Abstract
Phrenology was a nineteenth century endeavour to link personality traits with scalp morphology, which has been both influential and fiercely criticised, not least because of the assumption that scalp morphology can be informative of underlying brain function. Here we test the idea empirically rather than dismissing it out of hand. Whereas nineteenth century phrenologists had access to coarse measurement tools (digital technology referring then to fingers), we were able to re-examine phrenology using 21st century methods and thousands of subjects drawn from the largest neuroimaging study to date. High-quality structural MRI was used to quantify local scalp curvature. The resulting curvature statistics were compared against lifestyle measures acquired from the same cohort of subjects, being careful to match a subset of lifestyle measures to phrenological ideas of brain organisation, in an effort to evoke the character of Victorian times. The results represent the most rigorous evaluation of phrenological claims to date.
Keywords: Phrenology, MRI
1. Introduction
According to Franz Joseph Gall, the founder of phrenology, those of a mirthful disposition (i.e. those who like to laugh) should expect to find two prominent bumps on the forehead when compared to their more dour contemporaries (see Spurzheim, 1815). For nearly two centuries now, the academic community has openly mocked phrenology, yet the approach has seen moments of near redemption. In 1998 for example, electrical stimulation of the pre-SMA, a brain area near the “mirth” bump described by Gall, reportedly caused a patient to laugh (Fried, Wilson, MacDonald, & Behnke, 1998). More likely than not, Gall's association of this area with an “Organ of Mirthfullness” was accidental. It nonetheless frames the question empirically: does the local shape of the head reflect aspects of individual psychology?
A good reason to be sceptical about this is that the methodology behind phrenology was dubious even by the standards of the early 19th century. Phrenologists asserted the location of an “Organ of Amativeness” (describing “the faculty that gives rise to sexual feeling”) by probing the heads of “emotional” young women as well as the recently widowed; they hypothesised the location for an “Organ of Combativeness” by, inversely, searching for flat regions on the scalps of peaceable “Hindoos and Ceylonese” (Combe, 1835: 46). The phrenological approach therefore relied on tenuous and perhaps even offensive stereotypes about different social groups. Gall's science of “bump reading” would ultimately be abandoned as much for its fixation on social categories as for an inability within the scientific community to replicate its findings. These scientific failings would be exposed by anatomists like Paul Broca (1861) and Carl Wernicke (1874) who pioneered the alternative neuroscientific method of lesion–symptom mapping. Whereas lesion–symptom mapping described the brain directly, phrenology had to assume that scalp morphology correlated indirectly with local brain function. Even more damning: the results of lesion–symptom mapping contradicted those of phrenology. For instance, Broca and Wernicke identified lateral language areas in cortex roughly around the ear, where later phrenologists had asserted that the “Organ of Language” could be found below the eye (Spurzheim, 1815; see Supplementary Figure 1). In retrospect, phrenology's proposition that the brain is organised around functionally discrete modules was prescient. However, the idea that the brain's soft tissue might exert a significant effect on skull shape was, and is, nonsense. Or is it?
In this study, we sought to test the 19th century claims of phrenology by using 21st century scientific methods. We asked whether local changes in scalp morphology, measured reliably in almost six thousand subjects, do or do not correlate with the “faculties” that Gall described. For historical completeness, we also asked a second question: does local scalp morphology reflect the brain's underlying morphology? We asked this question because phrenologists believed that inspecting the outer surface of the head provided an indirect measure of brain shape, based on the assumption that the softness of the skull during development should allow it to yield under the pressure of locally expanding cortical structures (Catani and Sandrone, 2015, Catani and Thiebaut de Schotten, 2012, Finger, 1994). For data, we turned to the world's largest brain-imaging study, currently acquiring MRI and other data for 100,000 subjects (Alfaro-Almagro et al., 2017, Miller et al., 2016). We used all of the data from the first public release (5,724 subjects). The original scans were separated into parts representing the brain and parts representing the outer surface of the head: we focused on the outer surface of the head. By applying modern methods from neuroimaging—such as registration and normalisation, random field theory and mass univariate analysis—to the study of the cranium, we were able to search for statistical relationships between local head shape and lifestyle measures, which we took to reflect the “faculties” (or in modern terms “functions”) associated with phrenology. Although we did not expect to find any significant effects between lifestyle measures and head shape, we do believe it is important for scientists to test ideas, even unfashionable or offensive ones, and not be content by dismissing them out of hand. This study therefore represents the most rigorous evaluation of phrenological claims ever attempted and aims to offer either vindication, or the strongest objection yet against phrenology.
2. Methods
2.1. Data
We used anatomical brain-imaging data sampled from the UK Biobank Imaging study (http://imaging.ukbiobank.ac.uk). These data are representative of the largest neuroimaging study to date, aiming to acquire MRI and personal measures (including questionnaires and cognitive tests) for 100,000 subjects (Alfaro-Almagro et al., 2017, Miller et al., 2016). We used all available data from the first public release of 5,724 subjects (2,693 male, aged 45–78 years; mean = 62 years, standard deviation = 7 years; see Supplementary Figure 2).
2.2. Pre-processing
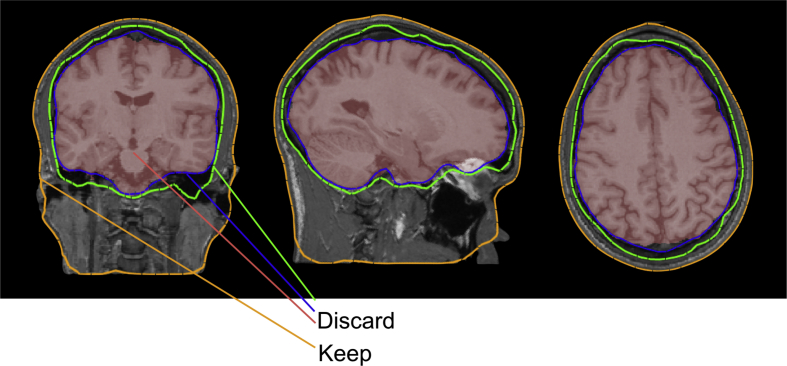
Each subject's T1-weighted structural scan was processed using the FSL Scalp Extraction Tool (SET) (Jenkinson et al., 2005, Smith, 2002). SET is used to produce an estimate of both inner and outer surfaces of the head (Fig. 2). Neuroimaging studies typically retain the extracted brain. For the main analysis, we discarded the brain in order to focus on the scalp surface.
Fig. 2.
Schematic of FSL's scalp extraction tool, which identifies various tissue boundaries (red = brain; blue = pial boundary; green = inner skull surface; orange = outer scalp surface). Phrenology is primarily focused on the scalp (outer surface of the head).
T1-weighted images were linearly aligned to a standard brain template (MNI152) using FLIRT (Jenkinson, Bannister, Brady, & Smith, 2002; Jenkinson & Smith, 2001), and the same transformations were applied to the vertex coordinates of the scalp surfaces of each subject. All scalp surfaces for all subjects were therefore aligned with one-to-one correspondence between the vertices, making it possible to compare scalps between subjects within a common space. In addition, we applied a hand-drawn mask to exclude surface vertices below the nose as these exhibited a high degree of between-subject variation, and were typically excluded by phrenologists (e.g. note the grey regions in Fig. 5).
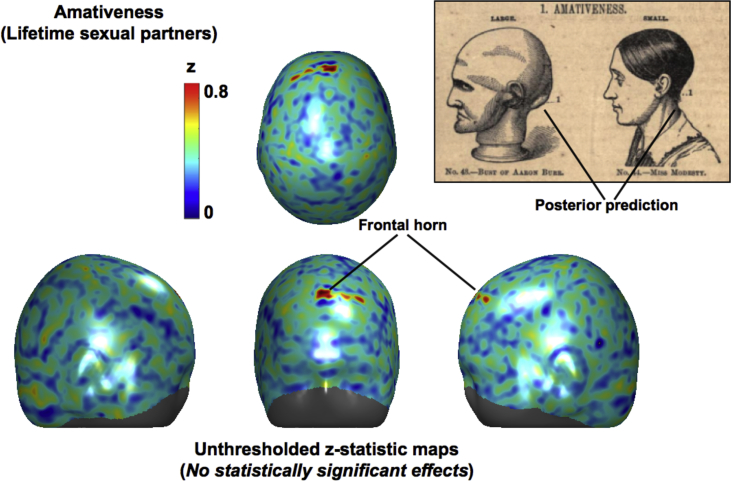
Fig. 5.
Illustration of over-interpreting null results. The scalp projections show an unthresholded z-statistic map of correlations between local scalp curvature and lifetime number of sexual partners, which has been overenthusiastically annotated with interpreted effects (i.e. the “frontal horn”). The results might be compared with those of the infamous “dead salmon” study, which highlighted the importance of correcting for multiple comparisons (Bennett, Baird, Miller, & Wolford, 2010). Please note that when thresholds for multiple comparisons were applied, none of the z-scores in this figure reached statistical significance. Also damning is the fact that the “frontal horn” area does not correspond to regions of interest predicted by 19th century phrenologists. The upper-right panel depicts a prediction for “Amativeness” on the opposite side of the skull (Fowler & Fowler, 1859).
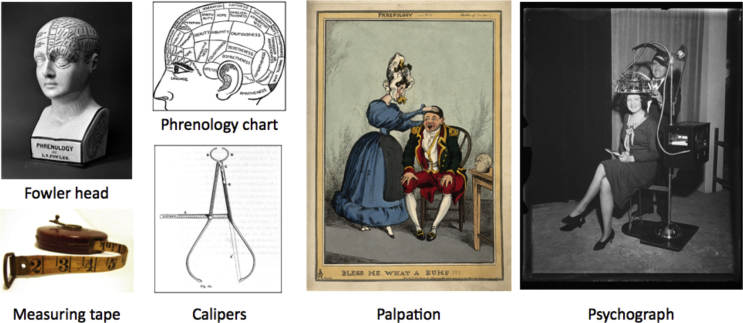
Although some phrenologists took global measures of the head using a measuring tape or calipers (Fig. 1), this practise was not unique. What was unique to phrenology was its emphasis on local head curvature, or “bumps”, encouraging us to focus here on the “bumps”. We calculated the mean (signed) curvature at each vertex of each individual surface projection (Peyré, 2011). This gave us 40,962 vertex measures per subject (see Fig. 4, panel A) which we smoothed using a 5 mm kernel (approximately half the width of a fingertip) effectively averaging over a larger surface area than the vertex. We then compared these automatically-extracted curvature measures against a set of lifestyle measures drawn from the same subjects.
Fig. 1.
Traditional tools of phrenology: Fowler head (L. N. Fowler, n.d.), phrenology chart (Unknown, n.d.), measuring tape (aussiegall, n.d.), calipers (Broca, 1871), palpation (Heath, 1830), psychograph (Harris & Ewing, 1931).
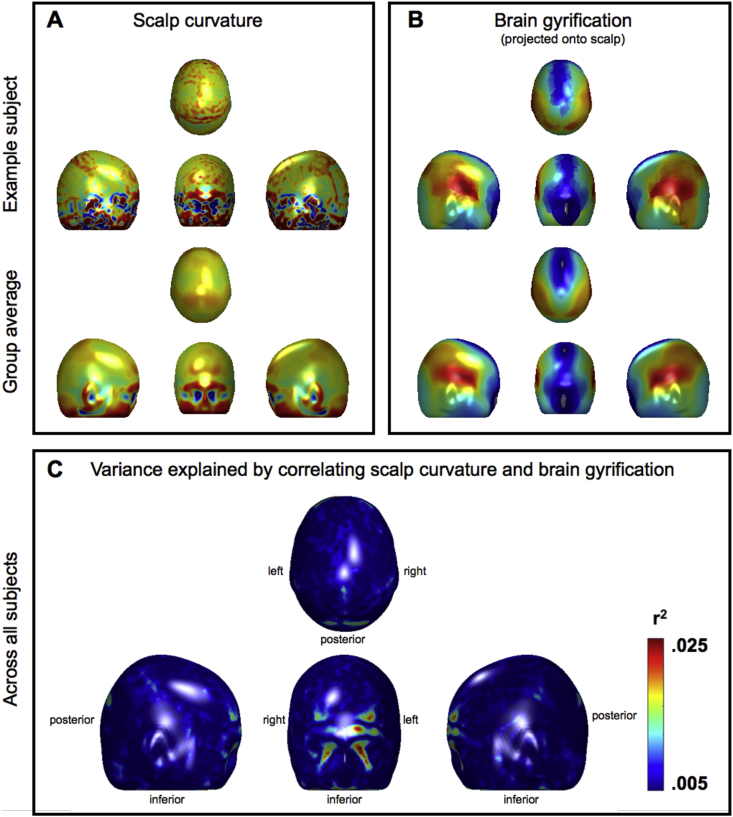
Fig. 4.
Scalp curvature, brain gyrification, and the variance explained by correlating the two. Panel A: Example scalp curvature data from a single subject (upper panel) and averaged over the entire cohort (lower panel). Red/Blue represents positive/negative (i.e. convex/concave) curvature values. Panel B: Example brain gyrification projected onto the scalp for a single subject (upper panel) and averaged over the entire cohort (lower panel). Red/Blue represents degree of gyrification (note large index values, in red, laterally over the Sylvian fissures). Panel C: Variance explained by correlating scalp curvature and brain gyrification. Note that the r2 values are very small; the “strongest” effects only explain about .025% of the variance (leaving 97.5% unexplained). The largest “effects” are also marginalised to the facial region, which is irrelevant to a great number of phrenological accounts and probably an artefact. All data have been projected onto the mean head surface.
2.3. Lifestyle measures and phrenological faculties
Philosophically, phrenology was organised around the metaphor of the brain as a collection of physical “organs” with identifiable functions such as “language” or “love”, or an “impulse to propagation”. In phrenology, these functions are referred to as “faculties”. Although these faculties diverge from the familiar functions mapped by neuroimaging in the 20th and 21st centuries, by focussing on identifiable functions the approaches do not differ in kind (Poldrack, 2010).
In addition to MRI, the UK Biobank Imaging study includes data from numerous questionnaires and cognitive tests, which we refer to collectively as “lifestyle measures”. Subject responses to these lifestyle measures could be binary (“Do you live with your parents?”) or integer-valued (“How many sexual partners have you had?”). Some integer-valued responses required closed-set answers (“How often do you eat beef?” given a range of options from 0 to 4, such that 0 means “never” and 4 means “I eat beef daily”). We used the lifestyle measures as proxies for 23 common phrenological “faculties” (Spurzheim, 1815).
Gall originally proposed 27 faculties (Eling et al., 2017, Gall, 1835). From these, we selected a subset of 23 faculties for which we found compelling lifestyle measures in the UK Biobank. For example, we associated the faculty of combativeness (argumentativeness) with lawyers, and we associated cunning with scientists. By connecting the faculty of “cunning” to our own profession, we follow a phrenological tradition evident for instance in Fowler and Fowler's (1859) choice to cite Gall's skull as an example of “Causality” (also referred to as “metaphysical perspicuity”—intended, it would appear, to be a good thing).
We give the full list of Faculties and associated lifestyle measures in Table 1, noting that: letter fluency (Faculty XIV) is the number of words starting with the letter “s” which the subject could produce in 1 min, and concept interpolation (Faculty XX) is a fluid-intelligence test which records one's capacity to solve problems that require logic or reasoning independent of acquired knowledge (where each subject had 2 min to complete as many questions as possible from the test). Four of Gall's faculties were excluded from our study because we could not find appealing proxies within the set of lifestyle measures. The excluded faculties were: XIII (recollection for persons), XXV (mimicry), XXVI (sense of god and religion), and XXVII (perseverance). We acknowledge that some of the associations may be less obvious. The link between Faculty XII (sense of locality) and the lifestyle measure “Time spent doing light physical activity” is an assumption that physically active people are more likely to get out of the house. Although keeping in mind that the lifestyle measures have important clinical and scientific uses, all associations here were made in a spirit of mirth.
Table 1.
Faculties and associated Biobank lifestyle measures.
| # | Faculty | Biobank lifestyle measure |
|---|---|---|
| I | Impulse to propagation (Amativeness) |
Lifetime number of sexual partners |
| II | Tenderness for the offspring or parental love (Philoprogenitiveness) |
People in the house related to participant (son/daughter/mother/father) |
| III | Friendly attachment or fidelity (Adhesiveness) |
People in the house not related to participant (husband/wife/partner/other) |
| IV | Valour, self-defence (Combativeness) |
Solicitor, lawyer, barrister, judge (job) |
| V | Murder, carnivorousness (Destructiveness) |
Beef intake |
| VI | Sense of cunning (Cunning) |
Scientist (job) |
| VII | Larceny, sense of property (Acquisitiveness) |
Number of vehicles in household |
| VIII | Pride, arrogance, love of authority (Self-Esteem) |
Banker (job) |
| IX | Ambition and vanity (Love of Approbation) |
Financial situation satisfaction |
| X | Circumspection (Cautiousness) |
Alcohol intake frequency |
| XI | Aptness to receive an education or the memoria realis (Eventuality and Individuality) |
Age completed full time education |
| XII | Sense of locality (Locality) |
Time spent doing light physical activity |
| XIV | Words, verbal memory (Words) |
Letter fluency |
| XV | Faculty of language (Language) |
Authors, writers (job) |
| XVI | Disposition for colouring, delighting in colours (Colouring) |
Photographers, painter (job) |
| XVII | Sense for sounds, musical talent (Tune) |
Music profession (job) |
| XVIII | Arithmetic, counting, time (Number) |
Mathematician (job) |
| XIX | Mechanical skill (Constructiveness) |
Hand grip strength (right) |
| XX | Comparative perspicuity, sagacity (Comparison) |
Concept interpolation |
| XXI | Metaphysical perspicuity (Causality) |
Clergy (job) |
| XXII | Causality, sense of inference (Mirthfulness) |
Writer, actor, comedian (job) |
| XXIII | Poetic talent (Ideality) |
Poet (job) |
| XXIV | Good nature, compassion, moral sense (Benevolence) |
Charity (job) |
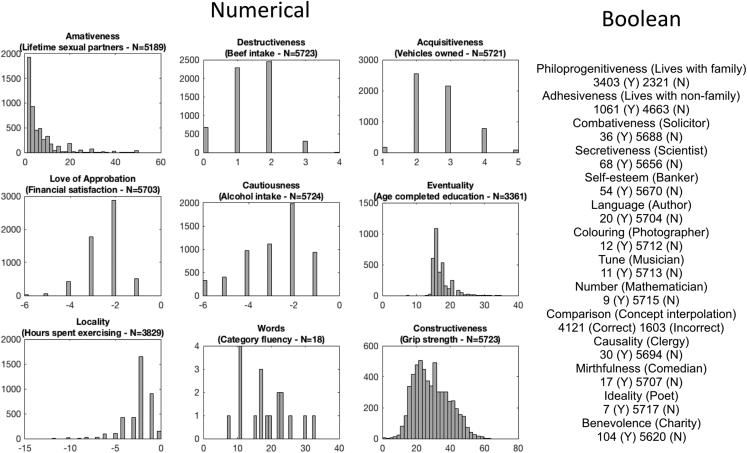
Fig. 3 summarises the distribution of responses obtained for the lifestyle measures for the available subjects. The numbers of subjects sampled for each category were rather large, except for the “job”-based lifestyle measures (see Table 1).We ultimately left these faculties out of the final phrenological analysis in which scalp morphology was correlated against lifestyle measures. For those interested, there is a paper exploring the lifestyle measures in greater detail (Miller et al., 2016).
Fig. 3.
Distributions of faculties (Biobank measures). We matched Gall's faculties against a set of personal measures that were acquired by the UK Biobank. Three subject measures (financial satisfaction, alcohol intake, and time spent exercising) were multiplied by −1 to correlate positively with the corresponding faculties. Amativeness had a long tail (values going up to 1,000); although these were cut out in the figure, no values were excluded from the GLM analysis. For the numerical faculties, N refers to the total number of subjects; for the Boolean faculties, N refers to the number of subjects who answered “no”.
2.4. Relating local scalp morphology to personal measures
Starting with phrenology's claim that bumps on the head relate to individual traits, we used multivariate regression to search for associations between local scalp curvature and lifestyle measures extracted from the Biobank. More concretely, we modelled vertex-wise scalp curvature against lifestyle measures including gender and age as nuisance regressors. For the binary measures, such as whether or not someone was a banker, the regression model could be set up as an unpaired t-test. For illustration purposes, all resulting t-statistics were converted to z-statistics (see Discussion). In total, we note that there were 14 binary lifestyle measures (faculties II, III, IV, VI, VIII, XV, XVI, XVII, XVIII, XX, XXI, XXII, XXIII and XXIV) and nine non-binary measures (faculties I, V, VII, IX, X, XI, XII, XIV, and XIX) all shown in Fig. 3. However, there were low numbers in some of the binary measures (faculties IV, VI, VIII, XV, XVI, XVII, XVIII, XIX, XXI, XXII, XXIII, XXIV), so these were omitted from the results.
We calculated vertex-wise p-values for the null hypothesis (that there should be no association between scalp curvature and lifestyle measures). In order to control for multiple comparisons across the scalp, which is something that phrenologists failed to the best of our knowledge to report, we used resel-based correction and Random Field Theory (Worsley et al., 1999, Worsley et al., 1992, Worsley et al., 1996) given a significance threshold of .05 (Bonferroni-corrected across the faculties tested).
2.5. Relating scalp morphology to local brain morphology
In order to test the second claim of phrenology, that bumps on the head should reflect the underlying shape of the cerebral cortex, we correlated each subject's local scalp curvature (described above) with a local index of brain gyrification (projected onto the scalp). This gyrification index was quantified using a surface ratio, corresponding to the amount of cortical surface packed within a limited spherical volume at every point on the cortex (Toro et al., 2008). If the phrenological hypothesis is true, we would expect a large negative correlation between scalp curvature and the gyrification index. We assume that the amount of cortical surface correlates with pressure under the skull but note that 19th century phrenologists imagined pressure to come from individual gyri instead. For data we extracted the cortical (pial) surface from each subject's T1-weighted scan using FreeSurfer (Dale, Fischl, & Sereno, 1999). In order to summarise the surface ratio of the cortex underlying each scalp vertex, we used the average surface ratio within a 20 mm sphere that was centred around the nearest cortical vertex. Once both measures (scalp curvature and cortical convolution) were mapped onto the scalp surface, we were able to correlate the two measures and answer the question of whether scalp morphology looks like a consistent proxy for underlying brain morphology.
3. Results
The phrenological analyses produced no statistically significant or meaningful effects.
4. Discussion
The present study sought to test in the most exhaustive way currently possible the fundamental claim of phrenology: that measuring the contour of the head provides a reliable method for inferring mental capacities. We found no evidence for this claim. First, we explored the effect on local scalp curvature of underlying brain gyrification given that phrenology assumes a relationship between head and brain morphology. We found that brain gyrification explains very little of the variance in local scalp curvature (Fig. 4). Second, we correlated local scalp curvature with a set of lifestyle measures interpreted as Victorian “faculties” (e.g. “lifetime number of sexual partners” was used as a proxy for the faculty of “Amativeness”, or the “impulse to propagation”). Despite the size of our sample and automation of our methods, we found no evidence to support phrenology's fundamental claim. The regions depicted on phrenological busts (Fig. 1) therefore should not be trusted. According to our results, a more accurate phrenological bust should be left blank since no regions on the head correlate with any of the faculties that we tested. But even below the level of statistical significance, we found historic phrenological predictions to be uninsightful. For example, Fig. 5 shows the unthresholded z-statistic map for correlations between local head curvature and lifetime number of sexual partners (“Amativeness”). Unsurprisingly, the “frontal horn” area that we point out does not correspond to ROIs proposed by phrenologists, which included areas at the back of the skull (Fowler & Fowler, 1859). For the reckless, zealous or simply curious reader, we include the remaining unthresholded z-statistic maps (none statistically significant) in the Supplementary Materials (Supplementary Figure 3 to Supplementary Figure 24). We did not analyse the relationship between lifestyle measures and brain morphology, since many such relationships are known and uncontroversial within 21st century neuroscience (Grogan et al., 2012, Maguire et al., 2000, Mechelli et al., 2004). What is peculiar about phrenology is its emphasis on the outer head (i.e. skull and scalp) as an indirect measure of the brain, and thus of personality and behaviour.
The strengths of our approach are the automation of head measurements from MRI data and number of subjects studied. Because the analysis methods were automated, the number of subjects studied could easily number in the thousands. By contrast, although phrenologists had access to quantitative tools like the measuring tape and caliper, and some attempt was even made to automate measurements as evidenced by the psychograph (Fig. 1), phrenology typically relied on “palpation” (the manual examination of subjects' heads, or 19th century digital technology). Reference materials including phrenology charts and Fowler heads (Fig. 1) were the results of underpowered studies based on anecdotal evidence (Eling et al., 2017).
Set against the strengths of our study, an apparent weakness is our use of 19th century “faculty psychology” with its description of human nature in idiosyncratic terms like “Amativeness” and “Philoprogenitiveness” (Poldrack, 2010), and grouping together of attributes like “eats meat” and “likes to kill”, which may strike some today as odd (Eling et al., 2017). Therefore it might be objected that we should have used a more recent ontology. However, phrenology's “faculty psychology” is not as different from current ontologies as it might first seem. One can readily find examples of 19th century faculties in the neuroimaging literature, albeit under different names (Table 2). We were also interested in grounding our study in Victorian concepts, despite an emphasis on 21st century methods. The lifestyle features that we selected also ranged over a wide number of behavioural and cognitive domains (e.g. motor skills, language, spatial awareness, decision making, etc.). So regardless of ontology, we hope to have covered many topics of interest.
Table 2.
Examples of nineteenth-century phrenology faculties in modern neuroimaging studies (of the brain). Adapted from Poldrack, 2010.
| Phrenological faculty | Modern neuroimaging equivalent | Associated regions | References |
|---|---|---|---|
| Impulse to propagation (Amativeness) |
Viewing of romantic lover vs. other individuals | Basal ganglia | Aron, Fisher, Mashek, Strong, & Strong, 2005 |
| Ambition and vanity (Love of Approbation) |
Activation for judgement about self vs. others | Medial prefrontal cortex | Ochsner et al., 2005 |
| Circumspection (Cautiousness) |
Activation correlated with harm avoidance | Nucleus accumbens | Matthews, Simmons, Lane, & Paulus, 2004 |
| Arithmetic, counting, time (Number) |
Activity correlated with arithmetic skill | Angular gyrus | Menon et al., 2000 |
As to the objection that phrenology was already a known dead-end scientifically, and that its claims did not need to be tested rigorously, it is indeed hard to find a time in history when phrenology was not seriously criticised. Even in 1815, the year that Spurzheim published his influential book on Gall's method, phrenology was dismissed by one reviewer as “a piece of thorough quackery from beginning to end” (Gordon, 1815). Not only did the reviewer take issue with the use of palpation as an indirect method for measuring the brain and its mental faculties, but he also objected to the idea the brain might be composed of multiple specialised components, writing the following (Gordon, 1815: 243):
“The cases in which portions of various sizes have been removed from almost all regions of this organ [the brain], without the slightest affection either of Intellect or Inclination, are numerous and most unequivocal.”
Despite Gordon's objection, this second idea, known today as “functional specialisation” or “segregation” (Tononi, Sporns, & Edelman, 1994), has proven central to our understanding of the brain since Broca's first celebrated case study (Broca, 1861). We mention this to highlight the importance of empiricism and of testing improbable sounding theories. We would argue that phrenology's claim, that the shape of the head might reflect brain function, is not a priori incoherent. It is certainly true that the shape of the head reflects mental capacities in extreme pathological cases such as hydrocephalus, where increasing head size could reflect progressive ventriculomegaly (Ridgway & Weiner, 2004). Even in the healthy population, adequate childhood nutrition might result both in increased intelligence scores and in parallel skull growth, such that one might detect a correlation between intelligence tests and local scalp curvature. The possibility of this outcome shows that the scalp-curvature hypothesis is not refutable by armchair methods alone but requires empirical testing. That said, we of course acknowledge that science cannot test all hypotheses, but rather, because of limited resources, scientists must choose assiduously between experiments (Feyerabend, 1975). It would not have been realistic, or perhaps even ethical, to acquire MRI for thousands of subjects with the purpose of testing a long-abandoned theory. However, one of many benefits of big data projects like the UK Biobank is that they provide resources for answering questions that might otherwise have remained untested, or even, because of limited resources, untestable.
Although written in a light-hearted spirit, this study demonstrates the feasibility of applying to cranial data the standard methods of neuroimaging (like registration, normalisation, random field theory, and mass univariate analysis). One potential application of these methods would be the clinical treatment of craniosynostosis. In extreme craniosynostosis cases, paediatric surgeons will separate the fused bones in a baby's head to increase the size of the cranial vault, thereby creating the necessary space for brain growth. Nonetheless, because of the inherent risks of surgery, there are many “border” cases that are not operated on, where the use of neuroimaging methods on the skull could be used to track (or maybe retrospectively evaluate) correlations between local head shape and cognitive development. These methods could be useful for deciding whether developmental impairments are sufficient to motivate similar “border” cases to be operated on in future. Incidentally, this sort of medical application is closer to the serious scientific and clinical motivation that originally animated the creation of the UK Biobank.
In summary, we hope to have argued convincingly against the idea that local scalp curvature can be used to infer brain function in the healthy population. Given the thoroughness of this study, it is unlikely that more scalp data would yield significant effects. Future work might focus on the inner (rather than outer) curvature of the skull, perhaps formalising a virtual method for creating endocasts, casts of fossilised skulls used to study the evolution of brains (Buchholtz and Seyfarth, 2001, Edinger, 1921). But we would advocate that future studies focus on the brain.
Contributors
OPJ and SJ conceived of the study over pints at our local pub, the White Hart; all authors contributed to data analysis and writing.
Funding
SJ is funded by UK Medical Research Council (MR/L009013/1). The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z).
Competing interests
All authors have completed the ICMJE unified disclosure form competing interest form at www.icmje.org/coi_ disclosure.pdf (available on request from the corresponding author) and declare no support from any organisation for the submitted work, and no financial relationships with any organisations that might have an interest in the submitted work in the previous three years.
Ethical approval
Not required.
Transparency statement
The authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Data sharing
The data are available online (http://www.ukbiobank.ac.uk/).
Acknowledgements
The data used in this work were obtained from the UK Biobank under Data Access Application Number 8107. We are grateful to the UK Biobank for making the resource data available, and are extremely grateful to all UK Biobank study participants, who generously donated their time to make this resource possible.
Reviewed 25 September 2017
Action editor Marco Catani
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.cortex.2018.04.011.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Alfaro-Almagro F., Jenkinson M., Bangerter N.K., Andersson J.L.R., Griffanti L., Douaud G. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 2017;166:400–424. doi: 10.1016/j.neuroimage.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A., Fisher H., Mashek D., Strong G., Strong H. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94(1):327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- aussiegall. (n.d.). Measuring time: An old measuring tape. Image at https://www.flickr.com/photos/14516334@N00/286709039 licensed under CC-BY-2.0.
- Bennett C.M., Baird A.A., Miller M.B., Wolford G.L. Neural correlates of interspecies perspective taking in the post-mortem Atlantic Salmon: An argument for proper multiple comparisons correction. Journal of Serendipitous and Unexpected Results. 2010;1(1):1–5. [Google Scholar]
- Broca P. Remarques sur le siège de la faculté du langage articulé; suivies d’une observation d’aphémie (perte de la parole) Bulletins de La Société Anatomique (Paris) 1861;6:330–357. 398–407. [Google Scholar]
- Broca P. C. Reinwald; Paris: 1871. Mémoires d’anthropologie. [Google Scholar]
- Buchholtz E.A., Seyfarth E.-A. The study of “fossil brains”: Tilly Edinger (1897-1967) and the beginnings of paleoneurology. Bioscience. 2001;51(8):674–682. [Google Scholar]
- Catani M., Sandrone S. Oxford University Press; Oxford: 2015. Brain Renaissance: From Vesalius to modern neuroscience. [Google Scholar]
- Catani M., Thiebaut de Schotten M. Oxford University Press; Oxford: 2012. Atlas of human brain connections. [Google Scholar]
- Combe G. Marsh, Capen & Lyon; Boston: 1835. Elements of phrenology. [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Edinger T. Über Nothosaurus. Ein Steinkern der Schädelhöhle. Senckenbergiana. 1921;3:121–129. [Google Scholar]
- Eling P., Finger S., Whitaker H. On the origins of organology: Franz Joseph Gall and a girl named Bianchi. Cortex. 2017;86:123–131. doi: 10.1016/j.cortex.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Feyerabend P. New Left Books; New York: 1975. Against method. [Google Scholar]
- Finger S. Oxford University Press; Oxford: 1994. Origins of neuroscience: A history of explorations into brain function. [Google Scholar]
- Fowler, L. N. (n.d.). Photograph: “Phrenology”, a ceramic head. Wellcome Library, London. Image at https://wellcomecollection.org/works/wz7p6v6b licensed under CC-BY-4.0.
- Fowler O.S., Fowler L.N. Fowler and Wells; New York: 1859. New illustrated self-instructor in phrenology and physiology; with over 100 engravings; together with the chart and character of. [Google Scholar]
- Fried I., Wilson C.L., MacDonald K.A., Behnke E.J. Electric current stimulates laughter. Nature. 1998;391:650. doi: 10.1038/35536. [DOI] [PubMed] [Google Scholar]
- Gall F.J. In: On the functions of the brain and each of its parts: With observations on the possibility of determining the instincts, propensities, and talents, or the moral and intellectual dispositions of men and animals, by the configuration of the brain and head. Lewis W., editor. Marsh, Capen and Lyon; Boston, MA: 1835. [Google Scholar]
- Gordon J. The doctrines of Gall and Spurzheim. Edinburgh Review. 1815;25:227–268. [Google Scholar]
- Grogan A., Parker Jones O., Ali N., Crinion J., Orabona S., Mechias M.L. Structural correlates for lexical efficiency and number of languages in non-native speakers of English. Neuropsychologia. 2012;50(7):1347–1352. doi: 10.1016/j.neuropsychologia.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, Ewing . US Library of Congress; 1931. Woman seated with a psychograph, a phrenology machine, on her head.https://www.loc.gov/item/2016879401/ [Google Scholar]
- Heath W. Wellcome Library; London: 1830. A smartly dressed woman examining the head of a military man. Image at https://wellcomecollection.org/works/gw9zk4th licensed under CC-BY-4.0. [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Pechaud M., Smith S. Poster session presentation at the meeting of the Eleventh Annual Meeting of the Organization for Human Brain Mapping, Toronto, Canada. 2005. BET2: MR-based estimation of brain, skull and scalp surfaces. https://www.elsevier.com/journals/cortex/0010-9452/guide-for-authors. [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Maguire E.A., Gadian D.G., Johnsrude I.S., Good C.D., Ashburner J., Frackowiak R.S.J. Navigation-related structural change in the hippocampi of taxi drivers. PNAS. 2000;97(8):4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews S., Simmons A., Lane S., Paulus M. Selective activation of the nucleus accumbens during risk-taking decision making. Neuroreport. 2004;15(13):2123–2127. doi: 10.1097/00001756-200409150-00025. [DOI] [PubMed] [Google Scholar]
- Mechelli A., Crinion J.T., Noppeney U., O'Doherty J., Ashburner J., Frackowiak R.S. Neurolinguistics: Structual plasticity in the bilingual brain. Nature. 2004;757 doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Menon V., Rivera S., White C., Eliez S., Glover G., Reiss A. Functional optimization of arithmetic processing in perfect performers. Cognitive Brain Research. 2000;9:343–345. doi: 10.1016/s0926-6410(00)00010-0. [DOI] [PubMed] [Google Scholar]
- Miller K.L., Alfaro-Almagro F., Bangerter N.K., Thomas D.L., Yacoub E., Xu J. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nature Neuroscience. 2016;19(11):1523–1536. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K., Beer J., Robertson E., Cooper J., Gabrieli J., Kihsltrom J. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Peyré G. The numerical tours of signal processing-advanced computational signal and image processing. IEEE Computing in Science and Engineering. 2011;13(4):94–97. [Google Scholar]
- Poldrack R.A. Mapping mental function to brain structure: How can cognitive neuroimaging succeed? Perspectives on Psychological Science. 2010;5(5):753–761. doi: 10.1177/1745691610388777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway E.B., Weiner H.L. Skull deformities. Pediatric Clinics of North America. 2004;51(2):359–387. doi: 10.1016/j.pcl.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurzheim J.G. Baldwin, Cradock, and Joy; London: 1815. The physiognomical system of Drs. Gall and Spurzheim; founded on an anatomical and physiological examination of the nervous system in general, and of the brain in particular; and indicating the dispositions and manifestations of the mind. [Google Scholar]
- Tononi G., Sporns O., Edelman G.M. A measure for brain complexity: Relating functional segregation and integration in the nervous system. Proceedings of the National Academy of Sciences. 1994;91(11):5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R., Perron M., Pike B., Richer L., Veillette S., Pausova Z. Brain size and folding of the human cerebral cortex. Cerebral Cortex. 2008;18(10):2352–2357. doi: 10.1093/cercor/bhm261. [DOI] [PubMed] [Google Scholar]
- Unknown (n.d.). brain-chart-diagram-face-fringe-2029363. Image at https://pixabay.com/p-2029363 licensed under CC0.
- Wernicke C. M. Cohn und Weigert; Breslau: 1874. Der aphasische symptomencomplex: Eine psychologische studie auf anatomischer basis. [Google Scholar]
- Worsley K.J., Andermann M., Koulis T., Macdonald D. Non-isotropic images. 1999. Detecting changes; pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K.J., Evans A.C., Marrett S., Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 1992;12(6):900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Marrett S., Neelin P., Vandal A.C., Friston K.J., Evans A.C. A unified statistical approach for determining significant voxels in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.