Abstract
Constant removal of sugars from the site of synthesis (i.e., leaves), in response to elevated sink (culm) demand, may perhaps prevent damping of photosynthesis, by sugar, and hence promote further sucrose accumulation in the culm. In this study, gibberellic acid (GA3) induced nearly 42.3% enlargement in cell size and about 39.3% increase in internodal length (sink capacity), 177% escalation in reducing sugar level (sink strength), amplified the expression of sucrose-metabolizing enzymes (sink demand), viz., 7.5-fold for SAI, 4.5-fold for CWI, sixfold for SPS, all demonstrating facilitation of augmented sucrose accumulation in the culm. The GA3-treated BO 91 cane (late maturing sugarcane variety) exhibited an elevated final sucrose concentration (40.54–41.6%) as compared to control (30.44–38.8%). The GA3-sprayed cane of early maturing Co J64 also showed such a boost, but it was lost by the end of maturity, perhaps due to inversion and/or the less effective GA3 treatment. Thus, results demonstrated the role of GA3 in augmenting sucrose content of cane culm, possibly by influencing source–sink dynamics in sugarcane.
Keywords: Source–sink, Sugarcane, Gibberellin, Sucrose, Gene expression, qRT-PCR
Introduction
Sugarcane houses a unique source–sink system wherein the mature culm serves as a large sucrose reservoir called the ‘sink’ and the leaves play the ‘source’ of photosynthetic sugars (Watt et al. 2014). The supply of photoassimilate works on a demand and supply basis wherein sucrose demand for growth and differentiation during plant development originates in the sink tissues and sucrose synthesis to meet this demand, occurs in the leaves of plants (Hussain et al. 2004). Changes in sink activity as plants grow and mature, result in change in the demand for photosynthate (viz., immediate utilization for growth and respiration or for storage too). The rate of phloem loading is accordingly adjusted, to accommodate the changing sink demand (Lalonde et al. 2003). Thus, the rate of sucrose synthesis in the source is governed by the rate of photosynthesis and also the rate of export from leaves (Battistelli et al. 1991).
Sucrose accumulation in sugarcane culm occurs against the concentration gradient powered by respiration-generated energy (Bieleski 1960). Sucrose has been reported to be present in the vacuole as well as the apoplastic space and symplast, in similar concentrations (Welbaum and Meinzer 1990). As the culm matures, sucrose concentration along the entire stalk increases, while the proportion of glucose and fructose decreases (Lontom et al. 2008). Hence, a sucrose gradient exists in the culm, with low levels in young internodes to more than 200 g kg−1 fresh weight in the mature internodes (Glasziou and Gayler 1972).
The quantum of sucrose accumulation is regulated by various enzymes involved in its synthesis and breakdown (Moore 1995), viz., invertases and synthases. SPS (sucrose phosphate synthase) is a key sucrose-synthesizing enzyme which regulates photosynthetic sucrose synthesis (Stitt et al. 1988). Photosynthetically produced sucrose present in the source mesophyll cells is either loaded symplastically via bundle sheath cells or exported to apoplasm and loaded into SE-CCC (sieve element-companion cell complex) by a proton-sucrose symporter (Riesmeier et al. 1994). Upon transportation and arrival of sucrose in the culm/stem, it can be catabolized by sucrose synthase (SuSy) or the three invertase isoforms, viz., soluble acid invertase (SAI; high in apoplast and vacuoles of young internodes, pH optimum: 4.4), cell wall bound acid invertase (CWI; pH optimum: 3.8), and neutral invertase (NI; in cytoplasm, pH optimum: 7.0) (Glasziou and Gayler 1972). The thus generated hexoses may be metabolized and fed into various biochemical pathways, or resynthesized into sucrose by SPS (Hatch et al. 1963). Sucrose synthase (SuSy) is also reported to partake in sucrose synthesis though it is primarily involved in sucrose degradation. As reported in sugarcane culms too (Moore 1995), during sink development, phloem unloading may initially occur via the symplastic route and later involve an apoplastic step also. The additional route for unloading is reasoned by the increased need for sucrose translocation due to growing sink demand. Sucrose transporters (SUTs) mediate sucrose influx at the source as well as its efflux at the sink. The sucrose unloaded in the stem parenchyma is cleaved by CWI, thus facilitating transport of monosaccharides through the plasma membrane. Surplus sugar (other than that required for growth, respiration and other metabolic processes) is resynthesized into sucrose by SPS and SS and stored in vacuole.
The idea of present study is derived from the fact that sugarcane culms have the potential to accommodate much more sucrose than has been attained till date. Sucrose accumulation in the culm can perhaps be improved by preventing/ delaying saturation of the sink demand, which will in turn induce greater sucrose production at the source, thus facilitating increased export of photosynthate supply through the phloem (Grof and Campbell 2005). Sugar feeding and cold-girdling treatments have demonstrated how sugar accumulation in leaves causes decrease in photosynthetic rates and chlorophyll content, and down-regulation of enzymes related to photosynthesis (Krapp and Stitt 1995), and sugar transporters (Chiou and Bush 1998), in several species including sugarcane (McCormick et al. 2006, 2008). McCormick et al. (2006) have demonstrated how increase in the sink demand brought about by partial shading, causes an increase in photosynthetic CO2 assimilation and electron transport rate of the sole unshaded source leaf over the duration of shading treatment. The immature culm exhibits a decrease in sucrose and increase in hexose sugar levels over the shading period due to increased sink demand.
Phytohormones like GA3 (gibberellic acid) have been reported to affect cell expansion thereby increasing sink size and sink strength, thus enhancing the competitive ability of the organ to draw assimilates (Iqbal et al. 2011). GA3 perhaps affects source–sink communication by stimulating assimilate transport and increasing phloem unloading of sucrose into the sink, establishing a more favourable sucrose gradient between sink and source (Cole et al. 1972). Gibberellins can thus be predicted to enhance sucrose accumulation in the sugarcane culm by heightening sink demand, subsequently increasing plant yield. In the present pursuit, the differences in sugar behaviour and gene expression of control and GA3-treated plants have been explored, to throw light on how GA (gibberellins) manipulation of regulatory components can help sustain high photosynthetic rates despite sufficient culm sucrose accumulation, thereby facilitating better sucrose yield. Also, a comparison between biochemical and molecular level response of high (CoJ 64) and low (BO 91) sucrose accumulating varieties to GA3, as drawn in this study, can display vital attributes that lead to high sucrose accumulation in the sugarcane culm.
Materials and methods
Plant materials
Planting was done in the last week of February, 2015, using three bud sets, of an early maturing, high sucrose accumulating (CoJ 64) and late maturing, low sucrose accumulating (BO 91) variety, at ICAR-Indian Institute of Sugarcane Research farms (26.78°N, 80.99°E, 111 msl) Lucknow, India. Employing two varieties with contrasting sucrose accumulation behaviour helped compare the role of GA3 in facilitating sucrose accumulation in an already high sucrose accumulator to that in a comparatively low sucrose accumulator and the differences involved therein. Gibberellic acid (GA3) was exogenously applied thrice, during the grand growth phase (July–September), at 1 month interval, in the form of a 35 ppm spray. Sampling of a non-sprayed plant was done on 0 DAS (0 day after spraying) in September, to serve as 0th day control and over the entire maturity (sucrose accumulation) phase, from October to January for CoJ 64 and October to March for BO 91. Each month, three control (non-sprayed) and three GA3-sprayed canes, of similar height and girth and reared under normal growth conditions, were sampled. Dividing each cane into three equal portions, tissue extracted from top and middle cane portions was used for RNA sampling, while juice from bottom, middle and top portions of the canes was used in the estimation of sugar. Sugar evaluation was also done in the freshly opened and photosynthetically most active LTM (last transverse mark) leaf of control and GA3-sprayed canes. RNA was also extracted from the LTM leaf for further molecular analysis.
SEM visualization
The internodal length and girth of three canes each, of control and GA3-treated type, was tracked weekly, for a month, after the last spray (i.e., through October). The middle portion (i.e., internode no. 10–14 in CoJ 64 and no. 9–13 in BO 91) of control and GA3-treated canes consistently showed greatest difference in length between corresponding internodes (Table 1). Tissue from a specific area in between the rind and pith of control and GA3-treated internodes that showed the maximum length difference, was visualized using scanning electron microscope. 5% w/v glutaraldehyde was employed as fixative and the culm tissue samples were incubated in it for 4 h at pH 7.0 in 0.05 M sodium cacodylate buffer. The samples were replenished with fresh fixative after 2 h and thereafter rinsed with buffer. Serial dehydration was carried out in ethanol (Busse et al. 2005). The samples were then subjected to critical point drying and were sputter-coated with palladium. These prepared samples were observed under JEOL 6490LV scanning electron microscope (SEM) with an accelerating voltage of 15 kV, to ascertain morphological difference between control and GA3 sprayed samples.
Table 1.
Difference in internodal length in middle region of control and GA3-treated canes
| Internode number | Sugarcane variety CoJ64 | Internode number | Sugarcane variety BO91 | ||
|---|---|---|---|---|---|
| Control | GA3 sprayed | Control | GA3 sprayed | ||
| Internodal length/girth (cm) | Internodal length/girth (cm) | Internodal length/girth (cm) | Internodal length/girth (cm) | ||
| 10 | 11.0/2.2 | 9.9/2.65 | 9 | 12.2/2.0 | 13.6/2.1 |
| 11 | 10.2/2.3 | 13.0/2.5 | 10 | 11.2/1.9 | 15.6/2.1 |
| 12 | 11.3/2.3 | 13.7/2.5 | 11 | 11.0/1.9 | 15.0/2.1 |
| 13 | 9.7/2.5 | 12.0/2.5 | 12 | 10.1/2.0 | 13.3/2.0 |
| 14 | 9.0/2.5 | 11.0/2.5 | 13 | 9.6/2.0 | 10.7/2.1 |
Reducing sugar and sucrose content
Standardized dilution of fresh cane juice and leaf extract was used to determine reducing sugar (RS) content in each, using Nelson’s method (Nelson 1944). For estimating total sugar content, 0.1 N HCl-mediated sucrose hydrolysis step was incorporated at the beginning of the RS estimation procedure. Absorbance was taken at 540 nm with observations made in triplicate for each of the samples. The values were calculated, taking the standard curve of glucose as reference. The sucrose content was estimated as a difference of the two values.
Total RNA isolation
Isolation of total RNA was done (using Trizol) from defined cane portions (middle and top) and LTM leaf, of control and GA3-sprayed canes of BO 91 and CoJ 64 variety. RNA quality was checked on 1% agarose gel and the RNA was quantified using nanodrop spectrophotometer (Quawell UV–visible spectrophotometer). The concentration of RNA samples was normalized, taking clue from the gel and nanodrop observations. The total RNA samples were treated with DNase I (Thermo Scientific, USA) to remove any possible DNA contamination.
Semi-quantitative RT-PCR analysis
The RNA normalization was verified using 25S rRNA gene primer pair (F5′ GCAGCCAAGCGTTCATAGC3′; R5′CCTATTGGTGGGTGAACAATCC3′) as internal control. Leaf RNA samples were subjected to a combined cDNA synthesis and reverse transcriptase reaction set up using one-step RT-PCR kit (Qiagen, India). Differential expression w.r.t. gene specific primers, viz., phosphoenolpyruvate carboxylase (PEPC) (F5′ATCAAGGAGAAACTGGATG3′;R5′TCAGGAAAGAACTAGACTGC3′) and sucrose transporter (F5′GTGCTCATCTGCATTGCTGT3′;R5′CTTGTGCCAATTGTTTGTGG3′), were analyzed with respect to controlled and GA3 perturbed source–sink conditions. RT-PCR was performed in PTC 200 thermal cycler (MJ Research/BioRad, USA). The amplified product was distinctly visualized on 1.6% agarose gel, using the gel documentation system (Alpha Innotech, USA).
Quantitative real-time PCR analysis
Using 2 µg of total RNA as starting material, first-strand cDNA synthesis was done, priming it with oligo-dT and using RevertAid H minus Reverse Transcriptase (Thermo Scientific), as per the manufacturer’s instructions. Gene-specific primers for SAI (F5′CAGAGGAACTGGATGAACGA3′; R5′CCGCTTGAAATGTCAATGTC3′), CWI (F5′ TCTGTACAAGCCAACCTTCG3′; R5′CCGCTTGAAATGTCAATGTC3′), SPS (F5′ CCCGAACATTGCAAGAATTA3′; R5′CTCCGCTCCTCTCTGTTACC3′) and SuSy (F5′ GGCTGTTGCCTGATGCTGTT3′;R5′TGCTCGGTTCCAATGACCTT3′) (Chandra et al. 2015), were utilized to quantitatively estimate their gene expression by real-time PCR analysis, carried out in 48-well plates on a Step One Real-Time PCR system (Applied Biosystems, USA) using SYBR Green PCR Master mix (Applied Biosystems, USA). The gene encoding 25S was used as reference (i.e., for calibration) in all reactions. Real-time PCR was carried out using the relative quantification method and expression ratios were computed from cycle threshold values to obtain or RQ values, depicting fold change.
Data analysis
The experimental data was recorded as the mean of three replicates derived from three individual plants for each sample and the results were noted as the means ± standard error. Two-sided Student’s t test was employed to determine the significance of difference between the mean values of control and GA3-treated plants. The statistical significance of differences in the effect of treatment was represented as P ≤ 0.05 (*), P ≤ 0.01 (**), and P ≤ 0.001 (***) being most significant.
Results and discussion
Morphological evidence
Gibberellins (GAs) like GA3 have been known to affect plant morphology by increasing shoot length. GAs either promote cell division or cell enlargement and the resultant increased cell number or cell size furnishes more sites for assimilate deposition. Thus, in sugarcane, the state of growth and immaturity is maintained for a longer duration in culm tissue of sprayed cane, and hence GAs can be seen to be instrumental in increasing the sink size and sink strength of the cane.
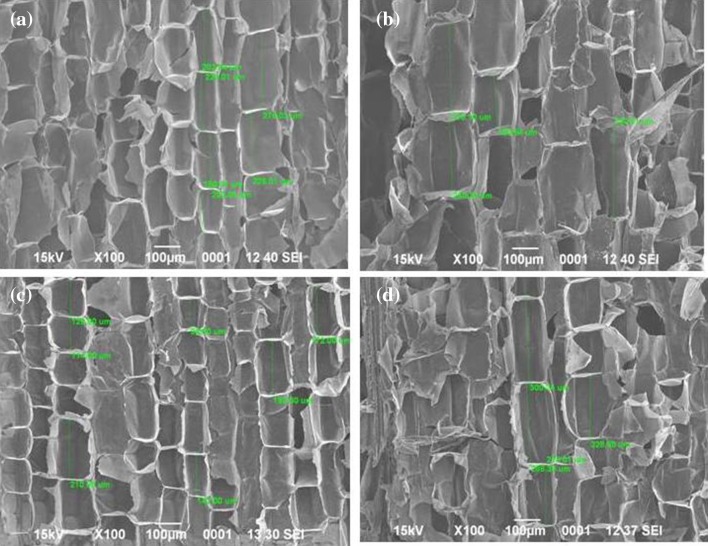
The current study ascertained the effect of GA3 spray on internodal length. Internodal length profiling of canes, done over a month after the last GA3 spray, showed visible differences among internodes of control and GA3-treated canes. Marked difference in length was especially observed in internodes 11–13 in CoJ 64 and 10–12 in BO 91, of control and GA3 treated canes (Table 1). The striking difference in the length of GA3-treated internodes shows the possible effect of GA3 in increasing the sink size and consequently the sink potential as well as sink demand. Internode no. 10 in BO 91 and 11 in CoJ 64 showed the maximum length difference (~ 27.45% in CoJ 64 and 39.3% in case of BO 91) between control and treated canes. Also, when visualized under SEM, tissue from these control and GA3-treated internodes exhibited large difference in cell size too. The noticeably larger cell size observed in the GA3-treated internodes, of both BO 91 (~ 19.2% bigger) and CoJ 64 (~ 42.3% bigger) canes (Fig. 1), further validates the role of GA3 in increasing cell size thereby enhancing sink size and sink capacity, making more room for sucrose accumulation.
Fig. 1.
Difference in the cell length as observed through SEM in culm tissue of control and GA3-treated samples of BO 91 (a, b) and CoJ 64 (c, d)
Even though the top portion is the actively growing part of cane, maximum difference in the length was observed between the middle region of control and GA3-treated cane. This is perhaps because it was the middle internodal portion that was actively growing while GA3 was sprayed and most effective (viz., July–September), and thus attained the maximum possible length. The top portion was still only developing in October and hence was unable to exhibit as visible a difference.
Biochemical evidence
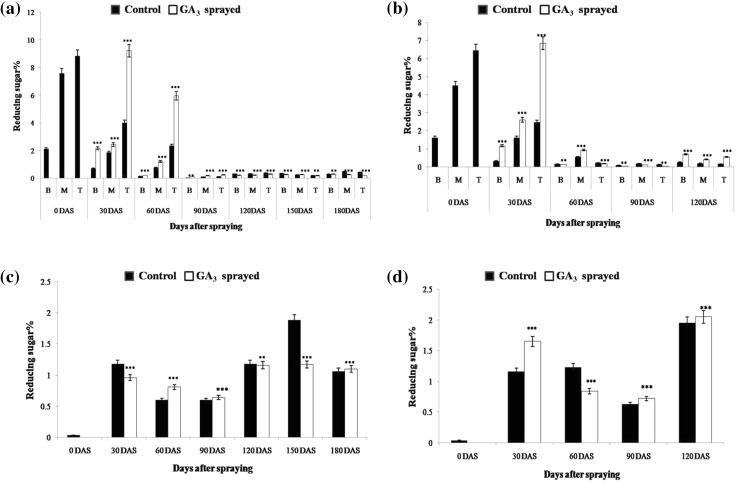
In compliance with earlier studies, the reducing sugar (RS) content of various (bottom, middle and upper) portions of both control and GA3-treated canes steadily decreased, while sucrose levels increased over the duration of study, depicting the progressing maturation of canes. A month after the last GA3 spray (30 DAS), the reducing sugar level in GA3-treated samples was observed to be drastically higher (9.23%) than that in control (4.01%), especially in the top internodes of both BO 91 and CoJ 64 (Fig. 2) canes, the values being far lesser in CoJ 64 (0.32% in control and 1.16% in GA3 treated). Gibberellins have been known to aid invertase activity in sink tissues, justifying the sharp rise in reducing sugar levels observed due to conversion of sucrose into hexoses. The heightened hexose level in GA treated sink tissues is considered responsible for their greater sink strength (Iqbal et al. 2011). Also, sucrose concentration fell substantially, especially in the GA3-treated elongating top internodes. A decrease in sucrose levels at the sink can be correlated to increased assimilate requirement (van Bel 2003) and elevated sink demand. Thus, gibberellins enhance sink strength, stimulating phloem loading and improving nutrient transport from source to sink tissues.
Fig. 2.
RS% in a, b internodal samples and c, d LTM leaf of control and GA3-sprayed BO 91 and CoJ 64 canes, respectively
Evidence suggests that leaf hexose concentration bears an inverse correlation with photosynthetic rate and communicated sink demand (McCormick et al. 2006). Concurrently, lower RS value was observed in the LTM leaf of GA3-treated (0.96%) BO 91 plant, as compared to control (1.18%). This may perhaps be linked to greater conversion of RS into sucrose, in answer to the developing high sink demand, it being lesser in the control plant. The GA3-treated leaf exhibited an elevated sucrose level (0.56%), affirming increased conversion of RS into sucrose, to meet up the stimulated phloem loading and transportation. The very low sucrose value in control leaf, possibly signifies that sucrose loading and transportation rate exceeds sucrose production rate. Conversely, a higher RS level observed in the GA3-treated leaf (1.65%) of CoJ 64, than in control (1.16%) which depicts the high photosynthetic rate developed to cater to the well-developed sink demand.
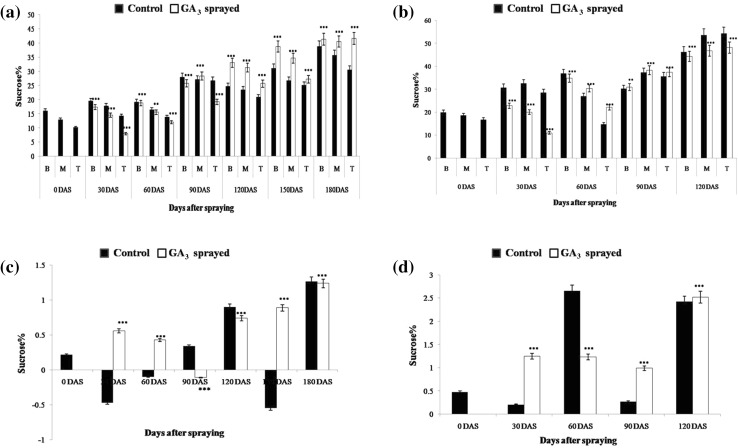
Observations made 60 DAS showed that the GA3-treated upper internodes of late maturing BO 91 still displayed prominently higher RS% (0.21%) as compared to control (0.14%), perhaps due to high sink strength. On the other hand, the control and GA3-treated samples of CoJ 64 showed comparable RS% values, depicting similar sink strength. This may be owing to gradual saturation of sink due to fading effect of GA3 and the early maturing nature of CoJ 64. The GA3-sprayed samples exhibited increased sucrose levels, compared to control ones, especially in upper internodes of CoJ 64 (Fig. 3). This validates higher sink potential and hence better sucrose accumulation in the GA-sprayed plants, as compared to control canes. The GA3-treated samples of BO 91 still exhibited lesser sucrose content as compared to control. This, in addition to the high RS values observed, points to the still prevailing high sink demand in the late maturing BO 91. Concomitantly, the RS levels fell in control and GA3-treated leaves, depicting their increased channelization into sucrose synthesis. However, the GA3-treated LTM leaf of BO 91 exhibited greater RS value than control, at 60 and 90 DAS, probably due to greater photosynthetic sugar production under stimulation of high sink demand; a pattern similar to that observed in early maturing CoJ 64 at 30 DAS. Parallely, higher RS (1.23%) and sucrose levels (2.65%) were observed in control than in GA3-treated leaves of CoJ 64 (0.84%; 1.23%), thus pointing to increased conversion of RS to sucrose and its enhanced transportation in GA3-treated leaves.
Fig. 3.
Sucrose% in a, b internodal samples and c, d LTM leaf of control and GA3-sprayed BO 91 and CoJ 64 canes, respectively
However, 90 DAS, a sudden dip in RS% values and a noticeable rise in sucrose% was observed in control and GA3-treated canes of both varieties (Figs. 2, 3). This may probably be due to the dip in ambient temperature in December and/or waning effect of the GA3 treatment done. However, lower RS% (0.04–0.11%) and higher sucrose% (30.96–38.32%) values were observed in the GA3-treated canes of CoJ 64 as compared to control canes (0.08–0.18%; 30.23–37.37%). Thus, as the early maturing CoJ 64 cane ripened, the GA3-treated cane displayed visibly greater sucrose accumulation under the effect of treatment. Nevertheless, GA3-treated BO 91 canes still exhibited higher RS% and lower sucrose% values than that in control samples, pointing to the still high sink demand. At this stage, 90 and 120 DAS, the leaves of late maturing BO 91 show a pattern resembling the sugar behaviour exhibited earlier (at 60 DAS) by early maturing CoJ 64’s leaves.
The higher sucrose level exhibited by GA3-treated CoJ 64 cane at 90 DAS, was however lost at 120 DAS. Both control and GA3-treated samples of CoJ 64 showed a rise in RS and drop in sucrose level perhaps illustrating an inversion in the cane due to early maturing nature of this variety. The leaf data too showed signs of saturation and inversion by displaying a pile up of RS and sucrose. Conversely, as time progressed, the RS levels showed a gradual drop in both control and GA3-treated leaves, perhaps due to decrease in photosynthetic rate with progressing maturity. January (120DAS) onwards, the leaves exhibited a rise in RS levels perhaps indicating accumulation of RS due to lesser conversion into sucrose, in the event of reduced demand from sink. Correspondingly, heightened sucrose levels depicted how sucrose hoarding at the source prevents further conversion from RS, pointing to gradually filling sink in CoJ 64. On the other hand, the GA3-treated BO 91 leaf too showed high RS content, but relatively lower than that in control, explaining the ongoing conversion into sucrose, due to greater sink demand.
Beyond this time, the reducing sugar level consistently dropped and the sucrose level stepped up in the GA3-treated canes of late maturing BO 91. At 180 DAS, remarkably higher sucrose concentrations were observed in the GA3-sprayed BO 91 culm samples (40.54–41.6%), as compared to control (30.44–38.8%). These observations support the idea that GA treatment perhaps promotes better sucrose accumulation in the sink by stimulating phloem loading/ unloading due to better sink strength and sink demand (Iqbal et al. 2011).
Molecular evidence
Quantitative real-time PCR analysis
In the current pursuit, real-time PCR was employed for quantifying the differential gene expression of various sucrose metabolizing enzymes, viz., SAI, CWI, SPS, SuSy, at the beginning (viz., October, 30 DAS) and towards the end (viz., January, 120 DAS) of maturation phase, among middle and top internodes of control and GA3-sprayed BO 91 and CoJ 64 canes. The result was interpreted in terms of log10 RQ values, where RQ = , depicting fold change, w.r.t. September control (0 DAS).
In terms of SAI expression
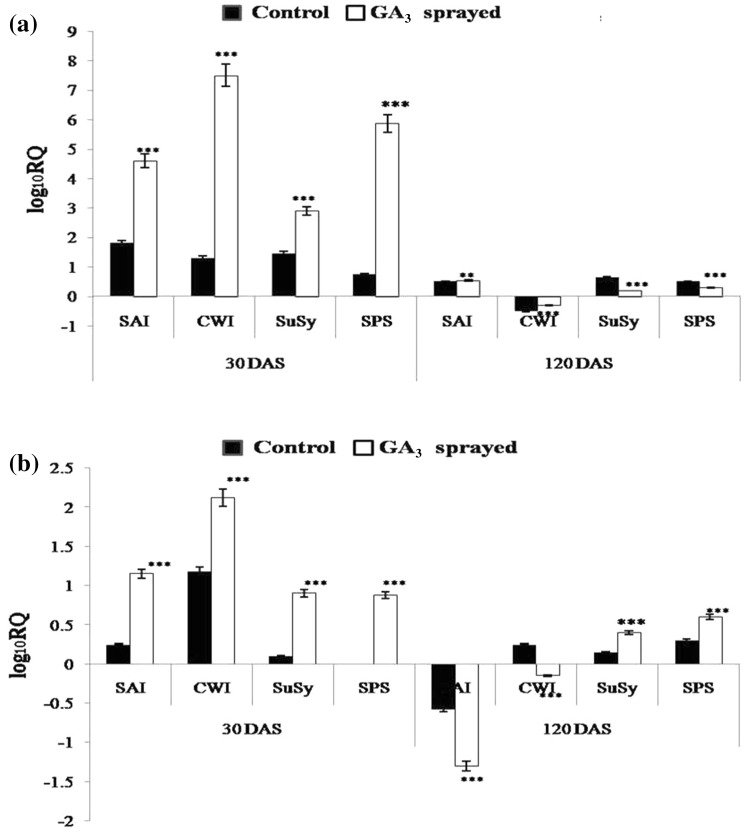
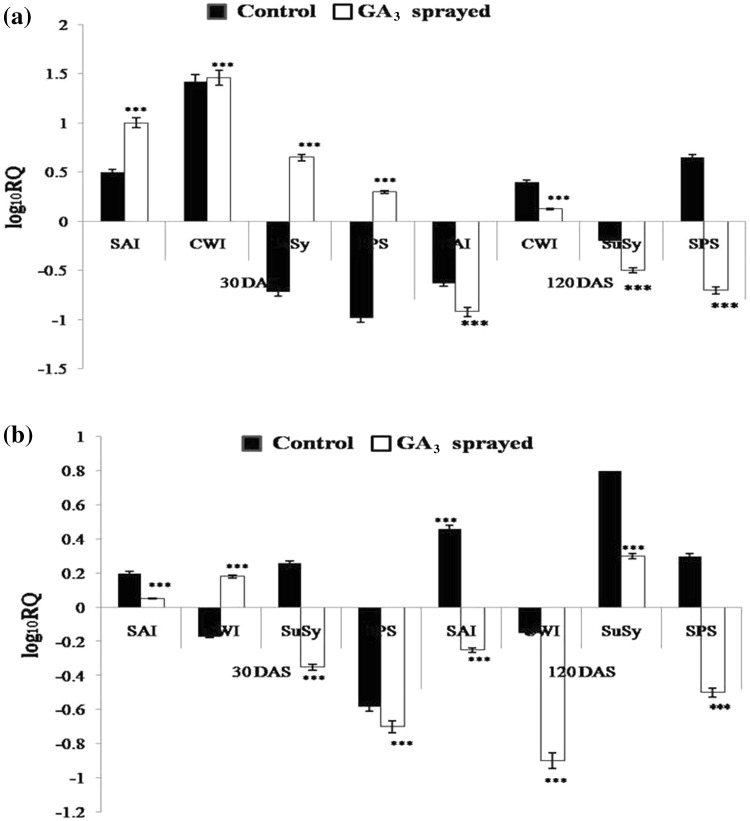
Many earlier studies have reported an increase in acid invertase activity under the effect of exogenous GA application (Iqbal et al. 2011), facilitating the breakdown of imported sucrose into hexoses. Zhu et al. (1997) too have reported that the activity of SAI (soluble acid invertase) is usually high in tissues that are rapidly growing, viz., immature internodes. In the current study too, SAI expression levels went up in October control and GA3-treated samples, as compared to September control of both BO 91 and CoJ 64. Since supplied GA perhaps prolongs the growth (immaturity) phase in culm tissue, the invertase levels in GA3-treated cane samples were relatively more heightened than in control, in top internodes of both, BO 91 and CoJ 64. As October marks the onset of maturity for BO 91, a 4.6-fold higher SAI expression was found in GA3-treated top internode while a significant but much lower 1.8-fold higher SAI expression was observed in the non-treated top internode sample, compared to September control (Fig. 4). This rise in SAI expression during early phase of maturity may be attributed to need for increased sucrose hydrolysis due to development of high sink demand. Nonetheless, GA3 samples exhibited strikingly higher SAI expression levels, more so in BO 91, perhaps due to development of higher sink strength under the influence of GA3 treatment. The high activity of SAI can be extrapolated to rapid hydrolysis of imported sucrose (as also qualified by the drastic rise in RS, especially in BO 91, in the same time frame) which is utilized in elongating cells during internodal elongation (Hatch and Glasziou 1963). This also points to the increased scope for sucrose translocation and unloading in the late maturing BO 91. On the other hand, since the early maturing CoJ 64 had relatively matured by October, a 1.15 times higher SAI expression was recorded in its top GA3-treated internode, as compared to September control. Accordingly, a 0.25-fold higher SAI expression was found in the top internode of control cane.
Fig. 4.
Quantitative analysis of differential expression (log10RQ) of SAI, CWI, SuSy, SPS in top internodal samples of control and GA3-treated a BO 91, b CoJ 64 canes, 30 DAS and 120 DAS
Though the BO 91 middle internode samples exhibited the expected higher SAI expression in GA3-treated ones (Fig. 5), contrarily, the GA3-treated middle internode of CoJ 64 showed a much lesser SAI expression than control. This perhaps points to the waning effect of GA3 in the nearly mature middle internode of CoJ 64. Early masking of vacuolar invertase activity has been found to be responsible for high sucrose accumulation in internode tissue of high sugar and early maturing cultivars (Dendsay et al. 1995). In congruence, the dipped SAI gene expression in the top internode of CoJ 64, affirms its high sucrose accumulating and early maturing nature. Since the GA3-induced rise in SAI expression was more prominent in BO 91 than in CoJ 64, it consequently indicates better sink strength development and perhaps corroborates the higher final sucrose concentration observed (in earlier section) in GA3-treated BO 91 samples over that in GA3-treated CoJ 64.
Fig. 5.
Quantitative analysis of differential expression (log10RQ) of SAI, CWI, SuSy, SPS in middle internodal samples of control and GA3-treated a BO 91, b CoJ 64 canes, 30 DAS and 120 DAS
SAI activity involved in sucrose unloading is shown to be reduced during maturation of stem storage tissue (Hawker and Hatch 1965). As maturation phase progressed, by January, the BO 91 samples became fairly matured. Thus, the SAI expression dropped in top internode of both control and GA3-treated samples of BO 91, however, still nearly 0.5 times higher than that in September (Fig. 4). The amount of sucrose accumulation in the internodes is said to be controlled by a SAI threshold level which when exceeded, prevents accumulation of sucrose in high amounts (Zhu et al. 1997). Internodes of the lower sucrose accumulating BO 91, perhaps exhibited SAI levels greater than this minimum threshold, thus indicating possibility of more sucrose uptake. Then again, CoJ 64 being early maturing, almost completely matured by January. Thus, SAI expression fairly dipped in the CoJ 64 top internodes, with more than 0.58-fold downregulation in control sample (as compared to September control) and an even greater, 1.3-fold downregulation in GA3-treated one, clearly indicating sufficiency since GA3 treatment has led to better sucrose accumulation.
It has been hypothesized that SAI and insoluble CWI influence sucrose accumulation in sugarcane during ripening; the activity being highest in the youngest internode and decreasing with internode age (Lontom et al. 2008). Having attained considerable maturity by January, the BO 91 middle internode samples exhibited a marked decline in SAI expression, with the control sample showing a 0.63-fold and the GA3-treated sample displaying a 0.92-fold downregulation, as compared to the September control. The middle portion of control CoJ 64 cane, however, showed a ~ 0.46-fold escalation in SAI level again, probably due to onset of inversion in the region, due to excessive maturity. The middle portion of GA3-treated CoJ 64 cane on the other hand, displayed a dipped SAI level, possibly indicating near complete maturity but still promoting a high rate of sucrose accumulation (Fig. 5).
In terms of CWI expression
Hawker and Hatch (1965) have described that in mature tissue, CWI cleaves unloaded sucrose to generate hexoses in the apoplastic space, that are actively transported across the parenchyma plasmalemma, thereby increasing the sink strength, facilitating phloem unloading into the sink and consequently augmenting the amount of sucrose stored (Ma et al. 2000). CWI activity has been reported in sugarcane culm tissues confirming the presence of an apoplastic unloading route (Glasziou and Gayler 1972). In one transcriptional level study in pea shoot, the expression of CWI gene has been shown to be induced after GA3 treatment (Wu et al. 1993). Here too, CWI showed augmented expression levels in early maturation phase, viz., October. In case of BO 91, the top internode of control cane showed a ~ 1.25-fold higher expression, while a huge 7.5-fold increase was observed in the GA3-treated ones, as compared to September control (Fig. 4). On the other hand, CoJ 64 did not show a very drastic difference in CWI expression, with the control sample exhibiting a ~ 1.25-fold and GA3-treated one, ~ 2.15-fold expression. The increased translocation under the effect of GA3, vouches the need for greater unloading in addition to the regular apoplastic unloading, and hence CWI expression can be reasoned to have gone up to facilitate the same. Thus, particularly more soaring CWI expression levels were observed in BO 91, possibly due to greater CWI contribution in apoplastic unloading. On the other hand, in CoJ 64 middle internode, CWI expression was ~ 0.2-fold upregulated in GA3 sample but ~ 0.2-fold downregulated in control sample, as compared to the September control. Much higher levels of CWI exhibited in GA3-treated samples as compared to control, validate the higher sink demand created by GA3 treatment, leading to better sucrose unloading. Thus, perhaps, CWI played a more prominent role in apoplastic unloading in GA3 treatment, here too, thereby making room for better sucrose accumulation.
In line with the obtained hexose pattern, the acid invertase occurring in the vacuole (SAI) and apoplastic space (CWI) of elongating internodes, have been known to disappear with increasing maturity of culm internodes (Albertson et al. 2001). As BO 91 cane gained maturity, the CWI expression in the top internodes waned considerably by January. CWI was downregulated, lesser in GA3-treated sample, perhaps promoting better sucrose accumulation and hence making provision for apoplastic unloading. Although, CWI expression was downregulated in GA3-treated sample of CoJ 64 too, however, it was upregulated in control, possibly due to onset of inversion. The middle portion samples further validated the role of CWI in determining sucrose accumulation in sugarcane. CoJ 64 almost completely matured by January and hence the control sample showed marked downregulation (0.15-fold) affirming its early maturing nature, while the GA3-treated one exhibited a 0.9-fold downregulation, indicating saturation of sucrose accumulation. Conversely, middle internode samples of control BO 91 cane still showed significant 0.5-fold upregulation in CWI expression (Fig. 5), depicting the still prevalent sucrose gradient and scope for sucrose unloading due to its late maturing nature. However, the corresponding GA3-treated one showed a minor 0.15-fold higher CWI expression, as compared to September control, signifying relatively greater sufficiency of sucrose and less need for transport (as also deciphered from the high sucrose values).
In terms of SuSy expression
Sucrose synthase (SuSy) is employed in an alternate pathway (other than that involving invertase) for conversion of imported sucrose into hexoses. (Nguyen-Quoc and Foyer 2001). Hence, a particularly high SuSy expression (~ 1.5-fold higher than September control) was observed in BO 91 control top internode sample (Fig. 4). Conversely, SuSy expression was ~ 0.075-fold in CoJ 64 top internode control sample, vouching greater sucrose accumulation in BO 91 at this stage. Lingle and Smith (1991) have reported that rapidly elongating internodes have higher SuSy activities than fully elongated internodes. Since GA treatment perhaps maintains a state of growth and immaturity in cane tissue for a longer while, SuSy expression was found to be ~ threefold and 0.9-fold higher in the still growing GA3-treated top internodes of BO 91 and CoJ 64 canes, respectively, as compared to September control. Thus, the high SuSy expression observed in GA3-treated top internodes of BO 91 and CoJ 64, in October, fully validates the correspondingly high RS values obtained. Similarly, a 0.65-fold higher SuSy expression was observed in October GA3-treated middle internode of BO 91, compared to September control (Fig. 5), pointing to the GA3-induced state of immaturity and high sink strength. Conversely, the control sample exhibited a ~ 0.7-fold downregulation as compared to September control, corroborating relative maturity in the tissue, lower down in cane. GA3-treated middle internode sample of CoJ 64 also displayed a ~ 0.35 fold down regulation while the control was 0.25-fold upregulated, perhaps due to early attainment of maturity, CoJ 64 being an early maturing variety.
In the latter maturation phase (January), SuSy expression noticeably decreased in the top internodes of both BO 91 and CoJ 64, depicting maturity. The GA3-treated internode exhibited a far lesser expression than control in BO 91, pointing to a more saturated state of sucrose accumulation. On the contrary, the control and GA3-treated middle internodes of CoJ 64 exhibited a noticeable upregulation in SuSy expression, perhaps due to inversion.
In terms of SPS expression
Sucrose phosphate synthase (SPS) is known to be the key enzyme for sucrose synthesis, playing a significant role in resynthesizing sucrose from the hexoses in the sink tissue (Huber and Huber 1996). Heightened SPS expression levels were observed in top internodes of BO 91 and CoJ 64 control canes in October, at the onset of maturity. This perhaps depicts entry of apoplastically unloaded sucrose, as hexoses, into parenchymatous cells and consequent resynthesis of sucrose by SPS. SPS activity has been demonstrated to alter sink capacity viz. increased sucrose unloading has been reported in tomato fruit under the effect of heightened SPS expression (Nguyen-Quoc et al. 1999). Thus, the high SPS levels (~ sixfold in BO 91 and ~ 0.85 fold in CoJ 64) exhibited by GA3-treated top internodes (Fig. 4), point to more active resynthesis into sucrose for storage and elucidate the heightened sink demand under the influence of GA3, justifying the higher sucrose accumulation observed biochemically. A 0.3-fold elevated SPS expression was observed in the GA3-treated middle internode of BO 91, as compared to September control (Fig. 5). Contrastingly, SPS expression in GA3 treated middle internode of CoJ 64 was 0.7-fold downregulated, compared to September control, possibly denoting relative maturation and sucrose saturation, thereby suggesting SPS peak in earlier months due to early maturing nature of CoJ 64. However, the corresponding control samples exhibited nearly single fold downregulation in BO 91 and ~ 0.6-fold in CoJ 64, perhaps depicting attainment of relative maturity and slowing of further sucrose transportation for storage, even under the influence of GA3. In January, SPS expression was still upregulated in the top control and GA3-treated samples of BO 91 and CoJ 64, still signifying scope for sucrose accumulation. SPS expression in the middle internodes of BO 91 was largely downregulated, displaying sufficiency of sucrose storage. However, SPS expression was found to be upregulated in control while it was downregulated in GA3-treated middle internode of CoJ 64, supporting the higher sucrose levels obtained biochemically too.
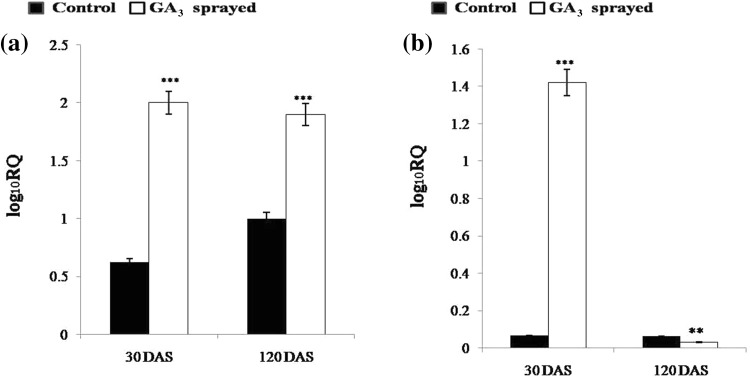
SPS activity stimulated under the effect of GA, is reported to enhance sucrose synthesis and its phloem loading (Iqbal et al. 2011). During October, transported sucrose was increasingly converted into RS in the culm, to maintain high sink demand. Thus, perhaps increased decanting of assimilate under the effect of increased sink demand resulted from SPS up regulation. Responding to the high sink demand, a notable twofold higher SPS expression was observed in BO 91’s GA3-treated LTM leaf and ~ 0.6-fold upregulation in the control one, as compared to September control (Fig. 6). A similar SPS expression pattern was also observed in CoJ 64 leaves too. Thus, the action of GA promotes sucrose synthesis within the leaf and partitioning, through their positive effect on SPS.
Fig. 6.
Quantitative analysis of differential expression (log10RQ) of SPS in LTM leaves of control and GA3-treated a BO 91, b CoJ 64 canes, 30 DAS and 120 DAS
Leaves of younger plants have been reported to typically assimilate at higher rates than older plants (Hartt and Burr 1967). This notion is affirmed by the observation that quantitative SPS expression was significantly downregulated in January as compared to that in October. The relative amount of sucrose accumulated by the sugarcane plant perhaps works by way of end point repression of photosynthesis. In the case of a mismatch between photoassimilate production at the source and rate of phloem loading, a negative feedback is perhaps triggered by the increasing sucrose content of leaf, which in turn affects SPS activity (Stitt et al. 1987). This shows how both control and GA3-treated LTM leaves of CoJ 64 showed diminished SPS expression, as compared to September control, perhaps because the completely matured CoJ 64 culm may have signalled sucrose sufficiency. By January, the late maturing BO 91 was still undergoing maturation and hence its control LTM leaf exhibited a single fold while the GA3-treated one showed a ~ twofold upregulation in SPS expression, signifying continued sucrose synthesis in these leaves. The larger fold expression in GA3-treated LTM leaf of BO 91 can perhaps be extrapolated to greater sucrose synthesis in response to the still high sucrose demand under the effect of GA3.
Semi-quantitative RT-PCR analysis
Source photosynthetic activity has been reported to be directly related to carbon demand of sinks in many plant species, including sugarcane, where a decrease in source photosynthetic assimilation rate occurs in response to diminished sink demand (Franck et al. 2006). Conversely, increased sink demand has been reported to caused a significant increase in photosynthetic rate and carboxylation efficiency (McCormick et al. 2006).
In terms of PEPC expression
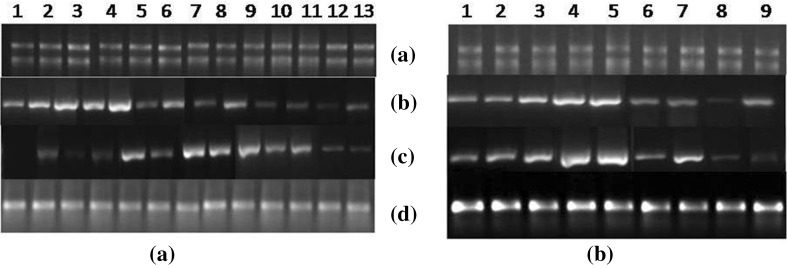
Phosphoenolpyruvate carboxylase (PEPC) is involved in the initial fixation of CO2 in mesophyll cells to form C4 dicarboxylic acids and hence influences the photosynthetic rate of C4 plants (Lian et al. 2014). GAs are presumed to stimulate photosynthetic activity and the increased photosynthetic sugar content is in turn used to encourage shoot growth (Iqbal et al. 2011). The marked increase in culm hexose sugars observed in October, in response to GA3 signifies the heightened sink demand which perhaps stimulates greater photosynthetic activity, in turn eliciting greater sucrose synthesis, and consequent greater loading at the source. The high RS level observed in GA3-treated leaves of CoJ 64 (inspite of simultaneous conversion into sucrose) in October and of BO 91 (Fig. 7), at 30 and 60 DAS, validate the high photosynthetic rate developed to meet up the high sink demand. This is further confirmed by the high PEPC expression in BO 91 samples of October, more so in GA3-treated LTM leaf, and even higher at 60 DAS. Source leaf photosynthetic activity in sugarcane has also been shown to be affected by the maturity of culm sinks; the associated accumulation of sucrose results in down-regulation of leaf photosynthetic rates (McCormick et al. 2008) perhaps due to sink sufficiency and diminished sink demand. This is reaffirmed by the gradually dropping RS levels observed and accounts for the waning PEPC expression obtained in the later months in both BO 91 and CoJ 64.
Fig. 7.
(a) Agarose gel depicting total RNA of A BO 91 and B CoJ 64 in normalized concentration (b) Differential expression of PEPC gene in control and GA3-sprayed LTM leaves of A BO 91 and B CoJ 64 (c) Differential expression of sucrose transporter gene in control and GA3-sprayed LTM leaves of A BO 91 and B CoJ 64 (d) RNA normalization verified using 25S rRNA gene primer as internal control. Lanes 1: LTM leaf of control cane on 0 DAS; Lanes 2–3: LTM leaf of control and GA3-sprayed cane on 30 DAS; Lanes 4–5: LTM leaf of control and GA3-sprayed cane on 60 DAS; Lanes 6–7: LTM leaf of control and GA3-sprayed cane on 90 DAS; Lanes 8–9: LTM leaf of control and GA3-sprayed cane on 120 DAS; Lanes 10–11: LTM leaf of control and GA3-sprayed cane on 150 DAS; Lanes 12–13: LTM leaf of control and GA3-sprayed cane on 180 DAS
In terms of SUT expression
SPS-synthesized sucrose forms the export material for transport from the source, phloem loading from the apoplasm, employing membrane transporters (Rae et al. 2005). Sucrose-H+ symporter (SUT) is among the most well-characterized sucrose transporters, employed in active apoplastic phloem loading (Kuhn and Grof 2010). The occurrence of SUT predominantly in the phloem and rise in its activity during peak sucrose production phase in leaves, suggest the role of sucrose transporter in phloem loading for transport (Lemoine et al. 1992). In the current study, the higher sucrose levels coupled with up regulated expression of SPS, in BO 91 GA3-treated leaf sample, probably point to lesser transportation initially, justifying dimmer SUT expression at 30 DAS (Fig. 7). The biochemical data viz. higher RS and greater levels of synthesized sucrose, in concert with higher SPS expression observed in GA3-treated samples of CoJ 64, over control, all support the pronounced expression of sucrose transporter in GA3-treated samples around 30 DAS in CoJ 64 and 60 DAS in BO 91. A greater dip in sucrose levels was observed in GA3-treated leaves of both BO 91 and CoJ 64, around 120 and 90 DAS, respectively, justified by the heightened sucrose transporter expression, perhaps due to increased transportation. A higher transport rate can also be predicted from the ambient cold temperature during cold months like December and January which favour sucrose accumulation. The increase in SUT levels in correspondence with dip in SPS levels in CoJ 64 LTM leaf validates the significant export of SPS-synthesized sucrose. As maturation phase reached culmination, the SUT expression gradually faded, denoting the diminishing sucrose transportation due to sucrose sufficiency.
Conclusion
Drawing analogy with BO 91, similar sugar and expression pattern were observed in CoJ 64 too, though over a shorter span of time. This qualifies the early maturing nature of CoJ 64. GAs reportedly stimulate acid invertase expression, causing increased hexose production in the elongating cells, through hydrolysis of imported sucrose. The consequent elevated sink demand prevents early saturation and draws more and more sucrose, thus boosting sucrose transport and phloem loading at the source. GA-dependent stimulation of SPS activity enhances sucrose synthesis and its export. Increased phloem loading causes the sucrose levels in the mesophyll to decline, perhaps enhancing photosynthetic activity and preventing its suppression by sugar accumulation. A significant outcome of this study was that the GA3-induced perturbation of source–sink communication was able to bring about an increase in final sucrose content, especially in BO 91. GA3-treated CoJ 64 also showed considerably higher sucrose levels at 90 DAS; however, the rise could not be retained upto 120 DAS, probably due to onset of inversion. These observations support the idea that GA treatment perhaps promotes better sucrose accumulation in the sink by stimulating phloem loading/unloading due to better sink strength and sink demand.
Acknowledgements
Kriti Roopendra is thankful to ICAR-Indian Institute of Sugarcane Research (IISR), Lucknow and Babasaheb Bhimrao Ambedkar University (BBAU), Lucknow, for providing all necessary facilities and guidance and to Council of Scientific and Industrial Research (CSIR) for their financial support (CSIR-NET SRF scheme).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Albertson PL, Peters KF, Grof CPL. An improved method for the measurement of cell wall invertase activity in sugarcane tissue. Aust J Plant Physiol. 2001;28:323–328. [Google Scholar]
- Battistelli A, Adcock MD, Leegood RC. The relationship between the activation state of sucrose-phosphate synthase and the rate of CO2 assimilation in spinach leaves. Planta. 1991;183:620–622. doi: 10.1007/BF00194285. [DOI] [PubMed] [Google Scholar]
- Bieleski RL. The physiology of sugarcane III. Characteristics of sugar uptake in slices of mature and immature storage tissue. Aust J Biol Sci. 1960;13:203–220. doi: 10.1071/BI9600203. [DOI] [Google Scholar]
- Busse JB, Lawrence ET, Riley AL. The effects of alcohol preexposure on cocaine, alcohol and cocaine/alcohol place conditioning. Pharmacol Biochem Behav. 2005;81:459–465. doi: 10.1016/j.pbb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Chandra A, Verma PK, Islam MN, et al. Expression analysis of genes associated with sucrose accumulation in sugarcane (Saccharum spp. hybrids) varieties differing in content and time of peak sucrose storage. Plant Biol. 2015;17:608–617. doi: 10.1111/plb.12276. [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR. Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA. 1998;95:4784–4788. doi: 10.1073/pnas.95.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DF, Dobrenz AK, Massengale MA. Effect of gibberellic acid on alfalfa (Medicago sativa L.) Crop Sci. 1972;12:674–676. doi: 10.2135/cropsci1972.0011183X001200050036x. [DOI] [Google Scholar]
- Dendsay JPS, Singh P, Dhawan AK, Sehtiya HL. Activities of internodal invertases during maturation of sugarcane stalks. Sugarcane. 1995;6:17–19. [Google Scholar]
- Franck N, Vaast P, Ge´ nard M, Dauzat J. Soluble sugars mediate sink feedback down-regulation of leaf photosynthesis in field-grown Coffea arabica. Tree Physiol. 2006;26:517–525. doi: 10.1093/treephys/26.4.517. [DOI] [PubMed] [Google Scholar]
- Glasziou KT, Gayler KR. Storage of sugars in stalks of sugarcane. Bot Rev. 1972;38:471–490. doi: 10.1007/BF02859248. [DOI] [Google Scholar]
- Grof CPL, Campbell JA. Molecular manipulation of sucrose metabolism in sugarcane. In: Solomon S, Grewal SS, Li Y-R, Magarey RC, Rao GP, editors. Sugarcane: production management and agro-industrial imperatives. Lucknow: International Book Distributing Company; 2005. pp. 507–521. [Google Scholar]
- Hartt CE, Burr GO. Factors affecting photosynthesis in sugarcane. Proc Int Soc Sugarcane Technol. 1967;12:590–609. [Google Scholar]
- Hatch MD, Glasziou KT. Direct evidence for translocation of sucrose in sugar cane leaves and stems. Plant Physiol. 1963;39:180–184. doi: 10.1104/pp.39.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD, Sacher JA, Glasziou KT. Sugar accumulation cycle in sugarcane. I. Studies on enzymes of the cycle. Plant Physiol. 1963;38:338–343. doi: 10.1104/pp.38.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker JS, Hatch MD. Mechanism of sugar storage by mature stem tissue of sugarcane. Plant Physiol. 1965;18:444–453. doi: 10.1111/j.1399-3054.1965.tb06907.x. [DOI] [Google Scholar]
- Huber SC, Huber JL. Role and regulation of sucrose phosphate synthase in higher plants. Ann Rev Plant Physiol Plant Mol Biol. 1996;47:431–444. doi: 10.1146/annurev.arplant.47.1.431. [DOI] [PubMed] [Google Scholar]
- Hussain A, Khan ZI, Ghafoor MY, et al. Sugarcane, sugar metabolism and some abiotic stresses. Int J Agric Biol. 2004;6:732–742. [Google Scholar]
- Iqbal N, Nazar R, Khan IR, et al. Role of gibberellins in regulation of source–sink relations under optimal and limiting environmental conditions. Curr Sci. 2011;100:998–1007. [Google Scholar]
- Krapp A, Stitt M. An evaluation of direct and indirect mechanisms for the ‘‘sink regulation’’ of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady state transcript levels after cold-girdling leaves. Planta. 1995;195:313–323. doi: 10.1007/BF00202587. [DOI] [Google Scholar]
- Kuhn C, Grof CPL. Sucrose transporters of higher plants. Curr Opin Plant Biol. 2010;13:288–298. doi: 10.1016/j.pbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Tegeder M, Throne-Holst M, et al. Phloem loading and unloading of sugars and amino acids. Plant Cell Environ. 2003;26:37–56. doi: 10.1046/j.1365-3040.2003.00847.x. [DOI] [Google Scholar]
- Lemoine R, Gallet O, Gaillard C, et al. Plasma membrane vesicles from source and sink leaves. Plant Physiol. 1992;100:1150–1156. doi: 10.1104/pp.100.3.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian L, Wang X, Zhu Y, et al. Physiological and photosynthetic characteristics of indica Hang2 expressing the sugarcane PEPC gene. Mol Biol Rep. 2014;41:2189–2197. doi: 10.1007/s11033-014-3070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle SE, Smith RC. Sucrose Metabolism Related to Growth and Ripening in Sugarcane Internodes. Crop Sci. 1991;31:172–177. doi: 10.2135/cropsci1991.0011183X003100010039x. [DOI] [Google Scholar]
- Lontom W, Kosittrakun M, Lingle SE. Relationship of acid invertase activities to sugar content in sugarcane internodes during ripening and after harvest. Thai J Agric Sci. 2008;41:143–151. [Google Scholar]
- Ma H, Albert HH, Paull R, Moore PH. Metabolic engineering of invertase activities in different subcellular compartments affects sucrose accumulation in sugarcane cells. Funct Plant Biol. 2000;27:1021–1030. doi: 10.1071/PP00029. [DOI] [Google Scholar]
- McCormick AJ, Cramer MD, Watt DA. Sink strength regulates photosynthesis in sugarcane. New Phytol. 2006;171:759–770. doi: 10.1111/j.1469-8137.2006.01785.x. [DOI] [PubMed] [Google Scholar]
- McCormick AJ, Cramer MD, Watt DA. Changes in photosynthetic rates and gene expression of leaves during a source–sink perturbation in sugarcane. Ann Bot. 2008;101:89–102. doi: 10.1093/aob/mcm258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PH. ) Temporal and spatial regulation of sucrose metabolism in the sugarcane stem. Aust J Plant Physiol. 1995;22:661–679. doi: 10.1071/PP9950661. [DOI] [Google Scholar]
- Nelson N. A photometric adaptation of Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- Nguyen-Quoc B, Foyer CH. A role for ‘futile cycles’ involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. J Exp Bot. 2001;52:881–889. doi: 10.1093/jexbot/52.358.881. [DOI] [PubMed] [Google Scholar]
- Nguyen-Quoc B, N’Tchobo H, Foyer CH, Yelle S. Overexpression of sucrose-phosphate synthase increases sucrose unloading in transformed tomato fruit. J Exp Bot. 1999;50:785–791. doi: 10.1093/jxb/50.335.785. [DOI] [Google Scholar]
- Rae AL, Grof CPL, Casu RE, et al. Sucrose accumulation in the sugarcane stem: pathways and control points for transport and compartmentation. Field Crops Res. 2005;92:159–163. doi: 10.1016/j.fcr.2005.01.027. [DOI] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB. Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J. 1994;13:1–7. doi: 10.1002/j.1460-2075.1994.tb06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Huber S, Kerr P. Control of photosynthetic sucrose formation. In: Photosynthesis MD, Hatch, Boardman NK, editors. The biochemistry of plants. New York: Academic Press; 1987. pp. 327–409. [Google Scholar]
- Stitt M, Huber SC, Kerr PS. Control of photosynthetic sucrose formation. In: Hatch MD, Boardman NK, editors. The Biochemistry of plants, a comprehensive treatise, photosynthesis. New York: Academic Press; 1988. pp. 327–409. [Google Scholar]
- van Bel AJE. Transport phloem: low profile, high impact. Plant Physiol. 2003;131:1509–1510. [PMC free article] [PubMed] [Google Scholar]
- Watt DA, McCormick AJ, Cramer MD. Source and sink physiology. In: Moore PH, Botha FC, editors. Sugarcane: physiology, biochemistry and functional biology. 1. Hoboken: Wiley; 2014. pp. 483–520. [Google Scholar]
- Welbaum GE, Meinzer FC. Compartmentation of solutes and water in developing sugarcane stalk tissue. Plant Physiol. 1990;93:1147–1153. doi: 10.1104/pp.93.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Mitchell JP, Cohn NS, Kaufman PB. Gibberellin (GA3) enhances cell wall invertase activity and mRNA levels in elongating dwarf pea (Pisum sativum) shoots. Int J Plant Sci. 1993;154:280–289. doi: 10.1086/297108. [DOI] [PubMed] [Google Scholar]
- Zhu YJ, Komor E, Moore PH. Sucrose accumulation in the sugarcane stem is regulated by the difference between the activities of soluble acid invertase and sucrose phosphate synthase. Plant Physiol. 1997;115:609–616. doi: 10.1104/pp.115.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]