Abstract
The relationship between brain development and clinical heterogeneity in autism (ASD) is unknown. This study examines the Social Responsiveness Scale (SRS) in relation to the longitudinal development of cortical thickness. Participants (N=91 ASD, N=56 TDC; 3-39 years at first scan) were scanned up to three times over a 7-year period. Mixed-effects models examined cortical thickness in relation to SRS score. ASD participants with higher SRS scores showed regionally increased age-related cortical thinning. Regional thickness differences and reduced age-related cortical thinning were found in predominantly right lateralized regions in ASD with decreasing SRS scores over time. Our findings emphasize the importance of examining clinical phenotypes in brain-based studies of ASD.
Keywords: Autism spectrum disorder (ASD), Social Responsiveness Scale (SRS), cortical thickness, brain development, longitudinal, autism severity
Individuals along the autism spectrum share common core impairments, but there exists a great deal of clinical heterogeneity among individuals. Although advances in neuroimaging methods and analyses have increased the number of studies investigating brain development in autism, the relationships between underlying brain structures and this clinical heterogeneity are still unknown. The present study investigates the brain basis of the clinical phenotype measured by the Social Responsiveness Scale (SRS) and how social difficulties change over time in ASD in relation to cortical brain structure.
A growing consensus is that many behaviors in individuals with autism spectrum disorder (ASD) result from atypical neural organization. Work from our group and others has examined cortical thickness as a potential avenue toward understanding atypical neural organization that may contribute to behaviors common among individuals. Studies examining cross-sectional samples, utilizing a single time point of MRI data, report widespread regions of thinner cortex in ASD (Chung et al. 2005; Ecker et al. 2013; Ecker et al. 2014; Foster et al. 2015; Hadjikhani et al. 2006; Hyde et al. 2010; Jiao et al. 2010; Misaki et al. 2012; Richter et al. 2015; Scheel et al. 2011; Wallace et al. 2010), regions of thicker cortex (Ecker et al. 2013; Foster et al. 2015; Hardan et al. 2006; Hyde et al. 2010; Jiao et al. 2010; Libero et al. 2014; Mak-Fan et al. 2012; Raznahan et al. 2012; Richter et al. 2015), or no cortical thickness differences from comparison groups (Ohta et al. 2016; Schaer et al. 2013). Despite the growing literature, there still lacks a consistent pattern of age-related cortical abnormalities in the ASD brain. It is unknown whether the absence of consistent findings may be due to sample sizes, different age ranges and developmental periods, or varying autism severity within and between samples. In addition, inferring developmental processes is limited with cross-sectional samples, due to different individuals being compared at different ages (Kraemer et al. 2000).
Longitudinal samples are advantageous in that the same individuals are examined over time within a dataset. Despite this advantage, few research groups have employed a longitudinal design to study cortical thickness development in ASD (Hardan et al. 2009; Hazlett et al. 2011; Schumann et al. 2010; Wallace et al. 2015; Zielinski et al. 2014). Studies of lobar thickness report no atypical age-related changes in young children (Hazlett et al. 2011), yet by later childhood and adolescence increased cortical thinning is found in the temporal and occipital lobes (Hardan et al. 2009). More recently, Wallace and colleagues examined two longitudinal scans from 17 ASD participants and 18 typical controls (age range 14-24 years) and found accelerated cortical thinning in the ASD group in the left posterior ventrolateral occipitotemporal and superior parietal cortex (Wallace et al. 2015). These findings complement work by our group on a large cross-sectional and longitudinal sample of 97 males with ASD and 60 typical controls scanned up to three times over a 7 year period (age of first scan 3-39 years; (Zielinski et al. 2014)). We found widespread increased cortical thinning in the ASD group in frontal (right paracentral, left pars opercularis, rostral middle frontal and frontal pole), temporal (right transverse temporal), parietal (bilateral postcentral, right superior parietal, left supramarginal), and occipital (bilateral cuneus, lingual, pericalcarine, and right lateral occipital) regions. Our findings suggest regionally specific abnormal cortical development in ASD that varied by developmental stage. For instance, some regions became thinner in ASD by adolescence whereas other regions did not become thinner until adulthood. Understanding the behavioral correlates of these brain-based cortical trajectories is the focus of the present study and fundamental for understanding the clinical course of the disorder.
The Social Responsiveness Scale (SRS, previously Social Reciprocity Scale; (Constantino et al. 2003; Constantino et al. 2000)) is a quantitative scale that measures the presence and extent of autistic social impairment and has been examined in both ASD and typical samples. The SRS is correlated with ADI-R scores and measures reciprocal social behavior and general behavioral problems that may underlie language difficulties and stereotyped behaviors common in autism (Constantino et al. 2003; Constantino et al. 2000; Hus et al. 2013). A report of longitudinal SRS scores in children with typical development or pervasive developmental disorder (PDD) describes modest improvement over time and the greatest improvement in those with the most severe scores at baseline (Constantino et al. 2009). These results are partially in line with a recent cross-sectional study by Wallace and colleagues (Wallace et al. 2017) where social communication impairments decreased with age in a typical sample, but these authors report increased impairments with age in the ASD group (early childhood vs adolescence and adulthood).
Despite the clinical utility of a quantitative measure of autism symptoms, studies of SRS in relation to cortical thickness in autism are limited. In a small sample of children with high functioning autism, higher SRS scores (more severe symptoms) were associated with increased thickness in left frontal and temporal regions, and lower scores associated with increased thickness in right frontal and left parietal regions (Richter et al. 2015). Interestingly, the SRS has been used to quantify autism traits in many typically developing samples and a number of regions in the frontal, temporal and parietal cortices show cortical thinning associated with higher SRS scores (Hedrick et al. 2012; Tu et al. 2016; Wallace et al. 2012). Although developed for behavioral research, the SRS has also recently been used as a phenotypic measure in genetic studies of autism (Lowe et al. 2015) and also of typical samples (Hedrick et al. 2012). Thus, SRS-brain relationships may inform future biological and genetic studies of not only those with ASD but also along the typical population.
The current report expands on our previous studies of longitudinal cortical thickness changes in autism by examining relationships between cortical thickness and SRS scores within typically developing participants and those with ASD. We also examine brain changes in relation to changing SRS scores over time to identify cortical regions differentially involved in improvement of social impairment in ASD. Our results aim to identify cortical regions that may be involved in the presentation and progression of the autism phenotype in ASD and typically developing individuals.
Methods
Participants
Behavioral and imaging data were collected as part of a longitudinal study at the University of Utah. The ASD group consisted of 91 males with ASD, age 3.4-36 years old at the time of their first scan, and a typically developing control group that included 56 males age 4-39 years at their first scan. As part of the longitudinal study, participants underwent MRI scanning up to three times over the course of seven years, for a total of 184 scans from ASD participants and 97 scans from typical controls available for analysis. There were no significant differences in number of MRI scans per participant (ASD=2.4, TDC=2.4, t=.05, ns), average inter-scan interval (ASD=2.8 years, TDC=2.9 years; t=.5, ns) or mean age across all MRI scans (ASD=16.4 years, TDC=16.7 years, t=.3, ns). Longitudinal cortical thickness changes in the larger ASD (n=97) and typically developing (n=60) groups were previously reported (see (Zielinski et al. 2014)). The present analysis included the subset of the larger sample with an SRS score collected during the course of the longitudinal study.
All study procedures were approved by the University of Utah IRB. At study intake, autism diagnosis [82% autism, 11% PDDNOS, 7% broad autism (defined in (Lainhart et al. 2006))] was based on the Autism Diagnostic Observation Schedule-Generic (ADOS-G (Lord et al. 2000)), Autism Diagnostic Interview-Revised (ADI-R; (Lord et al. 1994)), DSM-IV (American Psychiatric Association 1994) and ICD-10 criteria. 47% of the ASD group was taking psychotropic medications at some point during the study. Typically developing participants were also given the ADOS-G to screen for ASD behaviors. See Table 1 for additional demographic characteristics.
Table 1.
Demographic information for the ASD and typically developing groups.
| ASD (n=91) | TDC (n=56) | |||||
|---|---|---|---|---|---|---|
| mean (sd) | range | mean (sd) | range | t-test | p-value | |
| SRS Total Raw Score* | 100.7 (28) | 21 – 158 | 15.8 (11) | 0 – 51 | 21.2 | <.001 |
| FSIQ | 95 (21) | 49 – 137 | 117 (12) | 95 – 153 | −6.9 | <.001 |
| PIQ | 97 (18) | 50 – 129 | 115 (16) | 88 – 152 | −5 | <.001 |
| VIQ | 97 (23) | 51 – 145 | 114 (13) | 94 – 151 | −4.6 | <.001 |
| ADOS Communication | 4.8 (1.5) | 1–8 | .5 (.6) | 0–2 | 20.1 | <.001 |
| ADOS Social | 9.1 (2) | 4–14 | .7 (1) | 0–3 | 25.5 | <.001 |
| ADOS Total | 14.1 (3) | 6–22 | 1.2 (1) | 0–4 | 27.1 | <.001 |
| ADOS Severity Score | 8.3 (1.4) | 5–10 | 1.1 (.4) | 0–3 | 42.6 | <.001 |
SRS scores were averaged for those participants with multiple SRS scores.
Social Responsiveness Scale (SRS)
The Social Responsiveness Scale (SRS; (Constantino 2002)) was completed by a parent or close family member at study visits. All SRS raw scores collected over the course of the longitudinal study were included in our analyses (see Figure 1). We also examined the two SRS domain scores that map onto the DSM-5 criteria for autism spectrum disorder: a social communication/interaction score (SCI; sum of Awareness, Cognition, Communication, and Motivation subscales) and restricted/repetitive behavior score (RRB; Mannerisms subscale) (Frazier et al. 2014a; Frazier et al. 2014b; Frazier et al. 2012). We also examined change in SRS score over time in the ASD group by calculating SRS difference scores from the first visit and most recent follow-up visit for total raw SRS, SCI and RRB domain scores [SRS Difference Score = SRS raw score 2 (collected at Time 2 or 3) – SRS raw score 1 (collected at Time 1)]. Based on these difference scores, participants were subgrouped into those with increased score over time (Decline in social function) vs decreased score (Improve in social function), allowing for a direct comparison of cortical thickness between subgroups.. Two participants had no change in total SRS score over time and 5 individuals had no change in RRB scores so were excluded from their respective subgroup comparisons.
Figure 1.
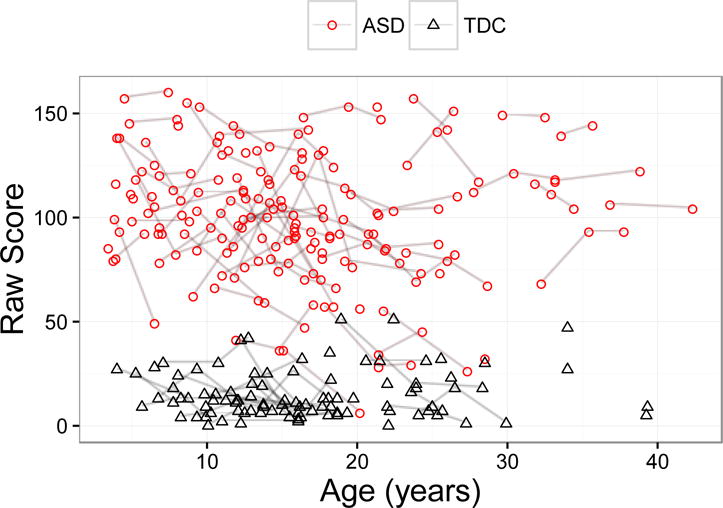
SRS Total Raw Scores in ASD and TDC (typically developing group). Each point represents an SRS score for a given participant and repeated scores for participants are connected by line.
Imaging protocol and cortical thickness estimation
T1-weighted 3D MPRAGE images were acquired on a Siemens Trio 3.0 Tesla MRI scanner. At time 1, an 8-channel, receive-only RF head coil was used (TI=1100ms, TR=1800ms, TE=2.93ms, flip angle=12°, FOV=256mm, slice thickness=1.0mm, 160 slices) and at time points 2 and 3, a 12-channel, receive only RF head coil was used (TI=900ms, TR=2200ms, TE=2.91ms, flip angle=9°, FOV=256mm, slice thickness=1.2mm, 160 slices). Imaging hardware and protocol changes from time point 1 to 2 and 3 were modeled in the statistical analysis. Extraction of cortical thickness estimates are described in detail in Zielinski et al., 2014. Briefly, Freesurfer image analysis suite v5.1.0 (http://surfer.nmr.mgh.harvard.edu) was used for cortical parcellation and thickness estimations using the Desikan-Killiany Atlas (Desikan et al. 2006), resulting in average cortical thickness in 34 cortical parcels per hemisphere.
Statistical analysis
Mixed-effects models were used to examine raw SRS scores in relation to longitudinal age-related changes in regional cortical thickness separately for the ASD and typical groups. Longitudinal cortical thickness was modeled with participants contributing the random effects, raw SRS score and age as fixed effects and the following covariates using lowest Akaike Information Criterion (AIC; (Akaike 1974)) value identifying the best-fitting model: SRS x age, age2, average whole brain cortical thickness, and imaging protocol. Mixed-effects models were also used to examine the SRS difference scores by comparing age-related changes in cortical thickness between the ASD participants with SRS scores that increased over time, or increasing autism behaviors, versus those whose scores decreased over time, or improved behaviors. The SRS Difference Group effect (decreased score vs increased score) and age were included as fixed effects and the following covariates examined for inclusion, using lowest AIC for model fit: Difference Group x age, age2, starting SRS score, average whole brain cortical thickness, and imaging protocol. Average whole brain cortical thickness was included as a covariate to control for whole brain changes. Analyses were performed in R 3.2.1 (R Core Team 2015) using the package FindMinIC (Lange et al. 2013). The FindMinIC package creates models using the fixed variables and all combinations of the covariates of interest, ranking the models based on minimum information criterion. All covariates were mean centered and to correct for multiple comparisons, FDR correction at q<0.05 was applied for each SRS analysis separately (SRS raw, SRS Difference Score, etc).
Results
SRS Total Score, SCI, RRB and Cortical Thickness
Total SRS raw score
Our first analysis identified cortical subregions with significant associations between cortical thickness and SRS total raw score. In ASD, higher SRS raw score, indicating more impaired functioning, was associated with thicker right frontal pole (t=2.2, p=.024), thinner right posterior cingulate (t=-2.5, p=.011) and thinner left inferior temporal gyrus (t=−2.1, p=.033). Higher scores were also associated with thicker left superior frontal (t=2.0, p=.046) and right temporal pole (t=2.0, p=.042), although these comparisons failed to survive FDR correction. Figure 2a shows regions of thicker or thinner cortex associated with SRS score in ASD. In the TDC group, higher SRS total score was associated with thicker left pars triangularis (t=2.0, p=.048) and thinner left insula (t=−2.1, p=.041), but neither result remained significant after FDR correction. Total raw SRS score was not associated with average whole brain cortical thickness for either group. Model estimates for both groups are presented in a Supplementary Table.
Figure 2.
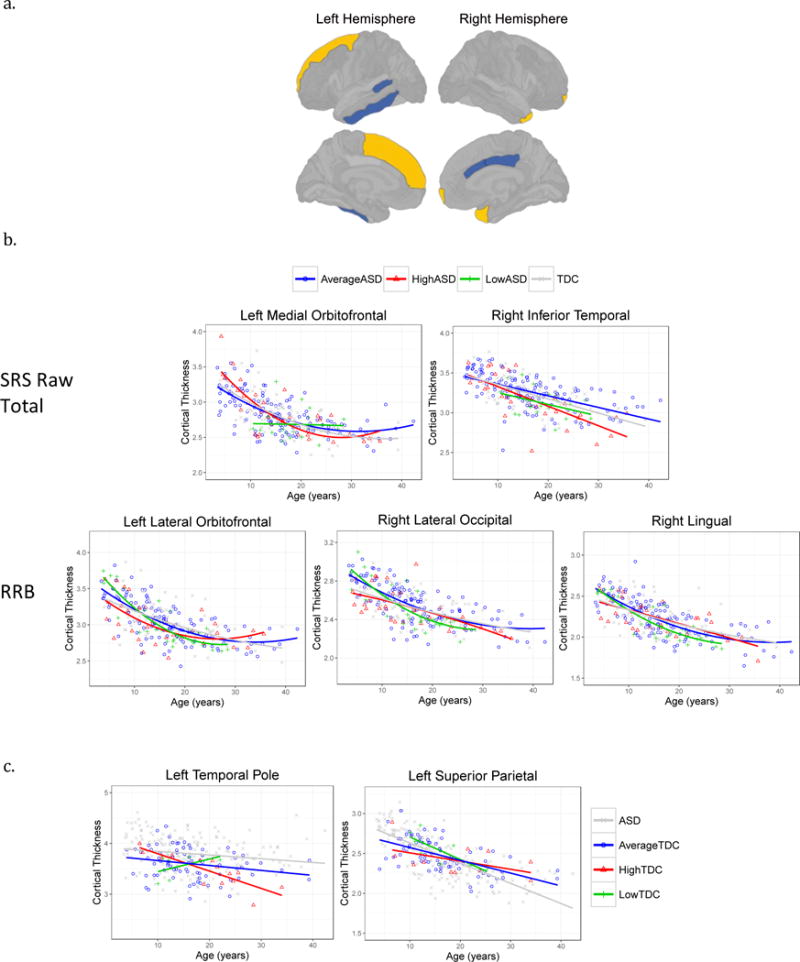
Regional cortical thickness associated with total raw SRS score, SCI and RRB domains.
2a. In the ASD group, higher SRS scores were associated with regions of thicker (light color) or thinner cortex (dark color). Higher scores were associated with thicker right frontal and temporal poles, and left superior frontal. Higher scores were associated with thinner left inferior temporal and bank of the superior temporal sulcus, and right caudal anterior and posterior cingulate.
2b. Scatterplots of cortical regions with significant SRS total score by age interactions in ASD. To visualization the interactions, individual participants and group fit lines are labeled according to ASD SRS subgroups: Average (within 1SD of ASD group mean), High (>1SD of group mean), Low (<1SD of group mean).
2c. Visualization of significant SRS score by age interactions in the TDC group only according to TDC SRS subgroups: Average (within 1 SD of TDC mean), High (>1SD of group mean), Low (<1SD of group mean).
SCI and RRB domains
Higher scores in both the SCI and RRB domains were associated with thinner left inferior temporal gyrus (SCI t=−2.2, p=.028; RRB t=−2.3, p=.021). Higher SCI score was associated with thicker left superior frontal (t=2.2, p=.025) and thinner right posterior cingulate (t=−2.8, p=.004) and higher RRB score with thicker right frontal pole (t=3.2, p=.001). Additional regions failed to remain significant after FDR comparison: higher SCI and thicker right temporal pole (t=2.1, p=.036) and thinner right caudal anterior cingulate (t=−2.1, p=.039); higher RRB and thinner left bank of the superior temporal sulcus (t=−1.9, p=.048). In the TDC group, all findings were left lateralized. Higher SCI was associated with thinner insula (t=−2.3, p=.023) and higher RRB was associated with thicker cuneus (t=2.9, p=.005) and pars opercularis (t=2.1, p=.042, missed FDR cutoff).
SRS by age interactions: SRS total raw, SCI, RRB
Significant SRS by age interactions, indicating age-related cortical thickness changes associated with SRS score were also found. In ASD, a SRS by age interactions in the left medial orbitofrontal region was driven by the RRB domain (SRS total t=−2.2, p=.027, RRB t=−2.7, p=.007) and right inferior temporal driven by the SCI domain (SRS total t=−2.3, p=.022; SCI t=−2.3, p=.020). To visualize the interaction between the two quantitative variables of SRS total score and age on cortical thickness, participants were classified into subgroups based on average SRS score collected during the study period. We used these average SRS scores to assign our participants into one of the following subgroups: Average SRS = within 1SD of group mean; High SRS = +1SD; Low SRS = -1SD. Figure 2b shows increased age-related cortical thinning in the ASD group with high SRS scores compared to the ASD participants with average and low SRS scores. RRB by age interactions were found in a number of regions: left lateral orbitofrontal (t=2.4, p=.016), superior frontal (t=2.3, p=.020), and right lateral occipital (t=3.4, p=.003) and lingual gyrus (t=2.5, p=.014). Scatterplots in 2b show increased age-related cortical thinning in the participants with low RRB scores. Additional SRS by age interactions that did not survive FDR correction are provided in the Supplementary Table.
In TDC, a significant SRS score by age interaction was found in the left temporal pole (SRS total t=−3.08, p=.004; SCI t=−2.8, p=.007; RRB t=−3.1, p=.003) and left superior parietal cortex for the SCI domain (t=2.3, p=.024). Figure 2c shows that the TDC participants with higher SRS scores have a greater degree of age-related regional cortical thinning in the left temporal pole and reduced age-related changes in the left superior parietal cortex.
Change in SRS Scores Over Time in Relation to Cortical Thickness in ASD
A subset of 65 individuals from the ASD sample had at least 2 SRS scores collected during the study period so were included in the Difference score analysis. As a group, Total raw SRS score decreased over time (mean at first SRS=103, second SRS=98, t=2.0, p=.04). Regional differences in both cortical thickness and age-related changes were found in the ASD subgroup comparison of those with decreased scores over time, or reduced autism behaviors, versus increased scores over time. No significant differences were found in age or starting SRS score between the participants with increasing Total raw SRS scores over time (n=25, mean age First SRS=14.3 years, mean First SRS=98) versus decreasing scores over time (n=38, mean age First SRS=15.6 years, mean First SRS=107). Compared to the ASD participants with increasing scores over time, the ASD participants with decreasing scores over time had thicker right pars opercularis (Total SRS raw group B=.07, t=2.6, p=.011; SCI group B=.06, t=2.1, p=.034, missed FDR correction) and thinner left pericalcarine (Total raw group B=−.1, t=−2.6, p=.009). Reduced RRB score over time was associated with thinner right supramarginal gyrus (B=−.07, t=−2.4, p=.018) and thinner caudal middle frontal (B=−.06, t=−2.0, p=.048), although the latter finding was no longer significant after FDR correction. Figure 3a displays regions of thicker or thinner cortex associated with decreased SRS severity over time.
Figure 3.
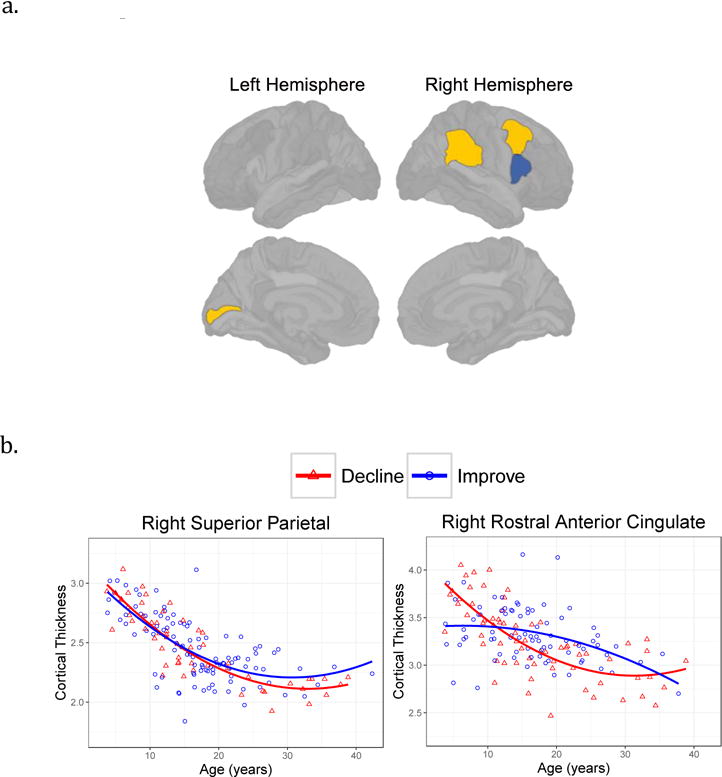
Regional cortical thickness associated with SRS difference scores.
3a. Cortical regions where participants with improved SRS scores over time had thicker (dark color, right pars opercularis) or thinner (light color, left pericalcarine, right supramarginal, right caudal middle frontal) cortex.
3b. Scatterplots of cortical regions with significantly different age-related changes according to Difference score group (Decline vs Improve). SCI scores were associated with right superior parietal and RRB associated with right rostral anterior cingulate.
Significant group by age interactions, indicating different cortical thickness age-related changes depending on whether the ASD participants had increased or decreased scores over time, were found for the SCI and RRB subscores only. As shown in Figure 3b, the ASD group with improved (or reduced) autism SCI behaviors showed reduced age-related cortical thinning in the right superior parietal (B=.008, t=2.3, p=.023) and those participants with reduced RRB behaviors had reduced age-related cortical thinning in the right rostral anterior cingulate (B=.014, t=2.3, p=.021). Additional Difference group by age interactions failed to survive FDR correction (SCI: left supramarginal B=−.006, t=−2.0, p=.041; RRB: left frontal pole B=−.014, t=− 1.9, p=.05).
Discussion
The present study examined a quantitative measure of autism behaviors in relation to cortical thickness development. We further examined our ASD sample to identify cortical regions that may be related to SRS domain severity and change in scores over time. Our study design thus allowed for a description of stable clinical-brain findings (regions where SRS scores were associated with thicker or thinner cortex), and dynamic clinical-brain findings (changing SRS severity over time was associated with cortical thickness and/or different developmental cortical trajectories). Our findings highlight regions that may be involved in behaviors measured with the SRS and emphasize the importance of including clinical phenotypes, and change in phenotype over time, in future brain-based studies of the disorder.
Stable SRS-Brain Relationships
The ease of administration of the SRS has made it a valuable measure to examine autism behaviors in ASD. A number of cortical regions were identified where SRS raw total and domain scores were associated with regions of thicker or thinner cortex, predominantly in the frontal and temporal lobes and cingulate cortex. We also identified increased age-related cortical thinning associated with higher SRS scores in the left medial orbitofrontal gyrus and increased left lateral orbitofrontal thinning associated with lower RRB domain scores. Previous cross-sectional studies have reported thinner orbitofrontal gyrus in children with ASD (Doyle-Thomas et al. 2013; Jiao et al. 2010; Richter et al. 2015) and higher ADI-R social scores associated with reduced thickness in the left orbitofrontal gyrus (Doyle-Thomas et al. 2013). Our results suggest that age-related developmental differences may also be present in this region. In addition, the finding of higher SRS scores associated with increased cortical thinning in the left medial orbitofrontal and inferior temporal lobe highlights the need to not only take into account clinical heterogeneity but also sample age in the interpretation of case- control comparison studies, as group differences may only be present at certain ages or developmental periods.
Dynamic SRS Changes Associated with Stable Brain Differences
The longitudinal nature of our dataset also allowed for the identification of dynamic clinical-brain relationships, such that we were able to subgroup individuals within our ASD sample based on their changing scores over the course of the study. The ASD subgroup analysis showed that those individuals whose scores decreased during the course of the study, or showed improved autism related behaviors, had regions of thicker or thinner cortex compared to the ASD group with increased severity of behaviors. Thicker right pars opercularis was associated with decreased Total score, a finding driven by the SCI domain. Our right-lateralized pars opercularis results are supported by behavioral studies that show a relationship between thinning in the pars opercularis and ADI-R Social + Communication scores (Hadjikhani et al. 2006) and reduced gray matter volume of the right pars opercularis associated with increased social communication and reciprocity impairments (Yamasaki et al. 2010).. Atypical development of the left pars opercularis, part of the inferior frontal gyrus language network, has also been shown previously in ASD. Increased thickness was found during childhood (Mak-Fan et al. 2012) while increased thickness reversed to thinner cortex in ASD only after controlling for age and intracranial volume (Libero et al. 2014). Both findings are in line with atypical age-related cortical thinning found in our larger ASD sample (Zielinski et al. 2014). Taken together, these findings suggest that an atypical frontal language network in ASD may contribute to social impairments, and suggest that increased right pars opercularis thickness may be a compensatory developmental change in some ASD participants that results in improved communication, and as a result improved social function and related behaviors.
Regions of thinner cortex were also found in those individuals who scores improved over time. Improved RRB domain score was associated with thinner right caudal middle frontal and supramarginal gyrus. In the left hemisphere, improved Total score was associated with thinner pericalcarine, a finding supported by a study by Blanken and colleagues who found lower SRS scores associated with thinner pericalcarine in boys (age 6-10 years; (Blanken et al. 2015)). The relationships found between regional thickness and clinical phenotype within ASD emphasizes the importance of understanding more about the early development of these regions and associated networks, as they may provide potential targets for therapies or interventions aimed at improving social function.
Dynamic SRS Changes Associated with Cortical Developmental Trajectories
We also found regions where age-related cortical thickness changes differed within the ASD group according to whether impairments increased or decreased during the study period. Reduced age-related cortical thinning was found in the right superior parietal cortex in those with improved SCI domain scores and right rostral anterior cingulate in those with improved RRB domain scores. Other research has identified cortical thickness changes associated with changing clinical profiles. Cortical thickness changes in the right precentral gyrus was recently associated with changing Vineland expressive communication scores in a lower functioning sample (Smith et al. 2016). Atypical gyrification in the left precentral gyrus was found in a cross-sectional sample of children with ASD; namely increasing gyrification with age in those with high SRS vs no change in gyrification in low SRS (Yang et al. 2016). The regional differences we found between our SRS subgroups underscores the importance of examining the SRS domain scores in future studies of ASD, as differing clinical phenotypes may be masked in just a total score comparison.
Typically Developing Findings
One of the clinical strengths of the SRS is the broad range of scores and ability to examine variation along the typical population. Although our study focused on ASD, we did find age-related changes in the left temporal pole associated with SRS score and left superior parietal associated with the SCI domain in the typical group. Our findings are in line with a study by Wallace and colleagues of a typically developing longitudinal cohort (age range 3-30 years; 323 participants; 54% male) who found thinner left superior parietal cortex associated with higher SRS scores (Wallace et al. 2012) and provide support for age-related changes that may also be present in this region. We also noted associations between higher SRS score and thicker left cuneus and pars triangularis and thinner left insula, although neither of the latter findings remained significant after multiple comparison correction. Our insula findings are partially in line with a small study of typical adolescents, where higher SRS scores were associated with thinner cortex in bilateral insula and right superior temporal gyrus (Tu et al. 2016). The continued application of this measure in genetic studies of typical development combined with results from the ASD field will aid in identifying shared and unique genetic and developmental markers that may have the potential for brain-based interventions.
Limitations
Our study is an important step in identifying cortical subregions involved in the progression of the autism behavioral phenotype but has a number of limitations. Our study design only consisted of high functioning, verbal male participants, so is not generalizable to minimally verbal or female populations. Another limitation is that only cortical thickness was examined. As neuroimaging methods and analysis continue to evolve, the combined analysis of cortical thickness along with other brain-based measures, such as gyrification and surface area, may be best able to identify stable and dynamic brain mechanisms. For example, studies have shown complex relationships between thickness and measures of cortical volume, surface area and gyrification (eg, (Wallace et al. 2013)) and regional gyrification has been associated with autism traits (Blanken et al. 2015; Schaer et al. 2013); thus a more thorough investigation into the clinical correlates of multiple cortical facets is warranted. Along these lines, we cannot discern the underlying biological mechanisms driving cortical thickness differences and thickness-SRS relationships and whether these are the same between participant groups. Finally, the longitudinal nature of our study design has many strengths but does have limitations. The SRS was the only longitudinal measure of autism severity collected over these time points and the original SRS was designed for parent report of children and adolescents up to age 18. Because of the need for consistent measurements over time, the original instrument was used for all participants at all timepoints, including those who had reached adulthood, and thus we only analyzed raw scores. Although the SRS is highly correlated with clinical measures of ASD (Constantino et al. 2003), the reliance on parent report can be problematic as it is unknown how different parents rate the severity of behaviors in question. Additionally, a number of our participants were taking psychotropic medications over the course of the study period and some changed medications over time. We do not know the short or long-term effects of psychotropic medication use on cortical thickness structure and longitudinal development. Moreover, we were not able to quantitatively assess how an individual’s treatment or therapies may be related to SRS score and change in score over time. Although, Constantino and colleagues did not find that treatment modalities predicted SRS change over time in a small sample of participants (n=40; (Constantino et al. 2009)), it is still unknown how psychotropic medications, therapy, intervention and treatment impact the structure of the developing brain and progression of the autism phenotype. Despite these limitations, our findings are an important step in understanding longitudinal brain correlates of autism-related behaviors from childhood into adulthood.
Conclusion
Although the SRS is considered a measure of autism behaviors related to social function, there are many components to social interactions where the inability to filter out relevant information (visual stimuli, reading emotional cues, auditory information) may prevent the learning of social cues and integration of appropriate social responses. Thus, the continued study of our ASD sample may identify important cortical regions outside of traditional “social” or “communication” networks that are important for filtering out, attending and responding to relevant information. Many studies show that the ASD brain is developmentally different from that of typical development, thus our examination of cortical thickness variation within an ASD sample is important for identifying regions where brain changes are associated with improved function that may not be found in a case-control comparison. Our current findings emphasize the importance of examining clinical phenotypes along the autism spectrum in future studies of brain structure and function and demonstrate the importance of examining longitudinal samples, as age-related developmental processes may mask or enhance group differences when only a single time point of data is included. A longitudinal study design provides a deeper understanding of group and individual differences in brain structure and development and will allow for more insight into the clinical progression of ASD and related brain based disorders.
Supplementary Material
Footnotes
Compliance with Ethical Standards
Conflict of interest. The authors declare that they have no conflict of interest.
Ethical Approval. All procedures in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinski declaration and its later amendments or comparable ethical standards.
Informed Consent. Informed consent was obtained from all individual participants included in the study.
References
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Blanken LM, et al. Cortical morphology in 6- to 10-year old children with autistic traits: a population-based neuroimaging study. Am J Psychiatry. 2015;172:479–86. doi: 10.1176/appi.ajp.2014.14040482. [DOI] [PubMed] [Google Scholar]
- Chung MK, Robbins SM, Dalton KM, Davidson RJ, Alexander AL, Evans AC. Cortical thickness analysis in autism with heat kernel smoothing. Neuroimage. 2005;25:1256–65. doi: 10.1016/j.neuroimage.2004.12.052. doi: S1053-8119(04)00754-2 [pii] 10.1016/j.neuroimage.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Constantino JN. The Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- Constantino JN, et al. Developmental course of autistic social impairment in males. Development and psychopathology. 2009;21:127–38. doi: 10.1017/S095457940900008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of autism and developmental disorders. 2003;33:427–33. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. Journal of developmental and behavioral pediatrics: JDBP. 2000;21:2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Doyle-Thomas KA, et al. Effects of age and symptomatology on cortical thickness in autism spectrum disorders. Research in autism spectrum disorders. 2013;7:141–150. doi: 10.1016/j.rasd.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, et al. Brain surface anatomy in adults with autism: the relationship between surface area, cortical thickness, and autistic symptoms. JAMA Psychiatry. 2013;70:59–70. doi: 10.1001/jamapsychiatry.2013.265. [DOI] [PubMed] [Google Scholar]
- Ecker C, et al. The effect of age, diagnosis, and their interaction on vertex-based measures of cortical thickness and surface area in autism spectrum disorder. Journal of neural transmission. 2014;121:1157–70. doi: 10.1007/s00702-014-1207-1. [DOI] [PubMed] [Google Scholar]
- Foster NE, et al. Structural Gray Matter Differences During Childhood Development in Autism Spectrum Disorder: A Multimetric Approach. Pediatric neurology. 2015;53:350–9. doi: 10.1016/j.pediatrneurol.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Ratliff KR, Gruber C, Zhang Y, Law PA, Constantino JN. Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the social responsiveness scale-2. Autism. 2014a;18:31–44. doi: 10.1177/1362361313500382. [DOI] [PubMed] [Google Scholar]
- Frazier TW, et al. Demographic and clinical correlates of autism symptom domains and autism spectrum diagnosis. Autism. 2014b;18:571–82. doi: 10.1177/1362361313481506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, et al. Validation of proposed DSM-5 criteria for autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:28–40e3. doi: 10.1016/j.jaac.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex. 2006;16:1276–82. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Libove RA, Keshavan MS, Melhem NM, Minshew NJ. A preliminary longitudinal magnetic resonance imaging study of brain volume and cortical thickness in autism. Biological psychiatry. 2009;66:320–6. doi: 10.1016/j.biopsych.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Muddasani S, Vemulapalli M, Keshavan MS, Minshew NJ. An MRI study of increased cortical thickness in autism. Am J Psychiatry. 2006;163:1290–2. doi: 10.1176/appi.ajp.163.7.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Archives of general psychiatry. 2011;68:467–76. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick A, et al. Autism risk gene MET variation and cortical thickness in typically developing children and adolescents. Autism research : official journal of the International Society for Autism Research. 2012;5:434–9. doi: 10.1002/aur.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Bishop S, Gotham K, Huerta M, Lord C. Factors influencing scores on the social responsiveness scale. J Child Psychol Psychiatry. 2013;54:216–24. doi: 10.1111/j.1469-7610.2012.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Samson F, Evans AC, Mottron L. Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Human brain mapping. 2010;31:556–66. doi: 10.1002/hbm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Chen R, Ke X, Chu K, Lu Z, Herskovits EH. Predictive models of autism spectrum disorder based on brain regional cortical thickness. Neuroimage. 2010;50:589–99. doi: 10.1016/j.neuroimage.2009.12.047. doi:S1053-8119(09)01336-6 [pii] 10.1016/j.neuroimage.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157:163–71. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, et al. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. American journal of medical genetics. 2006;140:2257–74. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange N, Fletcher PT, Zygmunt KM. FindMinIC: Find Models with Minimum IC R package version 1.6. 2013 http://cran.r-project.org/package=FindMinIC.
- Libero LE, DeRamus TP, Deshpande HD, Kana RK. Surface-based morphometry of the cortical architecture of autism spectrum disorders: volume, thickness, area, and gyrification. Neuropsychologia. 2014;62:1–10. doi: 10.1016/j.neuropsychologia.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Lord C, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord M, Rutter A, LeCouteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of autism and developmental disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lowe JK, Werling DM, Constantino JN, Cantor RM, Geschwind DH. Social responsiveness, an autism endophenotype: genomewide significant linkage to two regions on chromosome 8. Am J Psychiatry. 2015;172:266–75. doi: 10.1176/appi.ajp.2014.14050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak-Fan KM, Taylor MJ, Roberts W, Lerch JP. Measures of cortical grey matter structure and development in children with autism spectrum disorder. Journal of autism and developmental disorders. 2012;42:419–27. doi: 10.1007/s10803-011-1261-6. [DOI] [PubMed] [Google Scholar]
- Misaki M, Wallace GL, Dankner N, Martin A, Bandettini PA. Characteristic cortical thickness patterns in adolescents with autism spectrum disorders: interactions with age and intellectual ability revealed by canonical correlation analysis. Neuroimage. 2012;60:1890–901. doi: 10.1016/j.neuroimage.2012.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Nordahl CW, Iosif AM, Lee A, Rogers S, Amaral DG. Increased Surface Area, but not Cortical Thickness, in a Subset of Young Boys With Autism Spectrum Disorder. Autism research : official journal of the International Society for Autism Research. 2016;9:232–48. doi: 10.1002/aur.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. URL http://www.r-project.org/ [Google Scholar]
- Raznahan A, et al. Mapping cortical anatomy in preschool aged children with autism using surface-based morphometry. NeuroImage Clinical. 2012;2:111–9. doi: 10.1016/j.nicl.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J, et al. Reduced cortical thickness and its association with social reactivity in children with autism spectrum disorder. Psychiatry research. 2015;234:15–24. doi: 10.1016/j.pscychresns.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Schaer M, et al. Decreased frontal gyrification correlates with altered connectivity in children with autism. Frontiers in human neuroscience. 2013;7:750. doi: 10.3389/fnhum.2013.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel C, et al. Imaging derived cortical thickness reduction in high-functioning autism: key regions and temporal slope. Neuroimage. 2011;58:391–400. doi: 10.1016/j.neuroimage.2011.06.040. [DOI] [PubMed] [Google Scholar]
- Schumann CM, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:4419–27. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, et al. Cortical thickness change in autism during early childhood. Human brain mapping. 2016;37:2616–29. doi: 10.1002/hbm.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PC, Hsu JW, Lan CC, Liu CC, Su TP, Chen YS. Structural and functional correlates of a quantitative autistic trait measured using the social responsive scale in neurotypical male adolescents. Autism research : official journal of the International Society for Autism Research. 2016;9:570–8. doi: 10.1002/aur.1535. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010;133:3745–54. doi: 10.1093/brain/awq279. doi:awq279 [pii] 10.1093/brain/awq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, et al. Divergence of Age-Related Differences in Social-Communication: Improvements for Typically Developing Youth but Declines for Youth with Autism Spectrum Disorder. Journal of autism and developmental disorders. 2017;47:472–479. doi: 10.1007/s10803-016-2972-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, et al. Longitudinal cortical development during adolescence and young adulthood in autism spectrum disorder: increased cortical thinning but comparable surface area changes. Journal of the American Academy of Child and Adolescent Psychiatry. 2015;54:464–9. doi: 10.1016/j.jaac.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Robustelli B, Dankner N, Kenworthy L, Giedd JN, Martin A. Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 2013;136:1956–67. doi: 10.1093/brain/awt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, et al. Distinct cortical correlates of autistic versus antisocial traits in a longitudinal sample of typically developing youth. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:4856–60. doi: 10.1523/JNEUROSCI.6214-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, et al. Reduced gray matter volume of pars opercularis is associated with impaired social communication in high-functioning autism spectrum disorders. Biological psychiatry. 2010;68:1141–7. doi: 10.1016/j.biopsych.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Yang DY, Beam D, Pelphrey KA, Abdullahi S, Jou RJ. Cortical morphological markers in children with autism: a structural magnetic resonance imaging study of thickness, area, volume, and gyrification. Mol Autism. 2016;7:11. doi: 10.1186/s13229-016-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, et al. Longitudinal changes in cortical thickness in autism and typical development. Brain. 2014;137:1799–812. doi: 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


