Abstract
Purpose
To compare the effects of adding a progressive multimodal rehabilitation program to usual care (MRP + UC) versus UC alone on 1) functional mobility, strength, endurance and 2) ventilator weaning and discharge status of patients with ICU-acquired weakness (ICUAW) receiving prolonged mechanical ventilation (PMV).
Methods
Randomized pilot trial of an individualized MRP + UC versus UC in middle-aged and older ICU survivors with ICUAW receiving PMV. Outcomes compare changes in strength, mobility, weaning success and discharge home from a long-term acute care hospital (LTACH) between the groups.
Results
Eighteen males and 14 females (age 60.3 ± 11.9 years) who received PMV for ≥14 days were enrolled. Despite no significant differences between groups in the changes in handgrip, gait speed, short physical performance battery or 6-min walk distance after treatment, the MRP + UC group had greater weaning success (87% vs. 41%, p < 0.01), and more patients discharged home than UC (53 vs. 12%, p = 0.05). Post hoc analyses, combining patients based on successful weaning or discharge home, demonstrated significant improvements in strength, ambulation and mobility.
Conclusion
The addition of an MRP that improves strength, physical function and mobility to usual physical therapy in LTACH patients with ICUAW is associated with greater weaning success and discharge home than UC alone.
Keywords: ICU acquired weakness, Physical rehabilitation, Long term acute care hospital, Prolonged mechanical ventilation
1. Background
Approximately half of the patients admitted to intensive care units (ICUs) are over the age of 65 years [1] and develop some degree of muscle weakness [2]. This ICU - acquired weakness (ICUAW) is attributed to the inflammation, prolonged bed rest, medical/surgical interventions and therapeutics [3–5] associated with critical illness, and results in the loss of functional independence [6,7] and reduced quality of life [8]. These patients’ weakness is associated with greater disability [9], extended hospitalization [10], prolonged mechanical ventilation (PMV) [11,12] and increased post-ICU mortality [13,14] making their management challenging when transferred to long term acute care hospitals (LTACHs) for physical rehabilitation and ventilator weaning.
Although the management of critically ill patients emphasizes mobility-based rehabilitation early in the course of an ICU stay [15–19], efforts may be limited by severity of illness, level of sedation, muscle atrophy and weakness that delays rehabilitation until transition from the ICU to an LTACH. The few single arm studies examining the effects of short term, progressive physical rehabilitation on the functional and clinical outcomes of older PMV patients with ICUAW residing in LTACH facilities demonstrate improvements in pulmonary mechanics [20], limb strength [21], ambulation [22], and one-year survival [23]. To our knowledge there are no randomized trials examining the effectiveness of more intense, targeted physical rehabilitation on mobility function and more relevant clinical outcomes of successful ventilator weaning and discharge home in this chronically ill, disabled LTACH population.
We hypothesize that the addition of a multimodal rehabilitation program (MRP) that combines muscular strength and endurance training with functional retraining to usual care (UC) in a LTACH population can improve 1) functional mobility, strength, and endurance, and 2) ventilator weaning and discharge status of mechanically ventilated survivors of critical illness with ICUAW. To test this hypothesis, we performed a pilot study, randomizing these patients to either UC or MRP + UC to assess the feasibility and efficacy of the addition of the MRP on functional and clinical outcomes. As additional therapy, we posit that the MRP would either provide no additional improvements in function or clinical outcomes, or demonstrate that adding better, more frequent treatment results in better clinical outcomes and prognosis for recovery.
2. Methods
2.1. Patient characteristics
This single center, prospective pilot study was conducted in patients with ICUAW, aged ≥50 years receiving PMV on admission to an LTACH for continued medical care, rehabilitation and ventilator weaning. Those who met eligibility criteria (Table E1, Supplementary Materials) and provided informed consent underwent baseline functional assessments and were randomized to receive either UC or MRP + UC. Patients received serial functional assessments (identical to baseline testing) every 2 weeks that consisted of validated functional measures: 1) handgrip [24];2) Short Physical Performance Battery (SPPB) [25]; 3) 4 m walk gait speed [26]; 4) 6 min walk distance (6MWD) [27];5) manual muscle testing [28]; and 6) bedside assessments of basic functional mobility, including rolling, supine to sit, sitting, sitting for 5 min, sit to stand at bedside, sit to stand at chair, stand and pivot, standing for 1 min and ambulation. Each basic mobility maneuver was scored on a seven-level ordinal scale reflecting the amount of assistance the patient required to perform each task (0 = dependent, 1 = maximal assistance, 2 = moderate assistance, 3 = minimal assistance, 4 = contact guard, 5 = supervision, 6 = independent). Due to limited research staffing, only 2 patients could be enrolled in the MRP group at any given time; thus, 2 of the 32 patients that were enrolled were assigned to UC when research physical therapists (PTs) were not available.
PTs blinded to the interventions performed all baseline and follow-up assessments. To prevent bias, PTs other than those who performed the initial assessment conducted the training sessions in the MRP, while hospital PTs performed the UC rehabilitation. Patients received a maximum of eight weeks of MRP intervention training; their participation in the study was terminated early if they were discharged from the LTACH. This study was approved by the University of Maryland, Baltimore Institutional Review Board.
2.2. Multimodal rehabilitation program
The MRP is a progressive, patient-specific rehabilitation program that incorporates activities based upon each patients’ functional level, categorized into bed dependent, chair dependent and ambulatory groups. It was created based on our experience examining all maneuvers before initiation of the study in a cohort of 15 patients, of various levels of mobility ranging from bed dependent to chair dependent to ambulatory to determine its feasibility for each level of functional status. Based on this cohort’s tolerance and ability to perform each maneuver, we developed a core list of exercises that served as the basis of the MRP (Table 2). The MRP combines principles of physical therapy with exercise science to provide progressive functional retraining with the goal to increase muscular strength and cardiopulmonary endurance and progress the patient to a higher level of mobility. The muscle strengthening and endurance, aerobic conditioning, and functional mobility activities physiologically challenged patients within each functional category to progress to the next higher level of function. The muscular strength and endurance training are both closed and open kinetic chain to promote functional improvements based on the patients’ limitations. The duration of the MRP sessions was 45–60 min, 3 times/week at a different time of day from usual PT, occupational therapy, or recreational therapy sessions. Research PTs delivered the MRP, while the hospital therapists provided UC therapy; thus the MRP + UC group had more rehabilitation sessions/week Rehabilitation intensities were based on the patient’s heart rate, blood pressure, oxygen saturation and subjective reporting of exertion during activities using the Modified Borg Perceived Exertion Scale, or rating of perceived exertion (RPE), which uses a scale of 0 (no exertion) to 10 (maximal exertion) [29]. To promote training effects, the intensity, duration, and type of activity were modified during each PT session to an RPE of 3 to 5 to achieve a perception of moderate to vigorous exertion with prescribed rest periods that avoided full return to resting state according to the RPE. Supplementary oxygen and/or ventilator support was provided to patients during the MRP at a level equivalent to the highest oxygen level or ventilator settings required over the 72 h to meet their respiratory needs during rehabilitation.
Table 2.
Multimodal rehabilitation training program for older mechanically ventilated survivors of critical illness with ICUAW.
| Activity |
|
|||
|---|---|---|---|---|
| Muscle strengthening and power activities (functional) | Leg pressure Hip extension/abduction (supine) Closed kinetic terminal knee extension Ankle dorsiflexion Proprioceptive Neuromuscular facilitation Scapular depression Latissiumus pull downs Tricep extensions Hand putty |
Modified sit to stand Modified step-ups Hip extension/abduction (standing) Closed kinetic terminal knee extension Ankle dorsiflexion Proprioceptive Neuromuscular facilitation Shoulder flex/abduction Latissiumus pull downs Tricep extensions Hand putty |
Squats Step-ups Hip extension/abduction (standing) Latissimus pull downs Deltoid flies Tricep extensions Biceps Hand putty |
|
| Muscle endurance activities | Sitting edge of bed (30–60 seconds, rhythmic stabilization) Leg press (timed-30 seconds) Supine reverse leg raise, (timed-30 seconds) |
Restorator upper & lower extremity (timed-30–60 seconds) Standing balance: Unilateral stance Rhomberg Modified sit to stand Modified step up |
Stationary bicycle (timed-60–90 seconds) Upper body ergometry (timed 60–90 seconds) Squats Step-ups Modified military press Tricep extensions |
|
| Aerobic conditioning activities | Wheelchair mobility, restorator cycling for upper and lower body. | Stationary bicycle Upper body ergometry Pre gait activities |
Treadmill Stationary bicycle Upper body ergometry Ambulation |
|
Progression of mobility from left-most column (Bed Dependent) to right-most (Ambulatory).
Exercises categorized by row according to goals of therapy.
Muscle strengthening and endurance activities utilized elastic resistance bands and light weights.
The bed dependent patients required more than minimal assistance to complete supine to sit and sit to stand transfers. The MRP for these patients focused on closed kinetic, manually resistive functional exercises, and assisted functional activities such as transfers, edge of bed balance, pre-standing functional activities, strength, flexion and aerobic activities until they were able to transfer to a chair with minimal assistance, at which time they graduated to chair dependent.
The chair dependent patients required more than minimal assistance to complete standing, balance and pre-ambulation activities. The MRP for these patients focused on standing transfers and balance, pre-walking activities and progression of aerobic training. Modified closed kinetic exercises addressed strength, power and endurance aimed at retraining patients to transfer from bed to chair and initiate gait training. Joint mobilization, proprioceptive neuromuscular facilitation and manipulation treated range of motion deficits that restricted progression of functional mobility training. Modified closed kinetic exercise training focused on improving mobility, specifically progression from low mobility maneuvers (rolling, sitting up) to sitting and rising from a chair, independent standing and then ambulation. Patients were considered ambulatory when they were able to ambulate with minimal assistance for at least 25 ft. All groups also completed upper and lower body seated cycling to promote aerobic endurance and strength using a workload that permitted up to 20 min of cycling.
The MRP for the ambulatory patients focused on dynamic standing activities, pre-stair climbing and higher levels of muscle endurance training like squats, cycling and standing initially for 90 s and then to an RPE of 5. Additional emphasis was placed on gait, dynamic standing activities, and higher level functional activities like step-ups and standing hip extension/abduction maneuvers to strengthen muscles needed for walking. Patients who were ambulatory but still required LTACH care for complex post ICU medical management and continued ventilator weaning were still eligible for participation in this study if they met all study inclusion criteria.
2.3. Usual care rehabilitation
Usual care consisted of basic rehabilitation activities by LTACH PTs that exercised muscles in isolation without consideration of the physiological responses of intensity, effort, fatigue, quality of movement or endurance. Methods included training in sitting and balance, sit-to-standing, transferring from bed to chair, strengthening activities in and out of bed, and gait and assisted standing activities. However, this mobility training was not progressed systematically to achieve a training effect. Vital signs were usually monitored at the beginning and the end of the session, but not used to progress intensity, duration or recovery time. Oxygen was provided as needed to maintain oxygen saturation of ≥92% and meet their respiratory needs during therapy.
2.4. Analytical methods
Data are expressed as mean ± standard deviation, medians with interquartile ranges, or counts with percentages. Pearson’s chi square test or Fisher’s exact test, Student’s t-test, and the Wilcoxon rank-sum tests compared the proportion of patients successfully weaned, the median number of ventilator days, and discharge disposition (home, skilled nursing facility, acute care facility, remained in LTACH) of patients in UC and MRP groups (SAS 9.3, Cary NC, USA). Successful weaning from PMV was defined as the ability to tolerate tracheostomy collar for 48 h without mechanical ventilator support, or 7 consecutive days requiring only nocturnal ventilation for ≤8 h [30]. Patients not weaned prior to discharge from the LTACH or were transferred from the LTACH still requiring mechanical ventilation were assigned 56 ventilator days, corresponding to the maximum length of follow-up (eight weeks). Linear regression compared the change (final - baseline value = group + initial value) in the UC and MRP groups. Because many patients were discharged or transferred from the LTACH prior to the completion of the 8-week study period, the changes in functional measures were calculated using a last value carried forward paradigm, wherein the last measurement obtained, whether at 2, 4, 6 or 8 weeks, was used as the post-treatment value. Post-hoc analyses used ANOVA to determine whether there were significant differences in the changes in the basic mobility and functional measures of patients that successfully weaned or not and those who were discharged home or elsewhere, regardless of treatment group. All patients were included in the intent to treat analysis. A 2-tailed p value ≤0.05 indicated statistical significance.
3. Results
3.1. Baseline demographics, functional measures and physical therapy
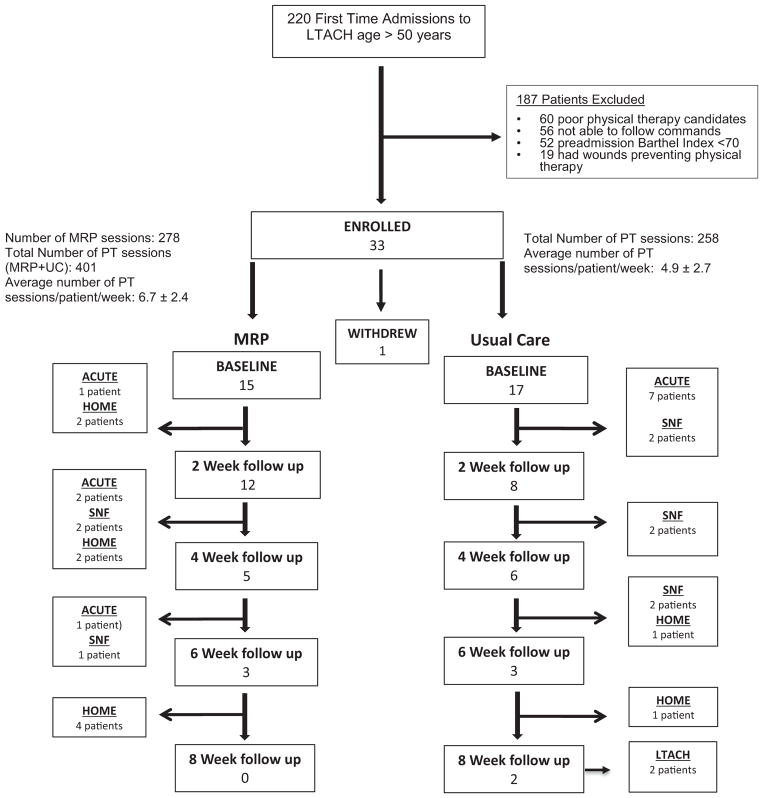
Eighteen male and 15 female patients 60.3 ± 11.9 years of age with comparable mean baseline characteristics were enrolled from August 2013 to March 2015 (Table 1). One patient withdrew shortly after signing consent (Fig. 1). All patients met 4 of 5 necessary criteria for ICUAW as defined by Stevens (Table E1, Appendix). At baseline, patients in both groups were similarly weak, except that women assigned the MRP had greater quadriceps strength than women assigned to UC (Table 3). The MRP + UC group had higher ambulatory and mobility function than the UC group, but this difference did not reach significance.
Table 1.
Baseline demographic characteristics of MRP and usual care groups.
| Demographics | Usual care N = 17 |
MRP N = 15 |
P-value |
|---|---|---|---|
| Gender | |||
| Male, n (%) | 10 (58.8%) | 8 (53.3%) | 0.96 |
| Age (years) | 63.1 ± 11.4 | 57.1 ± 12.0 | 0.16 |
| Body mass index (kg/m2) | 33.4 ± 14.0 | 33.9 ± 14.0 | 0.93 |
| (Range) | (23–44) | (19–59) | |
| Ambulatory, n (%) | 6 (35%) | 7 (47%) | 0.51 |
| Race | |||
| African American | 9 (53%) | 9 (60%) | 0.96 |
| White | 8 (47%) | 6 (40%) | |
| Barthel index | 90 ± 15 | 96 ± 8 | 0.16 |
| Albumin (g/dl) | 2.9 ± 0.6 | 3.1 ± 0.8 | 0.51 |
| Charlson comorbidity index | 4.3 ± 2.0 | 4.5 ± 3.0 | 0.36 |
All data expressed in mean ± standard deviation unless otherwise noted.
Fig. 1.
CONSORT Diagram depicting patient flow through the study including discharge disposition.
Table 3.
Baseline functional testing of Usual Care and MRP Groups.
| Functional testing | Usual care N = 17 |
MRP N = 15 |
P-value |
|---|---|---|---|
| Handgrip, kg | 12 ± 11 | 11 ± 9 | 0.78 |
| Manual muscle testing (lbs) | |||
| Quadriceps | |||
| Male | 24.8 ± 8.2 | 27.6 ± 8.0 | 0.47 |
| Female | 14.5 ± 5.5 | 23.4 ± 9.2 | 0.03 |
| Triceps | |||
| Male | 17.1 ± 3.4 | 18.9 ± 4.5 | 0.39 |
| Female | 9.8 ± 2.4 | 12.7 ± 4.5 | 0.57 |
| Mobility function | |||
| Gait speed, m/s | 0.11 ± 0.17 | 0.19 ± 0.23 | 0.27 |
| 6 minute walk distance (ft) | 16 ± 29 | 43 ± 79 | 0.20 |
| SPPB | 1.3 ± 2.2 | 2.4 ± 2.6 | 0.20 |
All data expressed in mean ± standard deviation unless otherwise noted.
The 15 patients in the MRP + UC group received 3 MRP sessions/ week in addition to their usual PT sessions. This resulted in more total sessions (401 vs. 258), but not a significantly greater number of sessions/week than UC (6.7 ± 2.4 vs. 4.9 ± 2.7, p = 0.06, Fig. 1). Of the 32 patients enrolled, 3 in the MRP + UC group and 9 in the UC were discharged from the LTACH facility prior to their 2-week assessment, thus providing only baseline data. Six patients were discharged from the MRP + UC group and 2 from the UC group between weeks 2–4, 2 patients from the MRP + UC group and 3 from the UC group between weeks 4–6, and 4 from the MRP + UC group and 1 from the UC group between weeks 6–8. Two patients from the UC group remained in the study for 8 weeks and remained in the LTACH.
3.2. Changes in mobility and strength
Those assigned to the MRP + UC group demonstrated greater increases in handgrip strength (3.6 ± 5.4 vs.1.3 ± 4.9 kg; p = 0.20), gait speed (0.13 ± 0.16 vs. 0.07 ± 0.20 m/s, p = 0.41), and 6MWD (40.6 ± 65.1 vs. 19.9 ± 54.2 ft., p = 0.33), but no appreciable difference in change in SPPB score (1.1 ± 2.3 vs. 1.4 ± 3.1 points, p = 0.87) compared to those in the UC group. None of these changes reached statistical significance.
3.3. Differences in ventilator weaning and discharge status
The patients in the MRP + UC group were on the ventilator for fewer days (median: 17 vs. 56 days, P = 0.07), and 13 of 15 in the MRP + UC group weaned successfully compared to only 7 of 17 in UC group (87% vs. 41%, p < 0.01). A higher proportion of patients in the MRP + UC group (8 of 15) also went home compared to the UC group (2 of 17; 53% vs. 12%, p = 0.05). A greater percentage of patients from the UC group were readmitted to ICUs compared to the MRP + UC group (41% vs. 27%, p = 0.39). Of the patients that were ambulatory at baseline in the intervention group all 7 patients (100%) were successfully weaned from PMV in the MRP + UC group compared to only 3 (3/6, 50%) in the control group at the end of their study participation (100% vs 50%, p = 0.07). Of the patients who were ambulatory at baseline 4 patients in the MRP + UC group were discharged home (4/7, 57%) compared to only 1 (1/6, 17%) in the control group at the end of their participation (57% vs 17%, p = 0.26).
3.4. Relationship of functional outcomes to weaning and discharge status
To identify the functional measures that predicted ventilator weaning and discharge home independent of group assignment, post-hoc subgroup analyses compared the changes in functional outcomes and mobility regardless of treatment group between the patients who successfully weaned from PMV to those who did not wean, and those who went home vs. those who were discharged elsewhere or remained in the LTACH after 8 weeks. The 20 patients from both groups (13 MRP, 7 UC) who were weaned from PMV had significantly greater increases in handgrip, gait speed and 6 MWD than the 12 patients who did not wean, and the 6.3 - fold greater change in SPPB score approached significance (Table 4). Furthermore, the 10 patients who went home (8 MRP, 2 UC) had significantly greater increases in handgrip, gait speed, 6MWD and SPPB than the 22 patients who were discharged elsewhere. Improvements in basic mobility and functional maneuvers of ambulation, standing, and handgrip strength were the strongest predictors of successful weaning and discharge home (Tables 4 and 5) Improvements in all other mobility measures except rolling and sitting predicted discharge home, but not weaning from PMV.
Table 4.
Post hoc analysis comparing changes in functional testing: Patients that successfully weaned vs. those who failed and Patients discharged home vs. other disposition.
| Functional testing | Not weaned N = 12 |
Weaned N = 20 |
P-value |
|---|---|---|---|
| Handgrip, kg | −0.2 ± 0.7 | 3.9 ± 6.1 | 0.04 |
| Gait speed, m/s | 0.02 ± 0.06 | 0.15 ± 0.21 | 0.05 |
| 6 MW distance (ft) | 2.5 ± 8.7 | 45.9 ± 70.7 | 0.05 |
| SPPB | 0.3 ± 0.6 | 1.9 ± 3.3 | 0.09 |
| Functional testing | Not home N = 22 |
Home N = 10 |
P-value |
|
| |||
| Handgrip, kg | 0.2 ± 1.8 | 7.1 ± 7.1 | <0.001 |
| Gait speed, m/s | 0.02 ± 0.06 | 0.27 ± 0.24 | <0.001 |
| 6 MW distance (ft) | 4.6 ± 18.4 | 84.5 ± 80.8 | <0.001 |
| SPPB | 0.2 ± 0.7 | 3.6 ± 3.9 | <0.001 |
All data expressed in mean ± standard deviation unless otherwise noted.
Table 5.
Association of changes in mobility and functional measures with weaning from PMV and discharge home.
| Activity | Weaning Odds ratio (95%CI) |
P-value | Discharge Home odds ratio (95%CI) |
P-value |
|---|---|---|---|---|
| Ambulation | 1.77 (1.06, 2.95) | 0.03 | 1.82 (1.15, 2.89) | 0.01 |
| Standing (1 min) | 1.68 (1.00, 2.81) | 0.05 | 1.80 (1.14, 2.83) | 0.01 |
| Stand and pivot | 1.50 (0.92, 2.43) | 0.10 | 2.03 (1.18, 3.49) | 0.01 |
| Sit to stand (chair) | 1.43 (0.90, 2.28) | 0.13 | 1.95 (1.17, 3.25) | 0.01 |
| Sit to stand (bed) | 1.30 (0.83, 2.03) | 0.25 | 2.23 (1.24, 4.04) | 0.01 |
| Sit edge of bed (5 min) | 0.97 (0.64, 1.48) | 0.89 | 1.60 (0.94, 2.73) | 0.08 |
| Sitting | 0.96 (0.62, 1.48) | 0.85 | 1.42 (0.88, 2.31) | 0.15 |
| Rolling | 0.87 (0.59, 1.27) | 0.46 | 1.00 (0.69, 1.46) | 1.00 |
| Handgrip | 1.62 (1.00, 2.64) | 0.05 | 1.76 (1.15, 2.70) | 0.01 |
| 6 MW distance | 1.03 (0.98, 1.08) | 0.20 | 1.03 (1.00, 1.07) | 0.05 |
| SPPB | 1.45 (0.89, 2.37) | 0.14 | 2.19 (1.15, 4.19) | 0.02 |
Odds ratio reports change in odds of weaning or going home for every 1 point change in independence score. Each basic mobility maneuver was scored on a seven-level ordinal scale reflecting the amount of assistance the patient required to perform each mobility measure (0 = dependent, 1 = maximal assistance, 2 = moderate assistance, 3 = minimal assistance, 4 = contact guard, 5 = supervision, 6 = independent).
4. Discussion
This study provides preliminary evidence that that the addition of a progressive, disability- targeted MRP to usual physical therapy is associated with higher rates of weaning success from PMV and discharge home in select middle-aged and older patients with ICUAW. Those who participated in the MRP demonstrated almost 3-fold greater improvements in handgrip strength, and 2-fold improvements in gait speed and 6MWD compared to the UC group, findings all of which trended toward statistical significance. In secondary analyses, significantly greater improvements in ambulation, standing and handgrip strength occurred in the patients weaned from PMV and those who went home than in those who did not, regardless of group assignment. This suggests that adding an MRP that combines principles of rehabilitation with exercise science into the care of patients with ICUAW in LTACHs is not only feasible and well tolerated, but also results in improvements in functional measures that are associated with better clinical outcomes and prognosis.
The clinical outcomes of successful weaning and discharge home likely occurred in the MRP + UC group because the patients got 3-fold stronger and showed 2-fold greater increases in ambulatory function than the UC group. This suggests that the responses were significant with respect to the most important clinical outcomes – weaning and discharge home, but the heterogeneous responses and small sample size limited power for the heterogeneous functional measures. However, the post-hoc assessment of the clinical improvements across MRP + UC and UC groups demonstrated these functional improvements were significantly greater in patients who successfully weaned or went home. Further, the ambulatory and basic mobility measures, as reflected by the 1.45 and 2.19 odds ratios seen for SPPB, more strongly predicted weaning and discharge home. This suggests that while the addition of this MRP to usual care is more efficacious than usual care alone in improving clinical outcomes of ventilator weaning and discharge home in older patients with ICUAW, those patients in the UC group who achieved these functional improvements could be weaned and discharged home. A larger sample size will be necessary to assess which functional gains are the best intermediate markers of clinical improvement in this population, and to determine the optimal frequency, duration and intensity of the delivered intervention. It is also likely that the outcome measures we chose (SPPB, 4-m gait speed, handgrip and 6MWD) have floor effects due to the severe weakness and functional impairment of this patient population, thus limiting the prognostic ability of these tests [14,31–34].
Several studies report the effects of incorporating more vigorous rehabilitation programs into the management of severely weak survivors of critical illness receiving PMV. One single arm, prospective study shows that PT focused on ambulation delivered to a cohort of 150 patients with ICUAW in which 69% received PMV improve walking function, muscle strength measures (Medical Research Council scores and handgrip), gait speed, and 6MWD [22]. Although the lack of a control group limits its generalizability, it is the largest study of its type utilizing a progressive PT program with repeated assessments every 2 weeks in patients with ICUAW. Two cohort studies, one prospective [35] in 77 patients and the other retrospective [36] in 49 ICU survivors requiring PMV show that rehabilitation interventions that incorporated mobility and strength exercises result in weaning rates of 74% and 100%, comparable to the 87% achieved with the current MRP. Three randomized controlled trials (RCTs) in mechanically ventilated patients with ICUAW compare the effect of rehabilitation interventions to usual care on clinical outcomes of weaning and discharge home. Two studies demonstrate weaning rates of 22% [23] and 75% [20] in the intervention groups, while the other did not report weaning as an outcome. These trials varied in the delivery of the rehabilitation intervention, ranging from 10 sessions lasting 15–25 min each, to 30 sessions lasting 30 min a session over 6 weeks. Our study planned to incorporate up to 24 sessions lasting 45–60 min each over a period of 8 weeks, but discharge home and to other facilities limited completion to <6 weeks in most patients. Similar to the current study, these three RCTs incorporated upper and lower limb strengthening exercises (light weights, resistance training) and mobility training (rolling, sitting at edge of bed, sit to stand, gait training when appropriate) and respiratory support during rehabilitation. Only one other study also incorporated cardiopulmonary training using upper and lower extremity cycle ergometry, as we did, to increase endurance [21]. Although the three RCTs included respiratory muscle training in their rehabilitation programs, we did not incorporate such training. Despite this, the proportion of patients weaned or discharged home in the combined MRP + UC group is superior to these 3 trials and surpasses the 54% weaned and 28% of patients discharged home in the largest multicenter outcomes study conducted in 1400 patients across 23 LTACH facilities nationwide [37]. Perhaps targeted respiratory muscle training is less important than the other elements of the MRP in improving weaning rates in mechanically ventilated patients with ICUAW, a view consistent with the latest pulmonary rehabilitation guidelines [38].
As our intervention group received both MRP and UC rehabilitation, it is possible that the combination of usual care PT with the MRP is necessary to provide additional functional and clinical benefits, that translate into better clinical outcomes. However, there was substantial variance in the number of group sessions that did not reach statistical significance, suggesting that the better clinical outcomes in the combined MRP + UC group are due to its progressive nature, higher intensity, and respiratory support, rather than the frequency of the rehabilitation sessions. The efficacy of the MRP added to UC in improving clinical outcomes for these debilitated LTACH patients is further supported by the differences in weaning and discharge outcomes observed in the patients in each group who were ambulatory at baseline, as more of these patients in the MRP + UC group weaned successfully from PMV and went home compared to those in the UC group. Furthermore, our findings in the LTACH setting differ from those of Morris et al. [39] who implemented an intensive physical therapy program in the ICU setting that incorporated sessions several times a day, including weekends. Despite the increased frequency and intensity of their program, there was no improvement in length of stay, the primary outcome. This further emphasizes that the nature of our MRP may be of greater importance in improving clinical outcomes in patients with ICUAW recovering from critical illness than the total number of therapy sessions. A larger randomized study will be needed to test this hypothesis.
Multidisciplinary strategies are needed to assess, prevent and manage pain, agitation, delirium, and weakness in patients requiring PMV cared for in LTACH facilities [40]. The results of this study suggest that an MRP that combines progressive physical rehabilitation with exercise is feasible, and can potentially promote ventilator weaning success, and increase rates of discharge home from LTACHs. Just as mobility-based physical rehabilitation improves outcomes early in the course of an ICU stay [15,41], the current results suggest such therapy also is of clinical benefit in the late recovery phase of disabled, older ICU survivors. Our results suggest that the implementation, rather than the timing of physical rehabilitation as originally thought (early vs. late in the course of critical illness), may be the key to promoting recovery.
The pilot nature and small sample size of this study limited our ability to detect significant differences in the comparison of functional outcomes between the MRP + UC and UC groups. As a small pilot study, this trial was limited in resources, as evidenced by the allocation of two enrolled patients to UC due to unforeseen staff limitations. We are encouraged by the outcomes of this pilot study, which suggest that a larger, definitive clinical trial evaluating the MRP for long term, ventilator dependent patients seems warranted.
This is based on several factors: 1) more physical therapists/personnel would have allowed more frequent measurement of functional and other quality of lifestyle parameters, as well as follow-up of patient outcomes after discharge from the LTACH; 2) the intermediate nature of outcome measures themselves (handgrip, SPPB, 4 gait speed, and 6 MWD) reduced their sensitivity (floor effect) to detect smaller, meaningful changes; and 3) other assessments, such as the Functional Status Scale for the ICU [42], the Chelsea Critical Care Physical Assessment [43], and the ICU mobility scale [44] may have provided additional insights into these patients’ functional status and response to our rehabilitation program, but were not yet validated in this population or available at the start of this pilot. Lastly, although feasibility of the MRP may be questioned since only 15% of those who met criteria enrolled into the study, we maintained strict inclusion and exclusion criteria, since we did not have ample therapists to insure patients’ safety if we enrolled more functionally impaired patients who might not safely tolerate the MRP. Thus, we excluded patients with cognitive impairment or survivors of catastrophic neurologic events such as intracranial hemorrhage, traumatic brain injury, and stroke who may be unable to voluntarily participate in comprehensive whole body functional assessments and physical rehabilitation. To provide greater generalizability of the MRP, staff and facility accommodations will be needed to safely enroll patients with functional limitations in individualized, lower level rehabilitation programs tailored specifically to their specific functional or neuromuscular impairments.
5. Conclusion
These findings suggest that the addition of an MRP, which combines strength, endurance, and mobility training, to usual rehabilitation programs for middle-aged and older survivors of critical illness with ICUAW in the LTACH setting is feasible, and improves weaning success from PMV and discharge to home compared to usual care. Thus, a larger, Phase II investigation of this intervention seems warranted to fine tune the intervention by identifying intermediate and long-term outcomes toward which rehabilitation should be directed, as well as the mechanisms that underlie the clinical improvements observed with this multimodal rehabilitation program.
Supplementary Material
Acknowledgments
Funding information
Dr. Verceles was supported by an NIH/NIA GEMSSTAR award (R03AG045100), a Pepper Scholar Award from the University of Maryland Claude D. Pepper Older Americans Independence Center (NIH/ NIA P30AG028747), a GRECC Special Fellowship in Geriatrics and a T. Franklin Williams Scholar Award, with funding provided by: Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Alliance for Academic Internal Medicine-Association of Specialty Professors and the American Thoracic Society Foundation. Drs. Wells, Sorkin, Terrin, and Goldberg also receive support from NIA P30AG028747.
List of abbreviations
- ICU
intensive care unit
- ICUAW
ICU acquired weakness
- LTACH
long term acute care hospital
- MRP
multimodal rehabilitation program
- PMV
prolonged mechanical ventilation
- SPPB
short physical performance battery
- UC
usual care
Footnotes
Clinicaltrials.gov identifier: NCT3195127.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2018.07.006.
Conflicts of interest
The authors report no conflicts of interest.
Authors’ contributions
Literature search: AV; Study concept and design: AV, MT, AG; Data acquisition or interpretation of data: AV, CW, JB, TJ, MT, JS; Drafting of the manuscript: AV, CW, AG; Critical revision of manuscript: All authors; Statistical analysis: AV, JS; Study supervision: AV, AG.
Drs. Verceles and Goldberg had full access to the data and take responsibility for its integrity and the accuracy of its analysis.
None of the authors had any conflicts of interest to report relevant to this study.
References
- 1.Milbrandt EB, Eldadah B, Nayfield S, Hadley E, Angus DC. Toward an integrated research agenda for critical illness in aging. Am J Respir Crit Care Med. 2010;182(8):995–1003. doi: 10.1164/rccm.200904-0630CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33(11):1876–91. doi: 10.1007/s00134-007-0772-2. [DOI] [PubMed] [Google Scholar]
- 3.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–67. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 4.Hermans G, Wilmer A, Meersseman W, Milants I, Wouters PJ, Bobbaers H, Bruyninckx F, Van den Berghe G. Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med. 2007;175(5):480–9. doi: 10.1164/rccm.200605-665OC. [DOI] [PubMed] [Google Scholar]
- 5.Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, de Jonghe B, Ali NA, Sharshar T. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37(10 Suppl):S299–308. doi: 10.1097/CCM.0b013e3181b6ef67. [DOI] [PubMed] [Google Scholar]
- 6.Heyland DK, Garland A, Bagshaw SM, Cook D, Rockwood K, Stelfox HT, Dodek P, Fowler RA, Turgeon AF, Burns K, et al. Recovery after critical illness in patients aged 80 years or older: a multi-center prospective observational cohort study. Intensive Care Med. 2015;41(11):1911–20. doi: 10.1007/s00134-015-4028-2. [DOI] [PubMed] [Google Scholar]
- 7.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 8.Svenningsen H, Langhorn L, Agard AS, Dreyer P. Post-ICU symptoms, consequences, and follow-up: an integrative review. Nurs Crit Care. 2015;22(4):212–20. doi: 10.1111/nicc.12165. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors associated with functional recovery among older intensive care unit survivors. Am J Respir Crit Care Med. 2016;194(3):299–307. doi: 10.1164/rccm.201506-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermans G, Van Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, Casaer MP, Meersseman P, Debaveye Y, Van Cromphaut S, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–20. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 11.De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30(6):1117–21. doi: 10.1007/s00134-004-2174-z. [DOI] [PubMed] [Google Scholar]
- 12.De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, Outin H, Sharshar T. Groupe de Reflexion et d’Etude des Neuromyopathies en R: Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35(9):2007–15. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 13.Sharshar T, Bastuji-Garin S, Stevens RD, Durand MC, Malissin I, Rodriguez P, Cerf C, Outin H, De Jonghe B. Groupe de Reflexion et d’Etude des Neuromyopathies En R: Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med. 2009;37(12):3047–53. doi: 10.1097/CCM.0b013e3181b027e9. [DOI] [PubMed] [Google Scholar]
- 14.Ali NA, O’Brien JM, Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, Almoosa K, Hejal R, Wolf KM, Lemeshow S, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178(3):261–8. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 15.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, Ross A, Anderson L, Baker S, Sanchez M, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–43. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 16.Morris PE, Griffin L, Berry M, Thompson C, Hite RD, Winkelman C, Hopkins RO, Ross A, Dixon L, Leach S, et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci. 2011;341(5):373–7. doi: 10.1097/MAJ.0b013e31820ab4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685–90. doi: 10.1001/jama.300.14.1685. [DOI] [PubMed] [Google Scholar]
- 18.Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, Wozniak AW, Colantuoni E, Ely EW, Rice TW, et al. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188(5):567–76. doi: 10.1164/rccm.201304-0651OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanni JM, Korupolu R, Fan E, Pradhan P, Janjua K, Palmer JB, Brower RG, Needham DM. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care. 2010;25(2):254–62. doi: 10.1016/j.jcrc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Chen YH, Lin HL, Hsiao HF, Chou LT, Kao KC, Huang CC, Tsai YH. Effects of exercise training on pulmonary mechanics and functional status in patients with prolonged mechanical ventilation. Respir Care. 2012;57(5):727–34. doi: 10.4187/respcare.01341. [DOI] [PubMed] [Google Scholar]
- 21.Chiang LL, Wang LY, Wu CP, Wu HD, Wu YT. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86(9):1271–81. doi: 10.2522/ptj.20050036. [DOI] [PubMed] [Google Scholar]
- 22.Mehrholz J, Muckel S, Oehmichen F, Pohl M. First results about recovery of walking function in patients with intensive care unit-acquired muscle weakness from the general weakness syndrome therapy (GymNAST) cohort study. BMJ Open. 2015;5(12):e008828. doi: 10.1136/bmjopen-2015-008828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Su CL, Wu YT, Wang LY, Wu CP, Wu HD, Chiang LL. Physical training is beneficial to functional status and survival in patients with prolonged mechanical ventilation. J Formos Med Assoc. 2011;110(9):572–9. doi: 10.1016/j.jfma.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Bohannon RW, Schaubert KL. Test-retest reliability of grip-strength measures obtained over a 12-week interval from community-dwelling elders. J Hand Ther. 2005;18(4):426–7. doi: 10.1197/j.jht.2005.07.003. (quiz 428) [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 26.Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) task force. J Nutr Health Aging. 2009;13(10):881–9. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 27.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. European respiratory journal]–>Eur Respir J. 1999;14(2):270–4. doi: 10.1034/j.1399-3003.1999.14b06.x. [DOI] [PubMed] [Google Scholar]
- 28.Fan E, Ciesla ND, Truong AD, Bhoopathi V, Zeger SL, Needham DM. Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med. 2010;36(6):1038–43. doi: 10.1007/s00134-010-1796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078–81. [PubMed] [Google Scholar]
- 30.MacIntyre NR, Epstein SK, Carson S, Scheinhorn D, Christopher K, Muldoon S. National Association for medical direction of respiratory C: management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128(6):3937–54. doi: 10.1378/chest.128.6.3937. [DOI] [PubMed] [Google Scholar]
- 31.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J. 2009;39(8):495–501. doi: 10.1111/j.1445-5994.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 33.Flaherty KR, Andrei AC, Murray S, Fraley C, Colby TV, Travis WD, Lama V, Kazerooni EA, Gross BH, Toews GB, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med. 2006;174(7):803–9. doi: 10.1164/rccm.200604-488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto-Plata VM, Cote C, Cabral H, Taylor J, Celli BR. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. European respiratory journal]–>Eur Respir J. 2004;23(1):28–33. doi: 10.1183/09031936.03.00034603. [DOI] [PubMed] [Google Scholar]
- 35.Clini EM, Crisafulli E, Antoni FD, Beneventi C, Trianni L, Costi S, Fabbri LM, Nava S. Functional recovery following physical training in tracheotomized and chronically ventilated patients. Respir Care. 2011;56(3):306–13. doi: 10.4187/respcare.00956. [DOI] [PubMed] [Google Scholar]
- 36.Martin UJ, Hincapie L, Nimchuk M, Gaughan J, Criner GJ. Impact of whole-body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33(10):2259–65. doi: 10.1097/01.ccm.0000181730.02238.9b. [DOI] [PubMed] [Google Scholar]
- 37.Scheinhorn DJ, Hassenpflug MS, Votto JJ, Chao DC, Epstein SK, Doig GS, Knight EB, Petrak RA. Ventilation outcomes study G: Post-ICU mechanical ventilation at 23 long-term care hospitals: a multicenter outcomes study. Chest. 2007;131(1):85–93. doi: 10.1378/chest.06-1081. [DOI] [PubMed] [Google Scholar]
- 38.Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, Make B, Rochester CL, Zuwallack R, Herrerias C. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(5 Suppl):4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 39.Morris PE, Berry MJ, Files DC, Thompson JC, Hauser J, Flores L, Dhar S, Chmelo E, Lovato J, Case LD, et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: a randomized clinical trial. JAMA. 2016;315(24):2694–702. doi: 10.1001/jama.2016.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balas MC, Devlin JW, Verceles AC, Morris P, Ely EW. Adapting the ABCDEF bundle to meet the needs of patients requiring prolonged mechanical ventilation in the long-term acute care hospital setting: historical perspectives and practical implications. Semin Respir Crit Care Med. 2016;37(1):119–35. doi: 10.1055/s-0035-1570361. [DOI] [PubMed] [Google Scholar]
- 41.Needham DM, Korupolu R. Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil. 2010;17(4):271–81. doi: 10.1310/tsr1704-271. [DOI] [PubMed] [Google Scholar]
- 42.Thrush A, Rozek M, Dekerlegand JL. The clinical utility of the functional status score for the intensive care unit (FSS-ICU) at a long-term acute care hospital: a prospective cohort study. Phys Ther. 2012;92(12):1536–45. doi: 10.2522/ptj.20110412. [DOI] [PubMed] [Google Scholar]
- 43.Corner EJ, Wood H, Englebretsen C, Thomas A, Grant RL, Nikoletou D, Soni N. The Chelsea critical care physical assessment tool (CPAx): validation of an innovative new tool to measure physical morbidity in the general adult critical care population; an observational proof-of-concept pilot study. Physiotherapy. 2013;99(1):33–41. doi: 10.1016/j.physio.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Perme C, Nawa RK, Winkelman C, Masud F. A tool to assess mobility status in critically ill patients: the Perme intensive care unit mobility score. Methodist Debakey Cardiovasc J. 2014;10(1):41–9. doi: 10.14797/mdcj-10-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.