Abstract
Objective
Think Health! ¡Vive Saludable! evaluated a moderate-intensity lifestyle behavioral weight loss program in primary care over 2 years of treatment. Final analyses examined weight change trajectories by treatment group and attendance.
Methods
Adult primary care patients (n=261; 84% female; 65% black; 16% Hispanic) were randomly assigned to Basic Plus (moderate-intensity; counseling by primary care clinician and a lifestyle coach) or (Basic; clinician counseling only). Intent-to-treat analyses used all available weight measurements from data collection, treatment, and routine clinical visits. Linear mixed-effects regression models adjusted for treatment site, gender, and age, and sensitivity analyses evaluated treatment attendance and the impact of loss to follow up.
Results
Model-based estimates for 24-month weight change from baseline were mean [95% CI] −1.34 kg [−2.92, 0.24] in Basic Plus and −1.16 kg [−2.70, 0.37] in Basic (net difference − 0.18 kg [−2.38, 2.03] p=0.874). Larger initial weight loss in Basic Plus was attenuated by a ~0.5 kg rebound at 12-to-16 months. Each additional coaching visit was associated with a 0.37 kg greater estimated 24-month weight loss (p=0.01).
Conclusions
These findings in mostly black and Hispanic female primary care patients suggest that strategies to improve treatment attendance may improve weight loss resulting from moderate-intensity counseling.
Keywords: African Americans, ethnic minorities, behavior modifications, primary care
INTRODUCTION
Obesity is a fundamental concern within primary care practice due to its adverse effects on prevention and management of chronic conditions such as high blood pressure, type 2 diabetes, and other aspects of health and well-being (1). Evidence that the most effective obesity treatment approaches occur outside of primary care settings or independently of practice staff has increased in the past decade (2). A role for primary care practices in supporting long-term weight management seems desirable, but models for how practices can best assist have proven difficult to establish.
The Think Health! study was designed to evaluate a moderate-intensity weight management approach involving counseling by primary care clinicians (physicians, except for 1 physician’s assistant) every 4 months supplemented with coaching by practice staff serving as lifestyle coaches(termed “Basic Plus” (3). Coaches provided counseling monthly for the first year, tapering to every other month in the second year. The comparison group was offered a low-intensity treatment involving only primary care clinician counseling (termed “Basic”). Think Health! was motivated by the particular need for obesity treatment programs that could be effective with black and Hispanic adults in accessible, routine health care settings. Nationally, obesity prevalence (BMI ≥ 30) is nearly 50 percent among non-Hispanic black adults and more than 40% in Hispanic adults, with higher prevalence among women than men in both ethnic groups (4). Grade 3 obesity (BMI ≥ 40) affects 16.5% of black women and 8% of Hispanic women (5). Evidence on effective weight management strategies in these populations is limited (6). Study recruitment, therefore, focused on practice sites with substantial proportions of black and Hispanic patients, and treatment was offered in both English and Spanish.
Interim results of Think Health!, based on a planned analysis after 1 year of treatment, have been reported (7). Basic Plus, compared with Basic, did not produce greater mean weight loss at 1 year (mean (SD weight change was 1.08 kg (−1.56 for Basic Plus vs. −0.48 for Basic; difference 1.08 kg; P = 0.093 based on a linear mixed-effects model), although more patients assigned to receive lifestyle coaching lost at least 5% of their baseline weight (23% vs. 10%, p = 0.022). Clinician and coach impressions of the treatment approach and its feasibility in their practice settings, based on interviews conducted after study completion, have also been reported (8). Here, we report the final, 2-year Think Health! outcomes. The main analyses focus on treatment group differences in patterns of attendance and weight change (the primary outcome) over 24 months, attained 24-month weight change, and the association of attendance at treatment visits with weight change among patients assigned to receive additional coaching.
METHODS AND PROCEDURES
Background
Study Design
Think Health! was a parallel group, randomized controlled trial (RCT) within primary care practices in Philadelphia, Pennsylvania. Key design and implementation elements are summarized here; detailed accounts are published elsewhere (3, 7). The objective was to assess the feasibility and effectiveness of a moderate-intensity lifestyle behavior change program (“Basic Plus”) compared with a lower-intensity version (“Basic”). Intensity was defined by frequency of in-person counseling contacts at the practice. The hypothesis was that Basic Plus would yield net greater mean weight loss than Basic when assessed at 1 and 2 years post randomization. Eligible individuals were ages 18 to 70 years, with BMI ≥27 kg/m2 and ≤55 kg/m2, who had been enrolled at one of the participating primary care practices for at least 1 year or had been seen at the practice at least twice. The target sample size was 240 patients, to be assigned 1:1 to Basic or Basic Plus, to detect a treatment group difference in weight change from baseline to 24 months of at least 2.4 kg (0.8 standard deviation of weight change) with 80% power. The study was approved by the institutional review boards of the University of Pennsylvania and Albert Einstein Healthcare Network.
Treatment approach
The treatment approach was adapted from a modification of the Diabetes Prevention Program lifestyle intervention (7, 9) (see Table 1). Treatment manuals and other printed materials were professionally designed in full color and provided in a logo tote bag at the first and subsequent clinician visits. The simplified treatment approach was designed to facilitate counseling by primary care clinicians and, in Basic Plus, by medical assistants or other ancillary staff serving as lifestyle coaches. Members of the research team provided clinicians and coaches with training in behavioral counseling and study logistics and arranged for them to complete an on-line human subjects research certification program. As in the Diabetes Prevention Program, topics covered included counseling to reduce caloric intake and increase calorie expenditure through physical activity, development and maintenance of stress management and behavior change skills, and overall weight loss motivation. Although the Basic program provided the same information, counseling frequency (only every 4 months) by clinicians was below the level of intensity expected to result in significant weight loss (10).
Table 1.
Features of the Think Health! ¡Vive Saludable! study intervention and comparison groups
| Basic Plus | Basic | |
|---|---|---|
Behavioral goals
|
✓ | ✓ |
Core treatment curriculum
|
✓ | ✓ |
Aids to enhance adherence
|
✓ | ✓ |
| Contact mode and frequency | ||
Primary care clinician
|
✓ | ✓ |
Lifestyle Coach – in person office visits
|
✓ | |
| Implementation Monitoring and Quality Control | ||
Protocol adherence by clinicians and practice staff
|
✓ | ✓ |
Lifestyle coaching
|
✓ | |
| Adherence Monitoring | ||
Attendance at clinician visits
|
✓ | ✓ |
Attendance at coaching visits-
|
✓ | |
Primary care practices
Primary care practices with substantial numbers of black or Hispanic participants were selected from a list of potential sites known to investigators or identified through word-of-mouth. Eligible practices had at least 1 interested clinician and at least 1 staff member for training as a lifestyle coach, and practices agreed to administrative and logistical requirements and compensation offered for participation. Preparation for participation included clinical and staff certification for involvement of research with human subjects, training for treatment delivery and completion of study forms, and developing administrative procedures for recruitment and study implementation. Providing each practice with a high-quality digital scale, installing a wall-mounted stadiometer for height measurements and training staff on their use facilitated standardization of study-related measurements taken at practices (by practice or research staff).
Participant enrollment and randomization
Potential study participants were identified from billing lists and clinician referrals during October 2007 through November 2008. Adults ages 18 to 70 years with BMI ≥27 and ≤ 55 kg/m2 were eligible if enrolled at the practice for at least 1 year or seen there at least twice. Exclusions were being pregnant or lactating; being non-ambulatory; taking systemic steroids, second generation anti-psychotics, or mood stabilizing agents; undergoing active cancer treatment; and having unstable cardiovascular disease or significant mental health conditions. The study coordinator implemented blinded randomization assignments provided electronically by a study biostatistician based on a 1:1 treatment allocation within each practice after stratification (by site) and permuted blocks with unequal block sizes on patient-level factors (age < or ≥35 years and gender).
Data collection
Weight, height, waist circumference, systolic and diastolic blood pressure, and questionnaires) were collected at practice sites by trained research staff, not blinded to treatment assignment, on 3 occasions: at eligibility determination (baseline); 1 year post-baseline; and either 2 years post-baseline or study closeout in May 2010. Length of follow up was determined by enrollment date, typically less than 2 years before a fixed study end date. Enrolling the target number of participants took longer than expected, while the study end date was inflexible due to a requirement of the funding mechanism that all funds be expended within 4 years of the June 2006 start date. Practice staff also took weight measurements at PCP or LC visits, and research staff extracted weights recorded in medical records when participants came to the practice for non-study visits during the time they were enrolled in Think Health!, including for inactive participants.
Questionnaires (English or Spanish) were self-administered with staff assistance as needed (3). Weight-related dietary behaviors were assessed and scored based on a 30-item version of a longer questionnaire used in a study of African American women (11). Caloric expenditure from physical activity was assessed with the Paffenbarger questionnaire (12). Perceived stress was assessed with Cohen’s 4 item measure (13), and the 8-item version of the Medical Outcomes Survey was used to assess Health Related Quality of Life (14). Having had a recent (prior 6 months) life change related to work, home, family, financial, or personal and social factors during was assessed with selected items adapted from the Holmes and Rahe Social Readjustment Rating Scale (with a “yes” response indicating stress) (15). A symptom checklist was used to monitor adverse events.
Treatment Implementation
As reported previously, treatment was implemented as planned in Year 1, allowing coaches flexibility to address more than one topic in a session or address topics out of sequence in line with patient interests and to account for missed visits (7). Scheduling systems and patient attendance influenced actual visit timing and completion. Two clinicians left practices in year 1, of which one was replaced, and there was some coach turnover but no subsequent turnover for either during year-2 implementation. The addition of bi-monthly automated telephone calls to both treatment arms in year 2 to enhance adherence was delayed due to technical issues. Calls were attempted with the 171 (66%) randomized patients who were defined as “active” (not withdrawn and attended a treatment visit within the last six (for Basic) or three months (Basic Plus)) at the end of treatment Year 1. Most (88%) attempted calls were completed, i.e., answered, and lasted long enough for the patient to hear the message or for the message to be left on an answering machine. The maximum number of calls received per person was three, depending on time in the study and call completion. The percentages of those randomized receiving 0, 1, 2, and 3 calls were 41, 34, 18, and 7%, respectively, in Basic, and similar in Basic Plus: 37, 28, 24, and 11%, respectively, with no difference by treatment group (Chi-square [df=3] =3.15; p=0.37).
Statistical Analyses
Total follow-up time was calculated by subtracting date of randomization from the date of the last available weight measurement (i.e., final data collection visit or latest treatment or medical record weight before month 24, or the date of withdrawal where applicable). We analyzed 1671 weight measurements in total (up to 7 per person): 559 taken by research staff; 959 taken by practice staff at PCP or LC visits; and 153 from medical records. During the interim data analysis, we had determined the acceptability of using weight measurements from treatment visits and medical records by comparing 1-year weights from these sources for participants who had a weight measurement in the same month by a research staff member (7). Intraperson differences were small and not biased toward either source, and mean differences between Basic (0.04 kg) and Basic Plus (0.14 kg) were similar and very small.
For body weight (primary outcome), we fit an array of potential models that allowed for complex trajectories of change over time, using all available weight measurements from any source, and compared them by the Akaike Information Criteria (AIC) (16). All models were fit by linear mixed-effects regression models with randomization stratification variables (primary care practice site, age group, and gender) as covariates and random intercepts for individuals and random slopes for time. Difference in weight change by treatment group was estimated by comparing the predicted differences over time, 24 months vs 0 months, using model-based expected values. A graphical representation of the model was prepared by plotting observed raw values using a locally weighted linear regression smoother (lowess) compared to the model-based predicted values. Percent weight change was also estimated from model-based predicted values for individual patients. Sensitivity of the model to loss to follow up was assessed by refitting the model with adjustment for baseline predictors of “dropout”, defined as having no weight measurement available from any source within the two months prior to the expected end of follow-up. Models for secondary outcomes (change in blood pressure and waist circumference) used the same approach as for weight but used up to 3 available measures (baseline, 1- and 2-year follow up), with additional models to adjust for baseline variables associated with missing data at these visits.
Treatment-visit attendance was used as a measure of dose, i.e., observed minus expected. We implemented an instrumental variable method for longitudinal data to examine the sensitivity of the primary result to lack of attendance (17, 18). These models estimated the effect of attendance at Basic Plus visits on mean weight change assuming attendance among those assigned to coaching visits (at ≥30%, ≥40%, or ≥50%) versus having no access to coaching visits (i.e., Basic).
We explored the association of number of coaching sessions attended with overall weight change considering only patients randomized to Basic Plus. We fit a linear mixed-effects model, as previously described with the interaction of number of coaching sessions and time as the factor of interest. The model adjusted for all baseline characteristics appearing in Table 2 and clinical site as potential confounders. In addition, to investigate baseline factors that might predict the number of coaching visits attended, we fit a generalized linear model with a negative binomial distribution and log link to account for overdispersion in the number of coaching sessions attended.
Table 2.
Baseline characteristics of randomized participants by treatment arm (n=261)
| Variable | Basic (n=137) | Basic Plus (n=124) |
|---|---|---|
| Race/ethnicity (%) | ||
| African American (non-Hispanic black) | 63.5 | 66.1 |
| Hispanic/Latino | 15.3 | 16.9 |
| Non-Hispanic white | 21.2 | 15.3 |
| Asian | 0 | 1.6 |
| Age Category (years) (%) | ||
| <35 | 16.8 | 16.1 |
| 35 to <45 | 23.4 | 19.4 |
| 45 – < 55 | 32.1 | 33.1 |
| 55+ | 27.7 | 31.5 |
| Education > 12 years education (%) | 66.4 | 72.6 |
| Female (%) | 82.5 | 86.3 |
| Employed fulltime (%) | 67.9 | 70.2 |
| Married/living with partner (%) | 48.2 | 40.3 |
| Sole caregiver for child/other relatives (%) | 13.9 | 21.8 |
| 3 or more persons living at home (%) | 35.8 | 29.8 |
| Mode of transportation (%) | ||
| Car | 65.7 | 72.6 |
| Public transportation | 27.0 | 23.4 |
| Other | 7.3 | 4.0 |
| Perceived stress (Cohen) (mean ± SD) | 14.83 ± 3.33 | 14.59 ± 3.17 |
| Recent major change in financial status (%) | 30.7 | 29.8 |
| Recent change in work status (%) | 32.1 | 51.6 |
| Recent change in home or family status (%) | 57.7 | 54.0 |
| Recent change in personal or social status (%) | 42.3 | 46.0 |
| Other recent change (%) | 7.3 | 10.5 |
| Current smoker (%) | 19.7 | 15.3 |
| Current drinker (%) | 33.6 | 32.3 |
| Excellent or very good self-rated health (%) | 13.1 | 16.1 |
| Any prior participation in a weight loss program (%) | 38.7 | 49.2 |
| Food Habits Score (mean ± SD) | 2.86 ± 0.35 | 2.87 ± 0.34 |
| High blood pressure(%) a | 12.4 | 14.5 |
| Blood pressure medication (%) | 41.6 | 48.4 |
| Body mass index (%) | ||
| < 35 kg/cm2 | 40.2 | 42.7 |
| 35–40 kg/cm2 | 28.5 | 25.8 |
| >= 40 kg/cm2 | 31.4 | 31.5 |
| Number of comorbid conditions (%) b | ||
| 0 | 37.2 | 29.8 |
| 1 | 33.6 | 41.9 |
| 2+ | 29.2 | 28.2 |
| Physician restriction on exercise (%) | 7.3 | 12.1 |
| Physical activity (%) | ||
| High | 21.9 | 25.0 |
| Moderate | 40.2 | 41.1 |
| Low | 38.0 | 33.9 |
SBP ≥ 140 or DBP ≥ 90
based on reported history of cardiovascular disease, high blood pressure, chronic obstructive pulmonary disease or asthma, diabetes, or musculoskeletal problems.
All analyses were performed using SAS (v 9.4) and Stata (v14.2). (See supplemental file for additional detail.)
RESULTS
Study Participants
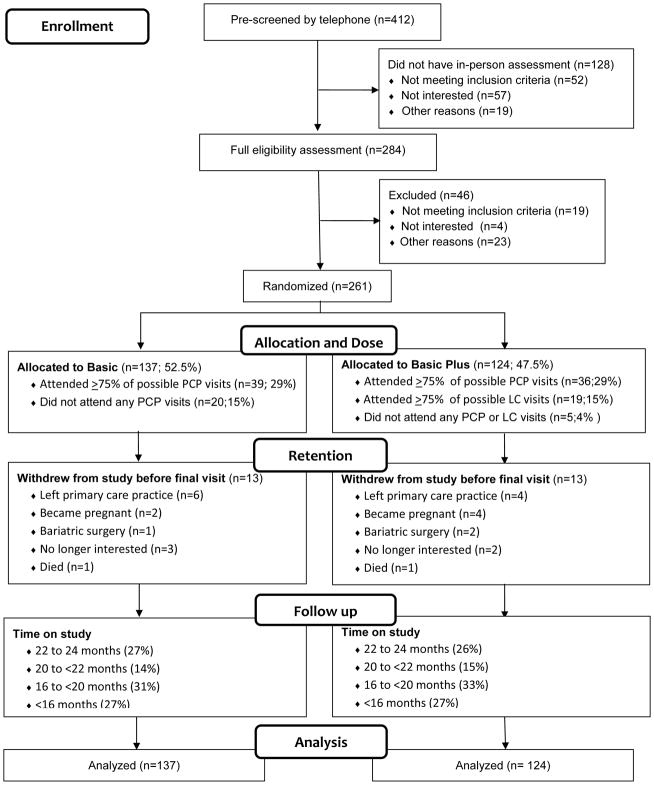
Participants were primarily female and mostly black or Hispanic. More than two-thirds had a high school education, and nearly one-third had two or more obesity-related conditions (Table 2). The most common comorbidities were high blood pressure (44%) and type 2 diabetes (18%) (3). Allocation was 52.5% and 47.5% to Basic and Basic Plus, respectively (Figure 1). A small number in each arm did not initiate treatment (20 in Basic and 5 in Basic Plus), and 13 in each arm withdrew from the study before the date of their final measurement. The proportions completing at least 75% of their possible clinician visits were similar across treatment arms (29%). The distribution of time on study reflects the fixed date of study closeout relative to randomization date. Not shown in Figure 1, final measurement visits were completed by 85 (62%) of Basic and 74 (60%) of Basic Plus participants. Follow-up waist and blood pressure measurements and questionnaire data were missing for those not completing the final measurement visit.
Figure 1.
Participant flow from pre-screening through study closeout
Attendance at Treatment Visits
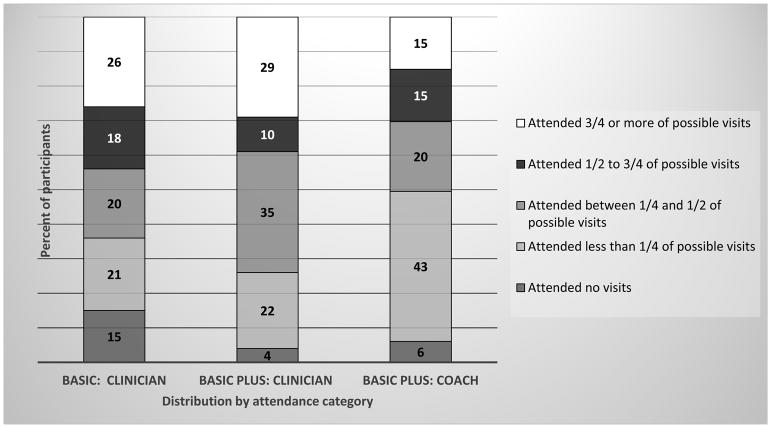
Figure 2 shows the distribution of attendance at treatment visits. Low attendance at clinician visits was more common among Basic than Basic Plus participants. A significant proportion of Basic Plus participants (43%) attended at least 1 but fewer than 25% of their possible visits. Among Basic Plus participants, change in financial status was associated with a higher level of attendance (mean [95% CI] difference in observed minus expected of 1.1 visits [0.1, 2.0]). Additionally, clinical site 3 had significantly less attendance compared to clinical site 1 (−2.7 visits [−4.2, −1.1]).
Figure 2.
Attendance at clinician counseling visits in Basic (low intensity) and Basic Plus (moderate intensity) and attendance at lifestyle coach counseling visits in Basic Plus
Primary Outcome
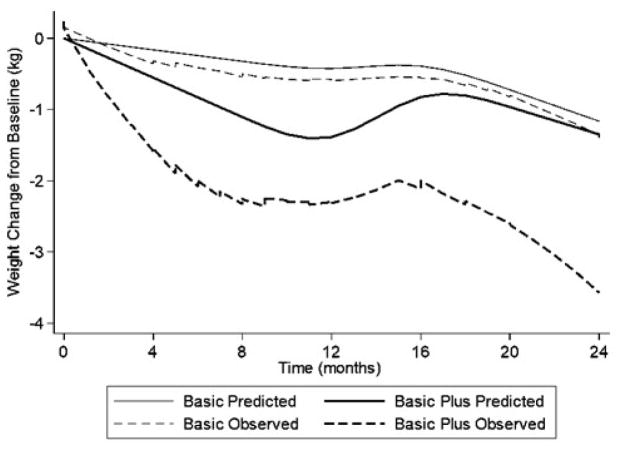
Table 3 shows model-based, intention-to-treat estimates of 24-month weight change from baseline by treatment group. Within- and between-group differences in mean weight change were not statistically significant. Mean [95% CI] weight change in Basic Plus was −1.34 kg [−2.92, 0.24], p=0.097, and the between-group difference was −0.18 kg [−2.38, 2.03], p=0.874. Figure 3 shows results of the exploratory analyses of weight loss trajectories over time. The observed slope of weight loss in Basic Plus was larger than in Basic but with a rebound between 12 and 16 months. In the fully adjusted model, this rebound was about 0.5 kg. The predicted (model-based) estimates of ≥ 5% weight loss at 2 years were 15.33% in Basic and 17.74% in Basic Plus (Chi-square [df=1] =0.28, p=0.60). Eighty-six (33%) participants were considered “dropouts” (49 in Basic and 37 in Basic Plus). A baseline recommendation by the participant’s clinician to restrict exercise and a reported change in financial status were associated with less “dropout. Adding these variables to the final linear mixed-effects model for weight change had a negligible effect on the estimated between-group difference (−0.21 kg [−2.41, 1.99] p=0.854).
Table 3.
Changes in clinical and behavioral measures from baseline to end of follow up
| Variable | Basic | Basic Plus | Treatment Group Difference in Change | |||
|---|---|---|---|---|---|---|
| Weight (kg) | Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean | 95% CI |
| Baselinea | 101.63 (20.94) | 100.69 (18.70) | ||||
| Change from baselineb | −1.16 | −2.70, 0.37 | −1.34 | −2.92, 0.24 | −0.18 | −2.38, 2.03 |
| P for change | 0.138 | 0.097 | 0.874 | |||
| Waist (cm) | ||||||
| Baselinea | 110.63 (15.56) | 112.15 (14.60) | ||||
| Change from baselinec | −0.68 | −2.43, 1.06 | −2.48 | −4.37, −0.60 | −1.79d | −4.36, 0.78 |
| P for change | 0.440 | 0.011 | 0.169 | |||
| Systolic BP | ||||||
| Baselinea | 119.68 (15.36) | 118.63 (15.77) | ||||
| Change from baselinec | 4.46 | 1.29, 7.62 | 8.14 | 4.78, 11.50 | 3.68e | −0.93, 8.3 |
| P for change | 0.006 | <.001 | 0.117 | |||
| Diastolic BP | ||||||
| Baselinea | 73.98 (10.40) | 74.54 (10.24) | ||||
| Change from baselinec | 4.37 | 2.14, 6.60 | 5.94 | 3.57, 8.31 | 1.57e | −1.69, 4.82 |
| P for change | <.001 | <.001 | 0.342 | |||
Baseline summary statistics for n=261, except for waist circumference where n=195
Model-based estimates of treatment effect over time using all measures for all randomized participants and are adjusted for site, gender, and age group.
Model-based estimates of treatment effect over time using available measures from 1-year and 2-year or final protocol follow- up visits
Participants with baseline BMI 35–40 (vs. BMI <35) were more likely to have a missing final waist measurement, and those with any prior participation in a weight loss program were less likely to have a missing final waist measurement. Adjusting for these variables resulted in a mean (95% CI) treatment group difference of 2.34 (4.86,0.17), P=0.169.
Participants reporting no change in financial status at baseline were more likely to have a missing final BP measurement. Adjusting for this variable resulted in a mean (95% CI) treatment group difference of 3.71 (−0.91, 8.3), P=0.115, for systolic BP and 1.58 (−1.68, 4.84), P=0.342, for diastolic BP.
Figure 3.
Observed (unadjusted) and predicted (adjusted) weight loss in Basic and Basic Plus over the 24-month study duration (locally weighted linear regression scatterplot smoothing). Predicted values are from linear mixed-effects regression models and reflect adjustment for randomization stratification variables (primary care practice site, age group, and gender) as covariates and random intercepts for each and random slopes for time. See text under Statistical Analyses for further detail.
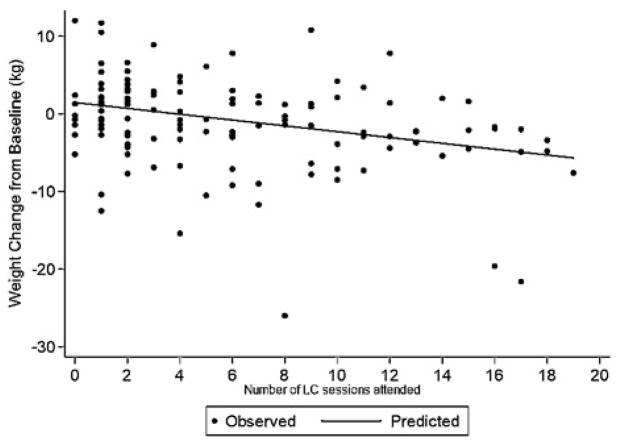
Weight change in Basic Plus was associated with the number of coaching visits attended (Figure 4). On average, for every additional coaching session attended, a 0.37 kg greater 24-month weight loss from baseline was observed (−0.375, 95% CI [−0.659, −0.091], p=0.01]. The association was also observed when attendance was modeled as the percent, rather than number of coaching visits attended: a 0.07 kg greater 24-month weight loss for a 1% increase in percent of possible visits attended (−0.069, [−0.119, −0.020], p=0.006). After adjustment for treatment attendance, the estimated additional weight loss among Basic Plus participants with higher attendance became larger (compared to results of an intention-to-treat analysis), although not significantly. For example, the attendance-adjusted analysis suggested a mean weight loss of 2.74 kg among Basic Plus participants who attended at least 50% of possible coaching visits.
Figure 4.
Association of weight change from baseline with the number of lifestyle coach visits attended (Basic Plus only; n=124)
Secondary Outcomes
There was no net treatment group difference in waist circumference or systolic or diastolic blood pressure (Table 3). Waist measurements, which had been initially removed from the protocol for feasibility reasons, were restored but after 66 participants had enrolled. There was a statistically significant increase in both systolic and diastolic blood pressure within treatment groups but no significant between group difference in blood pressure change.
Discussion
This study assessed the effects of a moderate-intensity weight loss program involving clinician counseling plus lifestyle coaching vs. a clinician counseling control in primary care practices. Model-based estimates indicated 2-year weight loss of about 1 kg in both arms (1.16 kg in Basic and 1.34 kg in Basic Plus) with no significant within- or between-group differences. Estimated weight losses of at least 5% of baseline weight) were observed for less than 1 in 5 participants in Basic (15%) and Basic plus (18%). Thus, not only was clinician-counseling alone (i.e., Basic) ineffective in facilitating meaningful weight loss, as expected, but the additional coaching, as implemented here, was also ineffective. The substantial rebound of about 0.5 kg in Basic Plus coincident with tapering of coaching frequency after 1 year offset an apparently greater initial weight loss in that arm.
Findings of modest weight change associated with moderate-intensity lifestyle behavioral change interventions are not uncommon in primary care studies (2, 19). This may apply particularly when counseling is delivered by clinicians or ancillary practice staff without some type of further enhancement. This interpretation is supported by findings of the POWER-UP study (20), in which several Think Health! investigators were involved (TAW, SKK, DBS, AGT). Compared to Think Health!, POWER-UP included more practices, enrolled only participants with at least 2 features of the metabolic syndrome, and enrolled proportionately fewer black (39%) and Hispanic (5%) participants. Two POWER-UP treatments were similar to Basic and Basic Plus. A third, “enhanced” treatment, arm provided both lifestyle coaching and meal replacements or weight loss drugs. Two-year weight loss in POWER-UP was 1.7 kg without and 2.9 kg with coaching, with no significant between-group difference (p=0.22), whereas the enhanced condition yielded a 4.6 kg mean weight loss, significantly larger than with clinician counseling alone (p =0.003). Other POWER collaborative group studies found significant effects of their interventions at 2 years (21, 22). Neither study relied on regular counseling by clinicians or practice staff with other duties, and both used remote contact (web-based, telephone, or interactive voice response) in all or part of the intervention. Also potentially relevant, controls in these studies were offered self-directed programs rather than minimal counseling as in Think Health! and POWER-UP.
Null findings or only modest weight loss are also common in weight loss data for black Americans (23). Approximately two-thirds of Think Health! participants were black, although the study was neither designed nor powered to support analyses by racial/ethnic subgroups. Black participants in Think Health! were included in an analysis of weight loss patterns in data for 604 black Americans (primarily women) pooled across three studies (24). One of the other two studies was conducted in association with primary care (25), and the other was in a university research setting (26). Moderate, clinically non-significant weight loss was the predominant pattern across all three studies—more of a weight maintenance than weight loss effect.
No behavioral measures were obtained during the initial post year-1 period when contact frequency was tapered. Including dietary, physical activity, and other measurements during tapering could facilitate understanding what takes place behaviorally during this period. A weight rebound (of about 4 kg) similar to that observed here was also observed in the above-referenced pooled analysis among those with modest (about 3 kg) or major (about 20 kg) weight loss in the first year, but not among those who lost gradually to about 2 kg over the 2-year period (24). Although initial weight loss is known to be the best predictor of final weight loss (27), a pattern of significant weight loss achieved more gradually but better maintained over the long term has been reported among black participants in other studies (28, 29).
The combination of relatively low attendance at coaching visits in Basic Plus, the significant, positive association of coaching visit attendance with weight loss, and analyses suggesting that the effect among those who attended at least half of their allowed sessions (compliers) might have lost close to 3 kg in comparison to compliers assigned to Basic rather than the intention-to-treat finding of about 1 kg supported the role of missed visits in the overall lack of significant effect of coaching. This finding, which was also suggested in the 1-year data (7) and is consistent with other published reports (20), is informative about why coaching was not effective but we are, unfortunately, unable to explain why attendance was so poor. Our belief that having a pre-existing relationship with the provider and practice would lead to high attendance was not supported. Identifying predictors of attendance was not a major focus of the study design, and only 1 of the baseline variables (financial status change—interpreted as a possible stressor) was associated with better attendance in Basic Plus. We did not assess practice-related variables that might have influenced attendance, although inquiries about this suggested that a role for scheduling problems (7, 8).
Findings for waist circumference and blood pressure (secondary outcomes), also indicated no benefit of coaching. Blood pressure increased in both groups, which would have been opposite to expectation had an effect on weight loss actually occurred (30). However, given the composition of the study sample, i.e., a high proportion of black Americans, who are at above-average risk of hypertension (5), the increase in blood pressure over time is not necessarily unexpected. Bennett et al. observed blood pressure increases in the Usual Care group of their primary care weight loss trial, which involved 71% black and 13% Hispanic participants recruited and treated in community health centers (21).
The diverse study population and community practice setting are strengths of this study given the limited data available on effective treatments with black and Hispanic adults in real-world settings. The inclusive health status eligibility allows for interpreting the data broadly with respect to demographically similar adults seen in primary care settings. The modeling approach, which used all available weight measurements, and the sensitivity analyses adjusting for missing data toward the end of follow up, facilitated a robust finding on the primary outcome despite data limitations. Censoring participants before a full 2 years of follow-up, combined with poor attendance at coaching visits, limited the ability to fully assess coaching effects when delivered at the intended dose. Statistical power for analyses of secondary outcomes and behavioral measures was limited by non-attendance at protocol follow-up visits and, for waist, at baseline.
In conclusion, the main finding of Think Health! is that adding moderate-intensity lifestyle coaching, against a background of infrequent clinician counseling was ineffective as a weight loss treatment over 2 years in this study population and with a pattern of low attendance. Focused inquiry into whether and how treatment attendance at primary care counseling visits can be improved is warranted. In addition, based on the results of the POWER studies, cited above, the potential for remote strategies using telephone, websites or mobile devices to generate equivalent or better results compared to in-person visits deserves further study.
Supplementary Material
Study Importance.
What is already known about this subject?
Models for primary care support of weight management are needed in general and particularly for black and Hispanic adults, who have above-average risks of obesity and severe obesity.
Augmenting clinician counseling with lifestyle coaching by practice staff is a potentially feasible approach.
What this study adds
A 2-year randomized controlled trial with mostly black and Hispanic female primary care patients compared weight loss counseling by primary care clinicians plus lifestyle coaches with clinician counseling only.
The availability of coaching did not result in significantly greater weight loss
Better attendance at coaching visits predicted larger weight losses among those assigned to receive coaching, suggesting a need for more focus on achieving adequate treatment dose.
Acknowledgments
Funding Agencies: The clinical trial was funded by the Pennsylvania Department of Health Commonwealth Universal Research Enhancement Program. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. Support was also provided by the NIH National Institute for Minority Health and Health Disparities (P60 MD000209-05). Partial salary support for A.G.T. and K.C.A. was provided by K12-HD043459-04 from the NIH National Institute of Child Health and Human Development. The two-year analyses reported here were supported by the NIH National Institute of Diabetes, Digestive, and Kidney Disease (R21DK089422). Dr. Morales’ effort was partly supported by the NIH National Institute of Mental Health (K01MH073903). The content does not necessarily represent the official views of these funding agencies.
We express appreciation to collaborating primary care practices, clinicians and staff and individual participants who made this study possible. We also acknowledge and thank other collaborators and staff who were engaged in various aspects of study: Dr. Susan Tan-Torres, Dr. Tina Harralson, Ms. Lisa Wesby, and Ms. Ronnie Kessler.
Footnotes
Clinical Trial Registration: Clinical Trials.gov Identifier NCT00959608
Disclosures: All grant funds for this study were to the authors through the University of Pennsylvania. Dr. Sarwer is a consultant to Allergan, BARONova, and Enteromedics. Dr. Wadden reports grants from Novo Nordisk and Eisai Pharmaceuticals and is a consultant to Weight Watchers and Novo Nordisk. The remaining authors declared no conflicts of interest.
References
- 1.Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med. 2017;376:1492. doi: 10.1056/NEJMc1701944. [DOI] [PubMed] [Google Scholar]
- 2.Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA. 2014;312:1779–1791. doi: 10.1001/jama.2014.14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumanyika S, Fassbender J, Phipps E, Tan-Torres S, Localio R, Morales KH, et al. Design, recruitment and start up of a primary care weight loss trial targeting African American and Hispanic adults. Contemp Clin Trials. 2011;32:215–224. doi: 10.1016/j.cct.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CLCM, Fryar CD, Flegal KM. NCHS data brief. 219. Hyattsville, MD: NCHS; 2015. Prevalence of obesity among adults and youth: United States, 2011–2014. [PubMed] [Google Scholar]
- 5.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: 2016. [PubMed] [Google Scholar]
- 6.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumanyika SK, Fassbender JE, Sarwer DB, Phipps E, Allison KC, Localio R, et al. One-year results of the Think Health! study of weight management in primary care practices. Obesity (Silver Spring) 2012;20:1249–1257. doi: 10.1038/oby.2011.329. [DOI] [PubMed] [Google Scholar]
- 8.Phipps E, Chacko LR, Fassbender JE, Allison KC, Sarwer DB, Wallace SL, Tan-Torres S, Bowman MA, Wadden TA, Kumanyika SK. Provider Views of the Feasibility and Utility of Lifestyle Obesity Treatment in Primary Care: Insights from the Think Health! Study. European Journal of Person Centered Health Care. 2015:3. [Google Scholar]
- 9.Tsai AG, Wadden TA, Rogers MA, Day SC, Moore RH, Islam BJ. A primary care intervention for weight loss: results of a randomized controlled pilot study. Obesity (Silver Spring) 2010;18:1614–1618. doi: 10.1038/oby.2009.457. [DOI] [PubMed] [Google Scholar]
- 10.U S. Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2009;150:551–555. doi: 10.7326/0003-4819-150-8-200904210-00009. [DOI] [PubMed] [Google Scholar]
- 11.Anderson CA, Kumanyika SK, Shults J, Kallan MJ, Gans KM, Risica PM. Assessing change in dietary-fat behaviors in a weight-loss program for African Americans: a potential short method. J Am Diet Assoc. 2007;107:838–842. doi: 10.1016/j.jada.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 14.Ware JEKM, Dewey JE, Gandek B. A Manual for Users of the SF-8 Health Survey. Lincoln, RI: 2001. [Google Scholar]
- 15.Holmes TH, Rahe RH. The Social Readjustment Rating Scale. Journal of psychosomatic research. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 16.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2. Wiley; Hoboken, N.J: 2011. [Google Scholar]
- 17.Nagelkerke N, Fidler V, Bersen R, Borgdorff M. Estimating the treatment effect in randomized clinical trials in the presence of non-compliance. Statistics in Medicine. 2000;19:1849–1864. doi: 10.1002/1097-0258(20000730)19:14<1849::aid-sim506>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Small DS, Ten Have JR, Joffe MM, Cheng J. Random effects logisitic models for analyzing efficacy of a longitudinal randomized treatment with non-adherence. Statistics in Medicine. 2006;25:1981–2007. doi: 10.1002/sim.2313. [DOI] [PubMed] [Google Scholar]
- 19.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med. 2009;24:1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett GG, Warner ET, Glasgow RE, Askew S, Goldman J, Ritzwoller DP, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med. 2012;172:565–574. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel-Hodge CD, Johnson CM, Braxton DF, Lackey M. Effectiveness of Diabetes Prevention Program translations among African Americans. Obes Rev. 2014;15(Suppl 4):107–124. doi: 10.1111/obr.12211. [DOI] [PubMed] [Google Scholar]
- 24.Morales KH, Kumanyika SK, Fassbender JE, Good J, Localio AR, Wadden TA. Patterns of weight change in black Americans: pooled analysis from three behavioral weight loss trials. Obesity (Silver Spring) 2014;22:2632–2640. doi: 10.1002/oby.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumanyika SK, Shults J, Fassbender J, Whitt MC, Brake V, Kallan MJ, et al. Outpatient weight management in African-Americans: the Healthy Eating and Lifestyle Program (HELP) study. Prev Med. 2005;41:488–502. doi: 10.1016/j.ypmed.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 26.Kumanyika SK, Wadden TA, Shults J, Fassbender JE, Brown SD, Bowman MA, et al. Trial of family and friend support for weight loss in African American adults. Arch Intern Med. 2009;169:1795–1804. doi: 10.1001/archinternmed.2009.337. [DOI] [PubMed] [Google Scholar]
- 27.Unick JL, Neiberg RH, Hogan PE, Cheskin LJ, Dutton GR, Jeffery R, et al. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity (Silver Spring) 2015;23:1353–1356. doi: 10.1002/oby.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumanyika SK, Espeland MA, Bahnson JL, Bottom JB, Charleston JB, Folmar S, et al. Ethnic comparison of weight loss in the Trial of Nonpharmacologic Interventions in the Elderly. Obes Res. 2002;10:96–106. doi: 10.1038/oby.2002.16. [DOI] [PubMed] [Google Scholar]
- 29.Espeland MA, Bray GA, Neiberg R, Rejeski WJ, Knowler WC, Lang W, et al. Describing patterns of weight changes using principal components analysis: results from the Action for Health in Diabetes (Look AHEAD) research group. Ann Epidemiol. 2009;19:701–710. doi: 10.1016/j.annepidem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leblanc ES, O’Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:434–447. doi: 10.7326/0003-4819-155-7-201110040-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.