Abstract
Background and Aims:
Pancreatic cancer organoids are tumor models of individualized human pancreatic ductal adenocarcinoma (PDA), created from surgical specimens and used for personalized treatment strategies. Unfortunately the vast majority of patients with PDA are not operative candidates. Creation of human PDA organoids at the time of initial tumor diagnosis is therefore critical. Our aim was to assess the feasibility of creating human PDA organoids by EUS fine-needle biopsy (EUS-FNB) in patients with PDA.
Methods:
Prospective clinical trial in patients referred to evaluate a pancreatic mass (ClincalTrials.gov: NCT03140592). EUS-FNA was performed for initial on-site diagnosis. Two additional needle passes were performed with a 22-gauge FNB needle for organoid creation. Primary outcome was successful isolation of organoids within 2 weeks of EUS-FNB (P0), confirmed by organoid morphology and positive genotyping.
Results:
Thirty-seven patients with 38 PDA tumors were enrolled. Successful isolation of organoids (P0) was achieved in 33 of 38 tumors, or 87%. Establishment of PDA organoid lines for ≥5 passages of growth (P5) was reached in 25 of 38 tumors, or 66%. In the single patient with successful P5 FNB-derived and P5 surgically derived organoids, there was identical matching of specimens. There were no serious adverse events. Two patients developed bleeding at the EUS-FNB puncture site requiring hemostasis clips.
Conclusions:
Pancreatic cancer organoids can be successfully and rapidly created by means of EUS-FNB using a 22-gauge needle at the time of initial diagnosis. Successful organoid generation is essential for precision medicine in patients with pancreatic cancer, in whom the majority are not surgically resectable.
Keywords: EUS, Organoids, EUS-FNB, Pancreatic Cancer, Pancreatic Adenocarcinoma
Background
Pancreatic ductal adenocarcinoma (PDA) is one of the most aggressive and lethal cancers among all cancer types, with relative 5-year survival rates of less than 8%.1-4 Unfortunately, the vast majority of patients (80%) are ineligible for the only curative treatment—surgical resection. Furthermore, PDA is therapeutically resistant to many of the current drug regimens. In order to develop a more thorough understanding of the biology of PDA, as well as to assess potential therapeutic targets, more biological material is needed from patient tumors for further molecular analysis. However, limiting the analysis of PDA tissue to only those patients that go for surgical resection (~20%) significantly reduces our ability to advance our knowledge of this disease; and studies that rely solely on surgical specimens alone may represent a biased sampling of all pancreatic cancers.
Organoids are small tissue fragments abstracted from larger organs (typically epithelial cells) that are grown into 3 dimensional (3D) cultures, so they may ultimately expand into ex vivo organ-like structures.5 Pancreatic cancer organoids are tumor models of individualized human PDA that can be rapidly generated from surgically resected neoplasms.6 Organoids simulate the full spectrum of a patient’s tumor, including stromal elements believed to be responsible for chemotherapy resistance.6-8 Unlike patient-derived xenografts which require relatively large amounts of tumor tissue and may take multiple months to establish in the host organism,9 human PDA organoids can be generated quickly and from smaller amounts of material (Figure 1). The organoids may then be used for personalized cancer treatment by testing potential therapeutic targets.
Figure 1.
A, Microscopic appearance of an early PDA organoid isolate; B, Gross appearance of a PDA organoid isolate after several cycles of propagation.
The majority of patients with suspicious pancreatic masses will undergo diagnostic EUS-guided fine-needle aspiration (EUS-FNA) or fine-needle biopsy (EUS-FNB) of the mass.3 EUS-FNB using a core needle has a theoretical advantage over FNA by providing increased amounts of tissue with preserved architecture, thereby making an initial positive diagnosis of PDA more likely. However, previous clinical trials comparing these two types of tissue acquisition needles have yielded conflicting results.10-15 A recent meta-analysis showed that FNA and FNB provided similar diagnostic yields, whereas FNB required fewer needle passes.16 In sampling pancreatic mass lesions, a low number of needle passes is critical so that additional biopsy specimens may be potentially obtained for ancillary molecular testing, without increasing the overall risk of procedural-related acute pancreatitis.17
The ability to create human PDA organoids by other means than surgically resected tissue has not been previously investigated. The utilization of FNB for creation of organoids at the time of initial cancer diagnosis has the potential advantage of providing precision medicine options for all patients with this disease—not just the minority that go for surgical resection. Additionally, EUS-FNB derived PDA organoids allow for a more global, unbiased understanding of tumor biology in pancreatic cancer, as this allows for tissue acquisition from the tumor before any neoadjuvant chemotherapy is provided.
The primary aim of this study was to assess the ability to generate human PDA organoids by means of EUS-FNB in patients presenting with a pancreatic mass suspected of PDA. Secondary aims were to evaluate patient safety with regards to additional needle passes used for organoid creation, as well as identical matching of organoids created from explanted tumors in those patients who proceeded to surgical resection.
Methods
Patient Selection
Patients considered eligible for this study included those over the age of 18 referred for endoscopic ultrasound (EUS) with tissue sampling of a pancreatic mass lesion suspected of PDA. Patients were excluded if they were unable to provide informed consent, were found to have a pancreatic lesion on EUS examination that was inconsistent with PDA (either before or after initial tissue sampling), were pregnant, had severe cardiopulmonary disease precluding a safe EUS procedure, or were unable to stop anticoagulation before EUS. Written informed consent was obtained for all patients before EUS. The study was approved by the Institutional Review Boards (IRB) at all participating centers: SUNY Stony Brook and Downstate Universities, and Cold Spring Harbor Laboratory (ClincalTrials.gov identifier: NCT03140592). All co-authors had access to and reviewed the study data. All co-authors approved the final manuscript.
Study Design and Procedural Details
This was a prospective pilot study conducted between February 2015 and April 2017. All EUS procedures were performed at Stony Brook University Hospital or SUNY Downstate Medical Center by one of five experienced endosonographers (J.B., J.C.B., S.N., D.T., S.V.). A standard curvilinear array echoendoscope (GF-UC140P-AL5 or GF-UCT180, Olympus America, Central Valley, Pa) was used in all patients. Initial diagnostic EUS-FNA examination was performed using a 25-gauge or 22-gauge standard FNA needle in most cases (Expect FNA Needle, Boston Scientific Inc., Marlborough, Mass). Rapid onsite evaluation (ROSE) for cytopathology with a technician and/or attending physician in the procedure room was used for each case. Once adequate cellularity was achieved with a positive preliminary diagnosis by ROSE, 1 to 2 additional needle passes by means of EUS-FNB were permitted for the purposes of organoid creation. EUS-FNB was be performed with a 22-gauge forward-acquiring FNB needle (SharkCore FNB Exchange System, Medtronic Inc, Minneapolis, Minn; or Acquire FNB Device, Boston Scientific Inc, Marlborough, Mass). After the FNB needle was inserted into the mass, several needle throws were performed using a “slow pull” technique with the inner stylet. Fanning each needle throw about the entire area of the mass was used in effort to obtain the best representative sample. If the endosonographer appreciated an adequate core tissue sample on gross visible inspection, only one pass for research was performed. If not, one additional FNB pass was undertaken. Because the risks of adverse event associated with FNA/FNB are believed to be increased with >7 needle passes (using standard needle sizes of 22-gauge and 25-gauge),3,4,17 only those patients in which adequate cellular material was achieved in ≤5 passes were permitted to continue participation in the study (and thus undergo 1-2 additional passes for research). As study enrollment progressed, the 22-gauge FNB needle was used for both initial diagnosis and for research purposes.
The FNB specimens were placed into basal organoid media, and immediately transported to the Stony Brook University Digestive Disease Research Tissue Procurement Facility (DDR-TPF) when performed at Stony Brook University Hospital. Within 24 hours the specimens then delivered to Cold Spring Harbor Laboratory (CSHL) for further processing and organoid generation. When performed at SUNY Downstate Medical Center, the FNB specimens were placed into basal organoid media and immediately transported to CSHL.
Organoid Generation and Maintenance
For those patients who eventually proceeded to surgical resection of their mass, organoids were created from the explanted tumor by methods previously described.6 For FNB specimens (Figure 2), tissues were mechanically dissociated in digestion media (5 mg/mL Collagenase XI, 10 μg/mL DNAse I, 10.5 μM Y-27632 in Human complete Feeding Medium) at 37 °C with mild agitation for 5 to 10 minutes. Dissociated cells and tissue fragments were plated with Matrigel and grown in Human Complete Feeding Medium (advanced DMEM/F12, HEPES 10 mM, Glutamax 1X, A83-01 500 nM, hEGF 50 ng/mL, mNoggin 100 ng/mL, hFGF10 100 ng/mL, hGastrin I 0.01 μM, N-acetylcysteine 1.25 mM, Nicotinamide 10 mM, PGE2 1 μM, B27 supplement 1X final, R-spondin1 conditioned media 10 % final, Afamin/Wnt3A conditioned media 50 % final.18 Successful isolation of organoid cultures was determined by outgrowth and proliferation of organoids within 2 weeks (P0). Organoid cultures were passaged by mechanical dissociation once every week or 2 weeks depending on growth rate. Successful establishment of organoid cultures was determined by the ability of organoids to reach passage 5 (P5).
Figure 2.
Organoid creation from EUS-FNB to the generation of 3D cultures.
Study Outcomes and Statistical Analysis
The primary outcome measure of this study was successful isolation of pancreatic ductal adenocarcinoma (PDA) organoids within 2 weeks of EUS-FNB procedure; referred to as P0. This was confirmed by organoid standard morphology and positive genotyping for known PDA mutations. Secondary outcomes included successful propagation of organoids for 5 additional passages, or cycles; referred to as P5. Additionally, for those patients that proceeded to surgical resection for their mass lesions, we assessed successful “matching” of organoids created from EUS-FNB, compared to those organoids created directly from the explanted tumor. Procedural adverse events were assessed. Basic descriptive statistical computation was performed using Vassar Stats (vassarstats.net).
Results
A total of 42 patients with a newly diagnosed, treatment naive pancreatic mass lesion were enrolled for study between February 2015 and April 2017. All of these patients underwent EUS-FNB of their lesions. The final diagnoses of the masses are shown in Table 1. Five patients were excluded because of a final histological diagnosis other than PDA. One patient had two separate lesions. In the final analysis, there were 37 patients with 38 newly diagnosed PDA.
Table 1.
Final diagnoses of pancreatic masses.
| Final Diagnosis | No. of Patients | No. of Lesions |
|---|---|---|
| Ductal adenocarcinoma | 37 | 38 |
| Neuroendocrine tumor | 2 | 2 |
| Lymphoma | 2 | 2 |
| Other | 1 | 1 |
| Total | 42 | 43 |
Table 2 outlines the patient demographics. Median patient age and BMI were 70 years, and 27 kg/m2, respectively. More than half the patients had a history of current or former tobacco use (51%), and 35% carried a diagnosis of Type II diabetes mellitus at the time of diagnosis. Table 3 shows the various characteristics of the tumors themselves. Most lesions were located in the pancreatic head region, with a median size of 3 cm. Initial positive preliminary diagnosis by ROSE was achieved in 3 needle passes for most cases (using either a 22-gauge or 25-gauge FNA needle, or a 22-gauge FNB needle). A grossly visible core specimen was achieved in 38 of 38 (100%) tumors after EUS-FNB for the research portion of the procedure. These core samples were all sent for organoid creation. More than one-third of patients (35%) underwent an ERCP procedure after their EUS examination.
Table 2.
Demographics of newly diagnosed, treatment naive PDA patients.
| Characteristics | Newly diagnosed PDA Patients (n = 37) |
|---|---|
| Median Age, years (range) | 70 (53 – 93) |
| Male sex (n, %) | 18 (49%) |
| Race (n, %) Non-Hispanic Caucasian Black/African American Hispanic Asian |
32 (86%) 3 (8%) 1 (3%) 1 (3%) |
| First degree relative with PDAC (n, %) Yes No Unknown |
5 (13%) 31 (84%) 1 (3%) |
| Previous cancer (n, %) | 8 (22%) |
| Chronic pancreatitis (n, %) | 3 (8%) |
| Diabetes mellitus, type 2 (n, %) | 13 (35%) |
| Tobacco (n, %) Current Ex-smoker Never smoker |
8 (21%) 11 (30%) 18 (49%) |
| Median Body Mass Index kg/m2 (range) | 27 (15 – 49) |
| Median CA 19-9 level, units/mL (range) | 321 (4.8 – 122,716) |
Table 3.
Characteristics of newly diagnosed, treatment naïve PDA tumors
| Characteristics | PDA tumors (n = 38) |
|---|---|
| Localization Head Body-tail |
23 (60%) 15 (40%) |
| Median largest diameter cm (range) | 3.0 (1.5 – 4.8) |
| Median number of needle passes for positive diagnosis (FNA and FNB) |
3 (1 – 5) |
| Number of needle passes for research (FNB only) One pass Two passes |
5 (13%) 33 (87%) |
| Grossly visible core present on FNB (n, %) | 38 (100%) |
| Concomitant ERCP following EUS examination (n, %) | 13 (35%) |
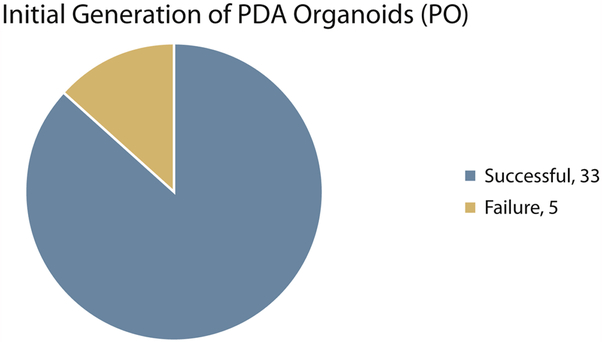
Successful isolation of pancreatic ductal adenocarcinoma (PDA) organoids within 2 weeks of the EUS-FNB procedure (P0) was noted in 33 of 38 tumors, or 87% (Figure 3). Five tumors (13%) failed to yield organoid isolates from EUS-FNB. This was due to the absence of viable epithelial cells from the FNB specimens upon receipt in the laboratory, despite a visible core tissue sample on gross examination in the endoscopy procedure room. Establishment of PDA organoid lines for ≥5 passages or cycles of growth (P5) was noted in 25 of 38 tumors, or 66% (Figure 4). They continue to undergo successful propagation at the time of manuscript preparation. Eight tumors (21%) failed to reach P5 status. There were 5 tumors that were intentionally frozen before P5 (but >P0) for use in ongoing additional research. If thawed and allowed to further propagate, the potential P5 success rate may be as high as 79%, or 30 of 38 tumors.
Figure 3.
Initial generation of PDA organoids within 2 weeks of EUS-FNB procedure (P0).
Figure 4.
Generation of established PDA organoids after ≥ 5 passages, or cycles of growth (P5).
Table 4 shows the patient follow-up for a median of 22 weeks after the EUS-FNB procedure. Eleven of 37 patients proceeded to the operating room for an attempt at surgical resection. Only 7 patients had a complete resection with curative intent (4 operations were aborted due to advanced disease). Thus, in total 30 of 37 (81%) patients did not receive surgical resection. Of the 7 surgical patients, 3 had neoadjuvant chemotherapy before resection, and therefore were excluded from assessing for a match between the FNB-derived organoid and the surgical explant-derived organoid. Four treatment naive patients remained for match assessment. One surgical explant-derive organoid failed to reach P5. Of the 3 remaining, 2 FNB-derived organoids failed to reach P5. The final patient had identical matching PDA organoids from both EUS-FNB and surgical resection.
Table 4.
Patient follow-up after EUS-FNB.
| Characteristics | Newly Diagnosed PDA Patients (n = 37) |
|---|---|
| Median follow-up (weeks) | 22 (1 – 103) |
| Chemotherapy post EUS-FNB (n, %) | 21 (57%) |
| Surgical resection following EUS-FNB (n, %) Resected without neoadjuvant treatment Resected after neoadjuvant treatment Not resected, locally advanced or metastatic |
4 (11%) 3 (8%) 30 (81%) |
| Deceased (n, %) | 10 (27%) |
There were no serious adverse events. One patient developed postprocedural acute pancreatitis, but was one of 13 who underwent a concomitant ERCP procedure immediately after the EUS-FNA/FNB. Two patients developed brisk bleeding at the EUS-FNB needle puncture site. Both required endoscopic hemostasis clips to completely stop the bleeding. There were no transfusion requirements, and no new hospital admissions as a result of the EUS-FNB procedure.
Discussion
Precision medicine refers to the ability to use patient-specific information (e.g. genomic or proteomic) in effort to guide the clinician in making customized diagnostic or therapeutic decisions. Often referred to as “personalized” medicine, it has particular importance in the field of pancreatic malignancy because the current treatment options are few in number and limited in overall efficacy.19 The primary example of precision medicine in pancreatic cancer today is next-generation sequencing (NGS). From a relatively small amount of additional tissue obtained from the primary pancreatic tumor, NGS allows for the assessment of millions of defined segments of the genome for the purpose of detecting various point mutations.20 This then enables “panel testing” for specific groups of mutations found in genes that are associated with pancreatic malignancy.21,22 The information can be used in the selection of various chemotherapy regimens known to be more effective in the presence of specific types of pancreatic tumors. NGS represents one form of precision medicine, and is currently in the early stages with regards to its overall potential in the treatment of this disease.
Organoids are an exciting new development for translational research and precision medicine in pancreatic cancer. They are essentially miniature tumor models of individualized human PDA that can be rapidly generated from surgically resected neoplasms.6 Organoids may simulate the full spectrum of a patient’s tumor, particularly if reconstituted with stroma and added fibroblasts.23 They may be used in 2 advantageous ways: (1) for basic scientific research on PDA tumor biology, and (2) for patient-specific chemotherapeutic drug sensitivity testing. This second method of organoid utilization represents a new form of precision medicine in pancreatic cancer, and may serve a complimentary role to next-generation sequencing (NGS). Furthermore, unlike patient-derived xenografts (PDXs), which may be technically difficult to transplant into the host organism, and can require up to 6 months to fully grow, 24-26 organoids may be quickly generated thereby making them more usable for a patient population with limited time.
Until now, however, PDA organoids have only been successfully created by means of extracting tumor tissue from the explanted specimen at the time of surgical resection.6 This severely limits the ability to use the organoids, because only a small portion of patients with the disease advance to the operating room for curative intent. The ability to successfully and reliably generate organoids in all patients with PDA represents a major advance in our ability to study and treat the disease. The creation of PDA organoids by means of EUS-guided tissue acquisition at the time of initial disease diagnosis is therefore critical.
In the current study, we successfully created pancreatic cancer organoids by means of EUS-guided FNB in most patients (87%) at the time they were first diagnosed with their tumor. The PDA organoids were rapidly created within 2 weeks of the EUS procedure. These early successful organoids then underwent attempts at further propagation with the goal of at least 5 total passages (P5). The purpose of this is to further enhance the micro-tumors in order to sustain prolonged survival and allow for evolving drug sensitivity testing (presumably throughout the course of a patient’s treatment). More than half of these organoids (66%) successfully reached P5 status, with an additional 5 tumors intentionally frozen and available for further propagation to P5. In the single patient with successful organoid generation from both the FNB specimen (P5) and the explanted tumor (P5), there was identical matching among the 2 specimens, thereby validating the feasibility and accuracy of FNB technique for organoid generation. Adverse events from 1 to 2 additional passes with the FNB needle appear to be limited to luminal bleeding at the puncture site in a small number of patients.
There are clear limitations of this study. First, this is a pilot study aimed to assess the feasibility and safety of EUS-FNB organoid creation, involving a limited number of patients only. Immediate and more consistently reliable organoid generation is needed in order to benefit patients at the start of their cancer treatment. Failure to reach P5 status in 100% of our samples likely occurred because the number of epithelial cells was low and/or the proliferation rate was slow. If the culture contains an overabundance of normal epithelial cells, proliferation may quickly arrest as the growth medium does not contain the necessary growth factor for normal pancreatic epithelium. Secondly, only a small fraction of patients that underwent EUS-FNB proceeded to surgical resection, and thus had explanted tumor available for organoid creation. As noted, successful “matching” of FNB organoids with surgical organoids is critical in providing validation of the FNB technique, and thus confirming that the FNB organoid is representative of the overall tumor biology. Further studies will compare the genomic and transcriptomic profiles of FNB-derived and surgically derived organoids.
The future direction of clinical research in pancreatic cancer organoids will continue to focus on rapid and reliable generation of organoids for all patients undergoing diagnostic EUS-guided tissue acquisition. This will enable early drug sensitivity testing for each patient’s tumor; thereby allowing physicians to design a precise chemotherapeutic treatment algorithm. Research using established organoids for such drug sensitivity testing is currently ongoing, and holds promising preliminary results (unpublished data, Tiriac et al). Additionally, as we continue to learn more about the performance of EUS-guided tissue acquisition (eg, optimal choice in needle type, 27 most desirable number of needle passes, 28 reduced need for ROSE depending on lesion type, 29 etc), these lessons can be translated to organoid generation so the most effective and least invasive means may be used. Ongoing translational endoscopic research with PDA organoids should focus on achieving a universal 100% P0 success rate; whereby all tumors can easily yield organoid isolates rapidly. In addition, a goal of >90% P5 success rate should be set, so to allow most patients to benefit from sustained isolates that may be used to study their own individualized tumor biology over the entire course of their disease. This concept of perfecting FNB-derived organoid creation is of great importance, as we will ultimately approach a time in which only a small number of needle passes are used for diagnostic cytology, and the remaining tissue acquired is needed for various pathways of precision medicine.
In conclusion, pancreatic ductal adenocarcinoma (PDA) is the ideal paradigm for creating organoids by means of FNB because (1) most patients will not undergo surgery; (2) all patients need a tissue diagnosis before systemic therapies may be initiated; and (3) only a small amount of tissue is needed for organoid creation. Our study demonstrates that PDA organoids can be successfully and rapidly created by means of EUS-guided core biopsy using a 22-gauge FNB needle at the time of initial tumor diagnosis. Successful organoid generation is essential for expanding the role of personalized medicine in patients with pancreatic cancer.
Acknowledgements
We would like to thank all members of the J.M.B. and D.A.T. laboratories for helpful discussions and suggestions throughout the course of this study. The authors would like to thank the Cold Spring Harbor Cancer Center Support Grant (CCSG, P30CA045508-29) shared resources. We are grateful to Junichi Takagi at Osaka University for sharing the Afamin/Wnt3A cell line. This project was also funded the NCI P20CA19299402 grant to J.M.B, D.A.T, E.L., and the Lustgarten Foundation, where D.A.T. is a distinguished scholar and Director of the Lustgarten Foundation–designated Laboratory of Pancreatic Cancer Research. D.A.T. is also supported by the Cold Spring Harbor Laboratory Association, the National Institutes of Health (NIH; 5P30CA45508-29, 5P50CA101955-07, 1U10CA180944-04, 5U01CA168409-5, 1R01CA188134-01, and 1R01CA190092-04), and the V Foundation. In addition, we are grateful for support from the following: Stand Up to Cancer/AACRPS09 (D.A.T.). Stony Brook pilot project grant (J.M.B.), ASGE ERA 71040(J.M.B.), SWOG ITSC 5U10CA180944-04 (D.A.T., H.T.).
This study was supported, in part, from the 2015 ASGE Endoscopic Research Award (ERA), NCI 5P20CA192994, and the Simons Foundation.
Abbreviations:
- EUS
endoscopic ultrasound
- FNB
fine-needle biopsy
- FNA
fine-needle aspiration
- PDA
pancreatic ductal adenocarcinoma
- NGS
next generation sequencing
- PDX
patient-derived xenograft
Footnotes
Study results were previously presented as an Oral Presentation at the ASGE EUS Topic Forum during Digestive Disease Week (DDW) 2017 in Chicago, IL.
Writing Assistance:
None
Disclosures:
None of the authors have any disclosures relevant to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nature reviews. Clinical Oncology 2010; 7: 163–172. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M Pancreatic cancer. NEJM 2010; 362:1605–17. [DOI] [PubMed] [Google Scholar]
- 3.Kim EY. Fine-needle biopsy: should this be the first choice in endoscopic ultrasound-guided tissue acquisition? Clin Endosc 2014; 47:425–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karadsheh Z, Al-Haddad M. Endoscopic ultrasound guided fine needle tissue acquisition: where we stand in 2013? World J Gastroenterol 2014; 20:2176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simian M, Bissell MJ. Organoids: A historical perspective of thinking in three dimensions. J Cell Biol 2017; 216:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boj SF, Hwang CI, Baker LA, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015; 160:324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boj SF, Hwang CI, Baker LA, et al. Model organoids provide new research opportunities for ductal pancreatic cancer. Mol Cell Oncol 2015; 3:e1014757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang CI, Boj SF, Clevers H, et al. Preclinical models of pancreatic ductal adenocarcinoma. J Pathol 2016; 238:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MP, Evans DB, Wang H, et al. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc 2009; 4:1670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang JY, Hebert-Magee S, Trevino J, et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc 2012; 76:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hucl T, Wee E, Anuradha S, et al. Feasibility and efficiency of a new 22G core needle: a prospective comparison study. Endoscopy 2013; 45:792–798. [DOI] [PubMed] [Google Scholar]
- 12.Lee YN, Moon JH, Kim HK, et al. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized parallel-group study. Endoscopy 2014; 46:1056–62. [DOI] [PubMed] [Google Scholar]
- 13.Kim GH, Cho YK, Kim EY, et al. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand J Gastroenterol 2014; 49:347–54. [DOI] [PubMed] [Google Scholar]
- 14.Strand DS, Jeffus SK, Sauer BG, et al. EUS-guided 22-gauge fine-needle aspiration versus core biopsy needle in the evaluation of solid pancreatic neoplasms. Diagn Cytopathol 2014; 42:751–8. [DOI] [PubMed] [Google Scholar]
- 15.Mavrogenis G, Weynand B, Sibille A, et al. 25-gauge histology needle versus 22-gauge cytology needle in endoscopic ultrasonography-guided sampling of pancreatic lesions and lymphadenopathy. Endosc Int Open 2015; 3:E63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy 2016; 48:339–49. [DOI] [PubMed] [Google Scholar]
- 17.Hewitt MJ, McPhail MJW, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 2012; 75:319–31. [DOI] [PubMed] [Google Scholar]
- 18.Mihara E, Hirai H, Yamamoto H, et al. Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/a-albumin. eLife 2016; 5:e11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diwakarla C, Hannan K, Hein N, Yip D. Advanced pancreatic ductal adenocarcinoma - complexities of treatment and emerging therapeutic options. World J Gastroenterol 2017; 23:2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei S, Lieberman D, Morrissette JJ, et al. Using “residual” FNA rinse and body fluid specimens for next-generation sequencing: An institutional experience. Cancer Cytopathol 2016; 124:324–9. [DOI] [PubMed] [Google Scholar]
- 21.Gleeson FC, Kipp BR, Kerr SE, et al. Characterization of endoscopic ultrasound fine-needle aspiration cytology by targeted next-generation sequencing and theranostic potential. Clin Gastroenterol Hepatol 2015; 13:37–41. [DOI] [PubMed] [Google Scholar]
- 22.Zutter MM, Bloom KJ, Cheng L, et al. The Cancer Genomics Resource List 2014. Arch Pathol Lab Med 2015; 139:989–1008. [DOI] [PubMed] [Google Scholar]
- 23.Ohlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017; 214:579–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubio-Viqueira B, Jimeno A, Cusatis G, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res 2006; 12:4652–61. [DOI] [PubMed] [Google Scholar]
- 25.Kim MP, Evans DB, Wang H, et al. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc 2009; 4:1670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krempley BD, Yu KH. Preclinical models of pancreatic ductal adenocarcinoma. Chin Clin Oncol 2017; 6(3):25. [DOI] [PubMed] [Google Scholar]
- 27.Nagula S, Pourmand K, Aslanian H, et al. Comparing EUS-fine needle aspiration and EUS-fine needle biopsy for solid lesions: A multicenter, randomized trial. Clin Gastroenterol Hepatol 2017; [Epub ahead of print], PMID: 28624647. [DOI] [PubMed] [Google Scholar]
- 28.Mohamadnejad M, Mullady D, Early DS, et al. Increasing Number of Passes Beyond 4 Does Not Increase Sensitivity of Detection of Pancreatic Malignancy by Endoscopic Ultrasound-Guided Fine-Needle Aspiration. Clin Gastroenterol Hepatol 2017; 15:1071–1078. [DOI] [PubMed] [Google Scholar]
- 29.Bhatia V, Varadarajulu S. Endoscopic ultrasonography-guided tissue acquisition: How to achieve excellence. Dig Endosc 2017. ; 29:417–430 [DOI] [PubMed] [Google Scholar]