Abstract
Metazoan eggs have a specialized coat of extracellular matrix that aids in sperm-egg recognition. The coat is rapidly remodeled after fertilization to prevent polyspermy and establish a more permanent barrier to protect the developing embryo. In nematodes, this coat is called the vitelline layer, which is remodeled into the outermost layer of a rigid and impermeable eggshell. We have identified three key components of the vitelline layer structural scaffold – PERM-2, PERM-4 and CBD-1, the first such proteins to be described in the nematode C. elegans. CBD-1 tethered PERM-2 and PERM-4 to the nascent vitelline layer via two N-terminal chitin-binding domains. After fertilization, all three proteins redistributed from the zygote surface to the outer eggshell. Depletion of PERM-2 and PERM-4 from the scaffold led to a porous vitelline layer that permitted soluble factors to leak through the eggshell and resulted in embryonic death. In addition to its role in vitelline layer assembly, CBD-1 is also known to anchor a protein complex required for fertilization and egg activation (EGG-1–5/CHS-1/MBK-2). We found the PERM complex and EGG complex to be functionally independent, and structurally organized through distinct domains of CBD-1. CBD-1 is thus a multifaceted regulator that promotes distinct aspects of vitelline layer assembly and egg activation. In sum, our findings characterize the first vitelline layer components in nematodes, and provide a foundation through which to explore both conserved and species-specific strategies used by animals to build protective barriers following fertilization.
Keywords: vitelline layer, C. elegans eggshell, fertilization, PERM-2 and PERM-4, CBD-1, zona pellucida
Graphical abstract
Introduction
Metazoan oocytes are covered by a layer of extracellular matrix that promotes species-specific recognition between egg and sperm. Following fertilization, the matrix is rapidly remodeled in order to prevent polyspermic fertilization and to protect the developing embryo from environmental insults (Wong and Wessel, 2008a). While this protective barrier goes by many different names – the fertilization envelope in sea urchin, the zona in mammals, the chorion in fish, and the eggshell in diptera and nematodes – the remodeling of the matrix utilizes evolutionarily conserved processes, including exocytosis of specialized secretory vesicles called cortical granules; activation of proteases, glycosidases, and cross-linkers; and assembly of carbohydrate- and glycoprotein-based matrices for structural support (Wong and Wessel, 2006).
The nematode eggshell is a multi-layered structure that provides mechanical support and environmental protection to the developing embryo (Christenson, 1950; Johnston and Dennis, 2011; Stein and Golden, 2015). It assembles in a step-wise manner using materials supplied by the oocyte. The outermost vitelline layer is thought to reside on the surface of the unfertilized oocyte, but little is known about its composition other than that it contains glycoproteins (Johnston et al., 2006; Natsuka et al., 2005). Just beneath the vitelline layer, the second layer forms upon fertilization through activation of chitin synthase (CHS-1), which polymerizes and deposits chitin beneath the vitelline layer, likely contributing to lifting of the vitelline layer from the zygote surface (Johnston et al., 2010; Maruyama et al., 2007; Zhang et al., 2005). Soon after, entry into anaphase of meiosis I triggers exocytosis of cortical granules that release their cargo of chondroitin proteoglycans (CPGs) to form the third eggshell layer (K. Sato et al., 2006; Bembenek et al., 2007; M. Sato et al., 2008; Olson et al., 2012). Following anaphase of meiosis II, the innermost layer of the eggshell assembles from fatty acid-based lipids to form the permeability barrier, which limits passage of small molecules and provides osmotic support to the embryo (Tagawa et al., 2001; Rappleye et al., 2003; Benenati et al., 2009; Olson et al., 2012). As one of the most impermeable structures in the animal kingdom (Anya, 1976; Christenson, 1950; Fairbairn, 1957), it is important to understand how the eggshell forms, the degree to which its construction mirrors that of extracellular barriers in other metazoans, and whether it can be exploited to combat parasitic nematode infection.
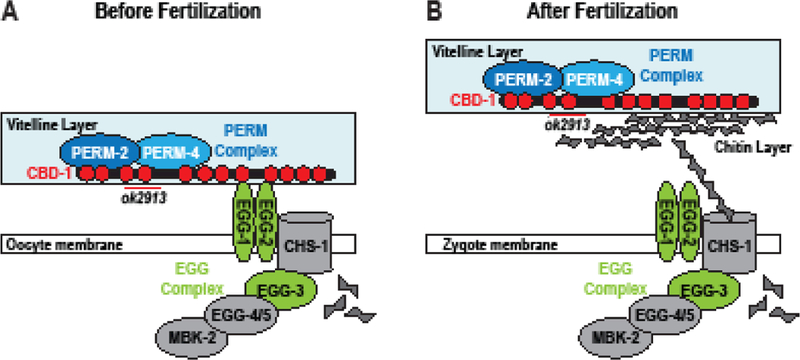
The outermost vitelline layer of the eggshell sits at an important junction. Proteins embedded in the vitelline layer are likely to play a role in sperm-egg recognition and/or binding, yet after fertilization must be rapidly remodeled in order to prevent polyspermic fertilization and to create the first line of defense in eggshell protection. A protein complex involved in C. elegans fertilization and polyspermy prevention was recently identified and shown to reside on the oocyte surface. Chitin-binding domain protein 1 (CBD-1) anchors the Egg Sterile (Egg) family members EGG-1 and EGG-2 to the cortex, where they have been proposed to play a role in fertilization (Johnston et al., 2010; Kadandale et al., 2005). CBD-1 also helps to anchor CHS-1, the EGG-3/4/5 proteins, and mini-brain kinase (MBK-2) to the oocyte cortex. Soon after fertilization, CHS-1 and the EGG-1/2/3 proteins are endocytosed, while EGG-4/5 and MBK-2 are released from the cortical tether into the cytoplasm to regulate the oocyte-to-embryo transition (Cheng et al., 2009; Maruyama et al., 2007; Stitzel et al., 2007), highlighting the importance of these proteins in egg activation, but not in eggshell vitelline layer remodeling. The fate of CBD-1 after fertilization is unclear; Johnston and Dennis (Johnston and Dennis, 2011) proposed that chitin polymer secreted at fertilization could bind the chitin-binding domains of CBD-1 and relieve its tether to the membrane-bound EGG-1–5/CHS-1/MBK-2 complex (hereafter called the EGG complex), but experiments to support this model have not yet been conducted. If CBD-1 is released from the zygote surface after fertilization, it is likely that proteins other than members of the EGG complex assist CBD-1 to remodel the nascent vitelline layer into the outermost layer of the eggshell.
To investigate vitelline layer formation in C. elegans, we examined proteins from a previous RNAi screen that were promising candidates for vitelline layer formation (Carvalho et al., 2011). We found that Permeable Eggshell protein 2 (PERM-2), PERM-4, and CBD-1 are essential components of the eggshell vitelline layer. Recruitment of PERM-2 and PERM-4 to the vitelline layer requires two chitin-binding domains in the N-terminus of CBD-1, but these two domains and the PERM complex itself are dispensable for the EGG complex, highlighting the dual role of CBD-1 in vitelline layer formation and egg activation. Disruption of PERM-2 and PERM-4 function by RNAi or null mutation resulted in leakage of soluble factors through the eggshell and caused embryonic lethality, indicating that PERM-2 and PERM-4 maintain the structural integrity of the vitelline layer that is important for establishing the impermeability of the nematode eggshell. In sum, this study characterizes the first set of bona fide vitelline layer proteins in C. elegans and lays the groundwork for future studies that compare vitelline layer remodeling in nematodes to other metazoans.
Materials & Methods
C. elegans strains
Worm strains used in this study are listed in Table S1, some of which were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). N2 (wild-type) (Brenner, 1974), AD189 (GFP::EGG-1) and RT495 (EGG-2::GFP) (Kadandale et al., 2005), AD200 (EGG-3::GFP) and AD238 (EGG-3::mCherry) (Maruyama et al., 2007), OD95 (GFP::PHPLC1δ1; mCherry::H2B) (Audhya et al., 2005), OD344 (mCherry::CPG-2) and OD367 (mCherry::CPG-1; GFP:: PH) (Olson et al., 2012), and VC2258 (cbd-1(ok2913) mutant) (C. elegans Deletion Mutant Consortium, 2012) were previously described. AG212 (CBD-1::mCherry) was a generous gift from David Levine and Andy Golden (NIH NIDDK). Strains were maintained at 20°C–25°C on nematode growth media (RPI, N81800) seeded with OP50 bacteria.
BLAST searches
Protein sequences for PERM-2 and PERM-4 were obtained from WormBase (www.wormbase.org version WS265) and used as a query in the NCBI blastp search engine to identify nematode homologs (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The protein sequence of each hit was used in a reciprocal blastp search that restricted the search set to the C. elegans genome. Only those nematode sequences that resulted in PERM-2 or PERM-4 as the top hit were considered as putative orthologs.
Plasmids and transgenic strains
PERM-2::mCherry (POM1) and PERM-4::mCherry (POM6) strains were generated via Mos1-mediated single copy insertion (MosSCI) as described (Frøkjær-Jensen et al., 2008). Gibson assembly (Gibson et al., 2009) was used to combine PCR products amplified by Q5 DNA Polymerase (NEB, M0491) with the primers in Table S2 using pBluescript, N2 genomic DNA, or pAA65 (McNally et al., 2006) as DNA sources for the vector backbone, perm-2 or perm-4, and mCherry sequences, respectively. AvrII (NEB, R0174) sites were introduced to facilitate subcloning from pBluescript into pCFJ151 that targets a Mos1 transposon site in chromosome II (Frøkjær-Jensen et al., 2008). pSO53 contained perm-2 coding sequence fused to a C-terminal mCherry tag under the control of 1.8kb of endogenous promoter and 1.2kb of 3’UTR regulatory sequences, while pSO58 contained similar sequences for C-terminally tagged perm-4 (1.5kb of endogenous promoter and 2.9kb of 3’UTR sequences). Paralyzed EG6429 adult worms (unc-119(ed3); ttTi5605 II) (Frøkjær-Jensen et al., 2014) were injected in the gonad with a mixture containing pCFJ601 (Mos1 transposase); pMA122 (peel-1) (Seidel et al., 2011); co-injection markers pCFJ90, pCFJ104, and pGH8; and either pSO53 or pSO58 (Frøkjær-Jensen et al., 2014; 2008). One week after injection the animals were heat shocked at 34°C for 2hr to activate peel-1 counter-selection, and moving worms were screened for absence of co-injection markers. Properly integrated lines were verified by DNA sequencing and outcrossed six times to N2 males.
The perm-2(pmn1) null allele (POM37) was created via CRISPR/Cas9-mediated gene editing according to the long-range homology-directed repair and self-excising cassette (SEC) method (Dickinson et al., 2015) using the oligos listed in Table S2. The pSO210 sgRNA/Cas9 plasmid to introduce a double-stranded break near the start codon of perm-2 was generated by site-directed mutagenesis of plasmid pJW1219 (Ward, 2015). The pSO212 repair template was designed to insert GFP in place of the perm-2 coding sequence, and was created by cutting pDD282 with ClaI/SpeI and performing HiFi DNA assembly (NEB, E5520) with PCR-generated left (557 bp) and right (608 bp) homology arms containing 30 bp of overlap with the pDD282 vector (Dickinson et al., 2015). Adult N2 worms were injected with a mixture containing pSO210, pSO212, and co-injection markers pCFJ90, pCFJ104, and pGH8. Hygromycin (5 mg/ml, Thermo Fisher, 10–687-010) was added to growth plates on day 3 post-injection to select for animals harboring the SEC, and one week later animals were screened for genomic edits through presence of the SEC (Rol phenotype) and absence of co-injection markers. Three independent lines were obtained, and one was selected for six outcrosses to N2 and SEC excision. Correct removal of perm-2 coding sequence and replacement with GFP was verified by sequencing through the edited region (Eurofins-Operon). All vectors for MosSCI and CRISPR/Cas9 cloning were obtained from Addgene.
RNA interference
Double-stranded RNA was prepared by PCR amplification of N2 genomic DNA using the oligos listed in Table S2, and in vitro transcription with Megascript T7 and T3 kits (Thermo Fisher, AM1334 and AM1338). RNA was purified by phenol-chloroform extraction and isopropanol precipitation. Pellets were solubilized in 1x soaking buffer (11mM Na2HPO4, 5.5mM KH2PO4, 2.1mM NaCl, 4.7mM NH4Cl) and annealed by incubation at 68°C for 10 minutes followed by 37°C for 30 minutes. Animals at the L4 or young adult stage were injected with 1 mg/ml dsRNA and grown for 6–30 hours at 20–23°C for imaging experiments, or recovered for 2 hours at room temperature and singled to individual plates for brood size and permeability assays. Control RNAi experiments were performed with 1 mg/ml of him-8 dsRNA or 0.5 mg/ml each of dsRNA targeting cpg-1 and cpg-2; neither control affects vitelline layer formation (Olson et al., 2012); (Olson et al., 2006). Short stretches of perm-2 dsRNA between 26–37 nucleotides share 87–93% identity with xbp-1, tcc-1, and Y39G10AR.11 (none of which exhibit penetrant embryonic lethality in genome-wide RNAi screens), while five regions between 19–21 nucleotides share 70–80% identity with perm-4, which could theoretically cause off-target RNAi effects. Short stretches of perm-4 dsRNA between 23–26 nucleotides share 88–91% identity with npp-8 and vab-1, while one region 21 nucleotides long shares 71% identity to perm-2.
Brood size and eggshell permeability assays
Mutant or injected L4-stage worms were singled onto individual plates and allowed to lay eggs for three days with transfer to a fresh plate each day. For permeability assays, growth plates were supplemented with 150 ug/ml Nile Blue A (Sigma, N0766). Twenty-four hours after removal of the adult worm, plates were scored for brood size (number of hatched larvae + unhatched clear embryos + unhatched blue embryos) (Carvalho et al., 2011).
Immunofluorescence
For immunofluorescence experiments, embryos were dissected on subbing solution-coated slides (4 mg/ml gelatin USP, 0.4 mg/ml chromalum, 1 mg/ml poly-L-lysine) in 0.75x egg salts (1x egg salts: 118 mM NaCl, 40 mM KCl, 3.4 mM MgCl2, 3.4 mM CaCl2, 5 mM Hepes pH7.4), freeze cracked, and fixed in −20°C methanol for 15 minutes as described previously (Olson et al., 2012). Following rehydration in PBS, slides were stained overnight with a 1:100 dilution of rhodamine-conjugated chitin-binding probe (New England Biolabs, discontinued) and 1 ug/ml DAPI (ThermoFisher, D1306) before being mounted in 0.5% p-phenylenediamine in 90% glycerol, 20 mM Tris pH 8.8. In some cases, the eggshell vitelline layer was removed prior to the freeze-cracking step by brief alkaline-bleach treatment (0.5 N NaOH, 2.5% bleach in M9 buffer). In all cases, a single central plane was imaged.
Live imaging microscopy
Whole worm imaging was performed by anesthetizing adults in a 2 ul drop containing 3 mg/ml tricane and 0.3 mg/ml tetramasole in M9 buffer on a multiwell test slide (MP Biomedicals, 096041505). Embryo imaging was performed by dissecting adults on a 24×50mm cover glass in a 3 ul drop of 0.75x egg salts surrounded by a thin ring of petroleum jelly, which prevented compression when overlayed with 22×22 mm cover glass (Olson et al., 2012). In some experiments, egg salts were supplemented with 6 uM FM4–64 or 2 ug/ml DAPI to verify eggshell permeability. All experiments involving cbd-1(ok2913) or perm-2(pmn1) worms were conducted with age-matched controls. Images of a single central plane were collected on an upright Nikon clipse 90i microscope equipped with a QuantEM 512SC CCD camera (Photometrics) using a 40× 0.75 NA Plan Fluor or 100× 1.40 NA Plan Apo VC objective lens and analyzed with either Nikon Elements or Fiji software (Schindelin et al., 2012).
Relative fluorescence intensity values in Fig. 2D were obtained by calculating Corrected Total Cell Fluorescence (CTCF) for a region of interest (ROI) drawn immediately outside the eggshell (total signal), an ROI surrounding the embryo (embryo signal), and a background ROI located between the embryo and eggshell. Eggshell signal was calculated as CTCFtotal – CTCFembryo. All values were normalized to total CTCF in control embryos. CTCF = (ROI integrated density – (ROI area * mean background fluorescence)). Variance values in Fig. 3C-E were measured by drawing a line along the boundary between two adjacent oocytes and obtaining plot profiles in Nikon Elements. The variance in log10 fluorescence intensity ratios between mCherry and GFP signals along the line was calculated to determine whether the mCherry-tagged vitelline layer protein exhibited clustering patterns similar to or different from the GFP::PH plasma membrane signal.
Figure 2. PERM-2 and PERM-4 localize co-dependently to the eggshell vitelline layer and require CBD-1.
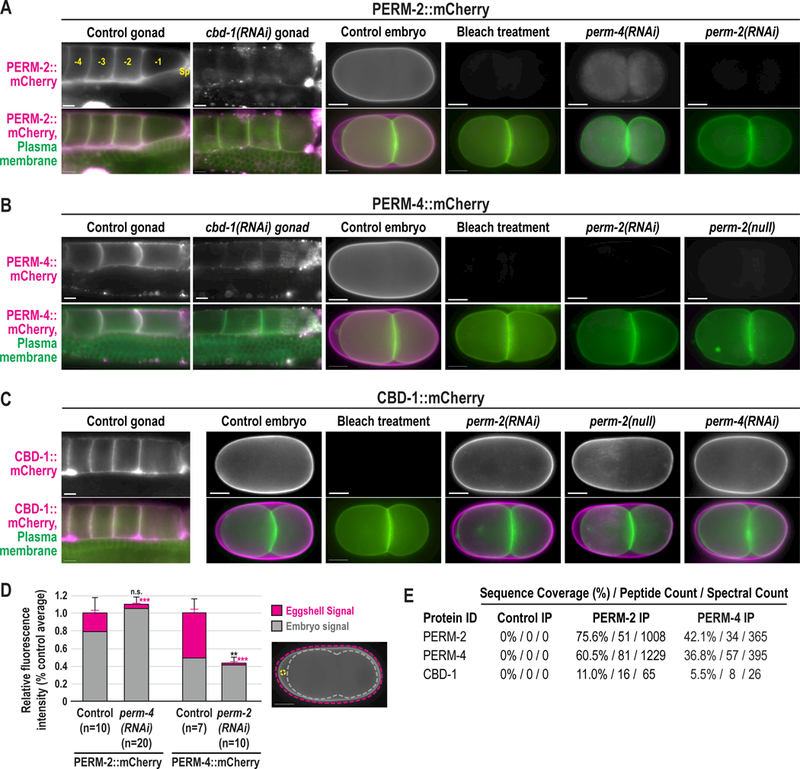
Widefield fluorescence images of gonads and embryos from control, RNAi-treated, or perm-2(null) worms that express a GFP-tagged plasma membrane probe (green) with either PERM-2::mCherry (A), PERM-4::mCherry (B), or CBD-1::mCherry (C) (magenta). Oocytes are numbered in (A) relative to the spermatheca (Sp), with the −1 oocyte the next to be fertilized. Some worms were treated with alkaline bleach, which removes the outermost vitelline layer but not the chitin or CPG layers of the eggshell. N=number of adults (not determined for some experiments), n=number of gonads or embryos. Scale bars, 10um. (D) Quantification of relative fluorescence intensity measurements of PERM-2::mCherry or PERM-4::mCherry found in the eggshell (pink bars) or embryo (gray bars), normalized to total fluorescence in controls. Fluorescence levels measured within regions of interest (ROIs) for total fluorescence (pink dotted line), embryo (gray dotted line), and background (yellow circle). Eggshell signal calculated as total fluorescence – embryo fluorescence. See methods for additional details on quantification. Illustrative image reproduced from panel A. n=number of embryos analyzed. Black error bars represent standard error of the mean (SEM) for total fluorescence (eggshell signal + embryo signal), pink error bars for eggshell signal only. ***p<0.0001 compared to controls by unpaired student’s t-test with unequal variance. (E) Mass spectrometry analysis of proteins co-immunoprecipitated from whole worm extract. Control IP, non-specific IgG beads incubated with PERM-2:mCherry extract. PERM-2 IP, RFP-Trap beads incubated with PERM-2::mCherry extract. PERM-4 IP, RFP-Trap beads incubated with PERM-4::mCherry extract. Table shows sequence coverage (percentage of protein length covered by identified peptides), peptide counts (total number of distinct peptides), and spectral counts (total number of spectra for all peptides) for PERM-2 (or PERM-2::mCherry), PERM-4 (or PERM-4::mCherry), and CBD-1 in the eluted fractions.
Figure 3. The PERM complex and EGG complex are structurally independent.
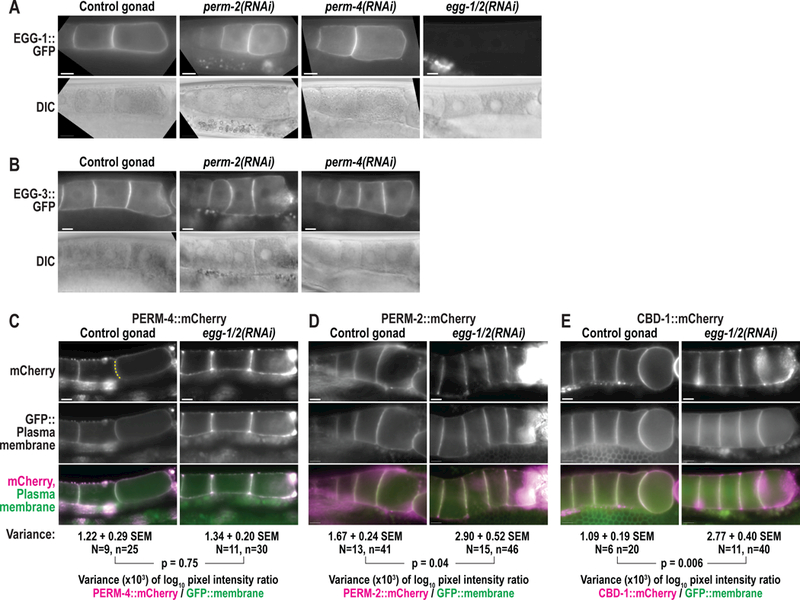
Widefield fluorescence and DIC images of gonads from control and RNAi-treated worms that expressEGG-1::GFP (A), EGG-3::GFP (B), PERM-4::mCherry (C), PERM-2::mCherry (D), or CBD-1::mCherry (E). N=number of adults, n=number of gonads. For (C)-(E), irregular distribution offluorescent markers was calculated as variance in log10 fluorescence intensity ratios between mCherry-tagged proteins and a GFP::plasma membrane marker along oocyte boundaries (dotted yellow line in C). SEM, standard error of the mean. N=number of gonads, n=number of oocyte boundaries. P-values calculated by unpaired student’s t-test with unequal variance. Scale bars, 10um.
Co-immunoprecipitation and protein identification by mass spectrometry
Crude extract was prepared from POM24 (PERM-4::mCherry; GFP::PH) and POM27 (PERM-2::mCherry; GFP::PH) adult worms by sonication in 10 mM Tris pH 7.5, 150 mM NaCl, 0.5 mM EDTA and 0.5% NP-40, and the soluble fraction was incubated with RFP-Trap magnetic agarose beads as recommended by the manufacturer (Chromotek). Immunoprecipitated proteins were eluted in 50 mM Tris pH8.5 containing 8M urea (Cheeseman et al., 2004) and processed for protein identification by mass spectrometry (Fonslow, 2014). Attempts to immunoprecipitate CBD-1::mCherry were unsuccessful.
For mass spectrometry analysis, samples were precipitated by methanol/chloroform. Dried pellets were dissolved in 8 M urea/100 mM TEAB, pH 8.5. Proteins were reduced with 5 mM tris(2-carboxyethyl)phosphine hydrochloride (TCEP, Sigma-Aldrich) and alkylated with 10 mM chloroacetamide (Sigma-Aldrich). Proteins were digested overnight at 37oC in 2 M urea/100 mM TEAB, pH 8.5, with trypsin (Promega). Digestion was quenched with formic acid, 5% final concentration. The digested samples were analyzed on a Q Exactive mass spectrometer (Thermo). The digest was injected directly onto a 15cm, 100um ID column packed with Aqua 3um C18 resin (Phenomenex). Samples were separated at a flow rate of 300nl/min on an Easy nLCII (Thermo). Buffer A and B were 0.1% formic acid in 5% acetonitrile and 0.1% formic acid in 80% acetonitrile, respectively. A gradient of 1–35% B over 180 min, an increase to 80% B over 40 min, and held at 90% B for 5 min of washing prior to returning to 1% B was used for 240 min total run time. The column was re-equilibrated with 10ul of buffer A prior to the injection of sample. Peptides were eluted directly from the tip of the column and nanosprayed directly into the mass spectrometer by application of 2.5kV voltage at the back of the column. The Q Exactive was operated in a data dependent mode. Full MS1 scans were collected in the Orbitrap at 70K resolution with a mass range of 400 to 1800 m/z. The 10 most abundant ions per cycle were selected for MS/MS and dynamic exclusion was used with exclusion duration of 15 sec.
Protein and peptide identification were done with Integrated Proteomics Pipeline – IP2 (Integrated Proteomics Applications). Tandem mass spectra were extracted from raw files using RawConverter (He et al., 2015) and searched with ProLuCID (Xu et al., 2015) against a protein database from www.wormbase.org (PRJNA13758.WS265) appended with common contaminants and RFP fusion proteins. The search space included all fully-tryptic and half-tryptic peptide candidates. Carbamidomethylation on cysteine was considered as a static modification. Data was searched with 50 ppm precursor ion tolerance and 600 ppm fragment ion tolerance. Identified proteins were filtered using DTASelect (Tabb et al., 2002) and utilizing a target-decoy database search strategy to control the false discovery rate to 1% at the protein level.
Statistics and protein predictions
Statistical analysis was carried out by unpaired student’s t-tests with unequal variance using GraphPad software (www.graphpad.com/quickcalcs/ttest1.cfm). O-linked mucin attachment sites were predicted based on consensus sequence (Chen et al., 2008). Chitin-binding domains were predicted by Prosite (prosite.expasy.org).
Critical reagents and resources are compiled in the Key Resources Table.
Results
PERM-2 and PERM-4 are required for eggshell integrity
PERM-2 and PERM-4 were previously identified in an RNAi screen for proteins required for eggshell formation in C. elegans (Carvalho et al., 2011). Reciprocal BLAST searches showed that PERM-2 and PERM-4 are widely conserved in free-living (Caenorhabditis, Diplosacpter, Pristionchus) and parasitic nematode genera (Ancylostoma, Necator, Haemonchus, Strongyloides, Loa, Brugia, and others), but little is known about their structure or function. PERM-2 and PERM-4 contain signal sequences and predicted O-linked mucin glycan attachment sites (Chen et al., 2008), so are likely to be secreted (Fig. 1A). While PERM-2 and PERM-4 lack characterized functional domains, multiple defined regions are highly conserved in their putative orthologs (Fig. 1A). To further investigate their function in eggshell formation, we analyzed the effect of PERM-2 and PERM-4 depletion on brood size and eggshell permeability. Inhibition of PERM-2 or PERM-4 by RNAi or null mutation significantly decreased brood size and increased eggshell permeability (Fig. 1B), indicating these proteins play an important role in maintaining the integrity of the C. elegans embryonic eggshell. Given that the perm-2(null) mutation had a less severe phenotype than RNAi inhibition (see methods for list of putative unintended targets), we decided to investigate onset of penetrance by analyzing brood size and permeability over the three successive days of adult fertility. Eggshell permeability defects became increasingly severe over time, particularly between days 1 and 2 of adulthood (Fig. 1C). Embryos depleted of PERM-2 or PERM-4 readily incorporated FM4–64 dye from the surrounding media into their plasma membranes, indicating that the eggshell permeability barrier was compromised (Fig. 1D). Depleted embryos completed several divisions before arresting around the gastrulation stage, a phenotype similar to that of proteins required to form the innermost permeability barrier of the eggshell (Olson et al., 2012). Simultaneous depletion of both PERM-2 and PERM-4 failed to enhance the phenotype, with embryos arresting at a stage similar to the single depletions (Fig. 1D). Whether embryonic arrest is caused by permeability defects or another factor such as polarity defects(Rappleye et al., 1999; Tagawa et al., 2001) remains unknown.
Figure 1. PERM-2 and PERM-4 are required for eggshell integrity.
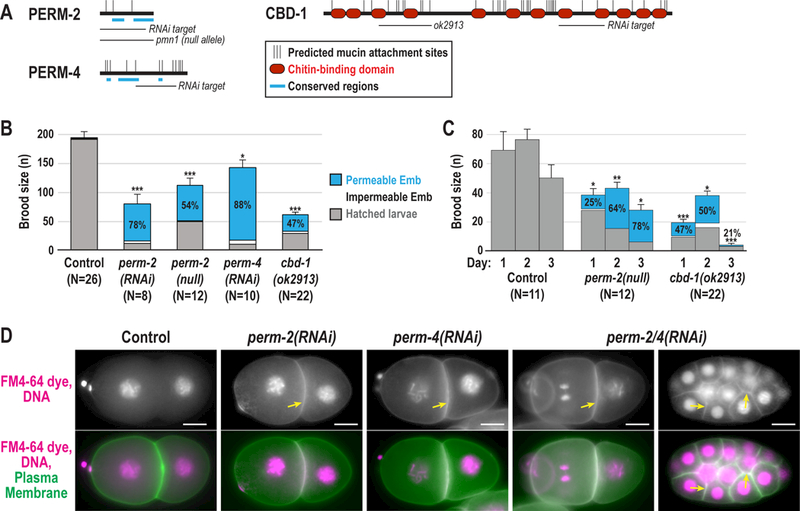
(A) Schematic representations of PERM-2, PERM-4 and CBD-1 showing the location of predicted O-linked mucin attachment sites (thin vertical black lines), chitin-binding domains (red ovals), conserved regions (thick blue lines), deletion alleles, and target regions for dsRNA-mediated interference (RNAi). Chitin-binding domains 3 and 4 of CBD-1 are disrupted by the in-frame ok2913 deletion allele. (B) Left, total brood size counts defined as the number of progeny produced from a single worm over a three-day laying period. Gray bars, hatched larvae; White bars, unhatched impermeable embryos; Blue bars, unhatched embryos permeable to Nile Blue dye, with percentage of brood showing the permeable phenotype indicated. (C) Daily brood size counts and phenotype distribution, as described in (B), for the perm-2(pmn3) null and cbd-1(ok2913) strains for three successive days after initial plating of L4-stage worms. For all graphs, data were pooled from 2–3 independent experiments per condition. Control worms were either N2 wild-type worms (deletion allele experiments) or injected with him-8 dsRNA (RNAi experiments); control data were pooled since brood size means and standard errors were similar (198±16 N2 N=11 vs. 188±13 him-8 dsRNA N=15). N=number of adults, n=number of progeny. Error bars represent standard error of the mean (SEM) for total brood size. *** p<0.0001, ** p<0.001, * p<0.05 compared to controls by unpaired student’s t-test with unequal variance. (D) Widefield fluorescence images of control and RNAi-treated embryos expressing mCherry-tagged histone H2B (magenta) and a GFP-tagged plasma membrane marker (green) that were dissected into FM4–64 dye (magenta). Arrows highlight plasma membrane stained with FM4–64 dye in permeable embryos (white in the merge). Control embryos, 0% permeable; all RNAi treated embryos, 100% permeable. Multicellular embryo shown for perm-2/4(RNAi) highlights its ability to complete multiple divisions before arresting. N=number of dissected adults, n=number of embryos. Scale bars, 10um.
PERM-2 and PERM-4 localization to the vitelline layer is co-dependent and requires CBD-1
Given that PERM-2 and PERM-4 inhibition resulted in a permeability barrier-like defect, we sought to determine whether these proteins localized to the permeability barrier. Surprisingly, mCherry-tagged PERM-2 and PERM-4 were found on the surface of oocytes in the gonad before fertilization and the embryonic eggshell after fertilization (Fig. 2A,B), a pattern consistent with the outermost vitelline layer of the eggshell rather than the innermost permeability barrier (Johnston and Dennis, 2011; Olson et al., 2012; Stein and Golden, 2015). The chitin-binding domain protein CBD-1 similarly localized to unfertilized oocytes in a previous study (Johnston et al., 2010) and was proposed to be a vitelline layer protein (Johnston and Dennis, 2011; Stein and Golden, 2015), so we also investigated its localization following fertilization. Similar to PERM-2 and PERM-4, CBD-1::mCherry also redistributed from the oocyte membrane to the embryonic eggshell after fertilization (Fig. 2C). Appearance of CBD-1::mCherry on the oocyte surface (−4.4 ± 1.1 oocyte, n=17) occurred slightly earlier than PERM-2::mCherry (−3.8 ± 1.1 oocyte, n=14, p=0.077) and PERM-4::mCherry (3.7 ± 0.8 oocyte, n=29, p<0.005). In order to verify vitelline layer localization, we treated PERM-2::mCherry, PERM-4::mCherry and CBD-1::mCherry embryos with alkaline bleach, which removes the outer vitelline layer from the eggshell while leaving the chitin layer, CPG layer, and permeability barrier intact ((Rappleye et al., 1999; Schierenberg and Junkersdorf, 1992); see also Fig. 5A). All three markers were successfully stripped from the eggshell following bleach treatment (Fig. 2A,B,C). We have thus characterized PERM-2, PERM-4, and CBD-1 as the first bona fide vitelline layer proteins in the C. elegans eggshell.
Figure 5. PERM-2 and PERM-4 promote structural integrity of the vitelline layer.
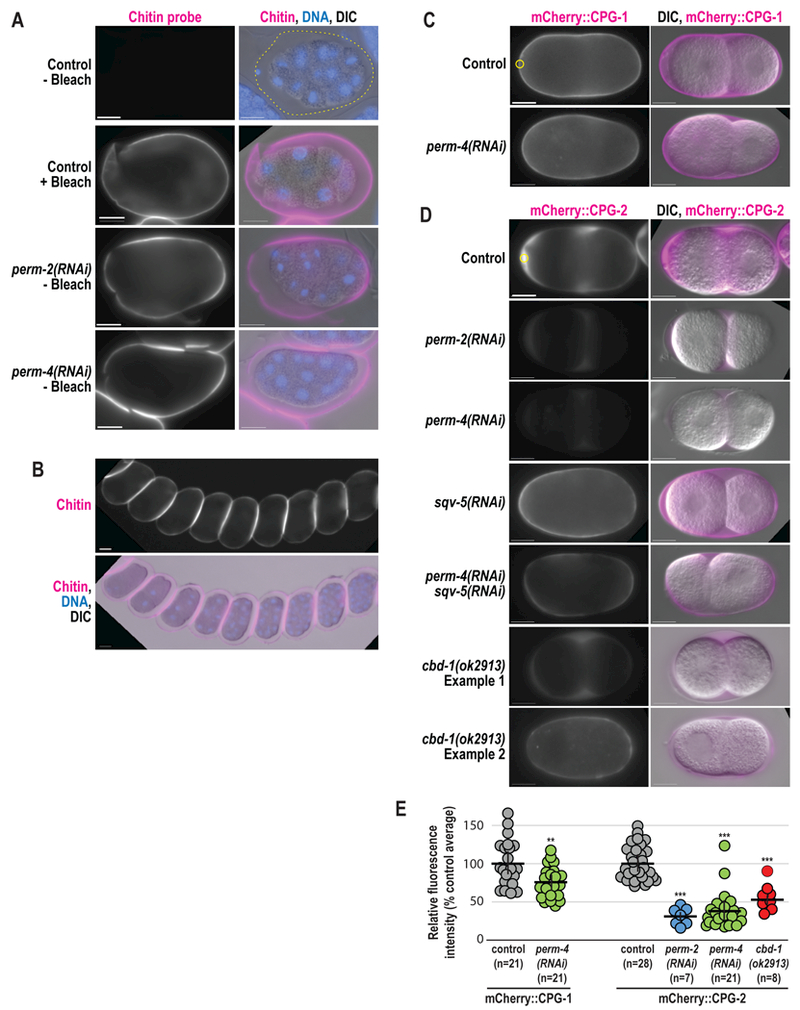
(A,B) Immunofluorescence of fixed embryos stained with a rhodamine-conjugated chitin-binding probe to mark the eggshell (magenta) and DAPI to mark DNA (blue). (C,D) Widefield fluorescence and DIC images of live embryos from control, RNAi-treated, and cbd-1(ok2913) mutant worms that express mCherry::CPG-1 (C) or mCherry::CPG-2 (D). n=number of embryos. Scale bars, 10um. (E) Quantification of relative fluorescence intensity of eggshell markers within a region of interest (ROI, yellow circles) normalized to the control average. Horizontal black bars represent the mean, vertical black bars the standard error of the mean (SEM). **, p=0.0033;***, p<0.0001 compared to controls by unpaired student’s t-test with unequal variance.
Due to their similar localization patterns, we hypothesized that PERM-2, PERM-4 and CBD-1 were part of an interdependent protein complex that provides the structural basis of the vitelline layer. In support of this idea, depletion of PERM-4 disrupted PERM-2::mCherry localization to the vitelline layer, with redistribution of all of the PERM-2::mCherry signal into intracellular compartments (Fig. 2A,D). Loss of PERM-2 via RNAi or null mutation likewise prevented vitelline layer targeting of PERM-4::mCherry (Fig. 2B,D), which was subsequently released into the gonad and uterine cavities (Fig. 2D and data not shown). These data show that PERM-2 and PERM-4 are co-dependent for recruitment and/or maintenance on the vitelline layer. Similarly, depletion of CBD-1 resulted in loss of PERM-2::mCherry and PERM-4::mCherry from the oocyte surface (Fig. 2A,B). However, inhibition of PERM-2 and PERM-4 had no effect on CBD-1::mCherry localization (Fig.2C). To explore whether the genetic interactions among PERM-2, PERM-4 and CBD-1 were due to a physical association, we performed co-immunoprecipitation (co-IP) experiments with RFP-Trap followed by protein identification by mass spectrometry. Co-IP of PERM-2::mCherry pulled down PERM-4 and CBD-1, while co-IP of PERM-4::mCherry pulled down PERM-2 and CBD-1. Members of the EGG complex were not identified in any co-IP experiments. Based on the combination of localization and interaction data, we conclude that CBD-1 functions at the top of the structural hierarchy to recruit and/or maintain PERM-2 and PERM-4 on the vitelline layer, and that PERM-2 and PERM-4 cooperate to maintain each other’s association with CBD-1 through physical association of members of the protein complex.
The PERM complex and EGG complex are mainly structurally independent
A previous study identified CBD-1 as an essential component of a protein complex on the oocyte membrane that organizes factors involved in fertilization and egg activation (Johnston et al., 2010). CBD-1 anchors EGG-1, EGG-2, and CHS-1 to the oocyte membrane and evenly distributes the downstream components EGG-3, EGG-4, EGG-5, and MBK-2 along the cortex (hereafter called the EGG complex) (Cheng et al., 2009; Parry et al., 2009). Given the role of CBD-1 in also recruiting PERM-2 and PERM-4 (hereafter called the PERM complex) to the oocyte surface, we sought to determine whether the EGG complex and PERM complex localize independently to CBD-1, or whether they are structurally interdependent. Depletion of PERM-2 or PERM-4 had no effect on membrane localization of EGG-1::GFP (Fig. 3A) or cortical localization of EGG-3::GFP (Fig. 3B) in unfertilized oocytes. Co-depletion of EGG-1 and EGG-2 likewise had no effect on the recruitment of PERM-4::mCherry to the oocyte surface (Fig. 3C). The distribution of PERM-2::mCherry showed subtle accumulation of irregular patches of signal between oocyte boundaries in the absence of EGG-1/ 2 (Fig. 3D). However, CBD-1::mCherry distribution was similarly affected (Fig. 3E), suggesting that EGG-1/2 depletion disrupted CBD-1::mCherry localization, which had an indirect effect on PERM-2::mCherry organization. These data suggest the PERM complex and EGG complex are, for the most part, structurally independent despite both complexes being organized by a common protein, CBD-1.
The PERM complex is organized by the N-terminus of CBD-1
Because our data showed that CBD-1 organizes two independent protein complexes at the oocyte surface, we next hypothesized that distinct regions of the CBD-1 protein may separately bind the PERM and EGG complexes. CBD-1 is a large protein (1319 aa, 144 kD) comprised of 12 chitin-binding domains interspersed by consensus sites for O-linked mucin chains (Fig. 1A) (Chen et al., 2008). The ok2913 deletion allele maintains the cbd-1 reading frame but disrupts chitin-binding domains #3 and #4 in the N-terminal half of the protein (C. elegans Deletionutant Consortium, 2012), allowing us to probe the effect of these chitin-binding domains on recruitment of the PERM and EGG complexes. While the cbd-1(ok2913) mutant exhibited a low brood size with significant eggshell permeability defects (Fig. 1B,C), it was able to produce limited numbers of fertilized embryos with which to conduct experiments. When the entire CBD-1 protein was depleted by RNAi, EGG-1::GFP failed to be delivered or maintained at the plasma membrane and instead was found in intracellular vesicles that accumulated in the cytoplasm and near the nuclear envelope (Fig. 4A), while EGG-3::GFP showed irregular distribution along the cortex rather than a continuous layer (Fig. 4B), as previously described (Johnston et al., 2010). However, the cbd-1(ok2913) mutant was able to effectively recruit EGG-1::GFP and EGG-3::mCherry to the oocyte membrane, maintained EGG-1::GFP localization during fertilization, and internalized EGG-3::mCherry with normal dynamics in recently fertilized embryos (Fig. 4A,B). By contrast, cbd-1(ok2913) mutants showed a dramatic decrease in maintenance of PERM-2::mCherry and PERM-4::mCherry at the oocyte surface and the vitelline layer of the eggshell (Fig. 4C,D), similar to the effect seen in cbd-1(RNAi) animals (Fig. 2A,B). In some instances, there was complete failure to recruit PERM-2::mCherry or PERM-4::mCherry (left embryo in Fig. 4C, gonad in 4D), while in other cases there was reduced and irregular recruitment of the marker (right embryo in Fig. 4C, embryo in Fig. 4D) or redistribution from the membrane surface to soluble regions where oocyte membranes failed to adhere (gonad in Fig. 4C). These results suggest that the N-terminus of CBD-1 helps to recruit and/or maintain the PERM complex, but not the EGG complex, on the oocyte before fertilization.
Figure 4. The N-terminus of CBD-1 recruits the PERM complex, but not the EGG complex.
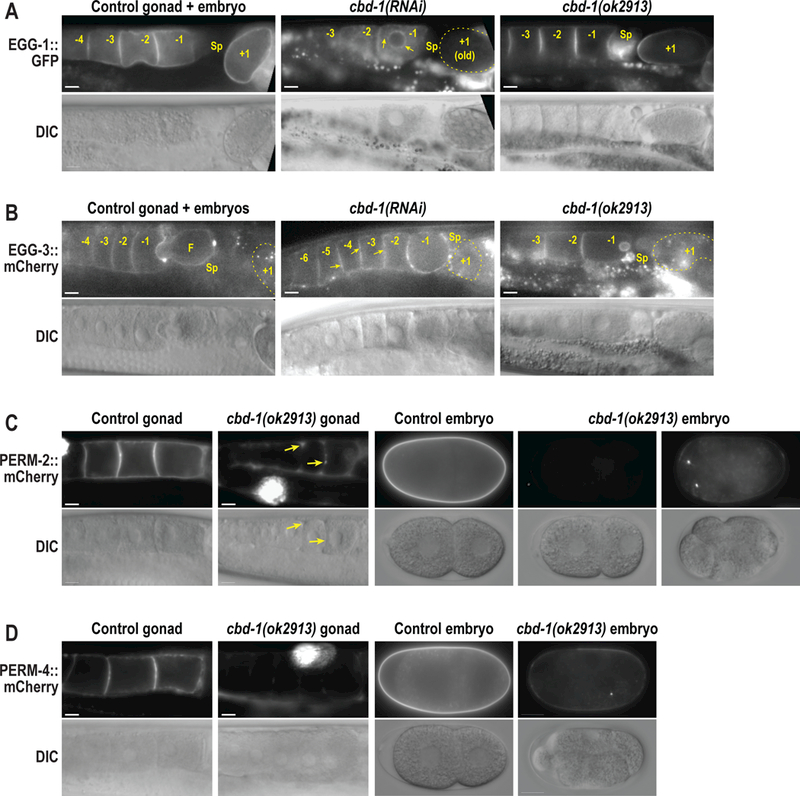
Widefield fluorescence and DIC images of gonads and embryos from control, RNAi-treated, or cbd-1(ok2913) worms that express EGG-1::GFP (A), EGG-3::mCherry (B), PERM-2::mCherry (C), or PERM-4::mCherry (D). Arrows in (A) indicate intracellular redistribution of EGG-1::GFP in cbd-1(RNAi) oocytes. Arrows in (B) highlight irregular and patchy distribution of EGG-3::mCherry along the oocyte cortex. Arrows in (C) highlight accumulation of PERM-2::mCherry in soluble regions where oocyte membranes fail to adhere. Oocytes and embryos are labeled relative to the spermatheca (Sp), with the −1 oocyte the next to be fertilized, and the +1 embryo (in some cases outlined in a dotted yellow line) recently fertilized and developing in the uterus. Small bright puncta in (A) and (B) represent autofluorescence of gut granules distinct from the EGG-1::GFP or EGG-3::GFP signal in the gonad. Large, bright signal in cbd-1(ok2913) mutant gonads in (C) and (D) is accumulation of secreted mCherry fusion protein in coelomocytes. The cbd-1(ok2913) allele disrupts the third and fourth chitin-binding domains of CBD-1 (see Fig. 1A). N=number of adults, n=number of gonads or embryos. Scale bars, 10um.
PERM-2 and PERM-4 promote structural integrity of the vitelline layer
Our existing model for C. elegans eggshell assembly states that the eggshell forms in a hierarchical manner, where external layers are essential for assembly of more internal layers (Olson et al., 2012). This model predicts that defects in the outermost vitelline layer would be catastrophic for eggshell assembly, as is observed in cbd-1(RNAi) embryos (Johnston et al., 2010). To test this prediction, we examined the structure and function of various markers for other eggshell layers following inhibition of PERM-2 and PERM-4. The chitin layer normally forms immediately beneath the vitelline layer and can be visualized with the external application of a chitin-binding probe (Zhang et al., 2005). Interestingly, depletion of PERM-2 or PERM-4 by RNAi resulted in enhanced staining of the chitin layer, similar to when the vitelline layer is removed by alkaline bleach treatment of wild-type embryos (Fig. 5A) (Zhang et al., 2005). The staining was intense enough that identical exposures of unbleached control embryos failed to detect chitin staining. These results suggest that the chitin layer can form in the absence of PERM-2 and PERM-4, but the chitin probe has greater access to underlying eggshell layers, likely due to an increase in permeability of the vitelline layer. Further evidence of abnormal vitelline layer structure was seen when embryos dissected from PERM-2 or PERM-4 depleted animals extruded from the uterus in long, interconnected chains (Fig. 5B), which is not observed in control animals. The “string of embryos” phenotype suggests that vitelline layer disruption results in changes to the eggshell surface that create sites of adhesion not normally present in wild-type embryos.
Given the increased permeability to externally-applied factors like the chitin probe, we next explored whether factors secreted from the embryo would be retained by a compromised vitelline layer. Soon after the chitin layer forms, cortical granule exocytosis releases the chondroitin proteoglycans CPG-1 and CPG-2. CPG-1 assembles beneath the chitin layer to form the third layer of the eggshell, while the majority of CPG-2 remains soluble and hydrates the perivitelline space between the eggshell and embryo proper (Olson et al., 2012). In embryos depleted of PERM-2 or PERM-4, mCherry::CPG-1 properly incorporated into the eggshell but at slightly lower levels (Fig. 5C,E). By contrast, mCherry::CPG-2 showed a significantly larger decrease in signal (Fig. 5D,E), suggesting either that CPG-2 was not secreted into the perivitelline space in the absence of PERM-2, or that the protein failed to be retained in the perivitelline space and leaked out of the eggshell. To discriminate between these hypotheses, we depleted Squashed Vulva 5 (SQV-5), the enzyme required to build chondroitin chains on the CPG core proteins, rendering mCherry::CPG-2 in an unglycosylated and less soluble state, but still able to be secreted (Olson et al., 2012). When SQV-5 and PERM-4 were co-depleted by RNAi, the insoluble form of mCherry::CPG-2 incorporated into the CPG layer of the eggshell rather than diffusing away (Fig. 5D), supporting the hypothesis that vitelline layer defects fail to retain soluble proteins in the perivitelline space between the eggshell and embryo. While the eggshells of PERM-2/4 depleted embryos retain some significant structural properties, the absence of these proteins results in a porous and improperly assembled eggshell.
We next examined mCherry::CPG-2 embryos carrying the cbd-1(ok2913) allele to test whether reduced levels of irregularly distributed PERM-2 and PERM-4 (see Fig. 4C,D) would have a similar effect on vitelline layer porosity as full PERM-2/4 depletions. mCherry::CPG-2 levels were reduced by 45% in cbd-1(ok2913) embryos, but the loss of signal was less severe than in PERM-2 or PERM-4 depletions (70% and 62%, respectively) (Fig. 5D,E). In 24 of 32 permeable embryos (75%), mCherry::CPG-2 was partially retained in the perivitelline space, indicating that a weakened vitelline layer was still able to prevent leakage of soluble proteins. Interestingly, in the remaining 8 of 32 embryos (25%), mCherry::CPG-2 was found in the eggshell proper rather than the perivitelline space, indicating that PERM-2 and PERM-4 remnants on the vitelline layer were partially able to restrict diffusion of large proteins. These data suggest that the vitelline layer exhibits variable degrees of porosity depending on existing levels of PERM-2 and PERM-4.
Discussion
The current work characterizes three key proteins required for vitelline layer formation, to our knowledge the first such proteins to be described in nematodes. Before fertilization, CBD-1 recruits PERM-2 and PERM-4 to the nascent vitelline layer on the oocyte surface via two chitin-binding domains in its N-terminus. CBD-1 also anchors the EGG/CHS complex to the oocyte cortex through a domain distinct from the PERM-2/4 recruitment domain (Fig. 6A). After fertilization, CBD-1 maintains its interactions with PERM-2 and PERM-4 as the vitelline layer is remodeled into the outer layer of the eggshell (Fig. 6B), while its interactions with the EGG complex are relieved (possibly through competition with newly synthesized chitin polymer (Johnston and Dennis, 2011)), followed by the EGG complex’s internalization and degradation (Maruyama et al., 2007; Parry et al., 2009; Stitzel et al., 2007). During vitelline layer formation and remodeling, PERM-2 and PERM-4 co-dependently maintain each other’s localization in order to establish a diffusion barrier and promote structural integrity of the eggshell.
Figure 6. Model for vitelline layer remodeling in C. elegans.
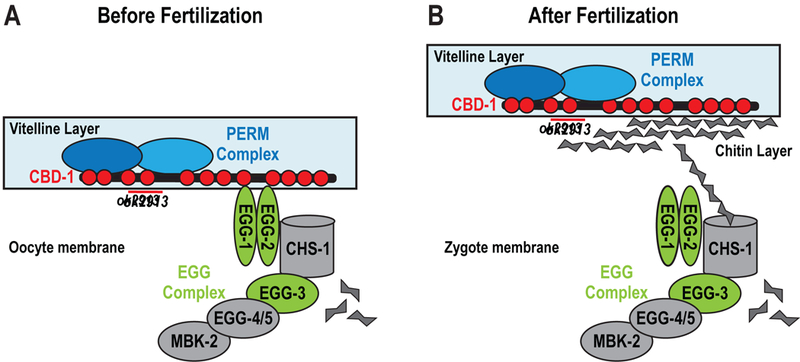
(A) Proposed structure of the vitelline layer before fertilization. CBD-1 (red) binds PERM-2 and PERM-4 (blue) through two N-terminal chitin-binding domains (red circles, deleted by the ok2913 allele). CBD-1 also stabilizes the EGG complex (green and gray) through domains distinct from those required for vitelline layer organization. Members of the EGG complex are required for fertilization and egg activation. Binding between PERM-2 and PERM-4 is indirectly supported by experimental data, but direct evidence is lacking. (B) After fertilization, synthesis of chitin may disrupt the interaction between CBD-1 and the EGG complex, allowing the vitelline layer to lift from the zygote surface. CBD-1 maintains association with the PERM complex in order to remodel the vitelline layer into a diffusion barrier that restricts entry and exit of large molecular weight compounds.
CBD-1 is a multifaceted regulator of vitelline layer assembly and egg activation
In the hierarchy of vitelline layer assembly, CBD-1 appears to function at the apex given (1) the severity of its phenotype compared to other components, and (2) its ability to recruit two functionally independent complexes via distinct domains. Embryos depleted of CBD-1 by RNAi produce a fragmented eggshell that slides off the embryo surface, exhibit polyspermic fertilization, and fail to undergo cell division (Johnston et al., 2010). By contrast, embryos co-depleted of PERM-2 and PERM-4 build rudimentary layers of the eggshell proper (vitelline, chitin, and CPG layers) and complete several rounds of cell division before arresting around the gastrula stage, suggesting the PERM complex assembles and functions downstream of CBD-1.
As a large protein with 12 distinct chitin-binding domains, CBD-1 has the potential to interact with multiple factors on the oocyte surface to promote distinct aspects of early embryogenesis. In support of this idea, the cbd-1(ok2913) deletion allele that removes chitin-binding domains 3 and 4 near the N-terminus disrupts localization of the PERM complex, but not the EGG complex. By contrast, depletion of the entire CBD-1 protein by RNAi disrupts both the PERM and EGG complexes (Fig. 4, (Johnston et al., 2010)). Similarly, the phenotype of cbd-1(ok2913) mutants is less severe than that of cbd-1(RNAi) embryos, with the former capable of producing enough viable embryos to propagate the homozygous mutant strain (Fig. 1B,C), and the latter exhibiting polyspermic fertilization (Johnston et al., 2010) followed by complete sterility within 8 hours of RNAi treatment (data not shown). While the cbd-1(ok2913) allele was useful in teasing apart regulation of the PERM and EGG complexes, more thorough characterization of the allele is warranted. Due to lack of protein detection methods, we were unable to determine if the CBD-1 domain mutant is stably expressed, or if the phenotypes are due to steady titration of small amount of functional protein, with the PERM complex more sensitive to CBD-1 levels than the EGG complex. The brood size data showing that ~50% of fertilized embryos are viable while ~50% are permeable over two successive days (Fig. 1C) lends support to the theory that the differential effects on the PERM and EGG complexes are due to the domain variant itself, rather than titration. Given that two adjacent chitin-binding domains of CBD-1 appear to specifically promote PERM complex assembly, it will be important to explore the domains necessary to recruit EGG-1/2 and CHS-1/EGG-3 to the oocyte membrane in order to identify structure-function relationships important for nematode fertilization and egg activation.
A particularly surprising aspect of this study is that CBD-1 links two different protein complexes that play very distinct roles at fertilization – structural support of the vitelline layer and zygote signaling. While extracellular matrix components such as laminins, cadherins, and proteoglycans are known to serve both structural and signaling roles(Bonnans et al., 2014; Boudreau and Bissell, 1998; Sekiguchi and Yamada, 2018), to our knowledge a similar mechanism has not been described for factors regulating egg coat formation and egg activation in fertilization models. A dual-function protein like CBD-1 may have evolved due to the unique mechanism used by nematodes to remodel the vitelline layer after fertilization. In many other animal models, fertilization initiates a calcium wave that stimulates egg activation through resumption of the cell cycle and metabolic processes, while also triggering cortical granule exocytosis to release structural building blocks and enzymes that remodel the egg coat(Whitaker, 2006). In C. elegans, the calcium wave does not trigger cortical granule exocytosis immediately after fertilization(Bembenek et al., 2007; Takayama and Onami, 2016), and thus remodeling of the egg coat relies on factors already residing in the vitelline layer. By complexing with PERM-2 and PERM-4, CBD-1 is well-positioned to provide the structural basis of the vitelline layer both before and after fertilization. The additional interactions between CBD-1, EGG-1/2, and CHS-1/EGG-3/4/5 help position the EGG complex members regularly along the oocyte membrane(Johnston et al., 2010), likely to promote timely interaction with sperm and the regular distribution chitin to quickly erect the polyspermy barrier. Through its association with CHS-1, CBD-1 is also well-positioned to bind chitin in order to quickly release the tether between the vitelline layer and the egg surface immediately after fertilization(Johnston and Dennis, 2011). While unique, the dual role of CBD-1 in promoting both structural features and signaling events at fertilization is strikingly efficient.
We also found it interesting that the PERM complex and EGG complex are, for the most part, quite independent (Fig. 3,4). The sole piece of data inconsistent with the simple model that CBD-1 organizes two entirely independent complexes is that EGG-1/2 depletion causes irregular distribution of PERM-2::mCherry along the oocyte surface (Fig. 3D). We believe the effect on PERM-2 is indirect, and in fact a consequence of the direct effect of EGG-1/2 depletion on CBD-1::mCherry localization (Fig. 3E). Our proposed model for PERM complex assembly (Fig. 6a) predicts that PERM-4 distribution would be similarly disrupted in EGG-1/2 depletions, but we found that PERM-4::mCherry localization remains unaffected. We therefore conclude that the PERM complex and EGG complex are functionally independent, but show slight structural dependence due to being organized by the common CBD-1 protein.
Dynamics of vitelline layer assembly
During vitelline layer assembly, CBD-1 appears to arrive at the surface of the −4/−5 oocytes just prior to, or around the same time as, PERM-2 and PERM-4 in order to serve as an anchor for the other vitelline layer components. Secretion of CBD-1 does not require the presence of PERM-2 or PERM-4, as CBD-1::mCherry localizes properly to the vitelline layer in the absence of both proteins (Fig. 2C). These data suggest that the association between CBD-1 and PERM-2/4 occurs at the oocyte surface rather than in the secretory pathway. By contrast, PERM-2::mCherry was found in cytoplasmic patches in oocytes and embryos depleted of PERM-4 (Fig. 2A,D),suggesting either that PERM-2 needs to bind PERM-4 in order to properly transit the secretory pathway, or that PERM-2 is internalized if it fails to be stabilized by PERM-4 on the oocyte surface. A reciprocal dependency does not hold true for PERM-4, as secretion of PERM-4::mCherry occurred normally in embryos depleted of PERM-2 or CBD-1, with no evidence of cytoplasmic accumulation (Fig. 2B,D). PERM-4::mCherry was unable to bind the eggshell in PERM-2 depletions (Fig. 2B) despite continued presence of CBD-1 (Fig. 2C), suggesting either that binding between PERM-4 and CBD-1 is indirect and mediated through PERM-2, or that PERM-2 and PERM-4 need to bind each other before being able to interact with CBD-1.
Interaction data from co-immunoprecipitation studies further support our model of a tripartite vitelline layer complex. While the PERM-2::mCherry co-IP was more robust than the PERM-4::mCherry co-IP, metrics within each dataset were largely comparable, particularly when protein size was taken into account (Fig. 2E). For example, PERM-4 is 1.6x larger than PERM-2 (35kD vs. 22kD, respectively), and PERM-4 peptide counts (number of unique peptides) and spectral counts (total number of spectra) were 1.1–1.7x higher in both IPs. These data suggest that PERM-2 and PERM-4 were present in roughly equal concentrations within each co-IP eluate. CBD-1 was present in both IPs but at significantly lower levels, suggesting either a weak interaction with PERM-2/4 or difficulty pulling down this very large protein that simultaneously interacts with the membrane-spanning EGG complex. We favor the latter hypothesis given our lack of success pulling down CBD-1 after multiple attempts and the absence of EGG complex members in co-IP eluates. While genetic and co-IP data suggest that PERM-2 and PERM-4 maintain each other’s localization at the vitelline layer through physical interaction, definitive evidence for a direct interaction is still lacking. Likewise, co-IP data support a model where PERM-2, PERM-4 and CBD-1 associate to form the vitelline layer, but the experimental approach does not allow us to distinguish whether interactions were detected within an intact cross-linked vitelline layer, or between individual proteins that interacted in solution. If PERM-2 and PERM-4 do interact while transiting through the secretory pathway, as proposed above, this could also explain why they were identified at much higher levels compared to CBD-1 in the co-IP eluates.
PERM-2 and PERM-4 promote structural integrity of the vitelline layer
The current model for C. elegans eggshell formation proposes that assembly occurs in a hierarchical fashion, where proper formation of outer layers is required for subsequent formation of inner layers (Olson et al., 2012). CBD-1 fits well with the model given that its inhibition prevents formation of the vitelline layer, chitin layer, CPG layer and permeability barrier ((Johnston et al., 2010) and Fig. 4C,D). However, we were surprised to find that inhibition of the two other vitelline layer components, PERM-2 and PERM-4, had little effect on formation of the chitin and CPG layers (Fig. 5), and only inhibited downstream assembly of the permeability barrier (Fig. 1). These data suggest that CBD-1 is the main contributor to vitelline layer architecture and is central to promoting chitin layer formation after fertilization, either by regulating the distribution of chitin synthase on the oocyte membrane and/or organizing chitin filament assembly via its chitin-binding domains (Johnston and Dennis, 2011). By contrast, the role of PERM-2 and PERM-4 appears not to involve chitin layer deposition, but rather to promote formation of a diffusion barrier that can restrict the entry of extracellular factors and maintain secreted factors within the region between the embryo and eggshell that can serve important structural or signaling roles. For example, an externally-applied chitin probe stained the chitin layer more intensely in perm-2(RNAi) and perm-4(RNAi) embryos than in control embryos with an intact vitelline layer (Fig. 5A), suggesting that the vitelline layer is more porous when PERM-2/4 are absent. Similarly, the failure of perm-2(RNAi) and perm-4(RNAi) embryos to retain CPG-2 in the perivitelline space resulted in failure to properly assemble the permeability barrier (Fig. 5D), explaining how a defect in the outermost vitelline layer can impact the innermost permeability barrier of the eggshell, without affecting formation of the intervening chitin and CPG layers.
Inhibition of PERM-2/4 not only caused defects within the eggshell layers, but also led to abnormalities at the eggshell surface. Inhibition of PERM-2/4 caused abnormal adherence at the eggshell surface, such as when embryos dissected from depleted worms were extruded attached to one another (Fig. 5B). This string of embryos phenotype suggests that absence of PERM-2/4 either uncovers adhesive components on the eggshell surface, or alternatively prevents the binding of uterine secretions that are normally thought to adhere to the outer eggshell as another layer of protection (Stein and Golden, 2015; Zimmerman et al., 2015). The latter hypothesis is attractive, since it could also explain the enhanced staining of the chitin probe through its ability to more easily penetrate the external eggshell layers. It will be important to explore the vitelline layer surface at the ultrastructural level to determine the degree of remodeling following fertilization, and determine which aspects are dependent on PERM-2 and PERM-4 function. These types of studies can be compared to those in human embryos, where ultrastructural analysis shows a striking remodeling event that converts a porous zona pellucida into an inter-connected, cross-linked structure after fertilization (Magerkurth et al., 1999; Nikas et al., 1994).
Mechanisms of vitelline layer remodeling include both evolutionarily conserved and species-specific features
In light of our findings on nematode vitelline layer assembly, we thought it interesting to consider which features are species-specific, and which share common principles across great evolutionary distance. Remodeling of the sea urchin vitelline layer into the fertilization envelope is one of the best understood and most visually dramatic examples in animals. Prior to fertilization, the sea urchin vitelline layer contains the egg bindin receptor proposed to interact with sperm, an extracellular isoform of the structural protein rendezvin, and the p160 protein that tethers the vitelline layer to the plasma membrane (Wessel and Wong, 2009). Fertilization triggers the exocytosis of cortical granules, which deliver structural building blocks and enzymes to aid in remodeling of the vitelline layer. For example, structural components including CUB-and LDLrA-domain proteins (e.g. proteoliasin, SFE1, SFE9, and additional rendezvin isoforms) associate in tandem arrays to expand the developing fertilization envelope, while the serine protease CGSP1 cleaves the p160 tether to release and lift the vitelline layer from the plasma membrane, aided by the hydration properties of sulfated glycosaminoglycans (Wessel and Wong, 2009). Finally, enzymes such as ovoperoxidase and transglutaminase crosslink structural proteins into a rigid fertilization envelope (Wong and Wessel, 2008b). Mining the C. elegans proteome for homologs of sea urchin fertilization envelope components failed to identify promising leads. While dozens of worm proteins contain CUB, LDLrA, and dual oxidase/peroxidase domains, none exhibit fertilization defects or eggshell permeability in genome-wide RNAi screens. Despite the lack of homology at the protein level, vitelline layer remodeling in worms and sea urchin do exhibit mechanistic similarities. For instance, in C. elegans, the vitelline layer is tethered to the embryo surface via interaction of the vitelline layer protein CBD-1 with the egg surface proteins EGG-1/2 and chitin synthase (CHS-1), similar to the p160 tether in sea urchin. In C. elegans, this tether is proposed to be reversed upon activation of chitin synthesis, with chitin competing for binding domains on CBD-1 to displace the EGG/CHS interaction (Johnston and Dennis, 2011). C. elegans requires an alternative mechanism to release the tether via substrate competition rather than protease delivery, since C. elegans cortical granules are not exocytosed upon fertilization, but rather 15 minutes later during anaphase of meiosis I. This is in contrast to sea urchin, where the CGSP1 protease is released from cortical granules immediately after fertilization, allowing rapid cleavage of the p160 tether.
The mammalian zona pellucida (ZP) is likewise analogous to the nematode vitelline layer. This thick extracellular coat resides on the surface of unfertilized oocytes and is remodeled following fertilization. The main structural components of the zona pellucida are ZP1–3 (ZP1–4 in humans), which contain conserved structural hallmarks called ZP domains (ZPD) (Wassarman and Litscher, 2016). In mammals, ZP proteins participate directly in sperm-egg binding (Bleil and Wassarman, 1980), which contrasts with C. elegans where depletion of CBD-1, PERM-2, or PERM-4 do not prevent fertilization. The other CBD-1 interactors, EGG-1 and EGG-2, are proposed to be involved in sperm-egg interaction and may fulfil this role (Kadandale et al., 2005), though additional sperm-egg recognition factors are still being sought. In addition to sperm recognition, vertebrate ZP proteins are involved in thickening the zona pellucida during oocyte maturation through polymerization of their ZPDs into cross-linked fibrillar structures (Darie et al., 2008; Jovine et al., 2002). Each ZPD contains ~260 amino acids, separated into ZP-N and ZP-C subdomains, with each subdomain stabilized by two disulfide bridges (Wassarman and Litscher, 2016). The C. elegans genome contains ~10 ZPD-containing proteins, but none are predicted to function during early embryonic development. While PERM-2, PERM-4 and CBD-1 do not share significant sequence homology with mammalian ZP proteins, it is interesting to note that each of the 12 CBD-1 chitin-binding domains is likewise stabilized by two disulfide bridges, reminiscent of the ZP-N and ZP-C domains. Likewise, two proteins of the sea urchin vitelline layer contain CUB domains that are stabilized through disulfide bonds (Wong and Wessel, 2008a), suggesting conservation of structural features across phyla. An interesting area of future research will be to investigate whether CBD-1, PERM-2 and PERM-4 form cross-linked fibrillar arrays to create a hardened eggshell, as is the case with the sea urchin CUB and LDLrA structural proteins and the mammalian ZPD proteins.
Our characterization of the first set of vitelline layer components in the nematode eggshell provides an opening to explore both widely-conserved and species-specific strategies used by animals during the formation of protective extracellular barriers, and could help identify novel therapeutic targets to fight parasitic nematode infection through destruction of this highly impenetrable barrier essential for embryonic viability.
Supplementary Material
Highlights.
PERM-2, PERM-4 and CBD-1 are key components of the C. elegans vitelline layer (VL)
CBD-1 organizes two protein scaffolds – the PERM complex and the EGG complex
The PERM complex and EGG complex are structurally and functionally independent
PERM-2/4 depletion resulted in a porous VL, eggshell adhesion, and embryonic death
The nematode VL has both evolutionarily-conserved and species-specific features
Acknowledgements
The authors thank members of the Fall 2012 Pomona College Advanced Cell Biology class (Claire Brickson, Jessica Chiang, Vivian Chou, Frances Hundley, Amy Li, David Morgens, Tyler Oe and Brian Wysolmerski) for initial investigations with PERM-2 and PERM-4; David Levine and Andy Golden for worm strain AG212; Giselle De La Torre, Katrina Dank, Jacob Brawer and Adia Ja’Nea James for technical assistance; and Matthew Marcello, Karl Johnson and Fabien Jammes for thoughtful comments on the manuscript. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Funding
This work was supported by Pomona College start-up funds (to SKO) and the Arnold and Mabel Beckman Foundation (to ZTW). JJM, JKD and JRY were supported by the National Institute of General Medical Sciences (8 P41 GM103533).
Abbreviations used in this paper
- CBD
chitin-binding domain
- CHS
chitin synthase
- co-IP
co-immunoprecipitation
- CPG
chondroitin proteoglycan
- dsRNA
double-stranded RNA
- EGG
egg sterile
- MBK
mini-brain kinase
- PERM
permeable eggshell
- RNAi
RNA interference
- SEC
self-excising cassette
- SQV
squashed vulva
- ZPD
zona pellucida domain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest
None
References
- Anya AO, 1976. Physiological aspects of reproduction in nematodes. Adv Parasitol 14, 267– 351. [DOI] [PubMed] [Google Scholar]
- Audhya A, Hyndman F, McLeod IX, Maddox AS, Yates JR, Desai A, Oegema K, 2005. A complex containing the Sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans. J Cell Biol 171, 267–279. doi: 10.1083/jcb.200506124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bembenek JN, Richie CT, Squirrell JM, Campbell JM, Eliceiri KW, Poteryaev D, Spang A, Golden A, White JG, 2007. Cortical granule exocytosis in C. elegans is regulated by cell cycle components including separase. Development 134, 3837–3848. doi: 10.1242/dev.011361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benenati G, Penkov S, Müller-Reichert T, Entchev EV, Kurzchalia TV, 2009. Two cytochrome P450s in Caenorhabditis elegans are essential for the organization of eggshell, correct execution of meiosis and the polarization of embryo. Mechanisms of Development 126, 382–393. doi: 10.1016/j.mod.2009.02.001 [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM, 1980. Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell 20, 873–882. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Chou J, Werb Z, 2014. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15, 786–801. doi: 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Bissell MJ, 1998. Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr. Opin. Cell Biol 10, 640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S, 1974. The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- elegans C Deletion Mutant Consortium, 2012. large-scale screening for targeted knockouts in the Caenorhabditis elegans genome. G3: Genes|Genomes|Genetics 2, 1415– 1425. doi:10.1534/g3.112.003830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Olson SK, Gutierrez E, Zhang K, Noble LB, Zanin E, Desai A, Groisman A, Oegema K, 2011. Acute Drug Treatment in the Early C. elegans Embryo. PLoS ONE 6, e24656. doi: 10.1371/journal.pone.0024656.t001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Niessen S, Anderson S, Hyndman F, Yates JR, Oegema K, Desai A, 2004. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes & Development 18, 2255–2268. doi: 10.1101/gad.1234104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-Z, Tang Y-R, Sheng Z-Y, Zhang Z, 2008. Prediction of mucin-type O-glycosylation sites in mammalian proteins using the composition of k-spaced amino acid pairs. BMC Bioinformatics 9, 101. doi: 10.1186/1471-2105-9-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KC-C, Klancer R, Singson A, Seydoux G, 2009. Regulation of MBK-2/DYRK by CDK-1 and the pseudophosphatases EGG-4 and EGG-5 during the oocyte-to-embryo transition. Cell 139, 560–572. doi: 10.1016/j.cell.2009.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson R, 1950. Nemic Ova. Introduction to Nematology (eds. Chitwood BG and Chitwood MG) Chapter XII, 175–190. [Google Scholar]
- Darie CC, Janssen WG, Litscher ES, Wassarman PM, 2008. Purified trout egg vitelline envelope proteins VEβ and VEγ polymerize into homomeric fibrils from dimers in vitro. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 1784, 385–392. doi: 10.1016/j.bbapap.2007.10.011 [DOI] [PubMed] [Google Scholar]
- Dickinson DJ, Pani AM, Heppert JK, Higgins CD, Goldstein B, 2015. Streamlined Genome Engineering with a Self-Excising Drug Selection Cassette. Genetics 200, 1035–1049. doi: 10.1534/genetics.115.178335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn D, 1957. The biochemistry of Ascaris. Exp Parasitol 6, 491–554. [DOI] [PubMed] [Google Scholar]
- Fonslow BR, 2014. Mass spectrometry-based shotgun proteomic analysis of C. elegans protein complexes. WormBook 1–18. doi: 10.1895/wormbook.1.171.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen S-P, Grunnet M, Jorgensen EM, 2008. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet 40, 1375–1383. doi: 10.1038/ng.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Davis MW, Sarov M, Taylor J, Flibotte S, LaBella M, Pozniakovsky A, Moerman DG, Jorgensen EM, 2014. Random and targeted transgene insertion in Caenorhabditis elegans using a modified Mos1 transposon. Nature methods 11, 529–534. doi: 10.1038/nmeth.2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA, Smith HO, 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature methods 6, 343–345. doi: 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- He L, Diedrich J, Chu Y-Y, Yates JR, 2015. Extracting Accurate Precursor Information for Tandem Mass Spectra by RawConverter. Anal. Chem 87, 11361–11367. doi: 10.1021/acs.analchem.5b02721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston WL, Dennis JW, 2011. The eggshell in the C. elegans oocyte-to-embryo transition. Genesis doi: 10.1002/dvg.20823 [DOI] [PubMed] [Google Scholar]
- Johnston WL, Krizus A, Dennis JW, 2010. Eggshell chitin and chitin-interacting proteins prevent polyspermy in C. elegans. Curr Biol 20, 1932–1937. doi: 10.1016/j.cub.2010.09.059 [DOI] [PubMed] [Google Scholar]
- Johnston WL, Krizus A, Dennis JW, 2006. The eggshell is required for meiotic fidelity, polar-body extrusion and polarization of the C. elegans embryo. BMC Biol 4, 35. doi: 10.1186/1741-7007-4-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovine L, Qi H, Williams Z, Litscher E, Wassarman PM, 2002. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat Cell Biol 4, 457–461. doi: 10.1038/ncb802 [DOI] [PubMed] [Google Scholar]
- Kadandale P, Stewart-Michaelis A, Gordon S, Rubin J, Klancer R, Schweinsberg P, Grant BD, Singson A, 2005. The egg surface LDL receptor repeat-containing proteins EGG-1 and EGG-2 are required for fertilization in Caenorhabditis elegans. Current Biology 15, 2222– 2229. doi: 10.1016/j.cub.2005.10.043 [DOI] [PubMed] [Google Scholar]
- Magerkurth C, Töpfer-Petersen E, Schwartz P, Michelmann HW, 1999. Scanning electron microscopy analysis of the human zona pellucida: influence of maturity and fertilization on morphology and sperm binding pattern. Hum. Reprod 14, 1057–1066. [DOI] [PubMed] [Google Scholar]
- Maruyama R, Velarde N, Klancer R, Gordon S, Kadandale P, Parry J, Hang J, Rubin J, Stewart-Michaelis A, Schweinsberg P, Grant B, Piano F, Sugimoto A, Singson A, 2007. EGG-3 Regulates Cell-Surface and Cortex Rearrangements during Egg Activation in Caenorhabditis elegans. Current Biology 17, 1555–1560. doi: 10.1016/j.cub.2007.08.011 [DOI] [PubMed] [Google Scholar]
- McNally K, Audhya A, Oegema K, McNally FJ, 2006. Katanin controls mitotic and meiotic spindle length. J Cell Biol 175, 881–891. doi: 10.1083/jcb.200608117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsuka S, Kawaguchi M, Wada Y, Ichikawa A, Ikura K, Hase S, 2005. Characterization of wheat germ agglutinin ligand on soluble glycoproteins in Caenorhabditis elegans. J. Biochem 138, 209–213. doi: 10.1093/jb/mvi117 [DOI] [PubMed] [Google Scholar]
- Nikas G, Paraschos T, Psychoyos A, Handyside AH, 1994. The zona reaction in human oocytes as seen with scanning electron microscopy. Hum. Reprod 9, 2135–2138. [DOI] [PubMed] [Google Scholar]
- Olson SK, Bishop JR, Yates JR, Oegema K, Esko JD, 2006. Identification of novel chondroitin proteoglycans in Caenorhabditis elegans: embryonic cell division depends on CPG-1 and CPG-2. J Cell Biol 173, 985–994. doi: 10.1083/jcb.200603003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SK, Greenan G, Desai A, Müller-Reichert T, Oegema K, 2012. Hierarchical assembly of the eggshell and permeability barrier in C. elegans. J Cell Biol 198, 731–748. doi: 10.1083/jcb.201206008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry JM, Velarde NV, Lefkovith AJ, Zegarek MH, Hang JS, Ohm J, Klancer R, Maruyama R, Druzhinina MK, Grant BD, Piano F, Singson A, 2009. EGG-4 and EGG-5 Link Events of the Oocyte-to-Embryo Transition with Meiotic Progression in C. elegans. Curr Biol 19, 1752–1757. doi: 10.1016/j.cub.2009.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappleye CA, Paredez AR, Smith CW, Mcdonald KL, Aroian RV, 1999. The coronin-like protein POD-1 is required for anterior-posterior axis formation and cellular architecture in the nematode Caenorhabditis elegans. Genes & Development 13, 2838–2851. doi: 10.1101/gad.13.21.2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappleye CA, Tagawa A, Le Bot N, Ahringer J, Aroian RV, 2003. Involvement of fatty acid pathways and cortical interaction of the pronuclear complex in Caenorhabditis elegans embryonic polarity. BMC Dev Biol 3, 8. doi: 10.1186/1471-213X-3-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Sato M, Audhya A, Oegema K, Schweinsberg P, Grant BD, 2006. Dynamic regulation of caveolin-1 trafficking in the germ line and embryo of Caenorhabditis elegans. Mol Biol Cell 17, 3085–3094. doi: 10.1091/mbc.E06-03-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Grant BD, Harada A, Sato K, 2008. Rab11 is required for synchronous secretion of chondroitin proteoglycans after fertilization in Caenorhabditis elegans. J Cell Sci 121, 3177– 3186. doi: 10.1242/jcs.034678 [DOI] [PubMed] [Google Scholar]
- Schierenberg E, Junkersdorf B, 1992. The Role of Eggshell and Underlying Vitelline Membrane for Normal Pattern Formation in the Early C. elegans Embryo. Roux’s Archives of Developmental Biology 202, 10–16. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nature methods 9, 676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel HS, Ailion M, Li J, van Oudenaarden A, Rockman MV, Kruglyak L, 2011. A novel sperm-delivered toxin causes late-stage embryo lethality and transmission ratio distortion in C. elegans. PLoS Biol 9, e1001115. doi: 10.1371/journal.pbio.1001115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi R, Yamada KM, 2018. Basement Membranes in Development and Disease. Curr. Top. Dev. Biol 130, 143–191. doi: 10.1016/bs.ctdb.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KK, Golden A, 2015. The C. elegans eggshell. WormBook 1–35. doi: 10.1895/wormbook.1.179.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzel ML, Cheng KC-C, Seydoux G, 2007. Regulation of MBK-2/Dyrk Kinase by Dynamic Cortical Anchoring during the Oocyte-to-Zygote Transition. Current Biology 17, 1545–1554. doi: 10.1016/j.cub.2007.08.049 [DOI] [PubMed] [Google Scholar]
- Tabb DL, McDonald WH, Yates JR, 2002. DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res 1, 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa A, Rappleye CA, Aroian RV, 2001. Pod-2, along with pod-1, defines a new class of genes required for polarity in the early Caenorhabditis elegans embryo. Developmental Biology 233, 412–424. doi: 10.1006/dbio.2001.0234 [DOI] [PubMed] [Google Scholar]
- Takayama J, Onami S, 2016. The Sperm TRP-3 Channel Mediates the Onset of a Ca(2+) Wave in the Fertilized C. elegans Oocyte. Cell Rep 15, 625–637. doi: 10.1016/j.celrep.2016.03.040 [DOI] [PubMed] [Google Scholar]
- Ward JD, 2015. Rapid and precise engineering of the Caenorhabditis elegans genome with lethal mutation co-conversion and inactivation of NHEJ repair. Genetics 199, 363–377. doi: 10.1534/genetics.114.172361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman PM, Litscher ES, 2016. A Bespoke Coat for Eggs: Getting Ready for Fertilization. Curr. Top. Dev. Biol 117, 539–552. doi: 10.1016/bs.ctdb.2015.10.018 [DOI] [PubMed] [Google Scholar]
- Wessel GM, Wong JL, 2009. Cell surface changes in the egg at fertilization. Mol Reprod Dev 76, 942–953. doi: 10.1002/mrd.21090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M, 2006. Calcium at fertilization and in early development. Physiological Reviews 86, 25–88. doi: 10.1152/physrev.00023.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JL, Wessel GM, 2008a. Renovation of the egg extracellular matrix at fertilization. Int J Dev Biol 52, 545–550. doi: 10.1387/ijdb.072557jw [DOI] [PubMed] [Google Scholar]
- Wong JL, Wessel GM, 2008b. Free-radical crosslinking of specific proteins alters the function of the egg extracellular matrix at fertilization. Development 135, 431–440. doi: 10.1242/dev.015503 [DOI] [PubMed] [Google Scholar]
- Wong JL, Wessel GM, 2006. Defending the zygote: search for the ancestral animal block to polyspermy. Curr. Top. Dev. Biol 72, 1–151. doi: 10.1016/S0070-2153(05)72001-9 [DOI] [PubMed] [Google Scholar]
- Xu T, Park SK, Venable JD, Wohlschlegel JA, Diedrich JK, Cociorva D, Lu B, Liao L, Hewel J, Han X, Wong CCL, Fonslow B, Delahunty C, Gao Y, Shah H, Yates JR, 2015. ProLuCID: An improved SEQUEST-like algorithm with enhanced sensitivity and specificity. J Proteomics 129, 16–24. doi: 10.1016/j.jprot.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Foster JM, Nelson LS, Ma D, Carlow CKS, 2005. The chitin synthase genes chs-1 and chs-2 are essential for C. elegans development and responsible for chitin deposition in the eggshell and pharynx, respectively. Developmental Biology 285, 330–339. doi: 10.1016/j.ydbio.2005.06.037 [DOI] [PubMed] [Google Scholar]
- Zimmerman SM, Hinkson IV, Elias JE, Kim SK, 2015. Reproductive Aging Drives Protein Accumulation in the Uterus and Limits Lifespan in C. elegans. PLoS Genet 11, e1005725. doi: 10.1371/journal.pgen.1005725 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


