Abstract
OBJECTIVE:
To determine the effects of a transcranial direct current stimulation (tDCS) intervention with the anode placed over the left dorsolateral prefrontal cortex (dlPFC) and cathode over the right supraorbital region, on cognition, mobility, and “dual task” standing and walking in older adults with mild-to-moderate motor and cognitive impairments.
METHODS:
A double-blinded, block-randomized, sham-controlled trial was conducted in 18 non-demented, ambulatory adults aged ≥65 years with slow walking speed (≤1.0m/s) and “executive” dysfunction (Trail Making Test B score ≤25th percentile of age- and education-matched norms). Interventions included 10, 20-minute sessions of tDCS or sham stimulation. Cognition, mobility, and dual task standing and walking were assessed at baseline, post-intervention and two weeks thereafter. Dual tasking was also assessed immediately before and after the first tDCS session.
RESULTS:
Intervention compliance was high (mean±SD=9.5±1.1 sessions) and no unexpected or serious side effects were reported. tDCS, compared to sham, induced improvements in the Montreal Cognitive Assessment (MoCA) total score (p=0.03) and specifically within the executive function sub-score of this test (p=0.002), and in several metrics of dual task standing and walking (p<0.05). Each of these effects persisted for two weeks. tDCS had no effect on the Timed Up-and-Go test of mobility or the Geriatric Depression Scale. Those participants who exhibited larger improvements in dual task standing posture following the first tDCS session exhibited larger cognitive-motor improvements following two weeks of tDCS (p<0.04).
INTERPRETATION:
tDCS intervention designed to stimulate the left dlPFC may improve executive function and dual tasking in older adults with functional limitations.
Keywords: tDCS, executive function, dual task standing, older adults
INTRODUCTION
Aging is often associated with concomitant declines in cognitive and motor function1,2. Standing and walking are cognitive-motor tasks essential to mobility and foundational to most daily activities. Moreover, these tasks are often completed while performing other activities, for example talking, reading signs, or making decisions. Such “dual tasking” interferes with performance on one or both tasks3,4. This interference, or “cost,” increases with advancing age5,6, is exaggerated in those with cognitive “executive” dysfunction7,8, and is predictive of future cognitive decline9 and falls10,11. Neuroimaging indicates that many cognitive-motor tasks, including standing and walking under dual task conditions,12,13 activate distributed cortical networks that include the left dorsolateral prefrontal cortex (dlPFC)—a brain region sub-serving executive function14. We thus contend that interventions designed to facilitate functional activation of the dlPFC and its connected neural networks may improve dual task performance, and thereby improve cognitive function and mobility in older adults.
Transcranial direct current stimulation (tDCS) offers a noninvasive and safe means of modulating cortical excitability15,16. In healthy adults, one 20-minute session of tDCS with the anode over the left dlPFC and the cathode of the right supraorbital region facilitates the excitability of this region17, enhances performance on tests of executive functions,18,19 and may improve the ability to perform two cognitive tasks concurrently20. Our team has demonstrated that in both younger and older adults, one session of this type of tDCS improves performance in tests of mobility21 and reduces the dual task costs to gait and postural control20, when tested immediately following stimulation. Repeated exposure to tDCS also induces longer-lasting increases in cortico-spinal excitability15 and cerebral perfusion22, and has been reported to enhance processing speed and working memory in older adults with mild cognitive impairment23. We therefore hypothesized that a multi-session tDCS intervention with the anode over the left dlPFC and the cathode of the right supraorbital region, as compared to sham intervention, would induce lasting improvements in dual task performance, cognition and mobility, in older adults with mild-to-moderate impairments in cognitive and motor function.
METHODS
Trial Design
A pilot, sham-controlled, double-blinded, block-randomized trial was conducted (NCT02436915). Participants completed a baseline assessment and were then randomized to receive a two-week tDCS or sham intervention. Follow-up assessments were completed post-intervention (i.e., two-three days following) and again two weeks later. On the first day of the intervention, participants completed a dual task standing and walking assessment before and after tDCS administration to further establish the immediate effects of tDCS on related outcomes, and to assess the potential predictive value of the acute effect of a single session of tDCS on the longer-term, cumulative effects of multiple tDCS sessions.
Participants
Men and women were recruited between 2014–2016 via local advertisements and letters to individuals identified via medical record review at Hebrew SeniorLife and the Beth Israel Deaconess Medical Center, both in Boston, MA, USA. Potentially eligible individuals, as determined via phone screen, completed an in-person screen. Written informed consent was obtained at the beginning of this visit. The study and all recruitment material was approved by the Hebrew SeniorLife Institutional Review Board.
Individuals were enrolled if they were aged ≥65 years and exhibited both slow gait defined by preferred 4m over-ground walking speed less than 1.0m/s24 and executive dysfunction defined by a Trail Making Test (TMT) B time below the 25th percentile of age- and education-based norms25, 26. TMT Part A required participants to connect numbered circles in sequential order as quickly as possible. Part B required participants to connect circles containing numbers or letters in alternating sequence. Participants were given up to 300 seconds to complete each part. If the participant was unable to complete the test in the allotted time, 300 seconds was used for analysis.
Exclusion criteria included an inability to ambulate without assistance from another person (canes or walkers allowed); a clinical history of stroke, Parkinson’s disease, normal pressure hydrocephalus, or other neurological condition; self-report of severe lower-extremity arthritis or physician-diagnosis of peripheral neuropathy; moderate-to-severe dementia defined by a Mini Mental State Exam score of 18 or lower27; contraindications to tDCS including use of neuro-active drugs, self-report of seizure within the past two years, or open wounds on the scalp16; severe depressive symptoms defined by score greater than 11 on the Geriatric Depression Scale short form28; or self-report of physician-diagnosed schizophrenia, bipolar disorder or other psychiatric illness.
Nineteen of 201 screened individuals were interested and eligible (Fig 1). Eighteen of 19 randomized participants completed the intervention. One participant withdrew from the sham intervention prior to follow-up. Reason for withdrawal was illness deemed unrelated to participation. No participants withdrew from the tDCS intervention.
Figure 1: CONSORT diagram.
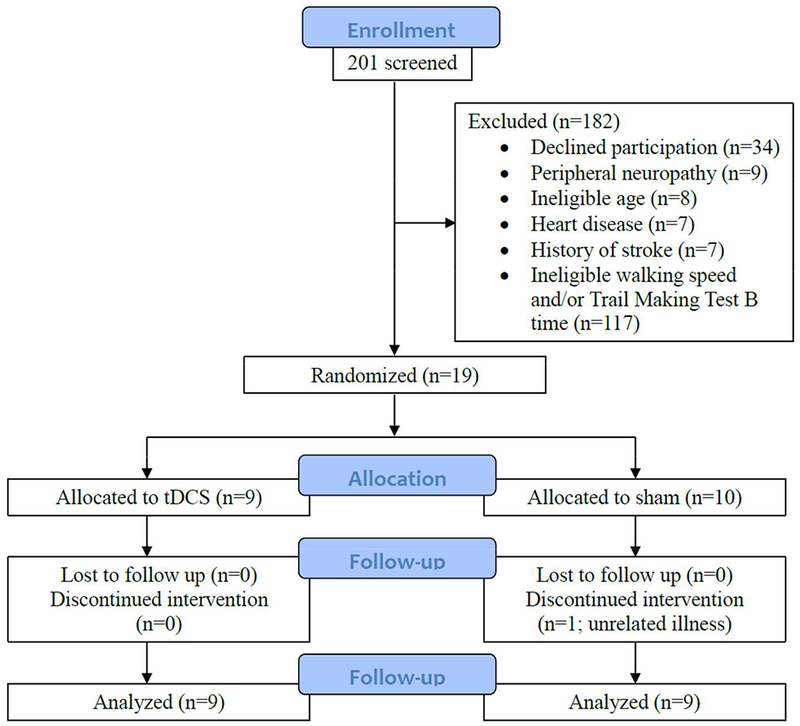
Interventions
Participants had an equal chance of being assigned to the tDCS or sham intervention. tDCS was delivered with the Starstim® system (Neuroelectrics Inc, Barcelona, Spain) connected to saline-soaked 35 cm2 synthetic sponge electrodes placed on the scalp. The anode (i.e., positive electrode) was placed over the F3 region of the 10/20 EEG electrode placement guide and the cathode (i.e., negative electrode) was placed over the right supra-orbital margin (Fp2)29 (Fig 2). This “montage” improves dual task standing and walking when tested immediately after stimulation in younger20 and older30 adults. tDCS consisted of 20 minutes of continuous stimulation at a maximum intensity of 2.0 mA. At the beginning of stimulation, current was automatically increased from 0.1mA, in 0.1mA increments over 60 seconds to minimize discomfort at stimulation onset16. During the first session, participants were instructed to notify the study personnel if and when they felt any uncomfortable sensations. The ramp-up procedure was stopped and for the remainder of this session and all sessions thereafter, tDCS was delivered at an intensity of 0.1mA below the highest level reached. At the end of each session, current was automatically ramped down to 0.0 mA over 60 seconds. For sham stimulation, the same electrode montage, ramp-up and session duration were used; however, current was automatically ramped down 60 seconds after ramp-up. This procedure was chosen because cutaneous sensations arising from tDCS diminish considerably within the first minute of stimulation31.
Figure 2: tDCS electrode placement and electrical current flow model.
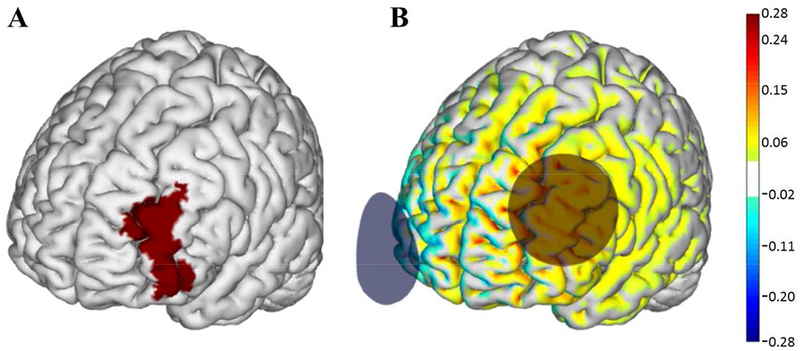
A) The left dorsolateral prefrontal cortex (dlPFC) (Brodmann Area 46) highlighted in red. B) Active tDCS was delivered at a maximum intensity of 2.0 mA with the anode (red circle) placed over the F3 region and the cathode (blue circle) over the Fp2 region according to the 10–20 EEG placement system. Warmer and cooler colors depict the normal component of the electrical field produced by this stimulation, modeled on a standard brain as described in Miranda et al (2013)50. Images courtesy of Neuroelectrics.
Participants and research staff administering tDCS were blinded to participant group assignment. Participants were assigned a code linked to their assigned intervention, as developed by the study statistician. Personnel uninvolved in tDCS administration preconfigured the tDCS and sham stimulation parameters for each code within the Starstim™ software. tDCS administrators were blinded to this code. Two separate codes were used for each condition to help ensure staff blinding. A “blinded” mode was then used such that tDCS parameters for each session were delivered by selecting the participant’s assigned code. At the end of the first tDCS session and every other session thereafter, participants completed a side effects questionnaire32. A blinding efficacy questionnaire was completed after the final tDCS session. Participants were asked to state whether they believed they received the tDCS or sham intervention and their confidence in this belief on a scale of 0–10, with 10 reflecting greatest confidence.
Baseline and Follow-Up Assessments
Cognition, mobility, and dual task performance were assessed at baseline, post-intervention, and again 2-weeks later. During the first visit, individuals completed screening tests to determine eligibility, including the MMSE33, 15-item short-form Geriatric Depression Scale28, 4m walk test34, and TMT tests A and B26. Eligible participants then completed the Montreal Cognitive Assessment (MoCA), the Timed Up-and-Go test of mobility, and a dual task paradigm. Follow-up testing included all of the above tests except the MMSE. If a cane or walker was used for any test, the participant used the same device for all similar trials at baseline and at each follow-up assessment.
The MoCA is a 30-point test of cognitive function that assesses visuo-spatial executive function, naming, attention, language, abstraction, delayed recall and orientation35. A different, validated version of the MoCA was used at each of the three assessments to minimize learning effects36. Version order was randomized across participants.
The TUG test was completed twice at each assessment and included rising from a chair, walking 3m, turning around, and returning to a seated position37.
The dual task assessment consisted of three trials in each of the following conditions: 1) single-task standing, 2) dual-task standing, 3) single-task walking, and 4) dual-task walking. Within dual task trials, participants were asked to stand or walk while audibly counting backwards by 3’s from a random 3-digit number between 200 and 999 provided prior to the trial. Trial order was randomized and no instructions were given regarding task prioritization. Serial subtraction was chosen because it induces meaningful dual task costs to gait and postural control38, activates a distributed cortical network including the left dlPFC39, is one of the most widely-used dual task paradigms4, and is reliable and minimally influenced by learning40.
Each 60-second standing trial was completed on a force plate (Model 9286AA, Kistler Instrument Corp., Amherst, NY) with eyes-open, arms at side and feet shoulder-width apart. Foot placement was traced on the first trial and this tracing was used in all subsequent trials. Before each trial, participants were reminded to stand as still as possible and focus their vision on a small “X” drawn on a wall at eye-level approximately three meters away. Walking trials were completed on a 60-foot oval indoor track with a 14-foot GaitRite mat (Franklin, NJ) along one side. Participants began each trial just prior to the gait mat and walked approximately 1.25 times around the track so as to pass over the mat two separate times. Participants were reminded to walk at their preferred speed and wore the same pair of their own shoes for all trials.
For each standing trial, transverse-plane postural sway (i.e., center-of-pressure) data were recorded by the force plate at 240Hz and filtered with a 10Hz low-pass Butterworth filter. For each walking trial, heel-strike and toe-off events were recorded (100Hz) by the GaitRite mat.
Study Outcomes
The four primary outcomes focused on global cognitive function measured by the MoCA total score (1), mobility measured by TUG time (2), and dual task performance defined by the cost (i.e., percent decrement in performance between single and dual task conditions) to both standing postural sway speed (3) and walking speed (4). Sway speed was defined as center-of-pressure path length divided by trial duration. Walking speed was determined by calculating the distance traveled from the first heel strike to the last toe-off on the GaitRite mat and dividing by elapsed time. For each trial, walking speed was calculated separately for each pass over the mat and then averaged. Each outcome was averaged across all trials of similar condition.
Secondary outcomes included 1) executive function defined as TMT Part B minus Part A, which adjusts the test for the motor speed and dexterity of the participant25, 2) the dual task cost to standing postural sway area (the area of an ellipse enclosing 95% of the center-of-pressure path), 3) the dual task cost to stride time (the average time between consecutive right heel strikes), 4) the dual task cost to stride time variability (stride time coefficient of variation), 5) postural control and gait outcomes in each condition separately, and 6) the GDS total score.
Statistical Analysis
Analyses were performed on data from the 18 participants who completed the intervention. Each of these participants completed both follow-up assessments. Analyses were performed using JMP software version 13 (SAS Institute, Cary, NC). Type-I error rate for this study was set at α=0.05 for all tests. Descriptive statistics were used to summarize intervention group characteristics and study outcomes. Potential between-group differences in demographics and baseline characteristics were assessed by unpaired t tests. Blinding efficacy was determined using Fisher’s exact test, which determined whether participants’ guesses of tDCS condition (after 10 sessions of tDCS) were correct to a greater degree than that expected because of chance.
The effect of the 10-session tDCS intervention on each study outcome was analyzed with a separate two-way repeated-measures ANOVA. Dependent variables were the percent change in each outcome from baseline to each follow-up visit. Model effects included intervention (tDCS, sham), follow-up visit (post-intervention, 2-week retention) and their interaction. Models were adjusted for age and sex. Tukey’s post-hoc testing was used to compare factor means of significant models. Effect sizes were calculated using Cohen’s d, with values greater than 0.8 taken to reflect large effects.
Observed effects of the tDCS intervention on study outcomes spurred two exploratory analyses. First, the effects of intervention on MoCA sub-scores were examined using separate ANOVA models similar to those described above. Second, linear regression was used to examine the relationships between A) MoCA scores and dual task performance at baseline across the entire cohort, and B) the percent change in each outcome following intervention specifically within the tDCS group. In the latter analysis, we used percent change data from baseline to the post-intervention and 2-week retention visits, and included follow-up visit as a model effect.
The immediate effects of the first tDCS session on dual task standing and walking performance were also analyzed using one-way ANOVAs. Dependent variables included the percent change from pre- to post-tDCS in the dual task cost to each standing postural control and gait outcome. Models were adjusted for age and sex. Secondary models examined the effects of tDCS on postural control and gait outcomes derived separately from single and dual task conditions. Finally, we used Pearson correlation to quantify the degree to which immediate effects of one tDCS session were associated with the longer-term effects of the multi-session tDCS intervention. These models were limited to data from those receiving tDCS and to those outcomes for which a single session of tDCS had a significant pre-post effect.
RESULTS
Baseline Participant Characteristics
The tDCS and sham intervention groups were similar in sex distribution, age, and BMI (tDCS: 5 females, mean±SD age=82±4 years, BMI=29±2 kg/m2; Sham: 5 females, age=79±4 years, BMI=31±2 kg/m2). Groups were also comparable in baseline performance in each study outcome (p>0.05; Table 1). The current cohort had mild-to-moderate functional impairments; as compared to published means from healthy cohorts of similar age, average TMT B – A times were approximately 100 seconds longer,41 TUG times were approximately 8 seconds longer,42 and walking speeds were approximately 0.4 m/s slower.43
Table 1:
Baseline function and the percent change in functional outcomes following a 2-week, 10-session tDCS intervention
| tDCS | Sham | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Baseline | Percent change from baseline: post-intervention | Percent change from baseline: 2-weeks | Baseline | Percent change from baseline: post-intervention | Percent change from baseline: 2-weeks | Intervention main effect: p value* | |
| Cognition | |||||||
| MoCA (total score) | 22 ± 4 | 8 ± 17% | 13 ± 16% | 22 ± 3 | 0 ± 8% | 0 ± 8% | 0.01 |
| TMT (Test B - Test A) | 153 ± 111 | −65 ± 25% | −38 ± 19% | 138 ± 54 | −17 ± 49% | −17 ± 58% | 0.54 |
| Mobility | |||||||
| TUG (sec) | 17 ± 6 | −3 ± 17% | −6 ± 17% | 16 ± 6 | 10 ± 14% | 3 ± 25% | 0.25 |
| 4-meter walk (m/s) | 0.7 ± 0.2 | 11 ± 23% | 13 ± 31% | 0.7 ± 0.1 | 8 ± 19% | 11 ± 14% | 0.79 |
| Dual task performance | |||||||
| Standing postural sway | |||||||
| Single task | |||||||
| - Speed (mm/s) | 27 ±11 | 0 ± 18% | −12 ± 23% | 25 ± 10 | 2± 28% | −5 ± 14% | 0.59 |
| - Area (mm2) | 719±242 | −3 ± 53% | 1 ± 58% | 737 ± 449 | 16 ± 28% | 5 ± 26% | 0.25 |
| Dual task | |||||||
| - Speed (mm/s) | 40 ± 28 | −18 ± 30% | −21 ±24% | 35 ± 17 | 25 ± 28% | 8 ± 28% | 0.0009 |
| - Area (mm2) | 901±448 | −36 ± 35% | −28 ± 38% | 926 ± 571 | 18 ± 27% | 28 ± 33% | 0.003 |
| Dual task cost | |||||||
| - Speed (%) | 46 ± 55 | −23 ± 24% | −11 ± 20% | 41 ± 44 | 16 ± 28% | 15 ± 31% | 0.004 |
| - Area (%) | 27 ± 79 | −33 ± 44% | −34 ± 24% | 34 ± 97 | 15 ±24% | 18 ± 23% | <0.0001 |
| Walking | |||||||
| Single task | |||||||
| - Speed (m/sec) | 0.7 ± 0.2 | 3 ± 11% | 10 ± 10% | 0.7 ± 0.2 | 3 ± 11% | 4 ± 5% | 0.47 |
| - Stride time (sec) | 1.3 ± 0.2 | −4 ± 8% | −7 ± 7% | 1.3 ± 0.2 | −4 ± 6% | −1 ± 7% | 0.33 |
| - Variability (COV) | 6.7 ± 2.0 | −16 ± 16% | −30 ± 8% | 7.1 ± 1.9 | −11 ± 31% | −10 ± 9% | 0.36 |
| Dual task (m/s) | |||||||
| - Speed (m/s) | 0.5 ± 0.3 | 18 ± 17% | 17 ± 15% | 0.5 ± 0.1 | 13 ± 19% | 10 ± 28% | 0.89 |
| - Stride time (sec) | 1.6 ± 0.7 | −8 ± 9% | −10 ± 9% | 1.5 ± 0.8 | −3 ± 10% | 0.1 ± 10% | 0.05 |
| - Variability (COV) | 9.8 ± 2.9 | −20 ± 15% | −33 ± 11% | 11 ± 3.3 | 4 ± 51% | 10 ± 32% | 0.02 |
| Dual task cost (%) | |||||||
| - Speed (m/s) | −28 ± 19 | 15 ± 17% | 6 ± 8% | −25 ± 16 | −14 ± 25% | 20 ±23% | 0.93 |
| - Stride time | 24 ± 11 | −24 ± 42% | −38 ± 70% | 18 ± 10 | 4 ± 14% | 7 ± 15% | 0.37 |
| - Variability (COV) | 46 ± 52 | −5 ± 67% | −20 ± 31% | 56 ± 35 | −11 ± 33% | 16 ± 36% | 0.08 |
Values indicate mean and standard deviation; MOCA = Montreal Cognitive Assessment; TMT = Trail making test; TUG = Timed up-and-go; COV = Coefficient of variation about average stride time;
p values are for the main effect of intervention on the percent change in each outcome from baseline to follow-up within 2-way repeated-measures ANOVAs adjusted for age and sex; Percent change mean values with a significant intervention main effect (p≤0.05) have been bolded.
Intervention Compliance, Side Effects and Blinding Efficacy
Intervention compliance was excellent (mean±SD=9.5±1.1 of 10 sessions; range=6-10 sessions) and similar between arms. Average intensity of tDCS did not differ between arm (tDCS: 1.9±0.3mA, range=1.7-2.0mA; Sham: 2.0±0.1mA, range=1.8-2.0 mA). All side-effects were mild and temporary (Table 2). One participant experienced an unrelated non-injurious fall during the intervention and no other adverse events were reported. The percentage of individuals who correctly guessed intervention assignment following intervention was 67% for those receiving tDCS and 33% for those receiving sham. This distribution did not differ between arm or from that expected by chance (Fisher’s exact test p=0.39). Confidence in these guesses was also similar between arms (tDCS=6.2±2.8 on a 10-point scale; sham=6.6±1.8; p=0.76).
Table 2:
The prevalence (i.e., percentage of all tDCS sessions) of self-reported tDCS side effects* by intervention arm
| tDCS | Sham | P value* | |
|---|---|---|---|
| Sensations under electrode | 65% | 70% | 0.83 |
| Skin redness | 32% | 16% | 0.07 |
| Headache | 0% | 5% | 0.49 |
| Neck pain | 0% | 2% | 1.00 |
| Sleepiness | 30% | 14% | 0.12 |
| Trouble concentrating | 5% | 7% | 1.00 |
| Acute mood change | 5% | 5% | 1.00 |
Side effects were recorded on the first tDCS session and every other tDCS session thereafter using a questionnaire adapted from Brunoni et al (2011)
Effects of the 10-session tDCS intervention
tDCS improved multiple aspects of cognitive and motor performance. ANOVA models adjusted for age and sex revealed a main effect of intervention on the percent change in MoCA total score (F=5.0, p=0.03, Cohen’s d=0.84) (Table 1 and Fig 3). Those receiving real tDCS, compared to sham, exhibited greater increases in MoCA performance. No main effect of followup visit was observed, indicating that the intervention effect on MoCA performance did not differ in magnitude between the post-intervention and 2-week follow-up visits. A similar intervention effect was present for the absolute change in MoCA total score (F=4.3, p=0.04, Cohen’s d=0.81). The increase in MoCA score following tDCS was 1.7±2.7 points post-intervention and 1.8±2.9 points at two weeks. The absolute change in MoCA score following sham was 0.0±2.0 points post-intervention and 0.1±1.9 at two weeks.
Figure 3: The effects of a 10-session tDCS intervention on each participant’s performance in the Montreal Cognitive Assessment (MoCA).
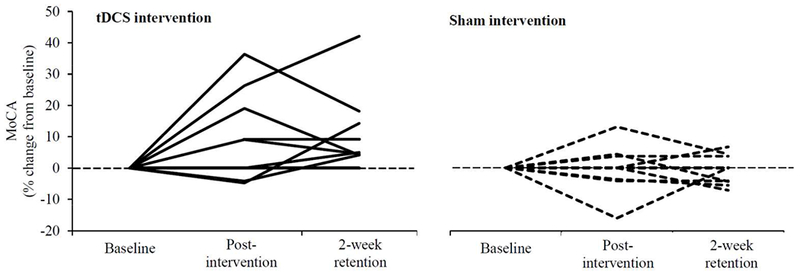
Participants completed a different version of the MoCA at each assessment. All but one participant who received the real tDCS intervention improved their performance on this test. At the group level, the tDCS intervention, as compared to sham, led to significant improvement in both the percent and absolute change in MoCA score from baseline, and these improvements persisted for at least two weeks (Table 1).
Analysis of MoCA sub-scores revealed that tDCS improved performance significantly only within the visuospatial executive function domain (F=11.9, p=0.002, Cohen’s d=1.05; absolute change: F=12.1, p=0.002, Cohen’s d=1.05). The absolute increase in this sub-score (out of five possible points) following tDCS was 0.8±0.4 points post-intervention and 1.1±0.6 points at two weeks. The absolute change in this sub-score following sham was −0.2±0.5 post-intervention and −0.2±0.4 at two weeks. The magnitude of intervention effect did not differ across follow-up visits. All other MoCA sub-scores were unchanged.
The tDCS intervention also mitigated dual task costs to standing postural sway. Intervention effects were present for the percent change in dual task costs to sway speed (F=10.6, p=0.004, Cohen’s d=1.12) and area (F=29.6, p<0.0001, Cohen’s d=1.40) (Table 1). For each outcome, tDCS induced greater percent reductions from baseline as compared to sham, and the magnitude of observed reductions did not differ between the two follow-up visits.
Further analysis revealed that neither tDCS nor sham intervention influenced postural sway when standing quietly. In contrast, intervention effects were observed for dual task postural sway speed (F=8.1, p<0.001, Cohen’s d=1.01) and area (F=10.8, p=0.003, Cohen’s d=1.12) (Table 1). The tDCS intervention, as compared to sham, induced greater reductions to both outcomes, and the magnitude of these reductions did not differ between the two follow-up visits.
The tDCS intervention improved several markers of gait, specifically when dual tasking. Significant intervention effects were observed for dual task stride time (F=4.3, p=0.05, Cohen’s d=0.99) and dual task stride time variability (F=6.1, p=0.02, Cohen’s d=0.99) (Table 1). Again, tDCS induced greater reductions in these outcomes, compared to sham, and the magnitude of these reductions did not differ between follow-up visits. tDCS did not influence gait outcomes within single task conditions, nor the dual task costs to these outcomes.
tDCS did not affect mobility as measured by the TUG test, depressive systems as measured by the GDS, or executive function as measured by TMT B. With respect to TMT, both intervention arms exhibited large performance improvements over the follow-up period as compared to baseline.
Relationships between MoCA and dual task performance
Across all participants at baseline, those with worse MoCA performance (lower total scores) exhibited higher dual task costs to postural sway area (r2=0.34, p=0.02). In the tDCS group, the percent improvement in the MoCA, from baseline to each follow-up visit, correlated with the percent reduction in sway area dual task cost at each respective visit (r2=0.62, p=0.005). No other significant associations between MoCA and dual task outcomes were observed.
Effects of one tDCS session
Participants completed the dual task assessment before and after their first tDCS session. Those receiving tDCS, as compared to sham, exhibited greater percent reduction in dual task cost to postural sway speed (F=6.2, p=0.03; Cohen’s d=1.08) and area (F=13.3, p=0.003; Cohen’s d=1.36) (Table 2). tDCS did not alter postural sway speed or area within single task conditions. Instead, tDCS as compared to sham resulted in greater percent reductions to these variables when dual tasking (speed: F=5.5, p=0.03; Cohen’s d=1.03; area: F=13.2, p=0.003; Cohen’s d=1.36). This single session of tDCS did not influence walking speed within either task condition.
Relationships between the effects of single and multiple tDCS sessions
Participants who exhibited greater reduction in the dual task costs to sway speed and area following the first tDCS session were more likely to exhibit greater reduction in these outcomes, as well as improvements in MoCA performance, at the 2-week follow-up (r2>0.60, p=0.04).
DISCUSSION
This pilot, randomized, sham-controlled trial suggests that a 10-session tDCS intervention with the anode over the left dlPFC and the cathode of the right supraorbital region is feasible and safe for older adults with impairments in gait and executive function. tDCS improved performance on the MoCA within the visuospatial executive function sub-score, and enhanced various aspects of gait and postural control that were retained for at least two weeks. Additionally, the first session of the tDCS intervention induced immediate improvements to dual task standing and walking performance, and, those who exhibited larger improvements following this session also exhibited larger improvements following the entire intervention.
The tDCS intervention improved MoCA performance on questions linked to executive function (i.e., short trail making test, drawing a cube, drawing a clock). This improvement was not likely due to practice effects, even though the TMT was also administered, as a different version of the MoCA was used at each assessment and improvements were limited to the tDCS group. This result is consistent with and extends multiple studies in healthier, younger cohorts reporting that one session of tDCS with the anode over the left dlPFC improves working memory19 and set-shifting44. As the current trial focused on very old adults with demonstrated executive dysfunction, it provides preliminary yet promising support that tDCS may also induce lasting improvements in executive function within more vulnerable populations.
Individuals who completed the tDCS intervention improved several aspects of postural control and gait, particularly when standing or walking within dual task conditions. Baseline dual task costs to standing and walking outcomes were greater in the current cohort compared to published reports of healthy older adults43. The observation that tDCS mitigated dual task costs to postural sway suggests that the intervention improved participant capacity to recruit available cognitive-motor resources45 and/or effectively allocate available resources to each task within the dual task paradigm46. Studies employing portable neuroimaging technology (e.g., functional near-infrared spectroscopy) are warranted to examine the effects of tDCS on brain activation during normal and dual task standing, in order to better understand tDCS-induced changes in brain function leading to functional improvement.
The tDCS intervention did not induce significant changes in walking speed or TUG performance. It did however result in shorter stride times and less stride time variability when walking while dual tasking. In older adults, these outcomes, especially when dual tasking, are greater in those with previous falls and in those with executive dysfunction8,47. The observation that tDCS with the anode over the left dlPFC and the cathode of the right supraorbital region improved these outcomes supports the involvement of the left DLPFC (and its connected neural networks) in the regulation of these important aspects of locomotor control and extends previous work by demonstrating the ability to change behaviors associated with this brain region.
All participants completed the dual task assessment before and after the first tDCS intervention session. Compared to sham, this session of tDCS immediately reduced the dual task costs to standing postural sway speed and area. These results are consistent with our previous studies in healthy younger and older adults20,30. We also observed that those who demonstrated larger effects on dual task performance following this single tDCS session exhibited larger improvements in cognitive-motor performance two weeks following the entire intervention. We therefore contend that the beneficial effects of tDCS may arise from modulation of frontal-executive systems linked to dynamic components of resource allocation. While this relationship needs to be confirmed, it also suggests that immediate after-effects of tDCS may be used to identify individuals who are most likely to respond favorably to longer interventions.
Our study was limited by a small sample size. Trials with larger samples and longer follow-ups are needed to confirm the observed effects of tDCS on cognitive-motor function. The use of an inactive sham protocol resulted in successful participant blinding to assigned intervention. It is possible that cognitive-motor improvements following tDCS did not arise from modulation of a specific brain region, but rather, a more general effect of stimulation on the brain. As modeled in Fig 2, the electrical field generated by tDCS likely influenced brain function beyond that of the left dlPFC. Future trials should consider comparing the effectiveness of the current tDCS intervention to “high-definition” montages that more focally target the left dlPFC, as well as to active tDCS targeting other brain regions or networks (e.g., sensori-motor cortices). Finally, age-related changes in brain anatomy and function likely influenced electrical current flow and may have contributed to inter-participant variance in intervention effectiveness48. We speculate that ‘personalized’ tDCS administered via an array of electrodes delivering current with parameters derived from modeling of individual MRIs49 will optimize current flow to the desired target and thus, improve the consistency of tDCS-induced benefits. Still, this small yet well-controlled study highlights the potential for tDCS to improve executive function and dual task performance even in older adults who have deficits in gait and cognitive function.
Table 3:
The immediate effects of a 20-minute session of tDCS on standing and walking with and without dual tasking (% change from baseline)
| tDCS | Sham | P value* | |
|---|---|---|---|
| Standing postural sway | |||
| Single task | |||
| - Speed | −3 ± 14 | −1 ± 18 | 0.89 |
| - Area | 4 ± 44 | 5 ± 12 | 0.49 |
| Dual task | |||
| - Speed | −15 ± 21 | 5 ± 12 | 0.03 |
| - Area | −32 ± 32 | 20 ± 25 | 0.003 |
| Dual task cost | |||
| - Speed | −14 ± 8 | 12 ± 17 | 0.003 |
| - Area | −41 ± 14 | 22 ± 39 | 0.0004 |
| Walking | |||
| Single task | |||
| - Speed (m/s) | 8 ± 3 | 3 ± 10 | 0.18 |
| - Stride time (s) | −1 ± 4 | −2±6 | 0.67 |
| - Variability (COV) | −33±13 | −10±16 | 0.12 |
| Dual task (m/s) | |||
| - Speed (m/s) | 11 ± 9 | 9 ± 13 | 0.78 |
| - Stride time (s) | −3 ± 4 | −2 ± 7 | 0.78 |
| - Variability (COV) | 4 ± 20 | −3 ± 25 | 0.25 |
| Dual task cost (%) | |||
| - Speed | 5 ± 11 | 3 ± 11 | 0.73 |
| - Stride time | 16 ± 16 | −7±28 | 0.25 |
| - Variability | −7±32 | 5±15 | 0.45 |
Values reflect the mean and standard deviation percent change from baseline values as reported in Table 1.
Acknowledgements
This work was supported by a Catalyst Award from the Falk Medical Research Trust, the Marcus Applebaum Pilot Award, a grant from the U.S.-Israel Binational Science foundation and grants from the National Institute on Aging (R01 AG041785 and R01 AG025037 to Dr. Lipsitz, K01 AG044543 to Dr. Manor, and T32 AG023480 to Dr. Lo). Biostatistical resources were provided by the Boston Claude D. Pepper Older Americans Independence Center (P30-AG013679). Dr. Pascual-Leone was partly supported by the Sidney R. Baer Jr. Foundation, the Football Players Health Study at Harvard University, and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). Dr. Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife, Boston, MA. Dr. Zhou was supported by the Milstein Medical Asian American Partnership (MMAAP) Foundation. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, or other funding sources.
Footnotes
Conflict of Interest:
Dr. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Magstim Inc., and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging.
References
- 1.Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev 2004;3(4):369–382. [DOI] [PubMed] [Google Scholar]
- 2.Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav R 2010;34(5):721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 2002;16(1):1–14. [DOI] [PubMed] [Google Scholar]
- 4.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord 2008;23(3):329–42–quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhaeghen P, Steitz DW, Sliwinski MJ, Cerella J. Aging and dual-task performance: a meta-analysis. Psychol Aging 2003;18(3):443–460. [DOI] [PubMed] [Google Scholar]
- 6.Beauchet O, Kressig RW, Najafi B, et al. Age-related decline of gait control under a dual-task condition. J Am Geriatr Soc 2003;51(8):1187–1188. [DOI] [PubMed] [Google Scholar]
- 7.Coppin AK, Shumway-Cook A, Saczynski JS, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing 2006;35(6):619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Springer S, Giladi N, Peretz C, et al. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord 2006;21(7):950–957. [DOI] [PubMed] [Google Scholar]
- 9.Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, et al. Association of Dual-Task Gait With Incident Dementia in Mild Cognitive Impairment: Results From the Gait and Brain Study. JAMA Neurol 2017;74(7):857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc 2012;60(11):2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirelman A, Herman T, Brozgol M, et al. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS ONE 2012;7(6):e40297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtzer R, Mahoney JR, Izzetoglu M, et al. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci 2011;66(8):879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan J, Blumen HM, Verghese J, Holtzer R. Functional connectivity associated with gait velocity during walking and walking-while-talking in aging: a resting-state fMRI study. Hum Brain Mapp 2015;36(4):1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szameitat AJ, Schubert T, Müller K, Cramon von DY. Localization of executive functions in dual-task performance with fMRI. J Cogn Neurosci 2002;14(8):1184–1199. [DOI] [PubMed] [Google Scholar]
- 15.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527 Pt 3(3):633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antal A, Alekseichuk I, Bikson M, et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol 2017;128(9): 1774–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill AT, Rogasch NC, Fitzgerald PB, Hoy KE. Effects of prefrontal bipolar and high-definition transcranial direct current stimulation on cortical reactivity and working memory in healthy adults. Neuroimage 2017;152:142–157. [DOI] [PubMed] [Google Scholar]
- 18.Javadi AH, Walsh V. Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimul 2012;5(3):231–241. [DOI] [PubMed] [Google Scholar]
- 19.Fregni F, Boggio PS, Nitsche M, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res 2005;166(1):23–30. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Hao Y, Wang Y, et al. Transcranial direct current stimulation reduces the cost of performing a cognitive task on gait and postural control. Eur J Neurosci 2014;39(8):1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou D, Zhou J, Chen H, et al. Effects of transcranial direct current stimulation (tDCS) on multiscale complexity of dual-task postural control in older adults. Exp Brain Res 2015; 166 (1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stagg CJ, Lin RL, Mezue M, et al. Widespread Modulation of Cerebral Perfusion Induced during and after Transcranial Direct Current Stimulation Applied to the Left Dorsolateral Prefrontal Cortex. J Neurosci 2013;33(28): 11425–11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meinzer M, Lindenberg R, Phan MT, et al. Transcranial direct current stimulation in mild cognitive impairment: Behavioral effects and neural mechanisms. Alzheimers Dement 2015;11(9):1032–1040. [DOI] [PubMed] [Google Scholar]
- 24.Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act 2015;23(2):314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corrigan JD, Hinkeldey NS. Relationships between parts A and B of the Trail Making Test. J Clin Psychol 1987;43(4):402–409. [DOI] [PubMed] [Google Scholar]
- 26.Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsyc 2000;22(4):518–528. [DOI] [PubMed] [Google Scholar]
- 27.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. JAm Geriatr Soc 1992;40(9):922–935. [DOI] [PubMed] [Google Scholar]
- 28.van Marwijk HW, Wallace P, de Bock GH, et al. Evaluation of the feasibility, reliability and diagnostic value of shortened versions of the geriatric depression scale. Br J Gen Pract 1995;45(393): 195–199. [PMC free article] [PubMed] [Google Scholar]
- 29.Boggio PS, Rigonatti SP, Ribeiro RB, et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychop 2008;11(2):249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manor B, Zhou J, Jor’dan A, et al. Reduction of Dual-task Costs by Noninvasive Modulation of Prefrontal Activity in Healthy Elders. J Cogn Neurosci 2016;28(2):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 2006;117(4):845–850. [DOI] [PubMed] [Google Scholar]
- 32.Brunoni AR, Amadera J, Berbel B, et al. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 2011;14(8): 1133–1145. [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12(3): 189–198. [DOI] [PubMed] [Google Scholar]
- 34.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332(9): 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 36.Costa AS, Fimm B, Friesen P, et al. Alternate-form reliability of the Montreal cognitive assessment screening test in a clinical setting. Dement Geriatr Cogn Disord 2012;33(6):379–384. [DOI] [PubMed] [Google Scholar]
- 37.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39(2):142–148. [DOI] [PubMed] [Google Scholar]
- 38.Hausdorff JM, Schweiger A, Herman T, et al. Dual-task decrements in gait: contributing factors among healthy older adults. J Gerontol A Biol Sci Med Sci 2008;63(12):1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirelman A, Maidan I, Bernad-Elazari H, et al. Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. J NeuroEng Rehabil 2014;11(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beauchet O, Freiberger E, Annweiler C, et al. Test-retest reliability of stride time variability while dual tasking in healthy and demented adults with frontotemporal degeneration. J NeuroEng Rehabil 2011;8(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashendorf L, Jefferson AL, O’Connor MK, et al. Trail Making Test errors in normal aging, mild cognitive impairment, and dementia. Arch Clin Neuropsychol 2008;23(2): 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther 2000;80(9): 896–903. [PubMed] [Google Scholar]
- 43.Hollman JH, McDade EM, Petersen RC. Normative spatiotemporal gait parameters in older adults. Gait Posture 2011;34(1): 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leite J, Carvalho S, Fregni F, Gon9alves 0F Task-specific effects of tDCS-induced cortical excitability changes on cognitive and motor sequence set shifting performance. PLoS ONE 2011;6(9):e24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boisgontier MP, Beets IAM, Duysens J, et al. Age-related differences in attentional cost associated with postural dual tasks: increased recruitment of generic cognitive resources in older adults. Neurosci Biobehav R 2013;37(8):1824–1837. [DOI] [PubMed] [Google Scholar]
- 46.Fischer R, Plessow F. Efficient multitasking: parallel versus serial processing of multiple tasks. Front Psychol 2015;6(Suppl. 1): 1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. YAPMR 2001;82(8):1050–1056. [DOI] [PubMed] [Google Scholar]
- 48.Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 2005; 15(11): 1676–1689. [DOI] [PubMed] [Google Scholar]
- 49.Datta A, Truong D, Minhas P, et al. Inter-Individual Variation during Transcranial Direct Current Stimulation and Normalization of Dose Using MRI-Derived Computational Models. Front Psychiatry 2012;3:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50..Miranda PC. Physics of effects of transcranial brain stimulation. Handb Clin Neurol 2013;116:353–366. [DOI] [PubMed] [Google Scholar]


