Abstract
Background:
There is an urgent need to provide access to cleaner end user energy technologies for the nearly 40% of the world’s population who currently depend on rudimentary cooking and heating systems. Advanced cookstoves (CS) are designed to cut emissions and solid-fuel consumption, thus reducing adverse human health and environmental impacts.
Study premise:
We hypothesized that, compared to a traditional (Tier 0) three-stone (3-S) fire, acute inhalation of solid-fuel emissions from advanced natural-draft (ND; Tier 2) or forced-draft (FD; Tier 3) stoves would reduce exposure biomarkers and lessen pulmonary and innate immune system health effects in exposed mice.
Results:
Across two simulated cooking cycles (duration~3h), emitted particulate mass concentrations were reduced 80% and 62% by FD and ND stoves, respectively, compared to the 3-S fire; with corresponding decreases in particles visible within murine alveolar macrophages. Emitted carbon monoxide was reduced ~90% and ~60%, respectively. Only 3-S-fire-exposed mice had increased carboxyhemoglobin levels. Emitted volatile organic compounds were FD<<3-S-fire ≤ND stove; increased expression of genes involved in xenobiotic metabolism (COX-2, NQO1, CYP1a1) was detected only in ND- and 3-S-fire-exposed mice. Diminished macrophage phagocytosis was observed in the ND group. Lung glutathione was significantly depleted across all CS groups, however the FD group had the most severe, ongoing oxidative stress.
Conclusions:
These results are consistent with reports associating exposure to solid fuel stove emissions with modulation of the innate immune system and increased susceptibility to infection. Lower respiratory infections continue to be a leading cause of death in low-income economies. Notably, 3-S-fire-exposed mice were the only group to develop acute lung injury, possibly because they inhaled the highest concentrations of hazardous air toxicants (e.g., 1,3-butadiene, toluene, benzene, acrolein) in association with the greatest number of particles, and particles with the highest % organic carbon. However, no Tier 0 to 3 ranked CS group was without some untoward health effect indicating that access to still cleaner, ideally renewable, energy technologies for cooking and heating is warranted.
Keywords: cookstoves, incomplete combustion, lung injury, oxidative stress, phagocytosis
1. Introduction
Air pollution is a global public health problem to which emissions from rudimentary cooking devices contribute significantly. Current appraisals predict that nearly 40% of the world’s population use solid-fuels such as wood, coal, charcoal, crop residues, animal dung, and other types of biomass burning for cooking, lighting, and heating (Anenberg et al., 2013). Burning of solid-fuels in simple inefficient stoves generates harmful emissions that contribute to poor indoor air quality and have detrimental impacts on human health (McCracken et al., 2012; Gordon et al., 2014). On a global basis, exposure to household air pollutants, including cooking-related emissions, have been linked to increases in morbidity and mortality causing an estimated 3.5+ million deaths annually (Lim et al., 2012; Lacey et al., 2017).
Associated acute health effects include respiratory and eye irritation, headache, cough, acute lower respiratory infection and severe pneumonia in children (Smith et al., 2011; Gordon et al., 2014). Longer term exposure is associated with stillbirths (Lakshmi et al., 2013), low birth weight, and preterm births (Malley et al., 2017); as well as increased prevalence of several chronic diseases including chronic obstructive pulmonary disease (COPD), diabetes, hypertension, cardiovascular disease, stroke, lung cancer and other types of neoplasia (IARC, 2010, McCracken et al., 2012; Martin et al., 2014; Gordon et al., 2014; Assad et al., 2015). These health effects are primarily observed in individuals exposed most directly to cookstove (CS) emissions for the longest durations, namely women cooking indoors and their children (Rumchev et al., 2007; Clark et al., 2009). Furthermore, these same emissions contribute to increases in atmospheric black and brown carbon and to greenhouse gases that are predicted to have adverse effects on regional and global climate patterns (Smith, 1994; Anenberg et al, 2013; Laskin et al., 2015). Taking all of these factors together, there is a great need for effective interventions to mitigate the undesirable consequences associated with the use of rudimentary cooking devices.
Serious efforts have been made to lessen the negative impacts of cooking-related biomass burning. One approach has been to develop advanced, more fuel-efficient, hence “cleaner cookstoves” (Kshirsagar & Kalamkar, 2014; Johnson & Chiang 2015). Advanced CS are intended to reduce emissions and minimize solid-fuel consumption, with the expectation to diminish adverse human health and environmental impacts (e.g., deforestation). Technological advancements for solid-fuel stoves are based chiefly on manipulating, enhancing, or redirecting air flow to maintain optimal oxygen for efficient fuel combustion throughout the cooking cycle (Kshirsagar & Kalamkar, 2014). We have previously developed and used standardized testing to compare fuel efficiencies of CS with differing designs (Jetter & Kariher, 2009). Under laboratory testing conditions (i.e., a 60-minute water boiling test), a rudimentary three-stone (3-S) fire emits high levels of carbon monoxide (CO) and particulate matter (PM) and is therefore rated as a Tier 0 cooking system. Advanced CS emit lower CO and PM concentrations, albeit with varying degrees of success, and thus receive Tier 1 to Tier 4 rankings (see S1 Table for metrics used for CS Tiers) (ISO 2011; Jetter et al., 2012; Still et al., 2015).
In addition to CO and PM, solid-fuel combustion generates complex mixtures of volatile and semi-volatile organic compounds (VOCs/SVOCs), and particles with adsorbed SVOCs — the proportions of which relate to the degree of incomplete combustion occurring (Traboulsi et al., 2017). VOCs/SVOCs emitted during relatively incomplete biomass burning include alkanes, alkenes, cyclic hydrocarbons, carbonyls, and assorted polyaromatic hydrocarbon (PAH) species (Brown et al., 1994; Lemieux et al., 2004; Jordan & Seen, 2005; IARC, 2010; Mutlu et al., 2016). On an individual basis, many of these compounds have been studied extensively and are known to be associated with effects such as acute cardiorespiratory irritation, lung and systemic oxidative stress, and cancer (Lemieux et al., 2004; Bates et al., 2015; IARC, 2016; Ye et al., 2017).
A limited number of field and epidemiological studies on health improvements derived from implementing advanced CS usage in homes are beginning to appear in the literature (Li et al., 2016). However, to date, perceived benefits for reducing acute respiratory conditions (e.g., childhood pneumonia) have been inconsistent (Smith et al., 2011; Bruce et al., 2013; Cundale et al., 2017; Mortimer et al., 2017). Equivocal findings appear to relate, in part, to real-world factors such as the poor quality of available solid-fuel, non-ideal operation of advanced CS (Wathore et al., 2017), inconsistent “adoption” of advanced CS despite availability, and stove stacking (i.e., continued use of rudimentary cooking systems along with advanced CS) (Ruiz-Mercado et al., 2013). Although results of field and epidemiologic investigations are highly important, they often lend little information to understanding the mechanistic outcomes of exposure to CS emissions.
Studies directly assessing health improvements derived from use of advanced CS in a controlled laboratory environment are lacking. Thus, to generate health effects data on CS emissions, we recently determined the mutagenicity emission factors (revertants/megajouldelivered, rev/MJd) of particles emitted during simulated cooking using a three-stone (3-S) fire (Tier 0), a natural-draft (ND, Tier 2), and a forced-draft (FD, Tier 3) stove burning dry red oak (Mutlu et al., 2016). Using organic extracts of emitted PM in the Salmonella mutagenicity assay, we found that the mutagenicity emission factor (rev/MJd) was reduced by 72.5 and 97.0% by the ND and FD stoves, respectively, compared to the 3-S fire. However, even this reduction resulted in the FD stove having a mutagenicity emission factor (expressed as rev/MJthermal) that was nonetheless on parity with that of diesel exhaust, a Group 1 (known) human carcinogen (IARC, 2014).
The present study was designed to further assess differential exposure and acute respiratory system effects of inhaled CS emissions. We exposed healthy female outbred (CD-1) mice to emissions from the same stoves and wood used by Mutlu et al (2016). We compared the results to those of mice inhaling filtered air. Assessments included: (1) characterization of CS emissions, (2) biomarkers of exposure, and (3) health effects related to altered behavior, pulmonary function, innate immunity, and development of lung injury, inflammation, oxidative stress, or upregulation of adaptive genes. We hypothesized that acute inhalation of solid-fuel emissions generated by FD and ND stove technologies would result in lower levels of exposure biomarkers and less pulmonary or systemic health effects compared to that of the 3-S fire. Our approach was also intended to provide mechanistic information as to which of the key components present in solid-fuel emissions elicited or were associated with the above health outcomes.
2. Materials and Methods
2.1. Experimental Animals
Young adult (8-wk-old) female CD-1 mice (18–21 g) were used to as surrogates of the women using indoor stoves. Mice were purchased from Charles River Laboratories (Raleigh, NC), and housed in groups of four in polycarbonate cages in an AAALAC-accredited, barrier-isolated, animal research facility (21 ± 1 °C, 50 ± 5% relative humidity, and 12:12-h light/dark cycle). Standard mouse chow (Prolab RMH 3000; LabDiet, St. Louis, MO) and water were provided ad libitum. All experimental procedures requiring laboratory animals were pre-approved and performed in accordance with the U.S. EPA NHEERL Institutional Animal Care and Use Committee recommendations.
2.2. Generation of Cookstove Emissions and Exposure Protocols
Based on the water boiling test protocol, dried red oak (≤ 6% moisture content on a wet basis) was used as the solid-fuel, and emissions were generated from a FD stove (Philips HD4012), a ND stove (Envirofit G-3000), and a traditional 3-S fire (as described by Jetter et al. 2012; http://cleancookstoves.org/technology-and-fuels/testing/protocols.html).
Then, to better link differences in the performance of stoves operated under controlled conditions to health outcomes, mice were exposed only to emissions generated during the cold start-up and high-power phase (in which 5 liters of water was heated from ~20°C to the boiling temperature) followed by a 45-minute low-power phase (in which the water temperature was maintained at ~3°C below the boiling temperature). The high- and low-power phases simulated a cooking event. Cookstoves were operated as intended by manufacturers with typical fuel and cooking pots, but emissions from actual use in the field may vary due to different operation and use of alternative fuels or cooking vessels. The fire was extinguished after each simulated cooking event, so mice were not exposed to emissions from smoldering wood, although human exposure to smoldering emissions may occur in actual use. After the first simulated cooking event, there was a 15-minute recovery period (with no exposure to emissions), followed by a second identical event. Altogether, mice were exposed for approximately ~3h including the start-up and high-power time required for the FD, ND, and 3-S fire (22, 34, and 37 minutes, respectively) included in two consecutive ~90-minute simulated cooking cycles (Figure 1A).
Figure 1.
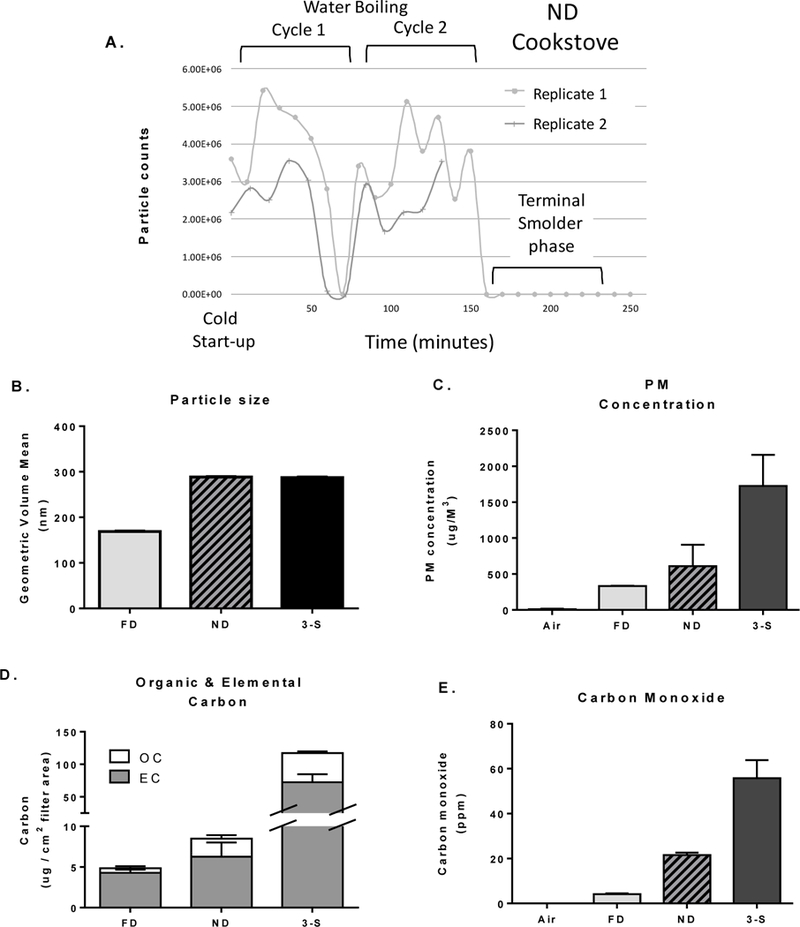
Characterization of cookstove emissions within the NOEC. (A) Example of real-time particle counts generated by the ND stove across the cold start-up and two consecutive water boiling cycles, replicate 1 and 2; (B) mean of the particle volume diameter (nm) assessed by SMPS; (C) mean PM concentrations (μg/M3) for each CS system; (D) relative elemental carbon (EC) and organic carbon (OC) contained in emitted PM; and (E) mean carbon monoxide concentrations (ppm). Figures depict the mean (± SD) of the two replicates for each stove week.
Separate (sham and exposed) whole-body inhalation exposures were performed by placing each mouse into a rat-sized nose-only exposure tube that was then inserted onto one of the two 64-port nose-only exposure chambers (NOEC) (Lab Products, Inc., Seaford, DE). The airflow rate per exposure port was ~1.5 L/minute to provide adequate mixing in the NOEC tube for each mouse. The primary emission stream was diluted 1:10 using a hood air flow rate of 150 cfm prior to diversion to the NOEC. The NOEC pulled the diluted emission atmosphere by use of an eductor that further diluted the emissions by 50% or 2:1. Because excessive CO concentrations could elicit varying degrees of asphyxiation (Quinn et al., 2009) thus limiting respiratory uptake or lung deposition of other toxicants (e.g., PM, VOCs, SVOCs), the intent was to prevent inhalation of CO ≥ 100 ppm because this has been shown to cause reduced physical activity, weight loss, and increased pulmonary barrier permeability in mice (Wilson et al., 2010). Each week, one solid-fuel stove type was evaluated using the same dilution system and final 1:20 dilution from the primary emission stack. For each stove type, two exposure replicates were performed each week in order to provide sufficient animals (n = 8/group) for assessing a wide variety of health endpoints immediately after exposure (0 h), and at 4 and 24 h post-exposure (PE). Each replicate included both air (sham) and CS emission-exposed mice.
2.3. Emissions Characterization
All exposure atmospheres were sampled directly from exposure ports on the NOEC. For each stove-week, continuous (real-time) gas and aerosol sampling for CO, nitrogen oxides (NOx), sulfur oxides (SOx), and oxygen (O2) were conducted during the inhalation exposures. A scanning mobility particle sizer (SMPS) (model 3080, TSI, Inc., Shoreview, MN) was used to determine semicontinuous size distributions and particle number concentrations. Particle mass concentrations (i.e., total filter PM mass/mean air flow rates) were also determined. Using a modified NIOSH 5040 method, thermal-optical analysis (TOA) was performed to determine the organic carbon (OC), elemental carbon (EC), and total carbon (TC) content in PM collected on the quartz filter samples as previously described (Hays et al., 2011). Using U.S. EPA Compendium Method TO-15 (1999), summa canisters were used to collect select air toxic VOCs in emissions midway through each CS exposure (collection period ~20 minutes) for subsequent off-line analysis by gas chromatography mass spectrometry. Carbonyls were sampled on DNPH cartridges (Sigma-Aldrich, St. Louis, MO) by Method TO-11A (1999).
2.4. Behavioral Assessments
All mice were observed individually for neurobehavioral or physiological changes during the last 30 minutes of exposures, while in their transparent rat exposure tubes. All mice were scored using a checklist that noted appearance (palpebral closure, piloerection, salivation, exophthalmos, altered respiration, facial swelling), behaviors (activities, sitting position, location within the tube), and toxicity (ataxia, tremor, seizure). This represented a snapshot of the behavior, as only one entry was made for each mouse. The observer could not be completely blind as to the treatment since the mice were exposed on different NOEC. Additionally, subsets of mice (n=8/group) were assessed for motor activity immediately after removal from the exposure tubes. Specifically, at 0 h PE, one subset of mice was individually placed in plastic shoebox cages in an isolated area for 30 minutes (with a small amount of Beta-Chip bedding). Cages were surrounded by photocells to record fine (repeated breaks of the same photobeam) and ambulatory (breaks of different beams) movements (cage rack system, San Diego Instruments, San Diego, CA). Habituation was tracked with twelve 2.5-minute intervals. A separate subset of mice was similarly assessed at 4 and 24 h PE.
2.5. Whole body plethysmography
Alterations in ventilation were assessed in conscious, unrestrained mice at approximately the same time of day to minimize diurnal effects on breathing patterns. Assessments were obtained via an eight-chambered mouse whole-body plethysmography (WBP) system using Buxco BioSystem XA software (Buxco Electronics, Wilmington, NC) to estimate tidal volume (VT), breathing frequency (ƒ), calculated minute volume (VT x ƒ = MV), and enhanced pause (Penh) — an index of airflow limitation and surrogate measure for bronchoconstriction (Hamelmann et al. 1997). For each stove-week, repeated assessments of the same subset of animals were performed at the following intervals: pre- (the day before), 0 and 4 h PE (the day of), and 24 h PE (the day after exposure). Ventilatory data at these four sampling intervals were collected for 30 minutes following a 5-minute acclimation period for each mouse in its individual WBP chamber.
2.6. Blood Carboxyhemoglobin
Using the submandibular bleeding technique described by Golde et al. (2005), whole-blood samples were acquired immediately upon removal of mice from their exposure tubes. Blood collection tubes were sealed and placed on ice to minimize loss of CO and other blood gases until analysis. Carboxyhemoglobin analysis was then performed using an IL-682 CO-Oximeter (Instrumentation Laboratory; Bedford, MA).
2.7. Hematopoietic parameters
Mice were euthanized via barbiturate overdose and anticoagulated (citrated) blood samples were collected via trans-diaphragmatic cardiac puncture. Hematological indices including total white blood cell (WBC) counts, total red blood cell (RBC) counts, % hematocrit, platelet counts, and lymphocyte (%) were measured using a Coulter AcT 10 Hematology Analyzer (Beckman Coulter Inc., Miami, FL).
2.8. Bronchoalveolar lavage fluid (BALF) collection and assessments
As previously described, immediately following blood collection, the thorax was opened and the trachea was cannulated (Gavett et al., 2015). The left main stem bronchus was occluded with a vascular clip and the left lung was transected and flash-frozen. The remaining right lung lobes were lavaged and then flash-frozen. BALF was used to assess total cell counts on an automated coulter counter (Z1 Beckman Coulter Life Sciences, Indianapolis, IN). Cytospin preparations (Cytospin-3 centrifuge, Shandon Inc.; Pittsburgh, PA) were used for cell differentials on slides stained with a modified Wright-Giemsa strain (Thermo Fisher Scientific, Waltham, MA) on an automated slide stainer (Hema-Tek 2000, Miles, Inc., Elkhart, IN). Using one slide set, cell differentials were enumerated based on counting 100–200 cells per slide. On the other set, animal number and exposure information were blinded to the observer. Evaluating four regions per slide, the number of particles visible within 50 – 100 macrophages were enumerated manually using 20- and 40X magnification on a Nikon Eclipse Ti inverted microscope. Results were expressed as the mean number of particles per macrophage evaluated (i.e., total particles observed/total macrophages assessed). Dark field microscopy images were obtained on a Nikon Eclipse Ti microscope. Following brief centrifugation, BALF supernatants were analyzed for biochemical alterations suggestive of cell activation [e.g., N-acetyl-β-D-glucosaminidase (NAG)], oxidative stress [e.g., gamma-glutamyl transpeptidase (GGT)], and lung injury or edema [e.g., increased lactate dehydrogenase (LDH), albumin, and total protein concentrations]. All biochemical determinations were performed using commercially available kits adapted for use on a Konelab 30 Clinical Chemistry Spectrophotometer Analyzer (Thermo Clinical Labsystems, Espoo Finland).
2.9. Alveolar Macrophage Phagocytosis and Phenotypic Analysis
Evidence of altered innate immunity was based on decreased nonspecific phagocytosis of fluorescent beads by alveolar macrophages as previously described (Burleson et al., 1987). In brief, 2.0 × 105 lung phagocytic cells obtained via lung lavage were incubated with fluorescent polystyrene beads (200 beads/cell; 1 μm beads, Polysciences, Inc.) for 2h and then cyto-centrifuged onto a glass slide for manual enumeration. The % phagocytosis (percentage of macrophages containing at least one bead) and the Absolute Phagocytic Index (API) (% of macrophages containing at least one bead X the average # of beads per positive cell) were determined for 200 cells/mouse. As a positive control group, each week a separate group of mice (n = 4 per week) were instilled with 2 μg of lipopolysaccharide (LPS; Escherichia coli endotoxin; 011:B4 containing 106 units/mg material, Sigma, St. Louis, MO) and similarly assessed at 4 h post-instillation.
Additionally, phagocytic cells obtained via lung lavage were examined for semiquantitative detection of intracellular cytokines at the single cell level using flow cytometry (O’Mahony et al., 1998; Schuerwegh et al., 2003). Access to the intracellular space was achieved by fixing cell membranes with 2% paraformaldehyde. Dead cells and debris were excluded from analysis by using forward scatter and 90° light scatter to establish a gate around viable cells. Macrophages were identified using antibodies for CD11b and F4/80 (Zhang et al., 2008). Antibodies (BD Biosciences) were used to assess differential expression in macrophage intracellular levels of IL-1β (pro-inflammatory) and IL-10 (anti-inflammatory) cytokines. Cells were analyzed with a LSR II flow cytometer (BD Biosciences) using FACS Diva software (BD Biosciences). Analysis of data was performed using FlowJo software (TreeStar, Inc., Ashland, OR).
3.0. Cytokines in bronchoalveolar lavage fluid
Pro-inflammatory cytokines (e.g., IL-1β, TNFα , IFNγ, KC-GRO, IL-4, IL-5, IL-6, IL-10, and IL-13) were measured in undiluted BALF supernatants collected at 4 h PE with a mesoscale Multi-Spot immunoassay kit for rodents (Meso Scale Diagnostics, Rockville, MD).
3.1. Lung Glutathione assessments
Levels of reduced glutathione (GSH) and glutathione disulfide (GSSG) were measured in homogenates derived from flash-frozen (non-lavaged) left lung tissue via an adapted HPLC-FLD dansylation method (Jones et al., 1998; Gan et al., 2005). In brief, a portion of the left lung lobe was homogenized in cold 4% perchloric acid (PCA) containing 0.2 M boric acid and 4 mM diethylenetriaminepentaacetic acid (Sigma, St. Louis, MO) at a constant ratio of 10 μl per 1 mg of lung tissue. After centrifugation (20 min, 4°C, 20,000 x g), cell-free supernatants were adjusted to pH 9.0 ± 0.2 and treated with dansyl chloride (Sigma Aldrich; St. Louis MO) to label the reduced and disulfide fractions. Following chloroform clean-up, the dansylated GSH and GSSG products of each sample were fluorescently quantified using an Agilent 1100 series HPLC-FLD (335 nm excitation; 515 nm emission; Agilent Technologies; Santa Clara, CA) and Supelco Discovery C18 column (Bellefonte, PA). Standard concentrations (5 to 500 μM) of GSH and GSSG (Sigma Aldrich; St. Louis MO) were prepared in parallel with each sample preparation. All data were acquired using Chemstation software (Agilent Technologies, Santa Clara, CA).
3.2. Gene Expression in the Lung
Total RNA was isolated from 20–30 mg of flash-frozen right lung tissue (post-lavage) using a Qiagen RNeasy RNA extraction kit (Valencia, CA). The purity was estimated using a Nanodrop ND-1000 spectrophotometer (Wilmington, DE). Real-time PCR was performed using TaqMan Gene Expression Assays for the following genes: Cyclooxygenase-2 (Cox-2; Mm00478374_m1), Cytochrome p450 1a1 (CYP1a1; Mm00487218_m1), Glutamate-Cysteine Ligase Catalytic subunit (GCLC; Mm00802655_m1), Hemeoxygenase-1 (HO-1; Mm00516005_m1), Interleukin-6 (IL6; Mm00446190_m1), Macrophage Inflammatory Protein-2 (MIP-2; Mm00436450_m1), NAD(P)H quinone dehydrogenase 1 (NQO1; Mm01253561_m1), and Tumor Necrosis Factor alpha (TNFα; Mm00443258_m1) (S2 Table) (Applied Biosystems, Foster City, CA). All target gene expression was normalized to β-actin mRNA expression (TaqMan Gene Expression Assay Mm00607939_s1) for each respective sample. An Applied Biosystems 7900HT Sequence Detection System was used to determine relative gene expression via real-time PCR reactions using TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA). Quantification of all relative gene expression was determined using the ∆∆CT method.
3.3. Statistical Analysis
First, for data reduction purposes, group differences in filtered air-exposed control animals across the three exposure weeks were assessed using one-way analysis of variance (ANOVA) with Holm-Sidak’s multiple comparisons test. Separate comparisons were performed for each time (i.e., 0, 4, and 24 h PE). For a given end point, if no significant group (week) differences were observed in air-exposed controls, control animal data for each time period were pooled. If significant group differences were observed in air controls, data were expressed as the % of the group mean for each week and results were then pooled. Data from FD, ND and 3-S fire-exposed mice were then compared to data from pooled air-exposed mice and to each other, for the corresponding time PE, using Holm-Sidak’s multiple comparisons test. Where Brown-Forsythe statistics indicated non-homogeneity of variances, data were reanalyzed by Kruskal-Wallis nonparametric ANOVA with Dunn’s multiple comparisons test (GraphPad Prism version 6.07). Group means ± SEM with significance of at least p < 0.05 are indicated in the resultant tables and figures below.
3. Results
3.1. Emissions Characterization
Characterization of exposure atmospheres at the exposure ports (i.e., animals breathing zone) revealed somewhat smaller mean particle size distributions, based on volume, for the FD stove compared to the ND stove and 3-S fire (Figure 1B). The smaller mean particle size for the FD stove is associated with its higher combustion efficiency. However, the geometric standard deviations for all particle size distributions for all stoves were greater than 2, indicating a bimodal size distribution. This shows that for each CS, one size distribution was above the mean particle size shown in Figure 1B, and one was below the mean particle size. In comparison to the traditional 3-S fire, considerable reductions were achieved in the PM concentrations emitted by the FD (80%) and ND (62%) stoves (Figure 1C). Depicted are the mean (± SD) of the two replicates performed for each stove week. The relative amounts of organic carbon (OC) and elemental carbon (EC) in emitted particles are depicted in Figure 1D. Notably, as stove combustion efficiency decreased, particle OC content increased. Specifically, the % of OC in particles from FD, ND, and 3-S fire emissions was 11%, 26%, and 38, respectively.
As a marker of stove combustion efficiency, CO measurements were highest in the 3-S fire emissions, averaging nearly 60 ppm; whereas the FD and ND stoves generated lesser concentrations, approximately 4 and 20 ppm, respectively (Figure 1F). Average temperature and relative humidity of the exposure tubes were maintained at ~22.2°C and ~39%, respectively. Concentrations of SOx and NOx species were < 1 ppm (data not shown).
Examination of the chemical speciation from representative samplings of select VOCs clearly demonstrated that the FD stove sample emitted the lowest concentrations of alkanes (e.g., n-hexane), alkenes (e.g., 1,3-butadiene), cyclic hydrocarbons or aromatics (i.e., benzene), and carbonyl species (e.g., acrolein), compared to the ND and 3-S samples (Figure 2A-D). However, there was no consistent trend as to which stove system, ND or 3-S fire, emitted the highest level of select aromatics or carbonyls. For many cyclic hydrocarbons, FD << 3-S fire < ND. With the exception of benzene, propylene, acetaldehyde, and formaldehyde, concentrations of the remaining VOCs were often substantially less than 50 parts per billion (ppb); while the FD stove often yielded values less than 5 ppb.
Figure 2.
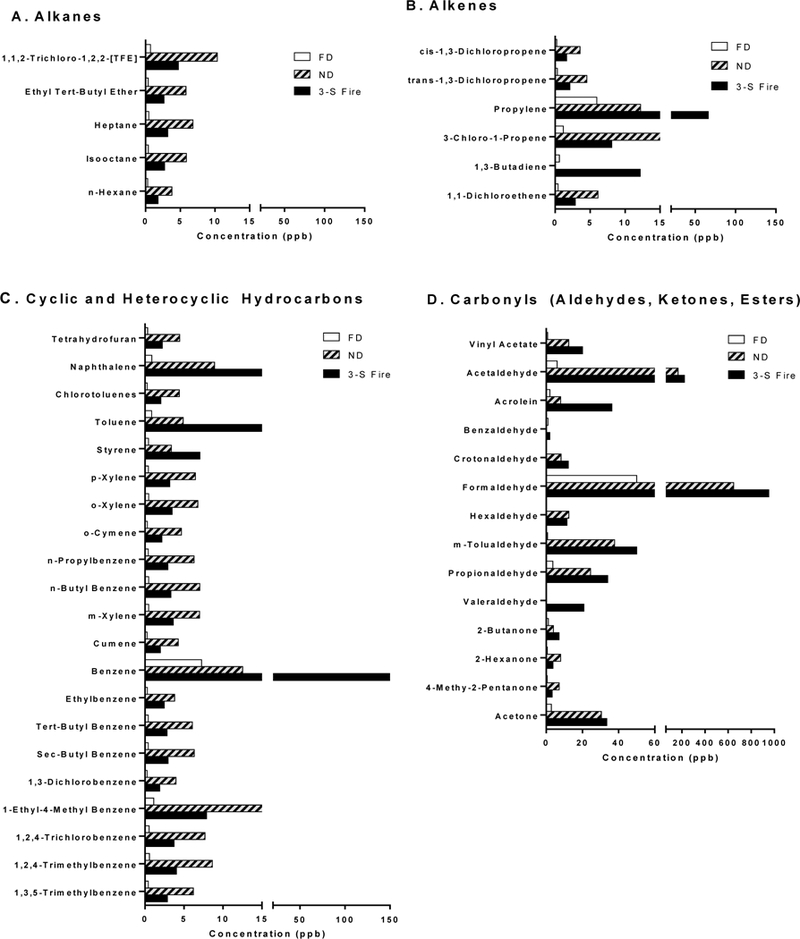
Volatile chemical speciation from representative canister collected samples of CS emissions. Mean concentrations (ppb) of two replicates for each stove week are depicted for (A) alkanes; (B) alkenes; (C) cyclic hydrocarbons or aromatics; and (D) carbonyl species based on emissions from the FD stove (white bars); ND stove (grey bars with hatching), or 3-S fire (solid black bars).
3.2. Health Effects and Biomarkers of Exposure
3.2.1. Behavioral Assessments and Motor Activity
No overt signs of toxicity or poor appearance (e.g., piloerection, labored respiration, ataxia) were observed during any of the CS exposures. There were no significant differences in behaviors for the FD and 3-S fire exposures. With the ND exposure, there were more mice sitting (not active) and more were located in the back of the tube (farthest away from the input source). This may represent lowered activity and avoidance of the exposure; however, due to unforeseen circumstances a different observer participated during this week only. Although there was cross-training and inter-rater reliability evaluations before testing, the control data for this exposure differed from the other two exposures. Thus, these treatment effects could be confounded by the differences in observers. Session totals of ambulatory and fine activity showed no significant differences across the study. With the 3-S fire exposure at time 0 h PE or FD exposure at 4 h PE, there were differences in habituation. In these cases, the treatment-by-interval factors were significant, yet no individual intervals showed differences in the two groups; visual inspection of the data suggested slightly faster habituation, but this effect was marginal (data not shown).
3.2.2. Whole body plethysmography (WBP)
Compared to air-exposed controls, no significant ventilatory alterations were detected in mice exposed to emissions from the FD, ND, or 3-S cooking systems either immediately (0 h PE), or at 4 and 24 h PE (data not shown). One minor difference was observed at 0 h PE; the average tidal volume of ND-exposed group was significantly less (~10%) than that of FD or 3-S fire-exposed mice. In general, the lack of ventilatory alterations is consistent with the minimal behavioral changes noted above. Results show that as anticipated inhalation of the diluted primary emission stream was not associated with excessive irritation (due to high VOC) nor overt respiratory depression (due to CO-related narcosis); therefore, we conclude that mice adequately inhaled volatile and PM components of the CS emissions during their exposures.
3.2.3. Biomarkers of exposure
Blood carboxyhemoglobin concentration and particulate burden of alveolar macrophages were two endpoints used to verify differential exposure to gaseous and PM components, respectively, of emissions across the CS groups. Because the half-life of CO in humans (3–4 h) is much longer than in mice (15 min) (Motterlini & Otterbein, 2010), mice were bled immediately upon removal from their exposure tubes. At 0 h PE, blood carboxyhemoglobin levels in 3-S fire-exposed mice were elevated significantly, compared to air-exposed mice or to mice exposed to emissions from the FD or ND stoves (Figure 3A). Notably, carboxyhemoglobin levels of the FD- or ND-exposure groups were not significantly different from those of control mice.
Figure 3.
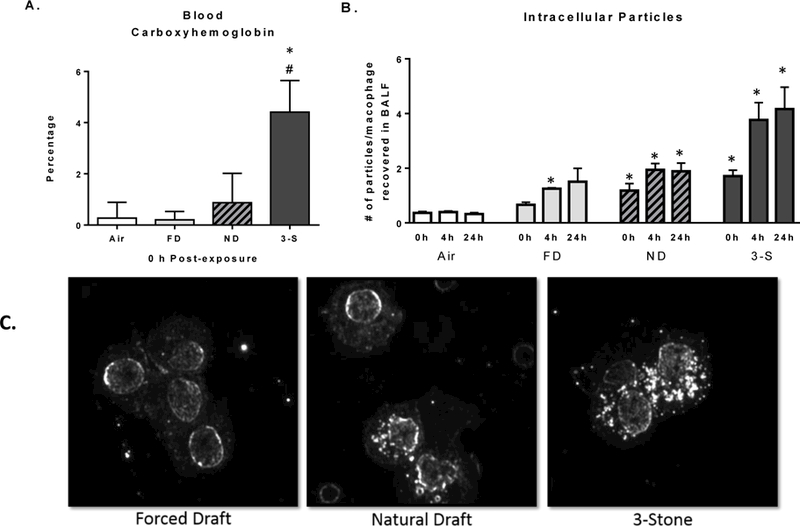
Biomarkers of exposure to CS emissions. (A) Blood % carboxyhemoglobin concentrations at 0 h PE; (B) number of particles visible in macrophages obtained via lung lavage at 0, 4, and 24 h PE; and (C) dark field microscopy (40X magnification) images show particles (white dots) within cell boundaries of phagocytic cells at 24 h PE. Data represent the mean (±SE) of n = 8 mice/group for the FD (light bars), ND (grey bars with hatches), and 3-S fire (solid black bars) exposed groups. *Significantly different than the air control group at the corresponding time PE. #Significantly different than all other CS groups at the corresponding time PE.
Despite exposures ending at 0 h PE, visual quantitative enumeration of particulate material within macrophages revealed that particle burden continued to increase across the 24 h timeframe examined (Figure 3B). There was considerable variability in the number of particles visible in phagocytes, even within the same lavage sample. Many PM-laden macrophages contained only 0–3 visible particles, whereas some had > 10, and clumping of PM made it difficult to define exact numbers. On average, the number of visible particles per phagocyte in the ND- and 3-S fire-exposed groups were significantly greater than air exposed-controls (Figure 3B). Qualitative examination of phagocytized particulates via dark field microscopy at 24 h PE similarly revealed visibly increasing particulate material (white dots) within the cellular boundaries of macrophages from exposed mice (FD << ND <3-S fire). The edge of the nuclear membranes were visible as elliptical rims of white within the cell (Figure 3C).
3.2.4. Complete blood counts
No CS exposure-related hematological effects were apparent at 0 h PE (Table 1). However, the 0 h hematocrits were reduced 10–15% compared to the latter time points due to prior blood collection for carboxyhemoglobin in this group of mice. At 4 h PE, the % hematocrit of the FD stove exposure group was mildly reduced (10%), whereas the % of lymphocytes in 3-S fire- and FD stove groups were mildly suppressed. Conversely, at 4 h PE, exposure to ND emissions was associated with a significant increase in the number of circulating WBCs. By 24 h PE, no further alterations were apparent. All changes were minor and of indeterminate health significance.
Table 1.
Comparison of changes in hematological indices.
| Exposure Group and Hematological Endpoints | n = | 0 h PE | 4 h PE | 24 PE |
|---|---|---|---|---|
| Hematocrit (%) | ||||
| Air controls (pooled) | 24 | 29.8 ± 0.53 | 35.1 ± 0.60 | 34.0 ± 0.59 |
| Forced Draft | 8 | 29.1 ± 0.86 | 31.4 ± 1.56* | 32.6 ± 0.89 |
| Natural Draft | 8 | 30.0 ± 0.95 | 33.6 ± 1.08 | 35.3 ± 1.24 |
| 3-Stone | 8 | 30.4 ± 1.01 | 34.5 ± 0.69 | 41.2 ± 0.65 |
| Circulating WBC counts (× 103/μL) | ||||
| Air controls (pooled) | 24 | 3.14 ± 0.16 | 2.55 ± 0.20 | 1.63 ± 0.11 |
| Forced Draft | 8 | 2.94 ± 0.25 | 1.55 ± 0.16 | 1.41 ± 0.11 |
| Natural Draft | 8 | 3.63 ± 0.22 | 3.38 ± 0.35*# | 1.69 ± 0.24 |
| 3-Stone | 8 | 3.64 ± 0.21 | 2.08 ± 0.23 | 2.13 ± 0.26 |
| Circulating Platelets (× 103/μL) | ||||
| Air controls (pooled) | 24 | 508 ± 32.4 | 597 ± 42.4 | 592 ± 35.7 |
| Forced Draft | 8 | 558 ± 57.3 | 690 ± 63.7 | 705 ± 81.5 |
| Natural Draft | 8 | 547 ± 34.2 | 764 ± 93.9 | 568 ± 45.0 |
| 3-Stone | 8 | 540 ± 76.0 | 550 ± 48.7 | 557 ± 53.6 |
| Lymphocytes (%) | ||||
| Air controls (pooled) | 24 | 59.0 ± 2.23 | 78.0 ± 1.19 | 70.9 ± 1.94 |
| Forced Draft | 8 | 54.8 ± 3.87 | 69.5 ± 1.66* | 76.3 ± 0.63 |
| Natural Draft | 8 | 61.1 ± 3.83 | 78.4 ± 1.48£ | 74.6 ± 1.72 |
| 3-Stone | 8 | 62.0± 4.44 | 71.6 ± 2.85* | 76.9 ± 2.21 |
Significantly different than the air control group at the corresponding time PE. p < 0.05.
Significantly different than the FD stove group at the corresponding time PE. p < 0.05.
Significantly different than all other CS groups at the corresponding time PE. p < 0.05.
3.25. Cellular and biochemical analysis of bronchoalveolar lavage fluid (BALF)
Cytological examination of cells recovered in BALF revealed that essentially all cells were alveolar macrophages. There was no indication that exposure to CS emissions resulted in pulmonary hemorrhage, sloughing of epithelial cells, or in an influx of inflammatory cells (e.g., neutrophils, eosinophils) at any time PE (data not shown). However, at 0 h PE, the total number of cells recovered in BALF from FD-exposed mice was significantly lower than air controls or 3-S fire-exposed mice; while the ND-exposed mice had an intermediate value (Table 2). Biochemical analysis of BALF of FD-exposed mice at 0 h PE further revealed a significant decrease in the concentration of NAG, a lysosomal enzyme often secreted by alveolar macrophages in response to phagocytosing particles (Mack et al., 1995). In 3-S fire-exposed mice, both albumin and total protein concentrations were significantly increased in BALF at 0 h PE, consistent with acute lung injury manifesting as minor increases in lung vascular or alveolar epithelial permeability. Negligible exposure effects were observed on BALF concentrations of LDH or GGT, except for the FD-group at 4 h PE. By 24 h PE, no exposure related differences were apparent.
Table 2.
Comparison of changes in bronchoalveolar lavage fluid end points.
| Exposure Groups | n = | 0 h PE | 4 h PE | 24 h PE |
|---|---|---|---|---|
| Total Cell Count (% of control) | ||||
| Air controls (pooled) | 24 | 100 ± 5.51 | 100 ± 5.34 | 100 ± 7.68 |
| Forced Draft | 8 | 65.5 ± 2.74* | 100 ± 6.67 | 93.5 ± 6.87 |
| Natural Draft | 8 | 87.8 ± 8.84 | 84.2 ± 4.73 | 90.1 ± 10.7 |
| 3-Stone | 8 | 110 ± 13.6£ | 104 ± 19.7 | 77.9 ± 12.2 |
| NAG (U/L) | ||||
| Air controls (pooled) | 24 | 7.19 ± 0.25 | 6.18 ± 0.47 | 7.38 ± 0.34 |
| Forced Draft | 8 | 5.27 ± 0.45*# | 5.82 ± 0.52 | 5.52 ± 0.68 |
| Natural Draft | 8 | 7.49 ± 0.74 | 8.47 ± 0.81 | 5.95 ± 0.49 |
| 3-Stone | 8 | 6.30 ± 0.28 | 7.80 ± 0.68 | 6.59 ± 0.76 |
| Albumin (μg/mL) | ||||
| Air controls (pooled) | 24 | 14.6 ± 0.81 | 15.6 ± 0.72 | 16.5 ± 0.63 |
| Forced Draft | 8 | 14.2 ± 1.00 | 16.6 ± 1.09 | 17.0 ± 0.90 |
| Natural Draft | 8 | 13.1 ± 1.41 | 15.1 ± 1.27 | 14.0 ± 1.45 |
| 3-Stone | 8 | 19.0 ± 1.40*# | 15.4 ± 1.17 | 16.1 ± 1.20 |
| Total Protein (μg/mL) | ||||
| Air controls (pooled) | 24 | 94.9 ± 2.82 | 99.5 ± 4.26 | 101 ± 4.08 |
| Forced Draft | 8 | 100 ± 6.58 | 103 ± 5.85 | 102 ± 12.1 |
| Natural Draft | 8 | 93.2 ± 6.47 | 114 ± 7.66 | 96 ± 7.42 |
| 3-Stone | 8 | 118 ± 12.0* | 106 ± 6.71 | 123 ± 5.53 |
| Lactate Dehydrogenase (U/L) | ||||
| Air controls (pooled) | 24 | 35.5 ± 1.21 | 35.3 ± 1.43 | 35.3 ± 1.43 |
| Forced Draft | 8 | 35.2 ± 1.97 | 37.3 ± 3.09 | 38.4 ± 4.46 |
| Natural Draft | 8 | 38.9 ± 2.18 | 36.5 ± 2.44 | 36.5 ± 3.70 |
| 3-Stone | 8 | 38.1 ± 3.16 | 32.7 ± 1.91 | 41.2 ± 2.69 |
| GGT (% of air control) | ||||
| Air controls (pooled) | 24 | 100 ± 3.20 | 100 ± 3.74 | 100 ± 5.92 |
| Forced Draft | 8 | 98.0 ± 7.80 | 142 ± 25.7* | 86.5 ± 8.68 |
| Natural Draft | 8 | 90.1 ± 2.00 | 104 ± 4.81 | 104 ± 6.21 |
| 3-Stone | 8 | 80.6 ± 10.7 | 111 ± 9.87 | 93.5 ± 3.00 |
Significantly different than the air control group at the corresponding time PE. p < 0.05.
Significantly different than the FD stove group at the corresponding time PE. p < 0.05.
Significantly different than all other CS groups at the corresponding time PE. p < 0.05.
3.2.6. Changes in lung macrophage intracellular cytokine expression and phagocytic function
Alterations in host defense were evaluated by assessing alveolar macrophage intracellular cytokine expression and non-specific phagocytic functionality at 0, 4 and 24 h PE. Macrophages recovered from 3-S fire-exposed mice showed significant increases in IL-1β content at 0 h (2.1-fold) and 4 h (2.3-fold) PE, however, data failed to demonstrate diminished phagocytic response (i.e., bead uptake) in this group (Table 3). By contrast, the ND-exposed mice showed significant reductions in the % of cells phagocytosing fluorescent beads, the calculated API, and concomitant (not statistically significant) trends towards reduced intracellular IL-1β (70%) and IL-10 (25%) at 4 h PE. Likewise, at 4 h PE, macrophages from LPS-treated mice (used as a positive control) had significant reductions in the % phagocytosis, API, and intracellular IL-10 content. Conversely, after LPS treatment, IL-1β expression was increased significantly (7-fold) (Table 3). Exposure to emissions from the FD stove was without effect on any of these parameters.
Table 3.
Comparison of macrophage phagocytosis and flow cytometry end points.
| Exposure Groups | n = | 0 h PE | 4 h PE | 24 h PE |
|---|---|---|---|---|
| % Phagocytosis (manual counts) | ||||
| Air controls (pooled) | 20–23 | 61.3 ± 1.84 | 64.1 ± 1.65 | 67.6 ± 2.40 |
| Forced Draft | 7–8 | 60.3 ± 4.26 | 66.7 ± 2.61 | 71.1 ± 3.65 |
| Natural Draft | 6–8 | 60.2 ± 1.40 | 46.5 ± 3.89*# | 65.3 ± 2.88 |
| 3-Stone | 5–8 | 54.0 ± 4.30 | 62.0 ± 3.70 | 54.6 ± 2.98£ |
| LPS-treatment | 11 | ND | 41.4 ± 2.82* | ND |
| Absolute Phagocytic Index (API) | ||||
| Air controls (pooled) | 20–23 | 156 ± 13.7 | 170 ± 11.4 | 219 ± 19.0 |
| Forced Draft | 7–8 | 168 ± 31.5 | 211 ± 25.2 | 224 ± 23.8 |
| Natural Draft | 6–8 | 137 ± 6.09 | 93.4 ± 19.5*# | 226 ± 23.9 |
| 3-Stone | 5–8 | 143 ± 32.6 | 169 ± 36.2 | 136 ± 24.1 |
| LPS-treatment | 11 | ND | 68.4 ± 12.0* | ND |
| IL-1β (flow cytometry-based) | ||||
| Air controls (pooled) | 23–24 | 2.96 ± 0.48 | 4.48 ± 0.70 | 2.68 ± 0.30 |
| Forced Draft | 8 | 1.26 ± 0.32 | 3.63 ± 0.69 | 2.99 ± 0.91 |
| Natural Draft | 8 | 3.56 ± 0.68 | 1.31 ± 0.28 | 5.63 ± 2.08 |
| 3-Stone | 8 | 6.38 ± 1.10*£ | 10.4 ± 2.27*# | 3.12 ± 0.36 |
| LPS-treatment | 12 | ND | 31.6 ± 1.98* | ND |
| IL-10 (flow cytometry-based) | ||||
| Air controls (pooled) | 23–24 | 57.6 ± 2.13 | 53.0 ± 4.41 | 58.0 ± 2.96 |
| Forced Draft | 8 | 52.9 ± 4.42 | 59.2 ± 4.37 | 60.6 ± 2.75 |
| Natural Draft | 8 | 53.9 ± 4.68 | 38.4 ± 7.09 | NA |
| 3-Stone | 8 | 62.6 ± 2.85 | 58.7 ± 5.64 | 55.4 ± 3.00 |
| LPS-treatment | 12 | ND | 32.0 ± 3.78* | ND |
Significantly different than the air control group at the corresponding time PE. p < 0.05.
Significantly different than the FD stove group at the corresponding time PE. p < 0.05.
Significantly different than all other CS groups at the corresponding time PE. p < 0.05.
ND, not determined. NA, not available.
3.2.7. Cytokines in bronchoalveolar lavage fluid
Based on the collective experience of the authors regarding particulate air pollutant investigations (e.g., diesel exhaust, concentrated air particles, wood smoke), the optimal time to detect increases in BALF cytokine levels after an acute exposure is ~4 h. Thus in this study, BALF samples obtained at 4 h PE were assayed for several pro-inflammatory mediators. Data revealed that only the 3-S fire-exposed mice showed significant increases in IL-1β, although the ND group had an intermediate value (Figure 4A). Only the FD group showed increased BALF concentrations of TNFα (Figure 4B). Exposure to CS emissions was without effect on BALF levels of KC-GRO, IL-4, IL-5, or IL-6 (data not shown). Concentrations of IL-10, IL-13, or IFNγ in BALF were below detection limits of the assay.
Figure 4.
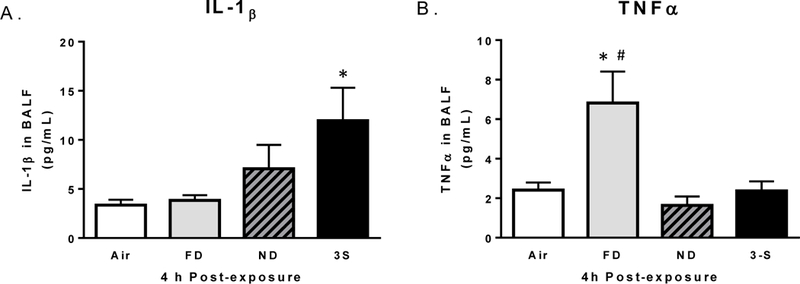
Cytokine concentrations of (A) IL-1β and (B) TNFα present in bronchoalveolar lavage fluid at 4 h PE. Data represent the mean (±SE) of n = 8 mice/group. *Significantly different than the air control group (p < 0.05). #Significantly different than all other CS groups (p < 0.05).
3.2.8. Relative change in lung glutathione content
Both reduced glutathione (GSH) and glutathione disulfide (GSSG) were examined in non-lavaged whole-lung homogenates because this glutathione redox pair is a well-established marker to evaluate oxidative stress. At 0 h PE, exposure to emissions from all three CS systems was associated with significant decreases in GSH (expressed as a percentage of air controls) (Table 4). GSH reductions were persistent at the 4 and 24 h PE evaluations in the FD and 3-S fire groups. Concurrent increases in GSSG were observed only in the FD stove group, both at 0 and 24 h PE (Table 4). Consistent with these findings, oxidative stress has been repeatedly cited as an outcome of exposure to wood smoke and other biomass emissions (Demling et al., 1994; de Carvalho et al., 2016).
Table 4.
Comparison of lung glutathione alterations.
| Exposure Groups | n = | 0 h PE | 4 h PE | 24 h PE |
|---|---|---|---|---|
|
Reduced Glutathione (% of air-exposed group) |
||||
| Air controls (pooled) | 20–24 | 100 ± 2.09 | 100 ± 2.97 | 100 ± 3.71 |
| Forced Draft | 8 | 72.6 ± 5.83* | 53.0 ± 5.34* | 70.5 ± 9.44* |
| Natural Draft | 8 | 82.1 ± 3.28* | 96.3 ± 3.92# | 80.4 ± 5.42 |
| 3-Stone | 4–8 | 73.2 ± 3.87* | 75.4 ± 6.25* | 69.5 ± 7.56* |
|
Glutathione Disulfide (% of air-exposed group) |
||||
| Air controls (pooled) | 20–24 | 100 ± 2.88 | 100 ± 2.93 | 100 ± 6.29 |
| Forced Draft | 8 | 120 ± 9.12* | 93.8 ± 3.76 | 148 ± 16.6*# |
| Natural Draft | 8 | 108 ± 2.56 | 114 ± 2.81£ | 106 ± 5.65 |
| 3-Stone | 4–8 | 100 ± 6.37 | 110 ± 8.13 | 91.4 ± 8.88 |
Significantly different than the air control group at the corresponding time PE. p < 0.05.
Significantly different than the FD stove group at the corresponding time PE. p < 0.05.
Significantly different than all other CS groups at the corresponding time PE. p < 0.05.
3.2.9. Lung adaptive gene expression changes
Because oxidative stress is often attributed as an initiating event for the up-regulation of genes related to pulmonary inflammation, antioxidant response, and xenobiotic metabolism, we examined the effects of CE exposure on steady state expression of eight target genes representing these pathways (summarized in S2 Table). Lung tissue homogenates collected at 0 h PE were used because preliminary analysis of select genes demonstrated maximal expression at this time (data not shown). At 0 h PE, exposure was without effect on lung gene expression of TNFα (a classical pro-inflammatory cytokine and pro-apoptotic mediator), IL-6 (a pleiotropic cytokine), or MIP-2 (a neutrophil chemoattractant) (Figure 5). Findings are consistent with lack of lung inflammatory cell influx in BALF at either 4 or 24 h PE.
Figure 5.
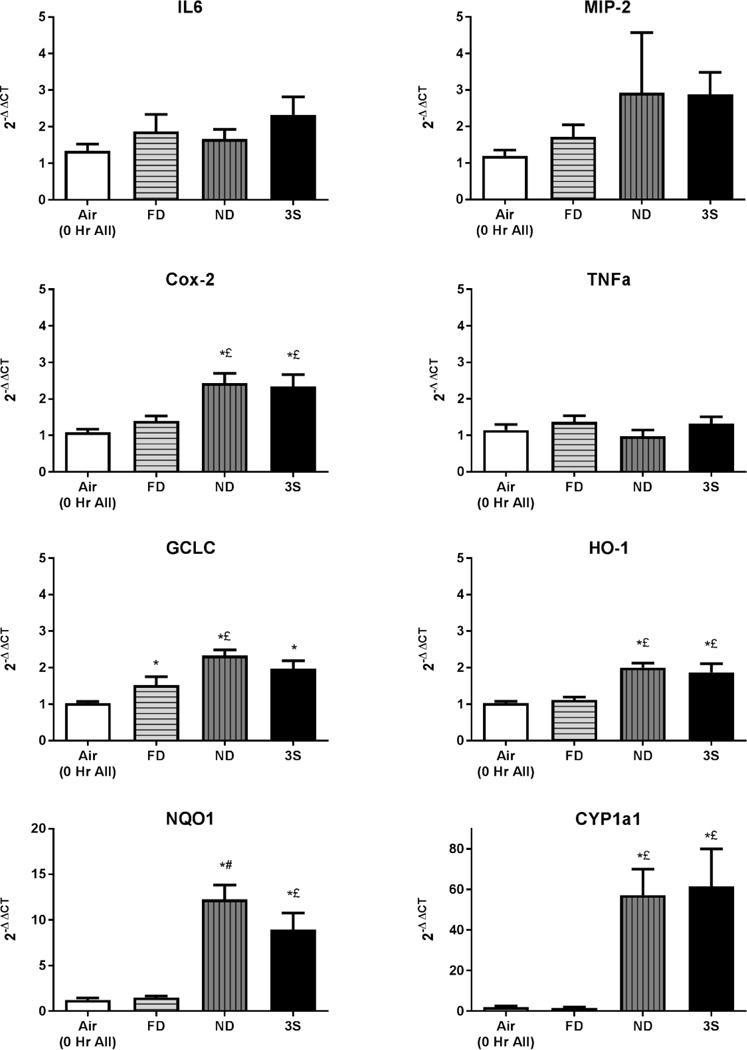
Alterations in steady-state expression of eight genes related to lung inflammatory pathways (TNFα, MIP-2, IL-6), antioxidant status (GCLC, HO-1), or xenobiotic metabolism (COX-2, NQO1, CYP1A1) were evaluated in lung tissue homogenates at 0 h PE. Results are normalized to β-actin, and represent the mean (±SE) of n = 8 mice/group for the FD (light bars with horizontal hatching), ND (grey bars with hatching), and 3-S fire (solid black bars). *Significantly different than the air control group at the corresponding time PE (p < 0.05). £Significantly different than the FD stove group at the corresponding time PE (p < 0.05). #Significantly different than all other CS groups at the corresponding time PE (p < 0.05).
Conversely, across all three CS-exposure groups, increases were detected in GCLC, which is the initial rate-limiting enzyme for glutathione synthesis (Figure 5). Moreover, increases in the ND group were significantly greater than the FD group. The oxidative capacity of PM from biomass burning is a well-established mechanism by which health effects may be elicited (Eiguren-Fernandez et al., 2010; Bates et al 2015). Expression of hemeoxygenase-1 (HO-1), a stress-response protein, was significantly elevated in lungs from the ND and 3-S fire groups, but not in the FD group, as compared to air controls. HO-1 increases have been reported in lung endothelial cells exposed to wood smoke extracts (Liu P-L et al., 2005). Likewise, significant increases were also detected for cyclooxygenase 2 (COX-2) expression in only the ND stove and 3-S fire-exposed groups. By far the greatest increases were observed in ND stove and 3-S fire-exposed groups for expression of genes linked to xenobiotic metabolism, namely NQO1 (10-fold) and CYP1a1 (50-fold); no such induction was observed in the FD stove group.
4. Discussion
Current assessments of cleaner cooking technologies have shown reductions in emitted air pollutants (i.e., CO and PM) as well as improved safety during use (Pilishvili et al., 2016; Ochieng et al., 2017; Yip et al., 2017; Pope et al., 2017), but relatively few studies have been able to demonstrate corresponding health benefits (Martin et al., 2014; Mortimer et al., 2017). We report for the first time results of a direct comparison of differential exposure and acute respiratory toxicological effects in healthy female mice inhaling solid-fuel emissions across three CS efficiency Tiers: 0, 2 and 3. We show that particles emitted from each stove were well within the respirable range, even for mice. Respirable fine or UF particles are considered especially harmful to human health, and are associated with development of cardiopulmonary diseases such as COPD and atherosclerosis (Traboulsi et al., 2017).
In comparing the PM and CO exposure concentrations of the present study (i.e., mean concentrations across two cycles of a standard water boiling test lasting ~3-h), levels reported for homes using rudimentary cooking devices are highly variable. Some studies describe maximum indoor PM levels of nearly 1000 μg/m3 in Sri Lankan homes (Chartier et al., 2017) and peak CO ≥ 30 ppm in homes in Kenya (Ochieng et al., 2017). Such concentrations are not unlike that used herein. Other studies report levels based on longer averaging periods. For example, the 48-h geometric mean fine PM and CO concentrations in kitchens of rural Kenya were ~600 μg/m3 and ~5 ppm, respectively (Yip et al., 2017); whereas in Honduran homes using traditional stoves, indoor geometric means for fine PM (8-h) and CO (1-h) were ~1000 μg/m3 and 14.3 ppm, respectively (Clark et al., 2010). Still lower median levels (~100 μg/m3) were reported in Guatemalan homes using wood-fueled chimney stoves (Weinstein et al., 2017). Of concern, despite the lower PM concentrations in the latter study, examination of urinary biomarkers in exposed pregnant women revealed increases in metabolites of several VOC (e.g., benzene, acrylonitrile, and PAH) – with maximum 1-hydroxypyrene levels in exceedance of occupational limits set for coke-oven workers (Weinstein et al., 2017).
As expected, emissions generated herein by the advanced ND (Tier 2) and FD (Tier 3) stoves yielded substantially less CO and PM compared to emissions from a rudimentary 3-S fire (with FD < ND < 3-S). Reduced CO and PM output was clearly and inherently linked to increasing fuel combustion efficiency of CS. Moreover, the percent (%) OC content of particles emitted by the ND and FD stoves decreased by 2- and 3-fold, respectively, compared to the 3-S fire (Figure 1C-F).
Interestingly, however, the relative concentrations of emitted select VOCs (as detected by the TO-15 and T0–11A methods) did not follow this simple pattern. Even though total VOCs were lowest for the Tier 3 (FD) stove, the relative abundance of these chemicals in emissions from the Tier 2 (ND) stove were often greater than the 3-S fire (Tier 0) (Figure 2). Notably, we observed significant but comparable increases in lung gene expression for NQ01, CYP1a1, HO-1, and COX-2 in both the ND and 3-S fire groups. Together, these data suggest that the acute alterations in gene expression were associated with exposure to the relatively higher VOC and/or particle OC content of emissions generated by the ND stove and 3-S fire. HO-1 induction in murine macrophages has previously been shown to occur after exposure to extracts of VOCs/SVOCs derived from ambient aerosols. Reportedly, induction was related to electrophilic capacities of the vapor-phase toxicants (Eiguren-Fernandez et al., 2010). Moreover, upregulation of the enzyme COX-2 affects not only xenobiotic metabolism, but also can influence cell proliferation and apoptosis, in other words, pathways involved in cell turnover, tissue repair, and tumorigenesis (Moraes et al., 2017). COX-2 upregulation may also modify immune and inflammatory responses in the lung, and has been linked to development of COPD (Shi et al., 2017).
4.1. Three-stone fire
In terms of linking health benefits derived from advanced CS usage to increasing combustion efficiencies of the CS, we took a snap shot across a wide variety of general health and respiratory system parameters that are often altered acutely following exposure to air pollutants. Starting with the Tier 0 cooking system, mice exposed to the 3-S fire inhaled the highest concentrations of notably hazardous air toxicants (e.g., 1,3-butadiene, toluene, benzene, and acrolein) as well as the highest concentration of particles with the highest OC content. We have shown previously that on an equal-mass basis, diesel particles with higher OC content are characteristically more toxic (e.g., in terms of inducing epithelial injury and HO-1 expression) (Manzo et al., 2010). Data also showed that mice exposed to the 3-S fire appeared to have the greatest number of particles present in their lung phagocytes. Furthermore, they showed a significant increase in alveolar macrophage IL-1β release, and were the only group to develop transient pulmonary edema. Gene expression in lung tissue showed rapid and significant increases in antioxidant and stress-response genes, along with robust increases for genes related to xenobiotic metabolism (i.e., NQO1 and CYP1a1). By 24 h PE, inhaled particles had yet to be cleared from lung phagocytes of 3-S fire-exposed mice, and based on continued reduction of lung GSH content (~30%), some degree of oxidative stress was ongoing.
The CYP1A1 gene encodes for the Phase I metabolizing enzyme cytochrome P450 1A1, which is known to be involved in metabolic processing of PAHs (Sen et al., 2007). As reported by Mutlu et al (2016), relative levels of PAHs (e.g., benzo-[a]pyrene) and oxy-PAHs (e.g., benzanthrone) in CS emissions were FD << ND < 3-S fire. Notably, when lung microsomes are exposed to carcinogens such as nitro– or amino-benzanthrones, not only are NQO1 and Cyp1A1 enzymes induced, these enzymes then appear to activate benzanthrones into species that form additional DNA adducts (Stiborova et al., 2008). In so doing, PAH-type carcinogens not only induce expression and activity of CYP1A1, but they also enhance their own genotoxic and carcinogenic potential.
Collectively, one interpretation of the above findings is that macrophage uptake of the large number of particles associated with inhaling emissions from a 3-S fire (or alternatively inhalation of particles with higher OC content), lead to upregulation and release of IL-1β from lung phagocytes. IL-1β is known to cause increased lung vascular permeability (Kolb et al, 2001). Enhanced IL-1β production has been documented in COPD. Further increases are common during COPD decompensation periods (Chung 2001). In turn, prolonged upregulation of IL-1β may induce other acute pro-inflammatory mediators (i.e., TNFα, IL-6, chemokines) (Lappalainen et al., 2005). However, the 3-S fire-exposed mice failed to show evidence of downstream pro-inflammatory cascades based on nominal change in lung TNFα, IL-6, or MIP-2 gene expression (0 h PE), unchanged TNFα levels in BALF (4 h PE), and lack of neutrophil influx (by 24 h PE). These findings could be related to their rather limited “two meal” emissions exposure. It may also be relevant that the 3-S fire-exposed mice inhaled the highest CO concentrations (≅ 60 ppm) and they were the only group to have detectable increases in blood carboxyhemoglobin. CO inhalation has been shown to selectively inhibit induction of key pro-inflammatory cytokines (e.g., TNFα) (Otterbein et al., 2000) and to attenuate neutrophil sequestration within the pulmonary vasculature of LPS-treated mice (Wilson et al., 2010). By inference, in the 3-S fire-exposed mice, inhalation of 60 ppm CO may have had anti-inflammatory effects that served to dampen downstream inflammatory cascades.
The lack of a strong inflammatory response despite continued lung oxidative stress is consistent with previous experimental studies examining the toxicity of hardwood smoke (Reed et al., 2006; Barrett et al., 2006). Competing effects are induced by the different chemicals and compounds present in solid-fuel smoke mixtures. On the one hand, continued inhalation of particles may perpetuate lung oxidative stress either directly (Bates et al., 2015) or via stimulating ROS production in lung macrophages (Eiguren-Fernandex et al., 2010), epithelial cells (Hawley & Volckens 2013), or endothelial cells (Liu et al. 2005). Meanwhile, continued exposure to high CO levels may suppress induction and/or release of key inflammatory mediators, thus blunting neutrophil influx and other classic indicators of lung inflammation. In so doing, repeated smoke exposure potentially results in silent, smoldering, yet persistent lung oxidative damage that over time culminates in development of COPD.
4.2. Natural-draft stove
For mice inhaling the ND stove emissions, data revealed several temporally related changes. At 0 h PE, mice showed somewhat reduced activity and lower tidal volumes (10%) compared to other stove-exposed mice. By 4 h PE, this group had significant increases in circulating WBCs, and this was the only group to show diminished uptake of fluorescent beads by lung macrophages — with reductions on par with that of the positive control (LPS-treated) mice. These results are consistent with that of Migliaccio et al (2013) demonstrating that pulmonary phagocytes from wood smoke-exposed mice had a decreased ability to phagocytize and mount an effective defense against infection with S. pneumoniae. In contrast to the other exposure groups, lung GSH levels in mice exposed to ND stove emissions appeared to recover by 4 h PE. It may be relevant that at 0 h this group exhibited the greatest increase in GCLC, the initial rate-limiting enzyme for glutathione synthesis. These mice also showed the greatest increase in gene expression for NAD(P)H quinone dehydrogenase 1 (NQO1) (Figure 5). The NQO1 gene is readily-inducible, thus providing greater levels of the NQO1 enzyme which has potent anti-oxidant and cytoprotective activities. By promoting 2-electron reductions of chemical species derived from aromatic compounds like benzene (e.g., quinones) or toluene (e.g., nitroaromatics), NQO1 may reduce levels of redox cycling chemicals, limiting their ability to generate reactive oxygen intermediates, and prevent further depletion of intracellular thiol pools (Dinkova-Kostova & Talalay 2010). Possibly through these actions, mice in the ND group were able to maintain relatively normal lung GSH levels by 4–24 h PE. In murine disease models, NQO1 has been shown to play a protective role against development of emphysema (Potts-Kant et al., 2012). Emphysema is a key pathologic feature of COPD. On the other hand, a report investigating PAH effects in a lung cancer cell line (A549 cells) demonstrated the NQO1 was not responsible for two-electron reduction of PAH-o-quinones, and did not offer protection against ROS formation from such compounds (Shultz et al., 2011). Relatedly, overexpression of NQO1 in human lung cancer cells is associated with poorer prognosis (Cui et al., 2014).
4.3. Forced-draft stove
Remarkably, mice exposed to the Tier 3, FD stove emissions did not show evidence of either acute lung injury or altered bead phagocytosis. Furthermore, this group failed to show induction of lung HO-1, NQO1 or CYP1a1 gene expression, consistent with their exposure to much lower VOC (and presumably SVOC and PAH) concentrations. Intriguingly, however, significantly fewer alveolar macrophages were recovered in BALF of the FD-exposed mice at 0 h PE, and there were corresponding decreases in NAG. Notably this was the only group to develop increased levels of TNFα in BALF. Macrophages are the major producers of TNFα and are also highly responsive to TNFα (Parameswaram & Patial 2010). One interpretation of our data is that phagocytosis of FD particles led to activation of macrophages, thus increasing surface adherence molecules such that they could not be recovered by gentle lung lavage. If this were the case, however, NAG levels should have been increased rather than decreased. Alternatively, because TNFα is a potent pro-apoptotic mediator, a more likely explanation is that phagocytosis of particles emitted by the FD stove resulted in acute upregulation of TNFα, thus eliciting apoptotic pathways in a subset of phagocytes, reducing the total number of alveolar macrophages in the lung at 4 h PE. Also at 4 h PE, mice in the FD stove group had somewhat lower numbers of circulating white blood cells (40% of air controls) and minor but statistically significant reductions in the hematocrit and % circulating lymphocytes. TNFα has also been shown to activate lung endothelial cells, up-regulating cell adhesion molecules on their surface (Kjaergaard et al., 2013). This could explain the increased margination of red and white blood cells (especially lymphocytes) in the lung microcirculation following exposure to the FD stove emissions. Exposure to particulate air pollution has been negatively associated with similar changes in RBC indices (Seaton et al., 1999).
Finally, data revealed that mice in the FD group were the only ones to have elevated γ-glutamyl transferase (GGT) activity in BALF. GGT is a cell membrane bound enzyme involved in extracellular glutamate transport to aid in synthesis of cellular glutathione. Increased GGT activity in BALF has been used as a marker of airway or alveolar cell injury related to oxidative stress. In concordance with this observation, lung GSH levels were significantly reduced (by 30 to 50% compared to air controls) from 0 h to 24 h PE, and moreover, they had coexisting increases in lung glutathione disulfide — indicative of more severe early and ongoing oxidative stress. In our previous study, we showed that particle extracts from FD stove emissions were the most mutagenic on an equal-mass basis (revertants/μg EOM) (Mutlu et al., 2016). Together, these data suggest that despite reduction in overall particle mass released by the Tier 3 stove, FD-stove emitted particles are still inherently quite toxic. Whether this relates to the somewhat smaller particle size fraction detected, to related increases in number of particles and/or their increased surface area, or to yet to be measured differences in vapor phase components emitted by the FD stove (e.g., electrophilic capacity) is beyond the scope of the current report.
4.4. Innate immunity
The above findings are consistent with reports associating exposure to solid-fuel CS emissions with modulation of the innate immune system and increased susceptibility to infection (Lee, 2015). Unlike most viral upper respiratory infections, bacteria tend to invade the lower respiratory tract (alveoli and lung parenchyma), thus alveolar macrophages play a central role in host defense against bacterial pathogens. Seemingly, owing to heightened macrophage apoptosis after exposure to FD stove emissions, significantly fewer lung phagocytes would be available to defend against bacterial invaders. PM exposure has also been shown to disrupt alveolar macrophage phagocytosis and intracellular killing (Lee, 2015), not unlike effects observed herein after exposure to the ND stove emissions. Studies of CS-using Malawian adults likewise showed that higher particle loading of macrophages was associated with impaired phagocytosis of pneumococci and mycobacteria (Rylance et al., 2015). Exposure may also disrupt lung barrier defenses and interfere with recruitment of neutrophils, effects similar to that observed in mice of the 3-S fire group. As the final effector cell involved in clearance of extracellular pathogens, inadequate neutrophil influx would significantly compromise host defense against infectious disease (Teng et al., 2017). Relatedly, results of the RESPIRE clinical trial in Guatemala showed that although introduction of kitchen chimney stoves did not result in reduced cases of pneumonia, the intervention was associated with a significant reduction in cases of physician-diagnosed, severe (hypoxemic), RSV-negative, pneumonia (Smith et al., 2011). This is important because severe RSV-negative cases are most likely of bacterial origin and have higher mortality. Encouragingly, exposure-response results from the RESPIRE trial predict that exposures below the intervention group mean would be associated with considerably lower risk for all acute lower respiratory infections as well as for severe pneumonia (Smith et al., 2011).
4.5. Limitations
We acknowledge that the current study had a number of limitations. First, only short-term exposures were examined (simulating CS emissions related to preparation of two consecutive meals) and the primary emissions were further diluted (approximately 1:20). Secondly, the exposures did not include the smolder phase of the cooking cycle, which for all CS, often emits the greatest peak CO and PM concentrations. Thirdly, red oak represents one of the cleanest burning solid-fuels used in advanced CS. Fourthly, all exposures were performed in healthy adult, outbred, mice. “Fifthly, mice were exposed to emissions from a carefully tended 3-S fire and from cookstoves operated as intended by manufacturers, but emissions measured under actual use conditions in the field are often worse than emissions measured under controlled conditions in the laboratory for both 3-S fires and select cookstoves [http://cleancookstoves.org/resources_files/stove-performance-inventory-pdf.pdf]. Lastly, the end points assessed in this study were acquired within 24 h of the initial exposure and later changes may have been missed. Possibly for these reasons, the overall exposure biomarker changes and health impacts produced across most of the end points examined were only of mild-to-moderate severity. These observations are nevertheless in agreement with the majority of the findings related to hardwood, wood smoke exposures. Based on extensive literature for hardwood emissions, sub-chronic to chronic exposure intervals at relatively high concentrations are necessary to consistently find adverse toxicological outcomes (Reed et al., 2006; Naeher et al., 2007; Hawley and Volckens 2013).
We further recognize that real-world household air pollution often includes unvented CS emissions that arise from all phases of the cooking cycle (i.e., start up, active heating, and smoldering), that emissions are often generated from poor quality solid-fuels, and that exposures entail repeated day-in, day-out cooking activities spanning years to decades. For these reasons, additional studies are in progress to further examine differential health impacts of inhalation exposure to emissions generated during high heat (active cooking or flaming conditions) vs. low heat (smoldering conditions), for emissions derived from alternative (non-red oak) biomass, and importantly, effects of repeated or subchronic exposure to solid-fuel emissions.
Moreover, because exposure to CS emissions increases the risk of developing pathological outcomes such as low birth weights and pulmonary infection in children, we are striving to expand our animal model approaches to include exposure during sensitive life stages (e.g., pregnancy and/or early life) and to incorporate co-morbidity factors (e.g., poor nutrition, infectious insults). By combining these approaches, we will be better able to assess and define health benefits likely to be attained for woman and children through broader usage of advanced solid-fuel “cleaner” cookstoves.
5. Conclusion
The present study was able to detect and characterize mild-to-moderate perturbations of several adverse respiratory health effects in mice after acute exposure to emissions from all three Tier 0, 2, and 3 cooking systems. We emphasize that exposure did not simply reveal identical outcomes with proportionately increasing effects as stove efficiency decreased (i.e., Tier 3 < Tier 2 << Tier 0). Rather, data indicated that each CS system emitted a unique emission profile, which in turn appeared to produce unique acute health perturbations in healthy adult mice. The 3-S-fire-exposed mice were the only group to develop acute lung injury, possibly because they inhaled the highest concentrations of hazardous air toxicants (e.g., 1,3-butadiene, toluene, benzene, acrolein), the greatest number of particles, and particles with the highest % organic carbon. Diminished macrophage phagocytosis was observed only in the ND group. Lastly, although lung glutathione was significantly depleted across all CS groups; the FD group showed the most severe, ongoing oxidative stress. Hence, no Tier 0 to 3 CS was without some untoward effect.
Based on results of the current investigation and that of our recent report on the mutagenicity of emission factors from these same stoves (Mutlu et al., 2016), as well as findings of several epidemiologic reports (Smith et al., 2011; Mortimer et al., 2017) — it is advisable that even with Tier 3 stoves, users should strive to burn the cleanest solid-fuels available and seek to incorporate adequate exterior ventilation of emissions. With these precautions, advanced solid-fuel CS usage may serve as a stepping stone to reduce household air pollution levels until greater access to Tier 4 stoves (i.e., liquid propane gas) or to electricity and electrical stoves are possible. This assumes that power plants will use low-sulfur coal and modern pollution control technologies (Cashman 2016). Clearly, there is a growing and concerted need to expand access to renewable and cleaner end user energy technologies for the nearly 40% of the world’s population living in impoverished conditions necessitating use of rudimentary cooking and heating systems.
Supplementary Material
Acknowledgments.
For their excellent technical expertise and assistance in performing the assays for this study, the authors thank: Pam Phillips (behavior and motor activity), James Lehmann (plethysmography), Richard Jaskot (hematology and carboxyhemoglobin assay), Judy Richards (clinical chemistries), Elizabeth Boykin (lung cell differentials), Mary Daniels (phagocytosis assays), Michael Henderson (macrophage cytokine expression), Alan Tennant (microscopy assistance), Colette Miller (cytokine assay) and Erica Stewart (data processing). We also thank Charly King, Todd Krantz, Seth Ebersviller, Bill Preston, and Michael Hayes for their outstanding expertise in conducting the inhalation exposures, monitoring, and emissions characterization. Lastly, we express our thanks to Drs. David DeMarini, Ozge Kaplan and Robert Luebke for critical review of this manuscript.
Footnotes
Competing financial interest declaration.
The authors report no conflicts of interest. This manuscript has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement for use.
References
- Anenberg SC, Balakrishnan K, Jetter J, Masera O, Mehta S, Moss J, Ramanathan V. 2013. Cleaner Cooking Solutions to Achieve Health, Climate, and Economic Cobenefits. Environ Sci Technol. 47:3944–3952. [DOI] [PubMed] [Google Scholar]
- Assad NA, Balmes J, Mehta S, Cheema U, Sood A. 2015. Chronic obstructive pulmonary disease secondary to household air pollution. Semin Respir Crit Care Med. 36(3):408–21. [DOI] [PubMed] [Google Scholar]
- Barrett EG, Henson RD, Seilkop SK, McDonald JD, Reed MD. 2006. Effects of Hardwood Smoke Exposure on Allergic Airway Inflammation in Mice. Inhalation Toxicology. 18:33–43. [DOI] [PubMed] [Google Scholar]
- Bates JT, Weber RJ, Abrams J, Verma V, Fang T, Klein M, Strickland MJ, Sarnat SE, Chang HH, Mulholland JA, Tolbert PE, Russell AG. 2015. Reactive Oxygen Species Generation Linked to Sources of Atmospheric Particulate Matter and Cardiorespiratory Effects. Environ Sci Technol. 49(22):13605–12. [DOI] [PubMed] [Google Scholar]
- Brown SK, Sim MR, Abramson MJ, Gray CN. 1994. Concentrations of Volatile Organic Compounds in Indoor Air – A Review. Indoor Air. 4:123–134. [Google Scholar]
- Bruce NG, Dherani MK, Das JK, Balakrishnan K, Adair-Rohani H, Bhutta ZA, Pope D. 2013. Control of household air pollution for child survival: estimates for intervention impacts. BMC Public Health. 13 Suppl 3:S8. doi: 10.1186/1471-2458-13-S3-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleson GR, Fuller LB, Menache MG, Graham JA. 1987. Poly(I):poly(C)-enhanced alveolar and peritoneal macrophage phagocytosis: quantification by a new method utilizing fluorescent beads. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY). 184:468–476. [DOI] [PubMed] [Google Scholar]
- Cashman S 2016. Life-Cycle Assessment of Cookstove Fuels in India and China. U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-15/325. [Google Scholar]
- Chartier R, Phillips M, Mosquin P, Elledge M, Bronstein K, Nandasena S, Thornburg V, Thornburg J, Rodes C. 2017. A comparative study of human exposures to household air pollution from commonly used cookstoves in Sri Lanka. Indoor Air. 27(1):147–159. [DOI] [PubMed] [Google Scholar]
- Chung KF. 2001. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 34:50s–59s. [PubMed] [Google Scholar]
- Clark ML, Peel JL, Burch JB, Nelson TL, Robinson MM, Conway S, Bachand AM, Reynolds SJ. 2009. Impact of improved cookstoves on indoor air pollution and adverse health effects among Honduran women. International Journal of Environ Health Research. 19:357–368. [DOI] [PubMed] [Google Scholar]
- Clark ML, Reynolds SJ, Burch JB, Conway S, Bachand AM, Peel JL. 2010. Indoor air pollution, cookstove quality, and housing characteristics in two Honduran communities. Environ Res. 110(1):12–8. [DOI] [PubMed] [Google Scholar]
- Cui X, Jin T, Wang X, Jin G, Li Z, Lin L. 2014. NAD(P)H:quinone oxidoreductase-1 overexpression predicts poor prognosis in small cell lung cancer. Oncol Rep. 32(6):2589–95. doi: 10.3892/or.2014.3494. [DOI] [PubMed] [Google Scholar]
- Cundale K, Thomas R, Malava JK, Havens D, Mortimer K, Conteh L. 2017. A health intervention or a kitchen appliance? Household costs and benefits of a cleaner burning biomass-fuelled cookstove in Malawi. Soc Sci Med. 183:1–10. doi: 10.1016/j.socscimed.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho FO, Felipe FA, de Melo Costa AC, Teixeira LG, Silva ÉR, Nunes PS, Shanmugam S, de Lucca Junior W, Quintans JS, de Souza Araújo AA. 2016. Inflammatory Mediators and Oxidative Stress in Animals Subjected to Smoke Inhalation: A Systematic Review. Lung. 194(4):487–99. doi: 10.1007/s00408-016-9879-y. [DOI] [PubMed] [Google Scholar]
- Demling R, Lalonde C, Picard L, Blanchard J. 1994. Changes in lung and systemic oxidant and antioxidant activity after smoke inhalation. Shock. 1(2):101–7. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Talalay P. 2010. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys. 501 (1):116–23. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiguren-Fernandez A, Shinyashiki M, Schmitz DA, DiStefano E, Hinds W, Kumagai Y, Cho AK, Froines JR. 2010. Redox and electrophilic properties of vapor- and particle-phase components of ambient aerosols. Environ Res. 110 (3):207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan J, Harper TW, Hsueh MM, Qu Q, Humphreys WG. 2005. Dansyl glutathione as a trapping agent for the quantitative estimation and identification of reactive metabolites. Chem Research in Toxicology. 18:896–903. [DOI] [PubMed] [Google Scholar]
- Gavett SH, Wood CE, Williams MA, Cyphert JM, Boykin EH, Daniels MJ, Copeland LB, King C, Krantz TQ, Richards JH, Andrews DL, Jaskot RH, Gilmour MI. 2015. Soy biodiesel emissions have reduced inflammatory effects compared to diesel emissions in healthy and allergic mice. Inhal Toxicol. 27(11):533–44. doi: 10.3109/08958378.2015.1054966. [DOI] [PubMed] [Google Scholar]
- Golde WT, Gollobin P, Rodriguez LL. 2005. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Animal. 34:39–43. [DOI] [PubMed] [Google Scholar]
- Gordon SB, Bruce NG, Grigg J, Hibberd PL, Kurmi OP, Lam KB, Mortimer K, Asante KP, Balakrishnan K, Balmes J. 2014. Respiratory risks from household air pollution in low and middle income countries. The Lancet Respir Med. 2:823–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 156:766–775. [DOI] [PubMed] [Google Scholar]
- Hawley B, Volckens J. 2013. Proinflammatory effects of cookstove emissions on human bronchial epithelial cells. Indoor Air.23:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays MD, Gullett B, King C, Robinson J, Preston W, Touati A. 2011. Characterization of carbonaceous aerosols emitted from outdoor wood boilers. Energy Fuel 25 (12), 5632–5638. [Google Scholar]
- IARC. 2010. Household use of solid fuels and high-temperature frying IARC Mong Eval Carcinog Risks Hum, Vol 95, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- IARC. 2012. Diesel and gasoline engine exhausts and some nitroarenes IARC Mong Eval Carcinog Risks Hum, Vol 105, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- IARC. 2016. Outdoor air pollution IARC Mong Eval Carcinog Risks Hum, Vol 109, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- ISO/IWA 11:2012. (en) Guidelines for evaluating cookstove performance. https://www.iso.org/standard/61975.html
- Jetter JJ, Kariher P. 2009. Solid-fuel household cook stoves: Characterization of performance and emissions. Biomass and Bioenergy. 33:294–305. [Google Scholar]
- Jetter J, Zhao Y, Smith KR, Khan B, Yelverton T, DeCarlo P, Hays MD. 2012. Pollutant Emissions and Energy Efficiency under Controlled Conditions for Household Biomass Cookstoves and Implications for Metrics Useful in Setting International Test Standards. Environ Sci Technol. 46:10827–10834. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Chiang RA. 2015. Quantitative Guidance for Stove Usage and Performance to Achieve Health and Environmental Targets. Environ Health Perspect. 123(8):820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, Carlson JL, Samiec PS, Sternberg P Jr, Mody VC Jr, Reed RL, Brown LA. 1998. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl deriv atives by HPLC. Clin Chim Acta 275(2):175–84. [DOI] [PubMed] [Google Scholar]
- Jordan TB, Seen AJ. 2005. Effect of airflow setting on the organic composition of woodheater emissions. Environ Sci Technol. 39:3601–3610. [DOI] [PubMed] [Google Scholar]
- Kjaergaard AG, Dige A, Krog J, Tønnesen E, Wogensen L. 2013. Soluble adhesion molecules correlate with surface expression in an in vitro model of endothelial activation. Basic Clin Pharmacol Toxicol. 113(4):273–9. doi: 10.1111/bcpt.12091 [DOI] [PubMed] [Google Scholar]
- Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. 2001. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 107(12):1529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Obata F. 2003. An optimized method for determination of intracellular glutathione in mouse macrophage cultures by fluorimetric high-performance liquid chromatography. Biomedical chromatography : BMC. 17:345–350. [DOI] [PubMed] [Google Scholar]
- Kshirsagar MP, Kalamkar VR. 2014. A comprehensive review on biomass cookstoves and a systematic approach for modern cookstove design. Renewable and Sustainable Energy Reviews. 30:580–603. [Google Scholar]
- Lacey FG, Henze DK, Lee CJ, van Donkelaar A, Martin RV. 2017. Transient climate and ambient health impacts due to national solid fuel cookstove emissions. Proc Natl Acad Sci U S A. 114(6):1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi PV, Virdi NK, Sharma A, Tripathy JP, Smith KR, Bates MN, Kumar R. 2013. Household air pollution and stillbirths in India: analysis of the DLHS-II National Survey. Environ Res. 121:17–22 [DOI] [PubMed] [Google Scholar]
- Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. 2005. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 32(4):311–8. [DOI] [PubMed] [Google Scholar]
- Laskin A, Laskin J, and Nizkorodov SA. 2015. Chemistry of Atmospheric Brown Carbon. Chem Rev. 115, 4335–4382. [DOI] [PubMed] [Google Scholar]
- Lee A, Kinney P, Chillrud S, Jack D. 2015. A Systematic Review of Innate Immunomodulatory Effects of Household Air Pollution Secondary to the Burning of Biomass Fuels. Ann Glob Health. 81(3):368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux PM, Lutes CC, Santoianni DA. 2004. Emissions of organic air toxics from open burning: a comprehensive review. Prog Energy Combust Science. 30:1–32. [Google Scholar]
- Li Z, Commodore A, Hartinger S, Lewin M, Sjödin A, Pittman E, Trinidad D, Hubbard K, Lanata CF, Gil AI, Mäusezahl D, Naeher LP. 2016. Biomonitoring Human Exposure to Household Air Pollution and Association with Self-reported Health Symptoms - A Stove Intervention Study in Peru. Environ Int. 97:195–203. doi: 10.1016/j.envint.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P-L, Chen Y-L, Chen Y- H, Lin S- J, Kou Yr. 2005. Wood smoke extracts induces oxidative stress-mediated caspase-independent apoptosis in human lung endothelial cells: Role of AIF and EndoG. Am J Physio Lung Cell Mol Physiol 289: L739–749. [DOI] [PubMed] [Google Scholar]
- Mack PA, Griffith JW, Riling S, Lang CM. 1995. N-acetyl-beta-D-glucosaminidase activity within BAL from macaques exposed to generic coal dusts. Lung. 173(1):1–11. [DOI] [PubMed] [Google Scholar]
- Malley CS, Kuylenstierna JC, Vallack HW, Henze DK, Blencowe H, Ashmore MR. 2017. Preterm birth associated with maternal fine particulate matter exposure: A global, regional and national assessment. Environ Int. 101:173–182. [DOI] [PubMed] [Google Scholar]
- Manzo ND, Slade R, Richards JH, McGee JK, Martin LD,Dye A. 2010. Susceptibility of inflamed alveolar and airway epithelial cells to injury induced by diesel exhaust particles of varying organic carbon content. J Toxicol Environ Health A;73(8):565–80. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Hollingsworth JW, Ramanathan V. 2014. Household Air Pollution from Cookstoves: Impacts on Health and Climate In: Global Climate Change and Public Health. New York, NY: Springer New York; p. 237–255. [Google Scholar]
- McCracken JP, Wellenius GA, Bloomfield GS, Brook RD, Tolunay HE, Dockery DW, Rabadan-Diehl C, Checkley W, Rajagopalan S. 2012. Household Air Pollution from Solid Fuel Use: Evidence for Links to CVD. Global Heart. 9//;7:223–234. [DOI] [PubMed] [Google Scholar]
- Migliaccio CT, Kobos E, King QO, Porter V, Jessop F, Ward T. 2013. Adverse effects of wood smoke PM(2.5) exposure on macrophage functions. Inhal Toxicol. 25:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes JL, Moraes AB, Aran V, Alves MR, Schluckbier L, Duarte M, Toscano E, Zamboni M, Sternberg C, de Moraes E, Lapa E Silva JR, Ferreira CG. 2017. Functional analysis of polymorphisms in the COX-2 gene and risk of lung cancer. Mol Clin Oncol. 6(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer K, Ndamala CB, Naunje AW, Malava J, Katundu C, Weston W, Havens D, Pope D, Bruce NG, Nyirenda M, Wang D, Crampin A, Grigg J, Balmes J, Gordon SB. 2017. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): a cluster randomised controlled trial. Lancet. 389(10065):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R, Otterbein LE. 2010. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 9(9):728–43. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- Mutlu E, Warren SH, Ebersviller SM, Kooter IM, Schmid JE, Dye JA, Linak WP, Gilmour MI, Jetter JJ, Higuchi M, DeMarini DM. 2016. Mutagenicity and Pollutant Emission Factors of Solid-Fuel Cookstoves: Comparison with Other Combustion Sources. Environ Health Perspect. 124(7):974–82. doi: 10.1289/ehp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, Smith KR. 2007. Woodsmoke Health Effects: A Review. Inhalation Toxicology. 19:67–106. [DOI] [PubMed] [Google Scholar]
- O’Mahony L, Holland J, Jackson J, Feighery C, Hennessy TP, Mealy K. 1998. Quantitative intracellular cytokine measurement: age-related changes in proinflammatory cytokine production. Clinical and experimental immunology. 113:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochieng C, Vardoulakis S, Tonne C. 2017. Household air pollution following replacement of traditional open fire with an improved rocket type cookstove. Sci Total Environ. 580:440–447. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. 2000. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 6(4):422–8. [DOI] [PubMed] [Google Scholar]
- Parameswaran N, Patial S. 2010. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 20(2):87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilishvili T, Loo JD, Schrag S, Stanistreet D, Christensen B, Yip F, Nyagol R, Quick R, Sage M, Bruce N. 2016. Effectiveness of Six Improved Cookstoves in Reducing Household Air Pollution and Their Acceptability in Rural Western Kenya. PLoS One. 11(11):e0165529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope D, Bruce N, Dherani M, Jagoe K, Rehfuess E. 2017. Real-life effectiveness of ‘improved’ stoves and clean fuels in reducing PM2.5 and CO: Systematic review and meta-analysis. Environ Int. 101:7–18. doi: 10.1016/j.envint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Potts-Kant EN, Li Z, Tighe RM, Lindsey JY, Frush BW, Foster WM, Hollingsworth JW. 2012. NAD(P)H:quinone oxidoreductase 1 protects lungs from oxidant-induced emphysema in mice. Free Radic Biol Med. 52(3):705–715. doi: 10.1016/j.freeradbiomed.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Quinn DK, McGahee SM, Politte LC, Duncan GN, Cusin C, Hopwood CJ, Stern TA. 2009. Complications of Carbon Monoxide Poisoning: A Case Discussion and Review of the Literature. Primary Care Companion to The Journal of Clinical Psychiatry. Prim Care Companion J Clin Psychiatry. 11(2):74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MD, Campen MJ, Gigliotti AP, Harrod KS, McDonald JD, Seagrave JC, Mauderly JL, Seilkop SK. 2006. Health Effects of Subchronic Exposure to Environmental Levels of Hardwood Smoke. Inhal Toxicol. 18:523–539. [DOI] [PubMed] [Google Scholar]
- Ruiz-Mercado I, Canuz E, Walker JL, Smith KR. 2013. Quantitative metrics of stove adoption using Stove Use Monitors (SUMs). Biomass Bioenergy. 57:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumchev K, Spickett JT, Brown HL, Mkhweli B. 2007. Indoor air pollution from biomass combustion and respiratory symptoms of women and children in a Zimbabwean village. Indoor Air. 17(6):468–74. [DOI] [PubMed] [Google Scholar]
- Rylance J, Chimpini C, Semple S, Russell DG, Jackson MJ, Heyderman RS, Gordon SB. 2015. Chronic Household Air Pollution Exposure Is Associated with Impaired Alveolar Macrophage Function in Malawian Non-Smokers. PLOS ONE.10:e0138762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerwegh AJ, De Clerck LS, Bridts CH, Stevens WJ. 2003. Comparison of intracellular cytokine production with extracellular cytokine levels using two flow cytometric techniques. Cytometry Part B, Clinical cytometry. 55:52–58. [DOI] [PubMed] [Google Scholar]
- Seaton A, Soutar A, Crawford V, Elton R, McNerlan S, Cherrie J, Watt M, Agius R, Stout R. 1999. Particulate air pollution and the blood. Thorax. 54(11):1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen B, Mahadevan B, DeMarini DM. 2007. Transcriptional responses to complex mixtures: a review. Mutat Res. 636(1–3):144–77. [DOI] [PubMed] [Google Scholar]
- Shi Z, Chen Y, Pei Y, Long Y, Liu C, Cao J, Chen P. 2017. The role of cyclooxygenase-2 in the protection against apoptosis in vascular endothelial cells induced by cigarette smoking. J Thorac Dis. 9(1):30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz CA, Quinn AM, Park JH, Harvey RG, Bolton JL, Maser E, Penning TM. 2011. Specificity of human aldo-keto reductases, NAD(P)H:quinone oxidoreductase, and carbonyl reductases to redox-cycle polycyclic aromatic hydrocarbon diones and 4-hydroxyequilenin-o-quinone. Chem Res Toxicol. 24(12):2153–66. doi: 10.1021/tx200294c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR. 1994. Health, energy, and greenhouse-gas impacts of biomass combustion in household stoves. Energy for Sustainable Development. 1:23–29. [Google Scholar]
- Smith KR, McCracken JP, Weber MW, Hubbard A, Jenny A, Thompson LM, Balmes J, Diaz A, Arana B, Bruce N. 2011. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 378(9804):1717–26. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Dracínská H, Mizerovská J, Frei E, Schmeiser HH, Hudecek J, Hodek P, Phillips DH, Arlt VM. 2008. The environmental pollutant and carcinogen 3-nitrobenzanthrone induces cytochrome P450 1A1 and NAD(P)H:quinone oxidoreductase in rat lung and kidney, thereby enhancing its own genotoxicity. Toxicology. 247(1):11–22. doi: 10.1016/j.tox.2008.01.018 [DOI] [PubMed] [Google Scholar]
- Still D, Bentson S, Li H. Results of Laboratory Testing of 15 Cookstove Designs in Accordance with the ISO/IWA Tiers of Performance. 2015. Ecohealth. 12(1):12–24 [DOI] [PubMed] [Google Scholar]
- Teng TS, Ji AL, Ji XY, Li YZ. 2017. Neutrophils and Immunity: From Bactericidal Action to Being Conquered. J Immunol Res. 9671604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traboulsi H, Guerri na N, Iu M, Maysinger D, Ariya P, Baglole CJ. 2017. Inhaled Pollutants: The Molecular Scene behind Respiratory and Systemic Diseases Associated with Ultrafine Particulate Matter. Int J Mol Sci. 18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA Compendium Method TO-15, 1999. U.S. EPA Compendium Method TO-15 Determination of volatile organic compounds (VOCs) in air collected in specially-prepared canisters and analyzed by gas chromatography/mass spectrometry (GC/MS; ) http://www.epa.gov/ttnamti1/files/ambient/airtox/to-15r.pdf [Google Scholar]
- U.S. EPA. 1999. Method TO-11A In Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, 2nd ed. EPA/625/R-96/010b. Cincinnati, OH: Office of Research and Development. [Google Scholar]
- Wathore R, Mortimer K, Grieshop AP. 2017. In-Use Emissions and Estimated Impacts of Traditional, Natural- and Forced-Draft Cookstoves in Rural Malawi. Environ Sci Technol. 7;51(3):1929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JR, Asteria-Peñaloza R, Diaz-Artiga A, Davila G, Hammond SK, Ryde IT, Meyer JN, Benowitz N, Thompson LM. 2017. Exposure to polycyclic aromatic hydrocarbons and volatile organic compounds among recently pregnant rural Guatemalan women cooking and heating with solid fuels. Int J Hyg Environ Health. pii: S1438–4639(16)30576–4. doi: 10.1016/j.ijheh.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MR, O’Dea KP, Dorr AD, Yamamoto H, Goddard ME, Takata M. 2010. Efficacy and safety of inhaled carbon monoxide during pulmonary inflammation in mice. PLoS One. 5(7):e11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Klein M, Chang HH, Sarnat JA, Mulholland JA, Edgerton ES, Winquist A, Tolbert PE, Sarnat SE. 2017. Estimating Acute Cardiorespiratory Effects of Ambient Volatile Organic Compounds. Epidemiology (Cambridge, Mass). 28:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip F, Christensen B, Sircar K, Naeher L, Bruce N, Pennise D, Lozier M, Pilishvili T, Loo Farrar J, Stanistreet D, Nyagol R, Muoki J, de Beer L, Sage M, Kapil V. 2017. Assessment of traditional and improved stove use on household air pollution and personal exposures in rural western Kenya. Environ Int. 99:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Goncalves R, Mosser DM. 2008. The isolation and characterization of murine macrophages. Current protocols in immunology. Chapter 14:Unit 14.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


