Age at independent walking (mos.) | Height (cm) | Weight (kg) | SB (min/day) | LPA (min/day) | MVPA (min/day) |
|---|---|---|---|---|---|---|
| Birth weight (g) | -0.030 | 0.148** | 0.125* | -0.039 | 0.012 | 0.052 |
| Age at independent walking (mos.) | -0.077 | -0.111* | 0.149** | -0.074 | -0.200*** | |
| Height (cm) | 0.722*** | 0.123* | -0.125* | -0.055 | ||
| Weight (kg) | 0.158** | -0.150** | -0.073 | |||
| SB (min/day) | -0.731*** | -0.575*** | ||||
| LPA (min/day) | 0.352*** |
* P < 0.05.
** P < 0.01.
*** P < 0.001.
SB, sedentary behavior; LPA, light physical activity; MVPA, moderate-to-vigorous physical activity.
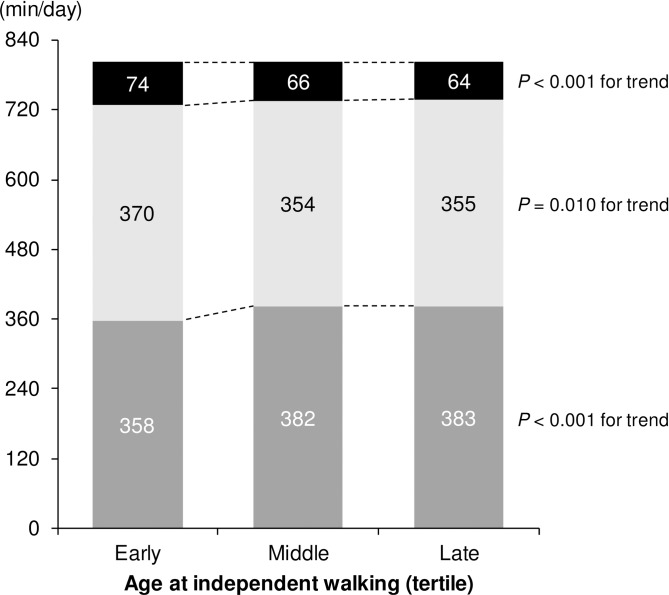
Table 3 presents the results of the multiple linear regression analyses used to examine the associations of age at independent walking with SB and PA. As shown in model 1, the age at independent walking was positively associated with SB (β = 0.13, 95% CI: 4.40, 17.37) and inversely associated with MVPA (β = -0.17, 95% CI: -6.72, -2.35) after adjusting for gender, months of age, interaction term (gender × months of age), schools, and accelerometer wear time. A further adjustment for birth weight and current weight made little difference in the relationships between age at independent walking and SB (β = 0.15, 95% CI: 5.43, 18.30) and MVPA (β = -0.18, 95% CI: -6.93, -2.56) (model 2 and Fig 1).
Table 3. Multiple linear regression analyses with SB or PA as the dependent variable.
| Independent variables | SB (min/day) | LPA (min/day) | MVPA (min/day) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B | β | P | B | β | P | B | β | P | |
| Model 1 | |||||||||
| Age at independent walking (mos.) | 10.88 | 0.13 | 0.001 | -6.35 | -0.10 | 0.022 | -4.54 | -0.17 | <0.001 |
| Model 2 | |||||||||
| Age at independent walking (mos.) | 11.86 | 0.15 | <0.001 | -7.12 | -0.11 | 0.010 | -4.74 | -0.18 | <0.001 |
Model 1: Adjusted for gender, months of age, interaction (gender × months of age), schools, and accelerometer wear time.
Model 2: As Model 1 plus birth weight and current weight.
B, unstandardized regression coefficient; β, standardized regression coefficient.
SB, sedentary behavior; LPA, light physical activity; MVPA, moderate-to-vigorous physical activity.
Fig 1. Estimated means of SB and PA among tertiles of the age at independent walking.
(Dark gray) SB. (Light gray) LPA. (Black) MVPA. Adjusted for gender, months of age, birth weight, current weight, interaction (gender × months of age), schools, and accelerometer wear time. SB, sedentary behavior; LPA, light physical activity; MVPA, moderate-to-vigorous physical activity.
When MVPA was introduced as a covariate in the model predicting SB (Table 4), the association with the age at independent walking was completely attenuated and ceased to be significant (β = 0.04, 95% CI: -1.92, 8.49) and MVPA was significantly associated with SB (β = 0.61, 95% CI: -2.05, -1.57).
Table 4. Multiple linear regression analysis with SB as the dependent variable.
| Independent variables | SB (min/day) | ||
|---|---|---|---|
| B | β | P | |
| Age at independent walking (mos.) | 3.29 | 0.04 | 0.215 |
| MVPA (min/day) | -1.81 | -0.61 | <0.001 |
Adjusted for gender, months of age, birth weight, current weight, interaction (gender × months of age), schools, and accelerometer wear time.
B, unstandardized regression coefficient; β, standardized regression coefficient.
SB, sedentary behavior; MVPA, moderate-to-vigorous physical activity.
We also examined the associations between age at independent walking and SB, LPA, and MVPA in boys and girls separately (S1–S3 Tables). As a result, similar results were obtained in both genders, except that the association between the age at independent walking and LPA was not significant in boys (β = -0.07, P = 0.335 for model 1; β = -0.09, P = 0.199 for model 2), while the association was still significant in girls (β = -0.12, P = 0.029 for model 1; β = -0.13, P = 0.024 for model 2) (S2 Table).
Discussion
This study was performed to examine the association between age at onset of independent walking and SB in children and to test whether this association is mediated by MVPA. The main finding of this study was that later age at independent walking was significantly (albeit weakly) associated with increased time spent in SB. To our knowledge, only one study by Wijtzes et al. (2013) has investigated the association between motor development and SB, and the investigators found no association between having a delayed gross motor development at one year and sedentary time in two-year-old toddlers [32]. As such, we believe that the present study is the first to show the link between an indicator of infant motor development and objectively measured SB. The difference in accelerometry may be a potential reason for the discrepancy between our findings and those of Wijtzes et al. (2013); They used a uniaxial accelerometer (ActiGraph) during one weekday and one weekend day in their study [32], while we used a triaxial accelerometer during seven days. The mean wearing days of accelerometer in our study (6.3 ± 1.0 days) was considerably greater than the minimum criteria (at least three days) which is required for reliable PA monitoring in young children [28,29]. Our results indicate that on average, each increase of one month in a child’s age at independent walking was associated with almost 12 min/day more of SB (Table 3, model 2). This may suggest important clinical implications, given that the age at which children first walk independently is distributed over more than a nine-month range [22].
However, an important possibility to be considered was that MVPA may play a mediator role in the observed association between age at independent walking and SB in model 2. To elucidate the substantial association between the timing of onset of independent walking and SB, we tested whether this association is mediated by MVPA. As a result, it was found that the association between the age at independent walking and the time spent in SB was completely mediated by MVPA, and MVPA was significantly associated with SB (Table 4). This means that the association between age at independent walking and SB in model 2 is caused by indirect effect through MVPA. Thus, it is plausible that the timing of onset of walking in infancy may influence the amount of MVPA in childhood, and then MVPA may cause the differences in sedentary time.
Independent walking, or upright and bipedal walking, a unique distinction of the human species, is the major motor developmental task during the first two years of life [33]. Age at onset of walking has often been used as an indicator of the progress of motor development in early life [14,17] and been shown to be associated with health risks in later life, such as bone strength [34] and blood pressure [35]. The age at which a child first walks independently varies widely from person to person and typically ranges from 8 months to 17–18 months, while 2.7% of children were not be able to walk independently at 24 months in a healthy sample [22]. Muscle strength and balance-control are thought to be rate-limiting factors for the onset of walking [33]; however, the timing of the onset of walking is modifiable. Exercise intervention such as stimulations to facilitate walking accelerate the development of walking [36]. Zelazo et al. (1972) showed that a few minutes of daily stepping practice with an upright posture over several weeks result in the onset of walking occurring 1.3–2.2 months earlier compared with control groups [37]. A more recent study has also shown that nutritional intervention improves gross motor development in small infants [38]. It is currently unclear whether these interventions to accelerate the gross motor development may have long-term benefits.
In the present study, the earlier age at onset of walking was associated with spending more time in MVPA in children (Table 3). This result is in line with previous studies showing that walking at an earlier age is associated with higher PA levels in infants and toddlers [17,18]. Although the detailed mechanisms by which the timing of onset of walking has an association with the amount of MVPA are not fully understood, some factors associated with child-rearing practices (e.g., encouragement to move by parents or caregivers) may carry over to the childhood years and therefore may increase MVPA. Another possibility is that potential motor proficiency may be associated with both the timing of the development of walking and the amount of MVPA. The link between higher motor proficiency and increased PA is widely known [39,40]. A few studies have shown a relation between earlier infant motor development and higher motor proficiency in later life [23,24,41]. Ridgway et al. (2009) reported that the age at which infants stand unaided and walking supported were associated with muscle strength, muscle endurance, and cardiorespiratory fitness at the age of 31 years [41]. Kuh et al. (2006) found that the age at which a child walked was a predictor of standing balance, chair rises [23], and muscle performance [24] at the age of 53 years. Prospective studies that include the assessment of factors associated with child-rearing practices and motor fitness would be beneficial to elucidate a causal association between the development of walking and PA patterns in later life.
In the case of analyzing by gender, the association between motor development and LPA was no longer significant in boys, while girls still showed a significant association. It is not clear why LPA was not predictive in boys, however, fewer sample size for boys would be partly related to these results and otherwise the patterns of LPA may be more strongly associated with other determinants such as psychological, social, and behavioral factors in boys.
The present study has several limitations. First, we relied on the parents’ recall for information on birth weight and the age at which the children walked independently. Registered birth weight has been shown to be in good agreement with birth weight recalled by mothers of school children age 8–11 years (interclass coefficient = 0.95, mean differences = 1.2 g) [42]. The reliability of age at walking recalled by parents of children six years of age and older is unknown [43]. As this variable is also based on parental reports rather than on an objective assessment, reporting bias may exist. In addition, this parameter is also accompanied by a problem of definition: independent walking has been variously defined as walking two or three steps without support [44] or walking at least five steps independently [45], or it was not specifically defined [34]. Therefore, recall bias, reporting bias, and the definition problem might potentially influence the data. Nevertheless, the 10th, 50th, and 90th percentile values for age at the onset of independent walking in this study (10.0, 12.0, and 15.0 mos., respectively) were almost the same as in the WHO Motor Development Study (10.0, 12.0, and 14.4 mos., respectively) [22] in that motor development was objectively assessed. Second, as this was a retrospective study, long term follow-up studies are needed to confirm the influence of development timing of onset of walking on PA and SB in later age. While this remains to be investigated, this study provides valuable information among infants with a later age at onset of independent walking.
Conclusion
In summary, the present study showed that a later age at onset of walking in infancy was associated with prolonged sedentary time in childhood. However, when MVPA was introduced as a covariate, this association was completely mediated by MVPA. MVPA was significantly associated with SB. These results indicate that a later age at onset of independent walking may have a negative influence on MVPA, with an associated increase in sedentary time in children. This suggests that the timing at which a child walked for the first time in early life may have long-term implications for subsequent activity patterns. Our findings also suggest that appropriate interventions which focus on increasing MVPA and thereby reducing SB may be beneficial in infants who demonstrate a later age at onset of independent walking.
Supporting information
* P < 0.05; ** P < 0.01; *** P < 0.001. SB, sedentary behavior; LPA, light physical activity; MVPA, moderate-to-vigorous physical activity.
(PDF)
Model 1: Adjusted for months of age, schools, and accelerometer wear time. Model 2: As Model 1 plus birth weight and current weight. B, unstandardized regression coefficient; β, standardized regression coefficient. SB, sedentary behavior; LPA, light physical activity; MVPA, moderate-to-vigorous physical activity.
(PDF)
Adjusted for months of age, birth weight, current weight, schools, and accelerometer wear time. B, unstandardized regression coefficient; β, standardized regression coefficient. SB, sedentary behavior; MVPA, moderate-to-vigorous physical activity.
(PDF)
Acknowledgments
The authors would like to thank the participants for their cooperation in the study. We also wish to thank the staff of the National Institute of Health and Nutrition for their help with the survey.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by JSPS KAKENHI Grant Number JP24500832 to CT (https://kaken.nii.ac.jp/en/grant/KAKENHI-PROJECT-24500832/) and JP13J07359 to TA (https://kaken.nii.ac.jp/en/grant/KAKENHI-PROJECT-13J07359/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.van der Ploeg HP, Hillsdon M. Is sedentary behaviour just physical inactivity by another name? Int J Behav Nutr Phys Act. International Journal of Behavioral Nutrition and Physical Activity; 2017;14: 1–8. 10.1186/s12966-016-0456-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose–response meta-analysis of cohort studies. Int J Epidemiol. 2011;40: 1382–1400. 10.1093/ije/dyr112 [DOI] [PubMed] [Google Scholar]
- 3.Diep L, Kwagyan J, Kurantsin-Mills J, Weir R, Jayam-Trouth A. Association of Physical Activity Level and Stroke Outcomes in Men and Women: A Meta-Analysis. J Women’s Heal. 2010;19: 1815–1822. 10.1089/jwh.2009.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedentary Behaviour Research Networ. Letter to the Editor: Standardized use of the terms “sedentary” and “sedentary behaviours.” Appl Physiol Nutr Metab. 2012;37: 540–542. 10.1139/h2012-024 [DOI] [PubMed] [Google Scholar]
- 5.Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary Behaviors and Subsequent Health Outcomes in Adults. Am J Prev Med. 2011;41: 207–215. 10.1016/j.amepre.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 6.Matthews CE, George SM, Moore SC, Bowles HR, Blair A, Park Y, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am J Clin Nutr. 2012;95: 437–45. 10.3945/ajcn.111.019620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biddle SJH, Pearson N, Ross GM, Braithwaite R. Tracking of sedentary behaviours of young people: A systematic review. Prev Med (Baltim). 2010;51: 345–351. 10.1016/j.ypmed.2010.07.018 [DOI] [PubMed] [Google Scholar]
- 8.Telama R. Tracking of physical activity from childhood to adulthood: a review. Obes Facts. 2009;2: 187–195. 10.1159/000222244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding D, Sallis JF, Kerr J, Lee S, Rosenberg DE. Neighborhood Environment and Physical Activity Among Youth. Am J Prev Med. 2011;41: 442–455. 10.1016/j.amepre.2011.06.036 [DOI] [PubMed] [Google Scholar]
- 10.Van Der Horst K, Paw MJCA, Twisk JWR, Van Mechelen W. A brief review on correlates of physical activity and sedentariness in youth. Med Sci Sports Exerc. 2007;39: 1241–1250. 10.1249/mss.0b013e318059bf35 [DOI] [PubMed] [Google Scholar]
- 11.Stierlin AS, De Lepeleere S, Cardon G, Dargent-Molina P, Hoffmann B, Murphy MH, et al. A systematic review of determinants of sedentary behaviour in youth: a DEDIPAC-study. Int J Behav Nutr Phys Act. 2015;12: 133 10.1186/s12966-015-0291-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildebrand M, Øglund GP, Wells JC, Ekelund U. Prenatal, birth and early life predictors of sedentary behavior in young people: a systematic review. Int J Behav Nutr Phys Act. 2016;13: 63 10.1186/s12966-016-0389-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Øglund GP, Hildebrand M, Ekelund U. Are Birth Weight, Early Growth, and Motor Development Determinants of Physical Activity in Children and Youth? A Systematic Review and Meta-Analysis. Pediatr Exerc Sci. 2015;27: 441–453. 10.1123/pes.2015-0041 [DOI] [PubMed] [Google Scholar]
- 14.Ridgway CL, Ong KK, Tammelin TH, Sharp S, Ekelund U, Jarvelin M- R. Infant Motor Development Predicts Sports Participation at Age 14 Years: Northern Finland Birth Cohort of 1966. Lucia A, editor. PLoS One. 2009;4: e6837 10.1371/journal.pone.0006837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattocks C, Ness A, Deere K, Tilling K, Leary S, Blair SN, et al. Early life determinants of physical activity in 11 to 12 year olds: cohort study. BMJ. 2008;336: 26–9. 10.1136/bmj.39385.443565.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoyama T, Tanaka S, Tanaka M, Okuda M, Inoue S, Tanaka C. Birth weight and infant motor development in relation to physical activity in childhood. Japan Journal of Human Growth Development Research. 2017;74: 9–18. 10.5332/hatsuhatsu.2017.74_9 [DOI] [Google Scholar]
- 17.Hnatiuk J, Salmon J, Campbell KJ, Ridgers ND, Hesketh KD. Early childhood predictors of toddlers’ physical activity: Longitudinal findings from the Melbourne InFANT Program. Int J Behav Nutr Phys Act. 2013;10: 1–9. 10.1186/1479-5868-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prioreschi A, Brage S, Hesketh KD, Hnatiuk J, Westgate K, Micklesfield LK. Describing objectively measured physical activity levels, patterns, and correlates in a cross sectional sample of infants and toddlers from South Africa. Int J Behav Nutr Phys Act. International Journal of Behavioral Nutrition and Physical Activity; 2017;14: 1–14. 10.1186/s12966-016-0456-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka C, Tanaka M, Okuda M, Inoue S, Aoyama T, Tanaka S. Association between objectively evaluated physical activity and sedentary behavior and screen time in primary school children. BMC Res Notes. 2017;10: 175 10.1186/s13104-017-2495-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prince SA, Saunders TJ, Gresty K, Reid RD. A comparison of the effectiveness of physical activity and sedentary behaviour interventions in reducing sedentary time in adults: a systematic review and meta-analysis of controlled trials. Obes Rev. 2014;15: 905–919. 10.1111/obr.12215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddique J, de Chavez PJ, Craft LL, Freedson P, Spring B. The Effect of Changes in Physical Activity on Sedentary Behavior: Results From a Randomized Lifestyle Intervention Trial. Am J Heal Promot. 2017;31: 287–295. 10.4278/ajhp.150129-QUAN-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Multicentre Growth Reference Study Group. WHO Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatr Suppl. 2006;450: 86–95. [DOI] [PubMed] [Google Scholar]
- 23.Kuh D, Hardy R, Butterworth S, Okell L, Richards M, Wadsworth M, et al. Developmental Origins of Midlife Physical Performance: Evidence from a British Birth Cohort. Am J Epidemiol. 2006;164: 110–121. 10.1093/aje/kwj193 [DOI] [PubMed] [Google Scholar]
- 24.Kuh D, Hardy R, Butterworth S, Okell L, Wadsworth M, Cooper C, et al. Developmental origins of midlife grip strength: findings from a birth cohort study. J Gerontol A Biol Sci Med Sci. 2006;61: 702–706. [DOI] [PubMed] [Google Scholar]
- 25.Hildebrand M, Kolle E, Hansen BH, Collings PJ, Wijndaele K, Kordas K, et al. Association between birth weight and objectively measured sedentary time is mediated by central adiposity: data in 10,793 youth from the International Children’s Accelerometry Database. Am J Clin Nutr. 2015;101: 983–990. 10.3945/ajcn.114.103648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hikihara Y, Tanaka C, Oshima Y, Ohkawara K, Ishikawa-Takata K, Tanaka S. Prediction Models Discriminating between Nonlocomotive and Locomotive Activities in Children Using a Triaxial Accelerometer with a Gravity-removal Physical Activity Classification Algorithm. PLoS One. 2014;9: e94940 10.1371/journal.pone.0094940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshima Y, Kawaguchi K, Tanaka S, Ohkawara K, Hikihara Y, Ishikawa-Takata K, et al. Classifying household and locomotive activities using a triaxial accelerometer. Gait Posture. England: Elsevier B.V; 2010;31: 370–374. 10.1016/j.gaitpost.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 28.Cliff DP, Reilly JJ, Okely AD. Methodological considerations in using accelerometers to assess habitual physical activity in children aged 0–5 years. J Sci Med Sport. 2009;12: 557–567. 10.1016/j.jsams.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 29.Penpraze V, Reilly JJ, MacLean CM, Montgomery C, Kelly LA, Paton JY, et al. Monitoring of Physical Activity in Young Children: How Much Is Enough? Pediatr Exerc Sci. 2006;18: 483–491. [DOI] [PubMed] [Google Scholar]
- 30.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51: 1173–1182. 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 31.Nanri H, Hara M, Nishida Y, Shimanoe C, Nakamura K, Higaki Y, et al. Dietary Patterns and Serum Gamma-Glutamyl Transferase in Japanese Men and Women. J Epidemiol. 2015;25: 378–386. 10.2188/jea.JE20140158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wijtzes AI, Kooijman MN, Kiefte-de Jong JC, de Vries SI, Henrichs J, Jansen W, et al. Correlates of Physical Activity in 2-Year-Old Toddlers: The Generation R Study. J Pediatr. 2013;163: 791–799.e2. 10.1016/j.jpeds.2013.02.029 [DOI] [PubMed] [Google Scholar]
- 33.Malina R. Motor Development during Infancy and Early Childhood: Overview and Suggested Directions for Research. Mot Dev Int J Sport Heal Sci J Sport Heal Sci. 2004;22: 50–66. 10.5432/ijshs.2.50 [DOI] [Google Scholar]
- 34.Ireland A, Muthuri S, Rittweger J, Adams JE, Ward KA, Kuh D, et al. Later Age at Onset of Independent Walking Is Associated With Lower Bone Strength at Fracture-Prone Sites in Older Men. J Bone Miner Res. 2017;32: 1209–1217. 10.1002/jbmr.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pillas D, Kaakinen M, Tzoulaki I, Netuveli G, Rodriguez A, Fung E, et al. Infant locomotive development and its association with adult blood pressure. Eur J Pediatr. Germany; 2014;173: 1309–17. 10.1007/s00431-014-2326-2 [DOI] [PubMed] [Google Scholar]
- 36.Adolph KE, Robinson S. The road to walking: What learning to walk tells us about development. Oxford Handb Dev Psychol. 2013;2: 1–39. 10.1093/oxfordhb/9780199958450.013.0015 [DOI] [Google Scholar]
- 37.Zelazo PR, Zelazo NA, Kolb S. “Walking” in the Newborn. Science. 1972;176: 314–315. 10.1126/science.176.4032.314 [DOI] [PubMed] [Google Scholar]
- 38.Torsvik IK, Ueland PM, Markestad T, Midttun Ø, Monsen A-LB. Motor development related to duration of exclusive breastfeeding, B vitamin status and B12 supplementation in infants with a birth weight between 2000–3000 g, results from a randomized intervention trial. BMC Pediatr. 2015;15: 218 10.1186/s12887-015-0533-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnett LM, Lai SK, Veldman SLC, Hardy LL, Cliff DP, Morgan PJ, et al. Correlates of Gross Motor Competence in Children and Adolescents: A Systematic Review and Meta-Analysis. Sports Med. 2016;46: 1663–1688. 10.1007/s40279-016-0495-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dencker M, Andersen LB. Accelerometer-measured daily physical activity related to aerobic fitness in children and adolescents. J Sports Sci. 2011;29: 887–895. 10.1080/02640414.2011.578148 [DOI] [PubMed] [Google Scholar]
- 41.Ridgway CL, Ong KK, Tammelin T, Sharp SJ, Ekelund U, Jarvelin M-R. Birth size, infant weight gain, and motor development influence adult physical performance. Med Sci Sports Exerc. 2009;41: 1212–1221. 10.1249/MSS.0b013e31819794ab [DOI] [PubMed] [Google Scholar]
- 42.Adegboye A, Heitmann B. Accuracy and correlates of maternal recall of birthweight and gestational age. BJOG An Int J Obstet Gynaecol. 2008;115: 886–893. 10.1111/j.1471-0528.2008.01717.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majnemer A, Rosenblatt B. Reliability of parental recall of developmental milestones. Pediatr Neurol. United States; 1994;10: 304–8. [DOI] [PubMed] [Google Scholar]
- 44.Equal Employment, Children and Families Bureau of the Ministry of Health, Labour, and Welfare in Japan. Report of the national growth survey on preschool children in 2010. 2011. pp. 19.
- 45.Wijnhoven TM, de Onis M, Onyango AW, Wang T, Bjoerneboe GE, Bhandari N, et al. Assessment of gross motor development in the WHO Multicentre Growth Reference Study. Food Nutr Bull. 2004;25: S37–45. 10.1177/15648265040251S105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
* P < 0.05; ** P < 0.01; *** P < 0.001. SB, sedentary behavior; LPA, light physical activity; MVPA, moderate-to-vigorous physical activity.
(PDF)
Model 1: Adjusted for months of age, schools, and accelerometer wear time. Model 2: As Model 1 plus birth weight and current weight. B, unstandardized regression coefficient; β, standardized regression coefficient. SB, sedentary behavior; LPA, light physical activity; MVPA, moderate-to-vigorous physical activity.
(PDF)
Adjusted for months of age, birth weight, current weight, schools, and accelerometer wear time. B, unstandardized regression coefficient; β, standardized regression coefficient. SB, sedentary behavior; MVPA, moderate-to-vigorous physical activity.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.