Abstract
Objectives/design:
As antiretroviral therapy (ART) rapidly expands in sub-Saharan Africa using new efficient care models, data on costs of these approaches are lacking. We examined costs of a streamlined HIV care delivery model within a large HIV test-and-treat study in Uganda and Kenya.
Methods:
We calculated observed per-person-per-year (ppy) costs of streamlined care in 17 health facilities in SEARCH Study intervention communities (NCT: 01864603) via micro-costing techniques, time-and-motion studies, staff interviews, and administrative records. Cost categories included salaries, ART, viral load testing, recurring goods/services, and fixed capital/facility costs. We then modeled costs under three increasingly efficient scale-up scenarios: lowest-cost ART, centralized viral load testing, and governmental healthcare worker salaries. We assessed the relationship between community-specific ART delivery costs, retention in care, and viral suppression.
Results:
Estimated streamlined HIV care delivery costs were $291/ppy. ART ($117/ppy for TDF/3TC/EFV [40%]) and viral load testing ($110/ppy for 2 tests/year [39%]) dominated costs versus salaries ($51/ppy), recurring costs ($5/ppy), and fixed costs ($7/ppy). Optimized ART scale-up with lowest-cost ART ($100/ppy), annual viral load testing ($24/ppy), and governmental healthcare salaries ($27/ppy), lowered streamlined care cost to $163/ppy. We found clinic-to-clinic heterogeneity in retention and viral suppression levels versus streamlined care delivery costs, but no correlation between cost and either retention or viral suppression.
Conclusions:
In the SEARCH Study, streamlined HIV care delivery costs were similar to or lower than prior estimates despite including viral load testing; further optimizations could substantially reduce costs further. These data can inform global strategies for financing ART expansion to achieve UNAIDS 90–90–90 targets.
Keywords: differentiated care, HIV antiretroviral therapy, micro-costing, streamlined care
Introduction
Treatment of HIV has expanded rapidly, with 19 million of 37 million HIV-positive persons worldwide (and 14 million of 26 million in sub-Saharan Africa) now receiving antiretroviral therapy (ART). However, HIV remains a global pandemic with almost 2 million new infections annually [1]. Nations are expanding progress towards the UNAIDS ‘90–90–90’ goals: 90% of HIV-positive individuals knowing their status, 90% of diagnosed individuals initiating ART, and 90% individuals on ART achieving viral suppression [2]. However, donor funding for ART expansion is anticipated to remain stable in coming years and in some areas may be declining [3]. This has created an urgent need for new innovative models of HIV care delivery that capture greater efficiencies and can serve far more patients at lower costs per patient.
Standard HIV care delivery is structured to serve broad, heterogeneous populations with easy to adopt, uniform procedures. The cost of standard HIV care delivery in Sub-Saharan Africa has declined over time with the advent of less expensive ART medications and improved clinical operations. Before 2009, HIV care delivery with ART and CD4+ cell count monitoring was estimated to cost between $643 and $1089 per person per year (ppy) [4]. Since 2009, HIV care delivery with CD4+ cell count monitoring has been estimated at $206–$924 ppy [5–10], while HIV care delivery with annual viral load (VL) monitoring has been estimated at $300–$628 ppy [11,12].
Differentiated care is a newer term used to describe models of HIV care that tailor clinical services to patients depending on their needs, improve clinic efficiency, and achieve strong and durable clinical outcomes [13]. Differentiated care models are diverse and include innovations such as pharmacy-only ART refill visits [8,14–16], clinic-based group visits with lay healthcare workers [12,17–21], community-based ART delivery [9,22–24], and community-based peer adherence support groups [25–30]. Each of these innovations has been implemented in sub-Saharan African settings to facilitate cost-effective ART scale-up. These innovations have been shown to reduce the time spent per patient, improve ART adherence, improve retention in care, and maintain durable viral suppression.
There are limited data on the costs and cost-effectiveness of differentiated ART delivery models. We previously estimated the cost of a streamlined HIV care delivery model in Uganda for patients with high CD4+ cell counts (>500 cells/μl) that included twice per year viral load monitoring as $320–$529 ppy [11]. In Kampala, Uganda, an every-two-month pharmacy-only visit program cost an estimated $520 ppy compared to $655 ppy for standard care, with an incremental cost-effectiveness ratio of $13,500/patient [8]. This program was deemed not cost effective as it exceeds the common cost-effectiveness threshold of three times annual per capita GDP (i.e. $2,116) [31]. In Jinja District, Uganda, a home medication delivery program cost $793 ppy versus $838 for facility-based refills [9]. In Cape Town, South Africa, a group visit program cost $300 ppy versus $374 ppy for standard care [12]. Further, in Tete, Mozambique, key informants reported cost savings as a primary benefit of community-based peer adherence support groups [25]. This literature is growing, but has not yet included cost analyses of comprehensive multicomponent models of streamlined HIV care delivered across multiple sites. Such data are needed to inform current discussions on the cost-effectiveness of differentiated care scale up.
The SEARCH Study is an ongoing cluster-randomized trial in 32 communities (approximately 10,000 persons each) in Uganda and Kenya of an intervention combining community-based HIV testing, linkage to care, and streamlined HIV and general medical care delivered in clinics. The SEARCH streamlined care model is a patient-centric approach that aims to meet patients’ needs, reduce transit time through services, boost efficiency for the patient and clinic, and achieve durable viral suppression [32–34]. Streamlined HIV care seeks to maximize the efficiency of highly trained clinical providers, in contrast to some differentiated care models that seek to shift care of stable patients away from clinical providers towards lay health providers and peers. After two years, the SEARCH intervention successfully achieved and exceeded the UNAIDS 90–90–90 targets for testing of more than 90% of adult community residents [35], initiating ART in more than 90% of HIV-positive persons, and achieving more than 90% viral suppression among persons on ART [34].
To inform current resource allocation policies on accelerating ART scale-up, we sought in this report to estimate costs associated with providing streamlined care in Ugandan and Kenyan HIV clinics in intervention communities of the SEARCH trial. We also sought to estimate costs of scaling up streamlined care models to capture increasing efficiencies that are possible at regional and national levels. Lastly, to assess healthcare value, we sought to explore variability across communities in their costs as compared with levels of retention in care and viral suppression.
Methods
Study setting and population
The SEARCH Study (NCT:01864603) is an ongoing cluster-randomized trial of a universal HIV test-and-treat intervention in 32 communities (approximately 10 000 persons each) across West Uganda, East Uganda, and Kenya. At baseline, communities receive a census and two week-long community health campaign with HIV testing (including CD4+ cell count and viral load measurement in HIV-positive persons) and testing for other diseases (including malaria, hypertension, and diabetes) for the entire population [34–36].
In the 16 SEARCH Study intervention communities, individuals diagnosed with HIV at baseline or at subsequent annual testing campaigns are offered immediate linkage to care and ART initiation at clinics in a streamlined model of HIV care. Our streamlined care model has been described previously [32,33]. Briefly, streamlined care is a multicomponent HIV care model designed to reduce structural barriers faced by patients, improve patient–clinician relationships, improve knowledge and decrease stigma. Central features include immediate ART initiation; a patient-centered, welcoming, empathetic environment; co-location of clinical, phlebotomy and medication dispensing services; viral load monitoring with structured viral load counseling; co-located care for noncommunicable diseases including hypertension and diabetes; quarterly (rather than monthly) clinic visits and ART dispensing; 24-h telephone access to a clinician; flexible clinic hours and locations for ART dispensation; and telephone appointment reminders and patient tracking following missed visits [32,33].
Ethics statement
The SEARCH Study was approved by institutional review boards at Makerere University College of Health Sciences (Kampala, Uganda), the Kenya Medical Research Institute (Nairobi, Kenya), the University of California, San Francisco, and the Uganda National Council for Science and Technology. SEARCH Study participants provided verbal informed consent in their preferred language.
Data collection
We collected information on resources required to support streamlined HIV care during 2-week site visits to 17 clinics located in the 16 SEARCH Study intervention communities from July 2015–June 2016 (second year of intervention) using previously published methods [11,37]. Study teams in the three regions (West Uganda, East Uganda, and Kenya) collected cost data using standardized tools. Teams recorded the economic value of utilized resources regardless of funding source. Costs were recorded as 2016 $USD. We ascertained costs in five key categories comprising streamlined care delivery: (A) personnel, (B) ART medications, (C) viral load testing (D) other recurrent goods and services and (E) fixed costs (see Supplemental Digital Content [SDC] 2 – Appendix, for detailed description of data sources).
Staff interviews
We interviewed SEARCH Study regional coordinators and assistant coordinators to ascertain types and amounts of SEARCH on-site resources (e.g. SEARCH clinic staff, ART medications) and off-site resources (e.g. SEARCH supervisory staff, viral load testing, transportation costs) needed for streamlined care. We interviewed health facility managers and ascertained resources needed for streamlined care, costs of these resources (e.g. staff salaries and benefits, unit costs for viral load testing supplies, and other recurring goods and services), and costs of resources lacking administrative records (e.g. utility/facility costs).
Administrative records review
We reviewed SEARCH administrative records to ascertain SEARCH staff salaries and benefits, costs of ART medications, and transportation costs for SEARCH staff and viral load test specimens. We reviewed health facility administrative records to obtain information on recurring goods and services (e.g. viral load and other laboratory testing supplies) consumed during the 2-week costing period.
Time and motion study
We conducted a time and motion (T&M) study in each clinic to establish the proportion of total work time clinic staff spent providing streamlined HIV care. Clinic personnel who either directly provided or supported streamlined HIV care completed 24-h time records daily during the 2-week observation period. Staff recorded information on the number of minutes spent: directly in-person with patients; on the phone with patients; working on tasks for patients; conducting nonclinical SEARCH Study research work; performing other work; and waiting for the next patient, idle or on scheduled break.
Patient visits
The number and types of patients seen during the 2-week observation was ascertained from clinic records. We noted patients receiving care for HIV, noncommunicable diseases or both conditions. To proportionally allocate resources consumed each day, we tallied numbers of enrolled SEARCH Study participants, non-SEARCH Study community members, and noncommunity members seen each day. In each study community, the number of SEARCH Study patients served and patient visits conducted during the previous 12 months was obtained from clinical records.
Retention in care and viral suppression in intervention communities
Retention in care was defined as 90 days or less late to a scheduled appointment 12 months after baseline study visit [38]. For each community, the probability of retention in care at 1 year was estimated via Kaplan–Meier survival estimates with censoring at the time of transfer or death.
Viral suppression was defined as HIV-1 RNA less than 500 copies/ml measured during annual HIV testing campaigns. For each community, we estimated the proportion of HIV-positive adult community residents with measured HIV RNA level were virologically suppressed.
Estimation of streamlined care costs
For each study community, we estimated annual per-patient streamlined care costs as a sum of the five cost categories: personnel, ART medications, viral load testing, other recurrent goods and services, and fixed costs (Supplemental Digital Content [SDC] 2 – Appendix).
Modeled scenarios of antiretroviral therapy scale-up costs
We modeled the cost of providing streamlined HIV care under a ‘base case’ scenario (outlined above), as well as three scenarios of increasing operational efficiency that could occur during a large scale-up of streamlined care, as follows:
Scenario A: Base case scenario costs, but with cost of first-line TDF/3TC/EFV antiretroviral medications set to lowest available costs based on United Nations Development Programme negotiated rates ($100 ppy) [39,40].
Scenario B: Costs were identical to Scenario A, except that model included the recommended standard one viral load test per year [41] (rather than two per year as provided in SEARCH), cost of viral load testing was set to Uganda and Kenya Ministry of Health (MOH) pricing of $24/test, and transportation of viral load specimens was considered absorbed into MOH processes (thus, noncontributory).
Scenario C: Costs were identical to Scenario B, except that staff salaries for all positions were estimated using 2016 MOH salary scales.
Clinic heterogeneity of cost versus retention in care and viral suppression metrics
For each clinic, we aggregated data on streamlined care costs (outlined above) and plotted these against our estimates of retention in care and viral suppression. We assessed overall correlation of streamlined HIV care delivery cost versus level of retention in care, as well as streamlined care cost versus level of viral suppression using Pearson's correlation coefficient.
Results
Health facility characteristics
We assessed streamlined HIV care in 17 health facilities in the 16 SEARCH Study intervention communities in West Uganda, East Uganda and Kenya (Table 1). Clinic staff (n = 140) participated in the T&M study. More staff members were involved in streamlined care provision in Kenya versus Uganda. During the 2-week observations, a total of 1313 patient visits occurred, with similar numbers across regions. Overall, 7707 HIV-positive patients had at least 1 streamlined HIV care visit from July 2015 to June 2016 in Kenya (n = 4954), West Uganda (n = 1807), and East Uganda (n = 946). Kenya had the highest mean number of annual visits per patient, followed by West Uganda and East Uganda (Table 1).
Table 1.
SEARCH intervention communities, health facilities, staff members, and patients included in costing activities.
| West Uganda | East Uganda | Kenya | Total | |
| SEARCH intervention communities | 5 | 5 | 6 | 16 |
| Health facilities assessed in SEARCH Study intervention communities | 5 | 5 | 7 | 17 |
| Health facility staff members assessed | 36 | 31 | 73 | 140 |
| HIV-positive SEARCH patients with visits during costing observation period | 460 | 443 | 410 | 1313 |
| HIV-positive SEARCH patients with ≥1 clinic visit in year of observation | 1807 | 946 | 4954 | 7707 |
| Number of visits per patient per year – mean | 4.6 | 4.0 | 5.3 | 5.0 |
Personnel effort toward streamlined care
We assessed the time spent by staff members to provide streamlined care. Consistent with the nurse-centered design of the streamlined care model, nurses spent the most time devoted to patient care, averaging 15.3 min per patient (see Table, Supplemental Digital Content [SDC] 1). Other clinic staff spent slightly less time per patient, including clinical officers (9.7 min), laboratory technicians (11.0 min), peer educators (11.1 min) and data/information officers (12.6 min; Table, SDC 1). The distribution of staff time differed across regions, with nurses spending more time per patient in West and East Uganda compared to Kenya.
Total average time spent per patient was lowest in West Uganda (36.3 min), was 68.4 min in East Uganda, and was highest in Kenya (103.6 min). In West Uganda, nurses and laboratory technicians provided most streamlined care services; in East Uganda, nurses dedicated more effort than clinical officers or laboratory technicians, but data/information officers also triaged patients and maintained patient files. In Kenya, data/information officers and other staff contributed to the triage and follow-up of patients, while peers supported education of patients.
Costs of streamlined care
Overall, provision of streamlined care cost an average of $291 ppy and varied across regions (average $299 ppy in West Uganda, $319 ppy in East Uganda, and $286 ppy in Kenya, Table 2). Costs were dominated by ART medications (40.4% of total), viral load testing (37.9% of total), and personnel/salary costs (17.6% of costs; Table 2). Recurring and fixed costs were minor contributors to overall costs. Personnel costs were highest in Kenya and lowest in West Uganda. ART medication costs were lower in Kenya ($99) than in Uganda ($150).
Table 2.
Observed per person annual cost of streamlined care delivery by study region.
| Cost category | West Uganda | East Uganda | Kenya | Overall (%) |
| Personnel | $25.62 | $44.01 | $64.50 | $51.08 (17.6) |
| ART | $150.36 | $150.36 | $99.00 | $117.35 (40.4) |
| Viral load testing | $110.17 | $110.36 | $110.12 | $110.29 (37.9) |
| Other recurring costsa | $9.41 | $7.60 | $5.33 | $5.33 (1.8) |
| Fixed costsb | $3.78 | $6.46 | $6.57 | $6.57 (2.3) |
| Total | $299.34 | $318.79 | $285.52 | $290.62 (100) |
ART, Antiretroviral therapy.
aRecurring costs included non-ART medications, utilities, and communications fees.
bFixed costs included equipment and facilities.
Modeled costs of optimized streamlined care models for scale-up
Figure 1 shows the actual observed costs of streamlined care (base care scenario), as well as modeled costs under three increasingly optimized care delivery scenarios. In scenario A (optimization to lowest available ART pricing), streamlined care costs in Uganda are reduced by $50.36 ppy; costs were not reduced in Kenya because ART is already available at the optimized $100 ppy price. In scenario B (annual optimized viral load testing and transportation), streamlined care cost is substantially reduced by $86 ppy in all regions. In scenario C (governmental staff salaries), streamlined care cost is reduced $4 ppy in West Uganda, $21 ppy in East Uganda, and $16 ppy in Kenya. In our maximally streamlined scenario, HIV care would cost $163 ppy overall, a 44% reduction from the $291 ppy base case estimate.
Fig. 1.
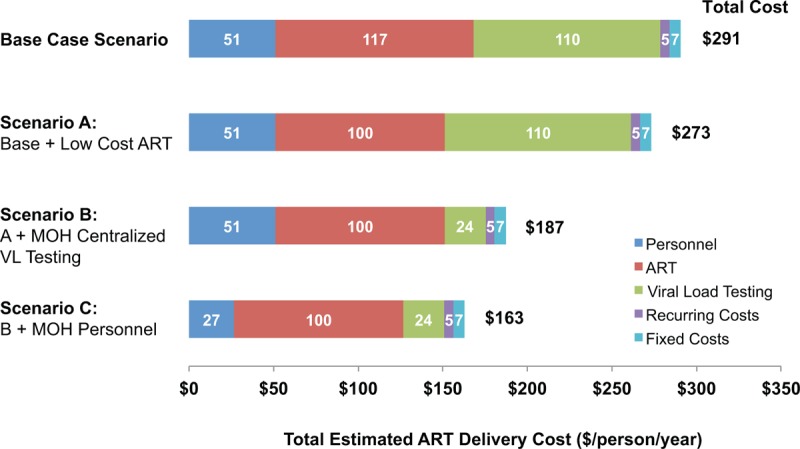
Annualized per-person estimated costs for observed and optimized scenarios of streamlined HIV care delivery.
Heterogeneity of cost, retention in care, and viral suppression across clinics
Figure 2 shows the heterogeneity of costs across health facilities and the relationship of cost to community levels of retention in care and viral suppression. The heterogeneity in costs across clinics and regions appeared to be driven mainly by variability in personnel costs for streamlined care. We did not observe correlation between streamlined care costs and the proportion of patients retained in care (R2 = 0.003) or virologically suppressed (R2 = 0.031; Fig. 3).
Fig. 2.
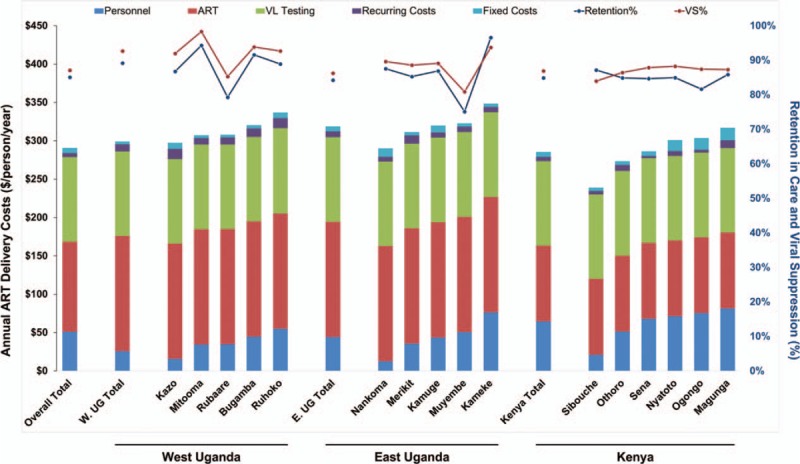
HIV care delivery costs in individual communities in West Uganda (n = 5), East Uganda (n = 5), and Kenya (n = 6).
Fig. 3.
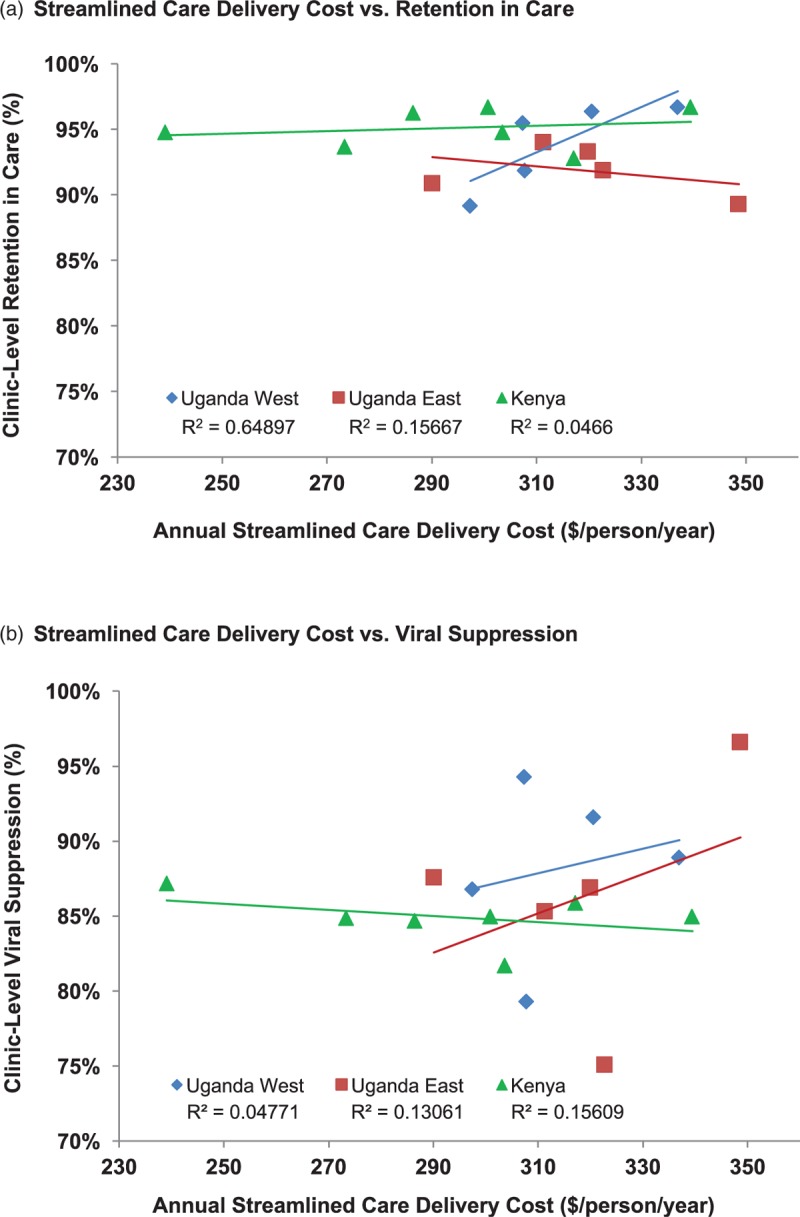
Community-specific streamlined care delivery costs versus retention in care and viral suppression.
Discussion
Streamlined HIV care delivery in the SEARCH test-and-treat trial cost an average $291 ppy, a cost similar to or lower than prevailing estimates despite adding viral load testing and care for noncommunicable diseases. In a series of modeled scenarios, we found that streamlined care costs can be lowered further during scale-up by using less expensive ART medications; optimizing viral load testing operations; and transitioning care to Ministry of Health (MOH)-salaried clinicians. Across clinics, we did not see correlation between the streamlined care costs and retention in care or viral suppression, suggesting that different clinics may have generated varying levels of healthcare value.
Our observed costs were similar to or lower than previous estimates for standard and differentiated care models across several PEPFAR-supported environments and programs ($206–$924 ppy) [5]. These encompass data from programs that primarily included CD4+ cell count monitoring and that were located in Uganda [8,9], Kenya [6], Ethiopia [7], and Zambia [10]. Our cost estimates are also lower than those for both standard and differentiated models of HIV care that include annual viral load monitoring, following more modern guidelines on key elements of HIV care ($300–$628 ppy). These included programs in both Uganda [11] and South Africa [12], two countries with well developed systems for viral load monitoring. Annual viral load testing and counseling are now recommended as core features of optimal service delivery [41]. Our streamlined care model included twice-a-year viral load testing and counseling as well as care for noncommunicable and general medical conditions, a comprehensive model that capitalizes on HIV infrastructure to deliver broader health services. Yet despite these additional expenses, costs compared favorably to standard models of care. Furthermore, we recently reported that this streamlined care model achieves strong clinical outcomes, including 89% retention in care among patients newly enrolled [38], and high viral suppression rates among stable adult community residents [33].
The lower cost of our streamlined care model may be related to several factors. First, our nurse-driven care allowed patients to transit rapidly through visits, decongesting clinics, using less clinician time, and allowing more patients to be served. Second, many clinics offered visits, phlebotomy and medication dispensation by single providers, reducing staff time spent delivering care and reducing wait time between providers. We previously reported that visits in our streamlined care model were over one hour shorter than standard government clinic visits [42]. Third, offering longer 3-month (versus 1–2 month) ART refills lengthened time between visits, reducing average daily load and time spent by staff. This strategy has shown positive outcomes [14–16], although issues with supply chain management can hamper implementation. Fourth, our inclusion of patients with higher CD4+ cell counts – a population less symptomatic and requiring less time-intensive monitoring – may have helped create and maintain efficiency.
Our study may inform ongoing discussions about continued scale-up of streamlined HIV care. Our modeled scenarios show that costs could be further reduced by: negotiating lower national ART costs, optimizing viral load testing operations, and employing MOH-salaried clinical staff to provide care. Ensuring lowest-price antiretroviral drugs only modestly reduced overall costs (6%), and the impact was greater in Uganda versus Kenya since ART prices were substantially lower in Kenya. Greater cost reductions (30%) occurred if viral load specimens reached a central laboratory via the MOH transport network (as is currently done in Uganda). This optimization may be important as newer point-of-care viral load technologies emerge and need integration into national streamlined care systems. Lastly, employing MOH-salaried clinic staff reduced costs modestly (8%). However, lower salaries must be cautiously approached as they have been linked to lower clinician performance and shortening of clinic hours [43], possibly challenging streamlined care adoption. Nevertheless, the fact that our model expanded services overall (with viral load testing and NCD care) while remaining cost-neutral and in some cases reducing costs, should create optimism that streamlined care models will be crucial for ongoing ART expansion.
We noted heterogeneity in streamlined care costs across regions. Understanding this variability is important as individual regions and nations consider the varying composition of their healthcare workforces, diverse patient needs, and services to offer. We observed higher overall ART delivery costs in Uganda, where streamlined care was more nurse-driven and provided by a smaller number of higher paid clinicians compared to Kenya, where care was provided by a wider range of providers. Task-shifting and task-sharing have been implemented to address workforce shortages. For example, nurse-initiated ART and use of peer health workers have both been highly cost-effective [18]. However, the most effective ratio of nurses, clinical officers, and lay staff remains unclear. Future analyses directly comparing intervention (streamlined) and control (standard) care delivery models in the SEARCH Study will explore this question. We also noted heterogeneity in the relative healthcare value delivered at different clinics: higher cost clinics were not associated with higher retention in care or viral suppression. Future work should investigate features of lower cost clinics that achieve higher viral suppression (i.e., higher value) and methods for propagating this success across entire clinic networks. Future work should also assess how provision of different, broader, and more integrated packages of services at clinics (i.e., services for TB, sexual and reproductive health, and noncommunicable diseases apart from diabetes and hypertension), will affect overall costs, and what potential exists for achieving even more substantial cost efficiencies in HIV care when services are combined. Several aspects of streamlined and differentiated care programs such as longer visit intervals with longer refill provision, co-location of services to achieve shorter yet effective visit times, and proactive care of healthier patient groups, can all increase available clinician time, allow for delivery of broader services, and capture greater efficiencies. These advances will be particularly important as momentum builds for designing broader systems to achieve universal healthcare delivery, especially systems that build upon successful HIV-focused infrastructure.
Our study is subject to certain limitations. First, streamlined care in the SEARCH Study was implemented as part of a broader HIV care system in which some patients received standard services alongside others receiving streamlined services. To isolate streamlined care costs, we conducted self-administered time-and-motion studies. However, accuracy of these assessments could be affected if staff members underestimated time needed for certain tasks or did not fully record break/waiting time. We minimized this risk by using simple tools allowing staff to fully capture downtime. Second, we did not capture which laboratory tests (other than viral load ) and medications (other than ART) were used in streamlined versus standard care; our analysis assumed similar usage patterns. Since streamlined care patients are often healthier, our costs of care may be overestimates. Third, we did not have documentation of certain facility costs including rent (part of fixed costs) or utilities (part of recurring goods and services costs); these were estimated via interviews with clinic managers. Despite these limitations, however, our overall cost estimates were within range of other prevailing estimates for HIV care delivery.
Our data from Ugandan and Kenyan clinics in the SEARCH Study demonstrate that streamlined HIV care that includes viral load testing and co-location of noncommunicable disease care can be delivered at costs similar to or lower than current prevailing ART delivery costs. Our results add to a nascent literature on the cost of streamlined HIV care models that include patients with high CD4+ cell counts who are a major focus of current expansion and scale-up of ART programs. These data can inform resource allocation decisions in the current era of rapidly expanding universal ART.
Acknowledgements
S.B.S., M.R.K, M.L.P., D.V.H. and V.J. designed the study. T.O., A.L., R.A., E.A., B.M., D.M., A.O., F.M., J.A., L.B.B., and D.K. conducted data collection, with critical input on its interpretation from S.B.S., T.O., L.B.B., L.B., M.R.K., M.L.P., D.V.H. and V.J. Manuscript drafted by S.B.S., M.L.P., D.V.H. and V.J., with input and revision from all co-authors.
Sources of Support and Acknowledgements: Research reported in this manuscript was supported by Division of AIDS, NIAID, of the National Institutes of Health under award number U01AI099959 and in part by the President's Emergency Plan for AIDS Relief, the Bill and Melinda Gates Foundation, and Gilead Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, PEPFAR, the Bill and Melinda Gates Foundation or Gilead Sciences. The SEARCH project gratefully acknowledges the Ministries of Health of Uganda and Kenya, our research team, collaborators and advisory boards, and especially all communities and participants involved.
Conflicts of interest
S.S., H.T., C.R.C., E.D.C., M.R.K., M.L.P., D.V.H. and V.J. have received grants from the National Institutes of Health. V.J. has received research grant support from Gilead Sciences. D.V.H. has received nonfinancial support (donation of study drug Truvada) from Gilead Sciences. All other authors declare no conflict of interests.
Supplementary Material
References
- 1.Joint United Nations Programme on HIV/AIDS. AIDS data. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2017. pp. 1–248. [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS. Ending AIDS: progress towards the 90-90-90 targets. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2017. pp. 1–198. [Google Scholar]
- 3.Henry J. Kaiser Family Foundation and UNAIDS. Financing the response to HIV in low- and middle-income countries: International Assistance from Donor Governments in 2015. Geneva, Switzerland: UNAIDS; 2016. [Google Scholar]
- 4.Galarraga O, Wirtz VJ, Figueroa-Lara A, Santa-Ana-Tellez Y, Coulibaly I, Viisainen K, et al. Unit costs for delivery of antiretroviral treatment and prevention of mother-to-child transmission of HIV: a systematic review for low- and middle-income countries. Pharmacoeconomics 2011; 29:579–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzies NA, Berruti AA, Berzon R, Filler S, Ferris R, Ellerbrock TV, et al. The cost of providing comprehensive HIV treatment in PEPFAR-supported programs. Aids 2011; 25:1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson BA, Bii M, Henly-Thomas S, McCoy K, Sawe F, Shaffer D, et al. ART treatment costs and retention in care in Kenya: a cohort study in three rural outpatient clinics. J Int AIDS Soc 2013; 16:18026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johns B, Asfaw E, Wong W, Bekele A, Minior T, Kebede A, et al. Assessing the costs and effects of antiretroviral therapy task shifting from physicians to other health professionals in Ethiopia. J Acquir Immune Defic Syndr 2014; 65:e140–e147. [DOI] [PubMed] [Google Scholar]
- 8.Babigumira JB, Castelnuovo B, Stergachis A, Kiragga A, Shaefer P, Lamorde M, et al. Cost effectiveness of a pharmacy-only refill program in a large urban HIV/AIDS clinic in Uganda. PLoS One 2011; 6:e18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaffar S, Amuron B, Foster S, Birungi J, Levin J, Namara G, et al. Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in Jinja, southeast Uganda: a cluster-randomised equivalence trial. Lancet 2009; 374:2080–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott CA, Iyer HS, McCoy K, Moyo C, Long L, Larson BA, et al. Retention in care, resource utilization, and costs for adults receiving antiretroviral therapy in Zambia: a retrospective cohort study. BMC Pub Health 2014; 14:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain V, Chang W, Byonanebye DM, Owaraganise A, Twinomuhwezi E, Amanyire G, et al. Estimated Costs for Delivery of HIV Antiretroviral Therapy to Individuals with CD4+ T-Cell Counts >350 cells/uL in Rural Uganda. PLoS One 2015; 10:e0143433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bango F, Ashmore J, Wilkinson L, van Cutsem G, Cleary S. Adherence clubs for long-term provision of antiretroviral therapy: cost-effectiveness and access analysis from Khayelitsha, South Africa. Trop Med Int Health 2016; 21:1115–1123. [DOI] [PubMed] [Google Scholar]
- 13.Grimsrud A, Bygrave H, Doherty M, Ehrenkranz P, Ellman T, Ferris R, et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J Int AIDS Soc 2016; 19:21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obua C, Kayiwa J, Waako P, Tomson G, Balidawa H, Chalker J, et al. Improving adherence to antiretroviral treatment in Uganda with a low-resource facility-based intervention. Glob Health Action 2014; 7:24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bemelmans M, Baert S, Goemaere E, Wilkinson L, Vandendyck M, van Cutsem G, et al. Community-supported models of care for people on HIV treatment in sub-Saharan Africa. Trop Med Int Health 2014; 19:968–977. [DOI] [PubMed] [Google Scholar]
- 16.Alamo ST, Wagner GJ, Ouma J, Sunday P, Marie L, Colebunders R, et al. Strategies for optimizing clinic efficiency in a community-based antiretroviral treatment programme in Uganda. AIDS Behav 2013; 17:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson L, Harley B, Sharp J, Solomon S, Jacobs S, Cragg C, et al. Expansion of the Adherence Club model for stable antiretroviral therapy patients in the Cape Metro, South Africa 2011–2015. Trop Med Int Health 2016; 21:743–749. [DOI] [PubMed] [Google Scholar]
- 18.Venables E, Edwards JK, Baert S, Etienne W, Khabala K, Bygrave H. They just come, pick and go.’ The Acceptability of Integrated Medication Adherence Clubs for HIV and Non Communicable Disease (NCD) Patients in Kibera, Kenya. PLoS One 2016; 11:e0164634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luque-Fernandez MA, Van Cutsem G, Goemaere E, Hilderbrand K, Schomaker M, Mantangana N, et al. Effectiveness of patient adherence groups as a model of care for stable patients on antiretroviral therapy in Khayelitsha, Cape Town, South Africa. PLoS One 2013; 8:e56088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khabala KB, Edwards JK, Baruani B, Sirengo M, Musembi P, Kosgei RJ, et al. Medication Adherence Clubs: a potential solution to managing large numbers of stable patients with multiple chronic diseases in informal settlements. Trop Med Int Health 2015; 20:1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimsrud A, Lesosky M, Kalombo C, Bekker LG, Myer L. Implementation and operational research: community-based adherence clubs for the management of stable antiretroviral therapy patients in Cape Town, South Africa: a cohort study. J Acquir Immune Defic Syndr 2016; 71:e16–e23. [DOI] [PubMed] [Google Scholar]
- 22.Vu L, Waliggo S, Zieman B, Jani N, Buzaalirwa L, Okoboi S, et al. Annual cost of antiretroviral therapy among three service delivery models in Uganda. J Int AIDS Soc 2016; 19 (5 Suppl 4):20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogt F, Kalenga L, Lukela J, Salumu F, Diallo I, Nico E, et al. Brief report: decentralizing ART supply for stable hiv patients to community-based distribution centers: program outcomes from an urban context in Kinshasa, DRC. J Acquir Immune Defic Syndr 2017; 74:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okoboi S, Ding E, Persuad S, Wangisi J, Birungi J, Shurgold S, et al. Community-based ART distribution system can effectively facilitate long-term program retention and low-rates of death and virologic failure in rural Uganda. AIDS Res Ther 2015; 12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasschaert F, Telfer B, Lessitala F, Decroo T, Remartinez D, Biot M, et al. A qualitative assessment of a community antiretroviral therapy group model in Tete, Mozambique. PLoS One 2014; 9:e91544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasschaert F, Decroo T, Remartinez D, Telfer B, Lessitala F, Biot M, et al. Adapting a community-based ART delivery model to the patients’ needs: a mixed methods research in Tete, Mozambique. BMC Public Health 2014; 14:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naslund JA, Dionne-Odom J, Junior Destine C, Jogerst KM, Renold Senecharles R, Jean Louis M, et al. Adapting and Implementing a Community Program to Improve Retention in Care among Patients with HIV in Southern Haiti: ‘Group of 6’. AIDS Res Treat 2014; 2014:137545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jobarteh K, Shiraishi RW, Malimane I, Samo Gudo P, Decroo T, Auld AF, et al. Community ART support groups in Mozambique: the potential of patients as partners in care. PLoS One 2016; 11:e0166444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Decroo T, Koole O, Remartinez D, dos Santos N, Dezembro S, Jofrisse M, et al. Four-year retention and risk factors for attrition among members of community ART groups in Tete, Mozambique. Trop Med Int Health 2014; 19:514–521. [DOI] [PubMed] [Google Scholar]
- 30.Auld AF, Shiraishi RW, Couto A, Mbofana F, Colborn K, Alfredo C, et al. A decade of antiretroviral therapy scale-up in Mozambique: evaluation of outcome trends and new models of service delivery among more than 300,000 patients enrolled during 2004–2013. J Acquir Immune Defic Syndr 2016; 73:e11–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Bank. GDP per capita (current US$). 2015. [Google Scholar]
- 32.Jain V, Byonanebye DM, Amanyire G, Kwarisiima D, Black D, Kabami J, et al. Successful antiretroviral therapy delivery and retention in care among asymptomatic individuals with high CD4+ T-cell counts above 350 cells/uL in rural Uganda. Aids 2014; 28:2241–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwarisiima D, Kamya MR, Owaraganise A, Mwangwa F, Byonanebye DM, Ayieko J, et al. High rates of viral suppression in adults and children with high CD4+ counts using a streamlined ART delivery model in the SEARCH trial in rural Uganda and Kenya. J Int AIDS Soc 2017; 20 Suppl 4:21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen M, Balzer L, Kwarsiima D, Sang N, Chamie G, Ayieko J, et al. Association of implementation of a universal testing and treatment intervention with HIV diagnosis, receipt of antiretroviral therapy, and viral suppression in East Africa. JAMA 2017; 317:2196–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chamie G, Clark TD, Kabami J, Kadede K, Ssemmondo E, Steinfeld R, et al. A hybrid mobile approach for population-wide HIV testing in rural east Africa: an observational study. Lancet HIV 2016; 3:e111–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain V, Petersen ML, Liegler T, Byonanebye DM, Kwarisiima D, Chamie G, et al. Population levels and geographical distribution of HIV RNA in rural Ugandan and Kenyan communities, including serodiscordant couples: a cross-sectional analysis. Lancet HIV 2017; 4:e122–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang W, Chamie G, Mwai D, Clark TD, Thirumurthy H, Charlebois ED, et al. Implementation and operational research: cost and efficiency of a hybrid mobile multidisease testing approach with high hiv testing coverage in East Africa. J Acquir Immune Defic Syndr 2016; 73:e39–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown LB, Havlir DV, Ayieko J, Mwangwa F, Owaraganise A, Kwarisiima D, et al. High levels of retention in care with streamlined care and universal test and treat in East Africa. Aids 2016; 30:2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.United Nations Development Programme. Breakthrough brings cost of HIV treatment to under $100 per patient per year. 2015. http://www.undp.org/content/undp/en/home/presscenter/pressreleases/2015/11/27/breakthrough-brings-cost-of-hiv-treatment-to-under-100-per-patient-per-year.html [Accessed 10 June 2018] [Google Scholar]
- 40.Medicines Sans Frontieres. Untangling the web of antiretroviral price reductions: 18th edition - July 2016. 2016. https://www.msfaccess.org/sites/default/files/HIV_report_Untangling-the-web-18thed_ENG_2016.pdf [Accessed 10 June 2018] [Google Scholar]
- 41.World Health Organization. Technical and operational considerations for implementing HIV viral load testing: access to HIV diagnostics. 2014:1-28. http://www.who.int/hiv/pub/arv/viral-load-testing-technical-update/en/ [Accessed 10 June 2018] [Google Scholar]
- 42.Shade SB, Chang W, Kahn JG, Mwai D, Mwangwa F, Kwarisiima D. SEARCH streamlined HIV care is associated with shorter wait times before and during patient visits in Ugandan and Kenyan HIV clinics. In: Program and abstracts of the 21st International AIDS Conference; July 2016; Durban, South Africa Abstract FRAE0203 2016. [Google Scholar]
- 43.Naburi H, Mujinja P, Kilewo C, Orsini N, Barnighausen T, Manji K, et al. Job satisfaction and turnover intentions among healthcare staff providing services for prevention of mother-to-child transmission of HIV in Dar es Salaam, Tanzania. Hum Resour Health 2017; 15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


