Structured Abstract
Background
Black Americans have greater rates, severity and resistance to treatment of hypertension than White Americans. The gut microbiota and its metabolites may contribute to this. This concept was tested in a pilot study.
Methods
Subjects with high (HBP, >140/80 mm Hg) and normal (NBP, <120/80mmHg) blood pressure (BP) provided stool and blood samples for whole genome sequencing (WGS) of gut microbiota and global untargeted metabolomics of serum. Patients were either black (B) with NBP (n=10 for WGS, 5 for metabolomics) and HBP (n=10 and 9, BHBP) or white (W) with NBP (n=20 and 13, WNBP) and HBP (n=12 and 8, WHBP).
Results
All four subject groups had distinct gut microbiota taxonomy by partial least squares discriminant analysis (PLS-DA). More importantly, linear discriminant analysis effect size showed marked differences in function of the microbiota of BHBP and WHBP (PLS-DA) with LDA scores <1. This included pathways for synthesis and interconversion of amino acids and inflammatory antigens. Similarly, metabolites differed (PLS-DA) with BHBP having significantly higher sulfacetaldehyde, quinolinic acid, 5-aminolevulinic acid, leucine and phenylalanine and lower 4-oxoproline and L-anserine.
Discussion
Combination analyses of functional gut metabolic pathways and metabolomics data in this small pilot study suggest that BHBP may have greater oxidative stress markers in plasma, greater inflammatory potential of the gut microbiome and altered metabolites with gut microbial functions implying insulin resistance. A fuller understanding of these potential differences could lead to race-based treatments for hypertension.
Keywords: hypertension, gut microbiota, global metabolites, racial disparities
Introduction
Black and white Americans have different incidence, severity and effective treatment of high blood pressure(HBP)1. Recent evidence highlighting gut microbiota alterations in HBP2 suggests potential racial differences in gut microbiomes may contribute to HBP disparities. We performed a pilot investigation of this relationship in Black (African descent) and White (European descent) Americans.
Methods
After IRB protocol approval and written informed consent, subjects provided fecal samples for whole genome sequencing(WGS) analysis of functional differences in gut microbiota, and blood for analysis of differential metabolites and gut ischemia markers. Black and white subjects were grouped by blood pressure (high blood pressure, HBP ≥ 140/80; normal blood pressure, NBP ≤ 120/80) at sampling. Systolic blood pressures between BHBP (151±4 mmHg) and WHBP (147±5 mmHg) were comparable: blood glucose was in normal range for all but 1 BHBP and 2 WHBP. Sample sizes were different between analyses as not every subject provided a blood sample. No differences by sex or age occurred but blacks had higher(p<0.05) body mass indexes.
WGS and Analysis
Stool was preserved (Quick-DNA™ Fecal/Soil Microbe Miniprep Kit; Zymo Research, Irvine, CA) and stored (−80°C ≤ 12 months). DNA was extracted, sequenced and analyzed in one batch (Wright Labs, LLC; Huntingdon, PA). DNA (1ng) underwent Nextera XT (Illumina, CA) tagmentation and library preparation with dual index barcoding. After quality assessment (high sensitivity bioanalyzer chip; Agilent, CA), equimolar library amounts were pooled and purified (QIAquick gel purification kit; Qiagen, CA) and sequenced (Illumina HiSeq4000) with a 2×150 index run. Raw read quality was assessed with FastQC, trimmed with Trimmomatic, using sliding window quality filtration at a 4-base average Q score of ≤ 28, and reads ≤ 120 basepairs discarded. Filtered reads were paired, concatenated and human DNA sequences removed (Kneaddata pipeline); unpaired reads were discarded. MetaPhlan quantified the samples’ bacterial taxonomic profiles, outputs were merged into a tsv table, then converted to a biom file for analyses. For functional gene profiles, filtered data were annotated (Uniref90 database within Humann2), regrouped (as KEGG orthology, KO) terms and counts per million normalized within Humann2. Partial least squares discriminant analysis (PLS-DA) of functional counts (METAGENassist) with interquartile range filtering. Variables with >85% zeros were removed and abundances normalized by autoscaling within METAGENassist. KOs were summarized in functional pathways using the KEGG database, relative abundances multiplied by 1 million and formatted as described3. LEfSe comparisons were made considering metacyc pathway data with “Race” as the main categorical variable (“Class”). Alpha levels of 0.05 were used for both Kruskal-Wallis and pairwise Wilcoxon tests. Linear Discriminant Analysis (LDA) scores >1 were displayed.
Metabolomics
Plasma samples underwent cellular extraction (Southeast Center for Integrated Metabolomics, SECIM). Global metabolomics profiling was performed (Thermo Q-Exactive Orbitrap mass spectrometer with Dionex UHPLC and autosampler). Samples were analyzed in positive and negative heated electrospray ionization with mass resolution of 35,000 at m/z 200 as separate injections and separated on an ACE 18-pfp 100 × 2.1 mm, 2 μm column with mobile phase A, 0.1% formic acid in water and mobile phase B, acetonitrile. This polar embedded stationary phase provides comprehensive coverage, but misses very polar species. Flow rate was 350 μL/min with 25°C column temperature. 4 μL was injected for negative and 2 μL for positive ions. MZmine (freeware) identified and aligned features, deisotoped, and performed gap filling of features missed in first alignment. Adducts and complexes were removed. Data were searched against SECIM internal retention time metabolite library. Values absent in 80% of data were removed. Missing values were imputed using k-nearest neighbor, quantile normalized, log2 transformed and autoscaled. Analyses were performed on combined positive and negative ions’ data using Metaboanalyst 3.0, an open source R-based program for metabolomics4. PLS-DA determined differential metabolites between groups and Student’s t-tests for differences between BHBP and WHBP, with p<0.05 considered significant.
Intestinal fatty acid binding protein (I-FABP) assays
Samples were measured by specific enzyme-linked immunosorbent assay (Quantikine, R&D Systems, # DFBP20). Inter-assay coefficient of variance was 6%.
Results
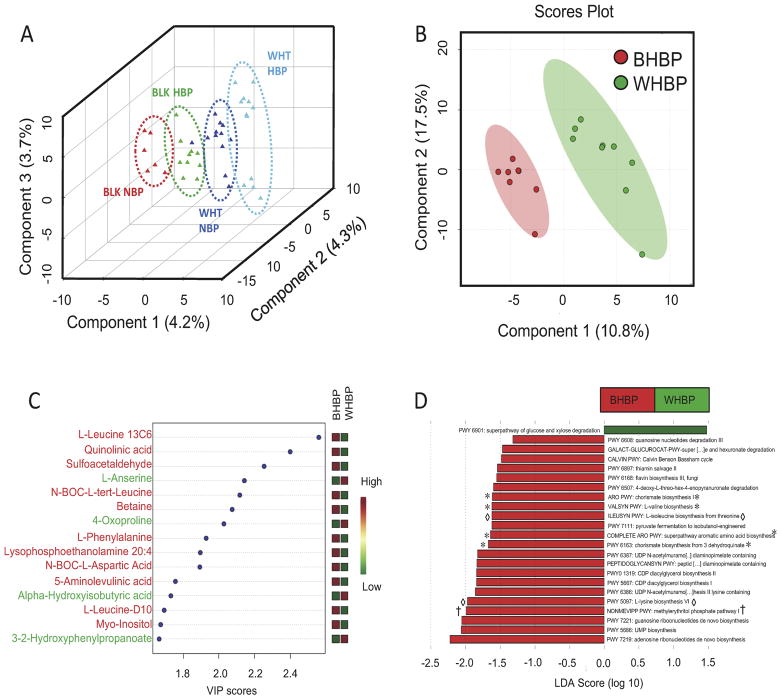
PLS-DA analysis of bacterial taxonomy derived from WGS of the 4 groups showed separation of populations (Figure 1A). Interestingly, the black HBP (BHBP) group was more closely related to the white NBP (WNBP); most divergent were white HBP (WHBP) and black NBP (BNBP). But, gut microbial taxonomy was different between BHBP and WHBP. In separate analyses of only hypertensive subjects, differential bacterial functional pathways (predominantly metabolic) driven by gut bacterial genomes were apparent (Figure 1D). For example, the KEGG pathway, methylerythritol phosphate pathway 1, was significantly enriched in microbiota of BHBP versus WHBP (LDA score = 2.0; † in Figure 1D).
Figure 1.
A. Partial least squares discriminant analysis (PLS-DA) of shotgun metagenomics determined bacterial populations in gut microbiota of blacks and whites with and without hypertension in this small cohort. Colored triangles represent subjects; black normal blood pressure (BLK NBP; red, n=6), black high blood pressure (BLK HBP; green, n=10), white normal blood pressure (WHT NBP; blue n=12), white high blood pressure (WHT HBP; aqua n=12). Dotted ovals are drawn as visual aids. B. PLS-DA scores plot representing known metabolites altered between black high blood pressure (BHBP; red, n=8) and white high blood pressure (WHBP; green, n=9) subjects. Pink and green ovals are 95% confidence interval ranges for BHBP and WHBP subjects, respectively. C. PLS-DA variable importance plot. A higher value indicates the importance of that metabolite in separating the groups shown in scores plot B. Metabolites named in red font are important variables in the African American (BHBP) group, and in green in the White American group (WHBP) D. Linear Discriminant Analysis (LDA) scores plot (log 10) of functional gene pathways overrepresented in gut microbiota of Black Americans with high blood pressure (BHBP; red bars) or White Americans with high blood pressure (WHBP; green bars) obtained from microbial whole genome sequence analysis of stool. * denotes pathways of de novo synthesis of amino acids, ◇ denotes pathways of interconversion of amino acids, † denotes microbial pathway for synthesis of the inflammatory antigens, isoprenoids.
PLS-DA analysis of global untargeted mass spectrographic analysis of plasma metabolites showed separation of metabolites by race with HBP in this study (Figure 1B). Variable importance plots highlighted differences (Figure 1C). Microbial and mammalian metabolites sulfoacetaldehyde5 and 5-aminolevulinic acid6 were significantly increased in BHBP compared to WHBP. Similarly, quinolinic acid, a tryptophan metabolite, was increased. In contrast, 4-oxoproline and L-anserine (carnosine metabolite) were higher in WHBP (Table 1).
Table 1.
| Metabolite | Black HBP | White HBP | P-value | VIP score |
|---|---|---|---|---|
| L-Leucine | 0.7258 ± 0.2036 | −0.6451 ± 0.2746 | 1.56E-03 | 2.56 |
| Quinolinic acid | 0.6804 ± 0.2754 | −0.6048 ± 0.2423 | 3.85E-03 | 2.40 |
| Sulfoacetaldehyde | 0.6390 ± 0.2350 | −0.5680 ± 0.2970 | 7.80E-03 | 2.25 |
| L-Anserine | −0.6077 ± 0.1458 | 0.5402 ± 0.3551 | 0.013 | 2.14 |
| Betaine | 0.5884 ± 0.1688 | −0.5230 ± 0.3530 | 0.016 | 2.07 |
| 4-oxoproline | −0.5752 ± 0.2875 | 0.5113 ± 0.2837 | 0.020 | 2.03 |
| L-Phenylalanine | 0.5471 ± 0.3037 | −0.4863 ± 0.2806 | 0.028 | 1.93 |
| 5-aminolevulinic acid | 0.4983 ± 0.3525 | −0.4429 ± 0.2477 | 0.049 | 1.76 |
| Alpha-hydroxyisobutyric acid* | −0.4910 ± 0.2537 | 0.4365 ± 0.3396 | 0.053 | 1.73 |
Values are means ± standard errors of the means of quantile normalized, log transformed areas of metabolic peaks in arbitrary units. VIP score derived from PLS-DA variable importance plot. Higher values indicate more importance of the metabolite in separating the subject groups.
bacterial metabolite, others are human- and bacterially-derived
BHBP associated more strongly with increased plasma leucine and phenylalanine than did WHBP, whereas WHBP had higher plasma levels of the microbial metabolite of valine, alpha-hydroxyisobutyric acid (Figure 1C, Table 1). BHBP patients’ microbiome was enriched in pathways for de novo synthesis (* Figure 1D) and interconversion (◇ Figure 1D) of amino acids. These, particularly leucine and phenylalanine, enter blood from the colon7. Betaine was also increased in BHBP compared to WHBP (Table 1). Finally, I-FABP was significantly increased in plasma of black compared to white HBP 2.2 ± 0.3ng/mL (n=9) vs 1.4 ± 0.2 ng/mL (n=8); mean ± SEM, P<0.05.
Discussion
These pilot data suggest that gut microbiome may contribute different features to HBP in blacks versus whites in our study cohort. Combined metabolite and microbial functions results suggest that black HBP is accompanied by higher oxidative stress, insulin resistance, and gut ischemia versus white HBP in our small study cohort, though these were not directly measured. Sulfoacetaldehyde, a taurine metabolite, is produced by polymorphonuclear cells during respiratory bursts8 and by the microbiome5. Taurine sequesters free radicals as it is metabolized to sulfoacetaldehyde. 5-aminolevulinic acid is substrate for ∂-aminolevulate dehydratase in heme synthesis. This enzyme is inhibited by pro-oxidants, lipid disturbances and type 2 diabetes (T2D)9 leading to increased 5-aminolevulinic acid, that further increases oxidative stress when oxidized at physiological pH to oxidants like hydrogen peroxide10. In WHBP, 4-oxoproline was higher versus BHBP (p=0.02); it is decreased by oxidative stress11. Quinolinic acid, a tryptophan metabolite, potent neuro-and gliotoxin and blood brain barrier modulator is increased by neuroinflammation, systemic immune activation and endotoxin12. The microbial methylerythritol phosphate pathway 1, generates intermediates that are proinflammatory microbial antigens, like 1-hydroxy-2 methyl-2-(E)-butenyl 4 diphosphate13, 14. This metabolite was not detected but as the metabolomic method used is insensitive for polar molecules we cannot discount the possibility that it was differential. Plasma sulfoacetaldehyde, 5-aminolevulinic acid, 4-oxoproline and quinolinic acid and enrichment of microbial antigen synthesis indicate oxidative stress and inflammation, processes associated with hypertension, and elevated in black versus white HBP in this cohort.
Potential insulin resistance in black HBP is suggested by decreased L-anserine15 and 4-oxoproline11, and increased betaine. Betaine, made by gut microbiota, is present in food and produced from choline in liver and kidney. It is protective against the pro-hypertensive actions of homocysteine, liver steatosis and dyslipidemia, but is a precursor of trimethylamine N-oxide, associated with hypertension, atherosclerosis and T2D15. Furthermore, increased branched chain and aromatic amino acids, particularly phenylalanine, isoleucine and tyrosine, carry enhanced risk for future development of T2D and are measurably different at least 12-years beforehand16, also suggesting insulin resistance in this BHBP versus WHBP patient population.
Hypertension in rodents is associated with decreased mesenteric blood flow17. I-FABP is released from ischemic human gut epithelium18 and linked to increased gut permeability, T2D19 and hypertension (unpublished data). IFABP differences in our BHBP subjects may indicate reduced mesenteric blood flow compared with WHBP. Together, these data reinforce differences between whites and blacks with HBP in this pilot study.
Limitations
A major limitation is small sample size, due to high cost of WGS; but WGS of gut microbiota in humans is a strength, being less biased and more robust for detecting function than 16S rRNA gene sequencing methodology. Another limitation is the relative inability of metabolomics methodology to detect lipid species, important in HBP. The small geographic area housing our study subjects reduces environmental influences on gut microbiome, however this may mean that the data lack broad applicability. Also, the data is cross-sectional rather than longitudinal and dietary considerations were not included. A final limitation is self-identification of race; however genetic testing, unavailable for this data set, was very closely linked to self-identification in other studies of our hypertensive patients20.
These pilot data provide initial information on a unique metabolic profile, partly dependent upon an altered gut microbiota, in blacks with HBP in a small cohort based in one USA location. A large-scale, multicenter study would validate these findings, determine the role of lipid metabolites and whether the profile is altered before HBP develops. If confirmed, race-based therapies, capitalizing on these differences, might be developed to decrease the racial disparities in HTN in the USA.
Highlights.
Black and white Americans with hypertension have different gut microbiomes
The gut microbiomes differ in composition and function
The gut microbiomes contribute to differing circulating metabolites
More oxidative stress, inflammation, gut ischemia in black than white hypertensives
Acknowledgments
Supported by NIH Grants HL33610 and HL132448
We gratefully acknowledge the help of Sara Croft, BA, Clinical Coordinators in Cardiovascular Division of College of Medicine at University of Florida, and the subjects of the study. The project was supported by NIH grants HL-33610, HL-132448 and U24DK-097209
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whelton P, Carey R, Aronow W, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of Cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017 Nov 7; doi: 10.1016/j.jacc.2017.11.006. pii: S0735-1097(17):41519-1. [DOI] [PubMed]
- 2.Richards EM, Pepine CJ, Raizada MK, et al. The gut, its microbiome, and hypertension. Curr Hypertens Rep. 2017;19(4):36. doi: 10.1007/s11906-017-0734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;2(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia J, Sinelnikov I, Han B, et al. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res. 2015 Jul 1;43(W1):W251–7. doi: 10.1093/nar/gkv380. Epub 2015 Apr 20.;43(W1):W251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondo H, Anada H, Osawa K, et al. Formation of sulfoacetaldehyde from taurine in bacterial extracts. J Biochem. 1971;69(3):621–3. [PubMed] [Google Scholar]
- 6.Dailey HA, Dailey TA, Gerdes S, et al. Prokaryotic heme biosynthesis: Multiple pathways to a common essential product. Microbiol Mol Biol Rev. 2017 Jan 25;81(1) doi: 10.1128/MMBR.00048-16. pii: e00048–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto M, Ooga T, Kibe R, et al. Colonic absorption of low-molecular-weight metabolites influenced by the intestinal microbiome: A pilot study. PLoS One. 2017 Jan 25;12(1):e0169207. doi: 10.1371/journal.pone.0169207. Jan 25;12(1):e0169207(Jan 25;12(1):e0169207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham C, Tipton K, Dixon H. Conversion of taurine into N-chlorotaurine (taurine chloramine) and sulphoacetaldehyde in response to oxidative stress. Biochem J. 1998 Mar 1;330(Part 2):939–45. doi: 10.1042/bj3300939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonfanti G, Ceolin RB, Valcorte T, et al. δ-Aminolevulinate dehydratase activity in type 2 diabetic patients and its association with lipid profile and oxidative stress. Clinical Biochemistry. 2011;44:1105–9. doi: 10.1016/j.clinbiochem.2011.06.980. [DOI] [PubMed] [Google Scholar]
- 10.Hermes-Lima M, Valle V, Vercesi A, et al. Damage to rat liver mitochondria promoted by delta-aminolevulinic acid-generated reactive oxygen species: Connections with acute intermittent porphyria and lead-poisoning. Biochim Biophys Acta. 1991;1056(1):57–63. doi: 10.1016/s0005-2728(05)80072-6. [DOI] [PubMed] [Google Scholar]
- 11.den Ouden H, Pellis L, Rutten G, et al. Metabolomic biomarkers for personalised glucose lowering drugs treatment in type 2 diabetes. Metabolomics. 2016 doi: 10.1007/s11306-015-0930-4. Epub 2016 Jan 6.;12(27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito K, Markey S, Heyes M. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience. 1992;51(1):25–39. doi: 10.1016/0306-4522(92)90467-g. [DOI] [PubMed] [Google Scholar]
- 13.Feurle J, Espinosa E, Eckstein S, et al. Escherichia coli produces phosphoantigens activating human gamma delta T cells. J Biol Chem. 2001 Oct 23;277(1):148–54. doi: 10.1074/jbc.M106443200. 2002. [DOI] [PubMed] [Google Scholar]
- 14.Sicard H, Fournie J. Metabolic routes as targets for immunological discrimination of host and parasite. Infect Immun. 2000;68(8):4375–7. doi: 10.1128/iai.68.8.4375-4377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–55. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T, Larson M, Vasan R, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011 Apr;17(4):53. doi: 10.1038/nm.2307. 2011 Epub 2011 Mar 20.;17(4):448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santisteban MM, Qi Y, Zubcevic J, et al. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2017;120(2):312–23. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derikx J, Schellekens D, Acosta S. Serological markers for human intestinal ischemia: A systematic review. Best Pract Res Clin Gastroenterol. 2017;31(1):69–74. doi: 10.1016/j.bpg.2017.01.004. Epub 2017 Feb 7. [DOI] [PubMed] [Google Scholar]
- 19.Cox A, Zhang P, Bowden D, et al. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 2017 Apr;4343(2)(2):163–166. 163–6. doi: 10.1016/j.diabet.2016.09.004. 2017 Epub 2016 Oct 10. [DOI] [PubMed] [Google Scholar]
- 20.Gerhard T, Gong Y, Beitelshees A, et al. Alpha-adducin polymorphism associated with increased risk of adverse cardiovascular outcomes: Results from GENEtic substudy of the INternational VErapamil SR-trandolapril STudy (INVEST-GENES) Am Heart J. 2008;156(2):397–404. doi: 10.1016/j.ahj.2008.03.007. Epub 2008 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]