Supplemental Digital Content is available in the text.
Abstract
Background:
There have been many technical and scientific advances over the last decade in peripheral nerve surgery. Human acellular nerve graft (HANA) has become increasingly popular but current practice patterns among hand surgeons have yet to be defined. Coding practices may not have kept up with this innovation. A 26 question survey of hand surgeons was performed to evaluate the adoption of HANA, and current coding and billing practices. The survey was sent to hand surgeons trained in orthopedic, plastic, general, and neuro surgery. The survey was designed and implemented by the Mayo Clinic Survey Center.
Results:
Four hundred sixty-one responses to the survey were received. Most respondents currently use HANA (70%). Of those surgeons who do use HANA, nearly all use it less than 10 times per month (98%). There was no significant difference in the use of HANA across different specialties. There was a significant difference in HANA use depending on practice type with higher use by those in group private practice (57%) compared with academic practice (28%), solo practice (12%), and other practice environment (3%). There was a significant difference in HANA use depending on the number of years in practice. Those in practice less than 5 years used HANA the most (32%), followed by > 20 years in practice (27%), 6–10 years in practice (16%), 16–20 years in practice (14%), and 11–15 years in practice (11%). When asked the Current Procedural Terminology code they would use to bill for the procedure of choice, the most common response was 64910 (nerve repair with synthetic conduit or vein allograft).
Conclusions:
HANA has surpassed nerve conduit as the traditional gold standard in our study with nearly 70% of hand surgeons using HANA in their practice and a greater percentage of respondents choosing HANA as their first choice to repair as compared with nerve conduit, nerve autograft, or vein graft. There remains confusion regarding appropriate billing practices for the use of HANA. Due to its common use, a Current Procedural Terminology code should specifically designated for the use of HANA in the hand.
INTRODUCTION
Peripheral nerve surgery has enjoyed a renaissance in the last 2 decades.1 The use of human acellular nerve allograft (HANA), the popularization of nerve transfers, and modern postoperative rehabilitation have improved outcomes for patients with devastating nerve injuries.1 HANA was approved by Food and Drug Administration (FDA) in 2007, and over the last decade its popularity has increased, particularly for short sensory nerve gaps.2–7 Advantages include ease of use, lack of donor-site morbidity, and decreased operative time.2–7 Although there are numerous studies demonstrating clinical outcomes of HANA,2–7 there have been no published studies of its use by hand surgeons. Furthermore, coding remains inconsistent as there is currently no specific Current Procedural Terminology (CPT) code for HANA use. A survey was designed to identify the prevalence and practice patterns of HANA use among hand surgeons.

MATERIALS AND METHODS
After institutional review board approval, a 26 question survey was designed and distributed to all members of the American Society for Surgery of the Hand and the American Association of Hand Surgery. These 2 societies represent the largest contingency of hand surgeons in the United States. The study was determined to be exempt by the Mayo Clinic Institutional Review Board 16-003609, and respondents consented to participate via an e-mailed written consent prompt. The survey was created and distributed electronically through the Mayo Clinic Survey Research Center using Qualtrics Survey Software. The survey questions included demographics, peripheral nerve surgery practice, HANA use, and a specific clinical scenario targeting the management of a digital nerve gap (see appendix, Supplemental Digital Content 1, which shows the entire survey instrument used in the collection of data from hand surgeons, http://links.lww.com/PRSGO/A790). Data were collected, and statistical analysis performed via the Mayo Clinic Survey Research Center. Responses for each field were tallied and a multivariate logistic regression model was used to identify factors associated with HANA use. All statistical analysis was performed using SAS version 9.4 software (SAS Institute Inc., Cary, N.C.), and values of P < 0.05 were considered statistically significant.
RESULTS
Demographics
Four hundred sixty-one responses to the survey were received of the 4,045 American Society for Surgery of the Hand and 1,131 American Association of Hand Surgery members. It was not possible to discern which survey recipients had dual memberships and therefore received the survey twice. The majority of respondents were trained in orthopedic surgery (76%) or plastic surgery (19%), followed by general surgery (5%) and neurosurgery (< 1%; Fig. 1A).
Fig. 1.
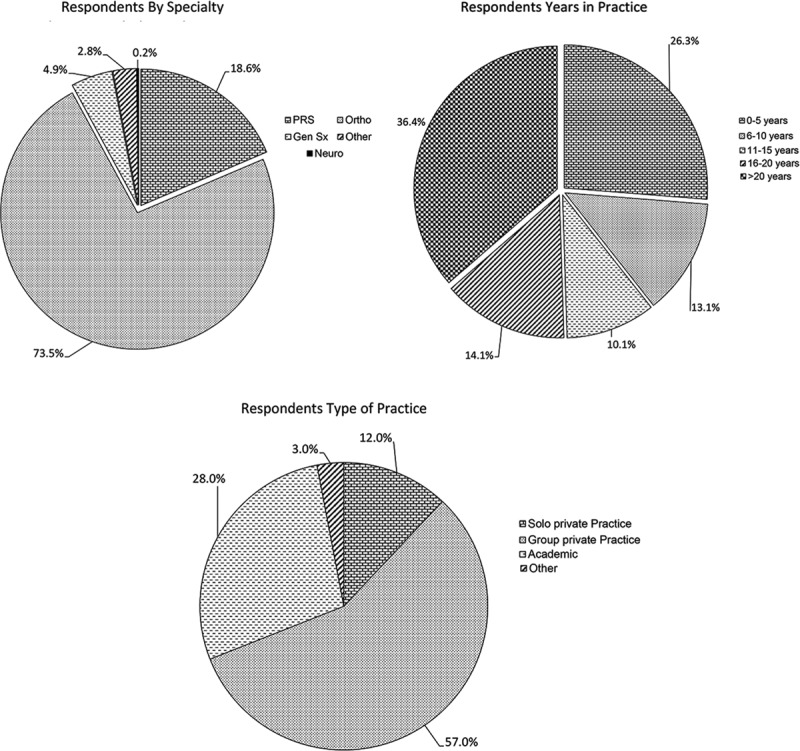
Respondents demographics. Professional characteristics of the survey population. A, Distribution of respondents by specialty. B, Distribution of years in practice of surgeons. C, Distribution of respondents by type of practice. (a) Respondents By Specialty; (b) Respondents Years in Practice; (c) Respondents Type of Practice.
Of the respondents, 39% were in practice less than 10 years, 25% 11–20 years and 36% greater than 20 years (Fig. 1B). Most respondents were associated with a group private practice (57%), followed by full-time faculty at an academic institution (28%), solo practice (12%), or other practice environment (3%; Fig. 1C). For those surgeons who completed a fellowship, 98% completed a hand surgery fellowship, 11% completed a peripheral nerve fellowship, and 7% completed a different fellowship (ie, microsurgery; Table 1). Some respondents completed multiple fellowships.
Table 1.
Prevalence of HANA Use Among Hand Surgeons

Peripheral Nerve Surgery Practice and HANA Use
When asked about the frequency of peripheral nerve surgeries performed, most perform fewer than 5 surgeries per month (47%; Table 1). Most respondents currently use HANA (70%). Of those surgeons who do use HANA, nearly all use it less than 10 times per month (98%). There was no significant difference in the use of HANA across different specialties (P = 0.41; Table 1). There was a significant difference in HANA use depending on practice type with higher use by those in group private practice (57%) compared with academic practice (28%), solo practice (12%), and other practice environment (3%; P = 0.0047). There was a significant difference in HANA use depending on the number of years in practice (P = 0.0001). Those in practice less than 5 years used HANA the most (32%), followed by > 20 years in practice (27%), 6–10 years in practice (16%), 16–20 years in practice (14%), and 11–15 years in practice (11%). There was a significant difference in HANA use depending on the number of peripheral nerve cases performed per month (P = 0.0367) with those performing less than 5 peripheral nerve cases per month having the highest percentage use (37%), followed by 6–10 cases (23%), 11–15 cases and > 20 cases (both 20%), and 16–20 cases (0%). Figure 1 shows the use of HANA depending on specialty, number of years in practice, number of nerve, and type of practice.
Clinical Scenario
A 35-year-old male presents with a dog bite to the right middle finger resulting in a traumatic laceration of the radial digital nerve at the level of proximal interphalangeal joint. After exploration and trimming here is a 2-cm gap of the nerve.
When presented with the clinical scenario above, 39% of respondents would use HANA, 35% nerve conduit, 21% nerve autograft, 3% vein graft as conduit, and 2% would utilize some other method (Fig. 2). When asked the CPT code they would use to bill for their procedure of choice, the most common response was 64910 (nerve repair with synthetic conduit or vein allograft, 49%), followed by 64890 (27%), 64831 (19%), 64999 (7%), 64834 and 64911 (each 1%). Table 2 demonstrates the frequency of CPT codes selected and CPT code selected by preferred nerve repair technique.
Fig. 2.

Method of nerve repair selected for a 2-cm digital nerve gap.
Table 2.
CPT Code by Procedure Chosen

Table 3.
Frequency of CPT Codes Chosen by Respondents

DISCUSSION
Nerve repair may date as far back as to the time of Hippocrates.8 The use of the operating microscope, knowledge of the internal topography of nerves, and clinical and basic science research have advanced the management of nerve injury, recovery, and repair.9 HANA is an important advancement in nerve surgery and offers an additional tool in the armamentarium of a hand surgeon. HANA was approved by the FDA in 2007. Since then, its use has increased in frequency and more data continue to emerge.2–7,10–12 HANA theoretically combines the off-the-shelf access of conduits with the structural axons of autograft. Advances in tissue processing in the last decade have overcome the immunogenicity associated with prior allografts, permitting regular use.4 Systematic reviews have demonstrated the success of HANA in reconstructing short gaps.4,13 Some recent studies suggest that larger gaps may also be reconstructed with HANA.6,14,15
Over 450 hand surgeons responded to our survey and were of similar distribution to national demographics and nearly 70% use HANA in their hand surgery practice. Nearly all use HANA less than 10 times per month. The use of HANA is commonplace in contemporary hand surgery practice, regardless of specialty, number of years in practice, type of practice, or fellowship training.
Although we had an even distribution of responses across experience levels, there was not an even distribution of HANA use. There was a bivariate distribution depending on the number of years in practice, with about 32% of those in practice less than 5 years using HANA and 27% of those in practice more than 20 years using HANA. The number of respondents in each bivariate group was similar. We anticipated that surgeons in practice less than 5 years would be more likely to use HANA because they would have completed at least a portion of their residency and fellowship training after FDA approval of HANA and were likely exposed to HANA during that time. We did not anticipate the increased use of HANA in those in practice > 20 years, and we do not have an explanation for this. Each of these bivariate groups (practice < 5 years versus > 20 years) uses HANA at nearly double the rate of those in practice 6–20 years. Academic practice surgeons (28%) may have lower usage of allograft because there are residents and fellows to harvest the nerve and close the donor site. Solo practitioners (12%) may use HANA most infrequently because those surgeons might work in a surgery center where allograft is not available, whereas those working in a group practice (57%) likely have a presence in a large hospital where allograft is stocked and readily available.
When presented with a case scenario of a 2-mm digital nerve gap, with the highest percentage of respondents choosing HANA (39%) as their repair method of choice, followed by nerve conduit (35%) and nerve autograft (21%). In this study, HANA surpassed nerve conduit traditional gold standard,1,16 nerve autograft in the scenario, but this is limited by a low response rate and variability of choice in different professional settings. There are 2 clinical studies sponsored by AxoGen, Inc., (Alachua, Fla.) and a literature review that suggests that HANA may be a better and more reliable option than nerve conduit.6,17,18 It is surmised that this is related to the inherent nerve structure within HANA when compared with the empty chamber of nerve conduits.
This study only looked at HANA use in a short 2-cm sensory nerve gap. The published data confirm that this is an appropriate use of HANA. Although there are some case studies in the literature,19,20 larger studies are needed to confirm the appropriateness of HANA use in mixed nerves or in nerve gaps longer than 3 cm.21–25
There has been some confusion regarding appropriate coding practice for the use of HANA. About half of respondents (49%) who stated that they would repair the nerve with HANA would use CPT code 64910 (nerve repair with synthetic conduit or vein allograft). Twenty-seven percentage would use CPT code 64890 (nerve graft, single strand, hand, < 4 cm). Because there is no CPT code specifically designated for the use of HANA, these are the alternatives that hand surgeons have identified. As demonstrated in Table 2, there is variability in coding for the various nerve repair techniques. Respondents who selected nerve conduit or nerve autograft had relative consistency in their coding choices, whereas those who selected HANA or vein graft did not. It would be useful to have a CPT code specifically designated for the use of HANA.
The limitations of this study include the low response rate and the singularity and simplicity of the clinical scenario. We did not look at other types of nerve repair including mixed nerves and longer nerve gaps. However, this is the first published article to shed light on the prevalence and practice patterns of HANA use among hand surgeons.
CONCLUSIONS
Over one-third of surgeons now report using HANA in their practice, and in the repair of a short sensory nerve gap, HANA was the repair of choice in respondents. There remains a large variability in the CPT coding for HANA.
Supplementary Material
Footnotes
Published online 6 August 2018.
Presented at the 2018 American Society For Peripheral Nerve January 14, 2018, in Phoenix, Arizona.
The above referenced application is approved by expedited review procedures (45 CFR 46.110, item 5). This approval is valid for a period of 3 year(s). The Reviewer conducted a risk-benefit analysis and determined the study constitutes minimal risk research. The Reviewer determined that this research satisfies the requirements of 45 CFR 46.111.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the the Division of Plastic Surgery at the Mayo Clinics.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Spinner RJ, Shin AY, Bishop AT. Advances in the repair of peripheral nerve injury. Neurosurgery. 2015;62:146. [DOI] [PubMed] [Google Scholar]
- 2.Cho MS, Rinker BD, Weber RV, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am. 2012;37:2340. [DOI] [PubMed] [Google Scholar]
- 3.Guo Y, Chen G, Tian G, et al. Sensory recovery following decellularized nerve allograft transplantation for digital nerve repair. J Plast Surg Hand Surg. 2013;47:451. [DOI] [PubMed] [Google Scholar]
- 4.Isaacs J, Browne T. Overcoming short gaps in peripheral nerve repair: conduits and human acellular nerve allograft. Hand (N Y). 2014;9:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y). 2009;4:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Means KR, Jr, Rinker BD, Higgins JP, et al. A multicenter, prospective, randomized, pilot study of outcomes for digital nerve repair in the hand using hollow conduit compared with processed allograft nerve. Hand (N Y). 2016;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taras JS, Amin N, Patel N, et al. Allograft reconstruction for digital nerve loss. J Hand Surg Am. 2013;38:1965. [DOI] [PubMed] [Google Scholar]
- 8.Belen D, Aciduman A, Er U. History of peripheral nerve repair: may the procedure have been practiced in Hippocratic School? Surg Neurol. 2009;72:190; discussion 193. [DOI] [PubMed] [Google Scholar]
- 9.Ray WZ, Mackinnon SE. Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol. 2010;223:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu DT, Strauch B. A prospective clinical evaluation of autogenous vein grafts used as a nerve conduit for distal sensory nerve defects of 3 cm or less. Plast Reconstr Surg. 1990;86:928. [DOI] [PubMed] [Google Scholar]
- 11.Sosin M, Weiner LA, Robertson BC, et al. Treatment of a recurrent neuroma within nerve allograft with autologous nerve reconstruction. Hand (N Y). 2016;11:NP5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strauch B, Ferder M, Lovelle-Allen S, et al. Determining the maximal length of a vein conduit used as an interposition graft for nerve regeneration. J Reconstr Microsurg. 1996;12:521. [DOI] [PubMed] [Google Scholar]
- 13.Sebastin SJ, Chung KC. A systematic review of the outcomes of replantation of distal digital amputation. Plast Reconstr Surg. 2011;128:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks DN, Weber RV, Chao JD, et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32:1. [DOI] [PubMed] [Google Scholar]
- 15.Tang P, Chauhan A. Decellular nerve allografts. J Am Acad Orthop Surg. 2015;23:641. [DOI] [PubMed] [Google Scholar]
- 16.Wang G, Lu S, Kuang Z, et al. [Experimental study on promotion of neurotropic reinnervation with chemically extracted acellular nerve allograft]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24:1288. [PubMed] [Google Scholar]
- 17.Isaacs J. Major peripheral nerve injuries. Hand Clin. 2013;29:371. [DOI] [PubMed] [Google Scholar]
- 18.Safa B, Buncke G. Autograft substitutes: conduits and processed nerve allografts. Hand Clin. 2016;32:127. [DOI] [PubMed] [Google Scholar]
- 19.Rinker B, Zoldos J, Weber RV, et al. Use of processed nerve allografts to repair nerve injuries greater than 25 mm in the hand. Ann Plast Surg. 2017;78:S292. [DOI] [PubMed] [Google Scholar]
- 20.Zhong H, Chen B, Lu S, et al. Nerve regeneration and functional recovery after a sciatic nerve gap is repaired by an acellular nerve allograft made through chemical extraction in canines. J Reconstr Microsurg. 2007;23:479. [DOI] [PubMed] [Google Scholar]
- 21.Bădoiu SC, Lascăr I, Enescu DM. Peripheral nerve allografting—why and how? Chirurgia (Bucur). 2014;109:584. [PubMed] [Google Scholar]
- 22.Colen KL, Choi M, Chiu DT. Nerve grafts and conduits. Plast Reconstr Surg. 2009;124:e386. [DOI] [PubMed] [Google Scholar]
- 23.Lin MY, Manzano G, Gupta R. Nerve allografts and conduits in peripheral nerve repair. Hand Clin. 2013;29:331. [DOI] [PubMed] [Google Scholar]
- 24.Rbia N, Shin AY. The role of nerve graft substitutes in motor and mixed motor/sensory peripheral nerve injuries. J Hand Surg Am. 2017;42:367. [DOI] [PubMed] [Google Scholar]
- 25.Rivlin M, Sheikh E, Isaac R, et al. The role of nerve allografts and conduits for nerve injuries. Hand Clin. 2010;26:435. [DOI] [PubMed] [Google Scholar]


