Abstract
Introduction
We assessed the impact of targeted therapies on healthcare resource use and compared treatment regimens used in patients diagnosed with metastatic renal cell carcinoma (mRCC).
Methods
Clinicopathological and administrative data of patients with mRCC from our institution were retrospectively collected from January 2000 to August 2014. Patients were divided into two groups based on the use of targeted therapies. Healthcare resource use (HCRU) data included non-scheduled total number of hospitalizations, total days hospitalized, emergency department visits, and healthcare professional consultations. Each variable was presented with absolute and relative values (i.e., per month of survival). Statistics relied on the use of t-student and Chi-square tests.
Results
Ninety-nine patients were included in the study; 60 were treated with targeted therapy. There were no statistically significant differences between the two groups for demographic features and clinicopathological stage. HCRU analysis revealed an absolute increase in the median number of healthcare consultants (6 vs. 4; p<0.01) and emergency department visits (1 vs. 0; p=0.02) for the targeted therapy group. However, analysis per month of survival showed the targeted therapy group had fewer consultants (0.33 vs. 0.40; p=0.04) and hospitalizations (0.09 vs. 0.13; p=0.03) than their counterpart. Population size, non-randomization, treatment selection bias, and heterogeneity were the main limitations of this study.
Conclusions
Monthly use of HCRU is lower in mRCC patients treated with targeted therapies. However, because of a greater overall survival, their absolute total HCRU will be higher than those not exposed to targeted agents.
Introduction
Kidney cancer is one of the most common types of cancer diagnosed worldwide. It is the third most common urological cancer among Canadian men and the most common among women.1 In 2017, approximately 6600 new cases were diagnosed in Canada.1 Of these cases, around 33% will be metastatic at diagnosis.2 Metastatic spread results in a five-year survival of 12%.2
During the last decade, the development of targeted therapies for metastatic renal cell carcinoma (mRCC) showed significant improvements over classic treatments of interferon (IFN)-alpha and interleukin (IL)-2 regarding overall survival (OS), progression-free survival, and patient-related outcomes, including side effects and health-related quality of life (HRQoL).3–7 However, annual costs associated with these treatments are estimated to be three to four times higher than treatments in the era before the use of targeted therapies.8–10
Most reports on targeted therapies have used oncological and patient-reported outcomes.3–7,11,12 Few studies have analyzed the impact of targeted therapies on healthcare resource use (HCRU).8,11 We believe HCRU could be an objective way to help assess overall quality of life of patients and costs associated with this treatment modality. Therefore, the primary objective of our study was to evaluate the impact of targeted therapies on HCRU in the mRCC population at our centre. Secondary objectives were to evaluate the effect of targeted therapies on OS and treatment regimens compliance with the 2015 Canadian consensus statement.13
Methods
Study design
This was an institutional review board-approved retrospective study that included all patients (≥18 years old) diagnosed with mRCC from January 1, 2000 to August 31, 2014. Data were retrieved until December 30, 2014. Newly diagnosed patients with mRCC of any histology subtype that received upfront palliative care, surgery, radiotherapy, targeted therapies, or combined modalities were included in the study. Patients with another primary cancer were excluded unless a metastasis biopsy confirmed the renal histology. The mRCC population was divided into two groups depending on the use of targeted therapies. Treatment allocations, followup schemes, and imaging use were based on patients’ and physicians’ preferences and represent a real-world treatment scenario of mRCC.
Data collection
Patients’ demographics, as well as oncological and HCRU data were collected using our centre’s institutional patient database. The hospital of the University of Sherbrooke is the only tertiary/quaternary healthcare provider in this region of Quebec; therefore, virtually all diagnosis, treatments, and followups are captured in this database. Demographic data included gender and age at diagnosis; oncological data included histology type, initial metastasis burden, and Motzer’s risk stratification.14 Histology types were collected either from the primary tumour or metastasis pathology report. Initial metastasis spread was devised into three subgroups: single metastasis, oligometastases (one to five metastases), and plurimetastases (six or more metastases).
HRCU variables were collected starting from the diagnosis of mRCC. They included non-scheduled, mRCC-related, total number of hospitalizations and emergency department visits, total number of days hospitalized, and number of healthcare consultants, including the palliative care team. Healthcare consultants included medical specialties and other health professionals (i.e., physical therapists, social workers, nutritionists, psychologists) involved in the care of mRCC patients. In addition to the absolute value of each HRCU variable collected, HCRU values per month of survival were calculated to evaluate the predisposition of each group of using healthcare resources.
Statistics
Median values and full ranges were used to present continuous variables, while categorical variables were presented using proportions. Categorical variables were compared with Chi-square or Fisher’s exact tests. Continuous variables were analyzed with student’s t-tests. Moreover, all tests were two-sided, with a significance level set at p<0.05. IBM SPSS Statistics for Windows, Version 22.0 was used for statistical analysis (released 2013, Armonk, NY: IBM Corp.).
Results
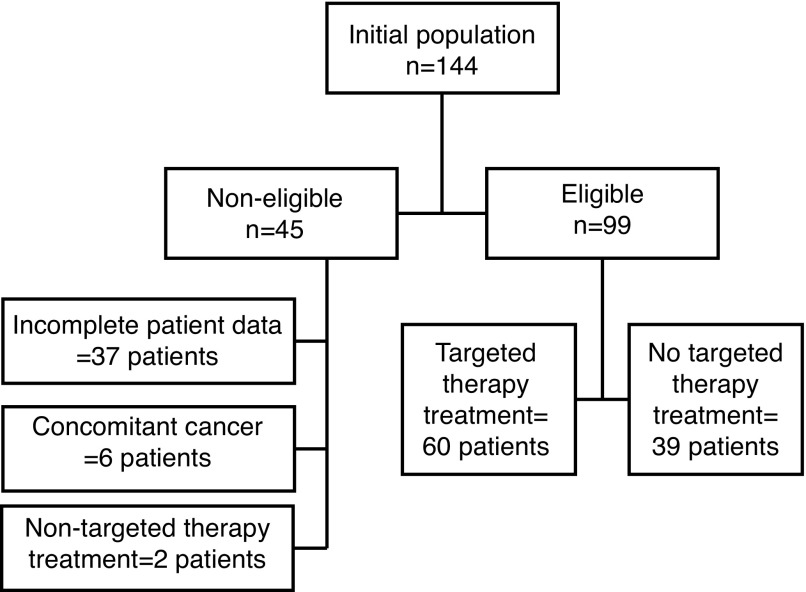
During the study period, 144 new cases of mRCC were diagnosed. Of those, 90 were included in the study. Exclusions were made for incomplete clinicopathological data and/or followup (n=37), concomitant non-RCC malignancy (n=6), and systemic non-targeted therapy treatment regimens (n=2) (Fig. 1). Sixty patients (60.6%) received targeted therapy. Median time from diagnosis to targeted therapy was 1.8 months (range 0–29.7). There were no statistically significant differences between the two groups for gender, age, histological subtype, initial metastatic spread, and Motzer’s risk criteria (p>0.05) (Table 1). Table 2 lists the reasons for the absence of targeted therapy treatment in the non-treated group. The two main reasons for the absence of targeted therapy were end of life/palliative status at diagnosis (n=10, 26%) and the use of alternative treatment modalities (n=9, 23%) for the metastasis, mainly surgery and radiotherapy.
Fig. 1.
Patient selection.
Table 1.
Demographic and initial oncological data based on the use of targeted therapy
| Targeted therapy group n=60 |
Absence of targeted therapy group n=39 |
p | |
|---|---|---|---|
| Gender | 0.6 | ||
| Male | 43 | 26 | |
| Female | 17 | 13 | |
| Age at diagnosis, median (range) | 63 (29–101) | 66 (20–90) | 0.092 |
| Histology type, n (%) | 0.11 | ||
| Clear-cell | 50 (83) | 29 (74) | |
| Chromophobe | 1 (2) | 2 (5) | |
| Papillary | 3 (5) | 1 (3) | |
| Mixed clear-cell/papillary | 3 (5) | 2 (5) | |
| Undifferentiated | 3 (5) | 3 (7) | |
| Sarcomatoid | 0 (0) | 2 (5) | |
| Metastatic burden, n (%) | 0.13 | ||
| Single metastasis | 7 (11) | 10 (26) | |
| Oligometastasis | 22 (37) | 9 (23) | |
| Plurimetastasis | 31 (52) | 20 (51) | |
| Motzer risk stratification scale, n (%) | 0.17 | ||
| Low | 13 (22) | 8 (20) | |
| Intermediate | 34 (57) | 11 (28) | |
| High | 10 (17) | 9 (23) | |
| Unknown | 3 (5) | 11 (28) |
Table 2.
Reasons for not receiving targeted therapy
| Number of patients, n (%) | |
|---|---|
| End of life state at diagnosis | 10 (26) |
| Metastasis treated with other modalities | 9 (23) |
| Patient’s will | 8 (20) |
| Insufficient information | 7 (18) |
| Non-eligible for treatment | 5 (13) |
Table 3 shows the results regarding HCRU. Targeted therapy use was associated with increased median number of consultants (6 vs. 4; p<0.01), median emergency department visits (1 vs. 0; p=0.02), and median OS (16.5 vs. 7.3 months; Breslow=0.01). However, when analyzed per month of survival, the targeted therapy group had less median number of consultants (0.33 vs. 0.40; p=0.04) and hospitalizations (0.09 vs. 0.13; p=0.03). No statistical significance was seen in the total number of hospitalizations (2 vs. 1; p=0.10) and number of days hospitalized (10 vs. 9; p=0.71). Table 4 shows that HCRU increases as the oncological prognosis worsens.
Table 3.
Median healthcare resource use based on the use of targeted therapy*
| Targeted therapy group n=60 | No targeted therapy group n=39 | p | |
|---|---|---|---|
| Number of hospitalizations | 2 (0–5) | 1 (0–5) | 0.10 |
| Number of hospitalizations per month of survival | 0.09 (0–0.79) | 0.13 (0–1.69) | 0.03 |
| Number of days hospitalized | 10 (0–72) | 9 (0–90) | 0.71 |
| Number of days hospitalized per months of survival | 0.48 (0–19.63) | 0.99 (0–20.22) | 0.21 |
| Healthcare consultants | 6 (1–14) | 4 (0–13) | <0.01 |
| Healthcare consultants per month of survival | 0.33 (0.029–2.18) | 0.40 (0–5.93) | 0.04 |
| Emergency department visits | 1 (0–6) | 0 (0–4) | 0.02 |
| Emergency department visits per month of survival | 0.052 (0–0.55) | 0 (0–1.69) | 0.44 |
| Overall survival | 16.5 (2.1–71.0) | 7.3 (0.8–73.9) | 0.02 |
Data presented as median (full range).
Table 4.
Median HCRU adjusted for Motzer risk stratification scale
| Low-risk | Intermediate-risk | High-risk | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Targeted therapy n= 13 | No targeted therapy n= 8 | Targeted therapy n= 34 | No targeted therapy n=11 | Targeted therapy n=10 | No targeted therapy n=9 | |
| Number of hospitalizations per month of survival | 0.04 | 0.08 | 0.08 | 0.20 | 0.21 | 0.33 |
| Number of consultants per month of survival | 0.26 | 0.13 | 0.26 | 0.59 | 0.57 | 0.67 |
| Number of consultants | 5 | 4 | 5 | 4 | 6 | 3 |
| Number of emergency department visits | 0 | 0 | 1 | 0 | 2 | 0 |
In the targeted therapy group, multiple regimens were used during the study (Table 5). Sunitinib was the most commonly used first-line therapy (75%). Second and third-line therapies were received by 40 (66.7%) and 13 (21.7%) patients, respectively, and consisted mainly of everolimus. Only five patients (8.3%) were treated with fourth-line therapy.
Table 5.
Treatment regimen description
| Line of therapy and medication used | Number of patients (%) |
|---|---|
| First-line | 60 (100) |
| Sunitinib | 45 (75) |
| Pazopanib | 8 (14) |
| Sorafenib | 2 (3) |
| Everolimus | 2 (3) |
| Temsirolimus | 2 (3) |
| Axitinib | 1 (2) |
| Second-line | 40 (66.7) |
| Everolimus | 20 (33.3) |
| Sunitinib | 9 (15) |
| Sorafenib | 9 (15) |
| Axitinib | 2 (3) |
| Third-line | 13 (21.7) |
| Everolimus | 5 (38) |
| Sunitinib | 3 (23) |
| Sorafenib | 3 (23) |
| Axitinib | 2 (16) |
| Fourth-line | 5 (8.3) |
| Sunitinib | 2 (3.3) |
| Sorafenib | 2 (3.3) |
| Axitinib | 1 (1.7) |
Discussion
In this study, we analyzed the impact of targeted therapy use on HCRU in the mRCC population at our centre. The study showed a statistically significant increase in total HCRU with the use of targeted therapies, specifically in terms of healthcare consultant involvement and number of emergency department visits. Non-adjusted clinical features may partly explain the HCRU differences, however, the relatively large OS rate difference between the groups may in itself explain the whole HCRU gap (16.5 vs. 7.3 months; Breslow=0.02). Interestingly, the OS rates recorded in our study represent a real-life scenario and survival rates differ from those observed in randomized controlled trials. For example, the COMPARZ study reported median OS of 29.3 and 28.4 months in its two groups; therefore, HCRU may be even higher in selected populations.15,16 To evaluate the effect of OS on HCRU, we analyzed HCRU values per month of survival. Patients without targeted therapy showed higher HCRU in terms of hospitalizations and number of consultants per month of survival. These results acknowledge the importance of considering the OS on HCRU and also suggest a decrease in HCRU per month of survival with the use of targeted therapies. This also implies that healthcare costs will follow the same trend as HCRU for these patients.17,18
Two studies directly assessed HCRU among mRCC patients.7,10 The COMPARZ non-inferiority clinical trial comparing pazopanib vs. sunitinib self-reported healthcare use during followup appointment for HRQoL assessment. This included monthly medical visits not related to the study, telephone consultations, number of days hospitalized, and number of emergency department visits for the first six months of followup. Similarly, Hansen et al did a post-doc evaluation of the COMPARZ study comparing HCRU between the two groups for the whole followup period.8 They used the study data regarding healthcare providers, laboratory and radiology use, hospitalizations, procedures, and pharmacy services. The combined results of these studies showed a statistical decrease of less than 0.03 emergency and radiology visits per month of survival in favour of pazopanib compared to sunitinib. Albeit statistically significant, the difference in HCRU seen between the groups of these studies is relatively small and may be explained by the fact that both groups received targeted therapies. Also, these studies lacked objective results, since the data used were self-reported by patients and included well-selected patients for clinical trial. Our study relies on data directly from patients’ computerized files, thus reflects objective information. Importantly, our study includes unselected patients.
Although patients included in this study were treated before the publication of the 2015 Canadian consensus statement on the management of advanced kidney cancer, a secondary endpoint was to evaluate concordance with these recommendations.13 The patients included in this study were treated according to current standards, with sunitinib and pazopanib being the two most commonly used first-line therapies and everolimus as the second-line treatment. All these molecules have Level 1 evidence status supporting their use. Since third- and fourth-line treatment regimens have not yet been clearly established, the use of targeted therapies not previously used or clinical trials are the only options, which is compatible with what patients receive in this study.
Our study has several limitations. The small sample size may have limited the detection of small differences between groups and may under power the analysis. The retrospective nature of the study represents another limitation of this study, but to our knowledge, there are no prospective studies that assessed HCRUs in mRCC in a real-world scenario. Further, the heterogeneity of the groups may also undermine the validity of the comparisons. For example, within the non-treated group, some patients were considered for palliative care only, while others had curative intent procedures. Another limitation was that real costs for HCRU and imaging use were not available for all patients to add an economic perspective to the use of healthcare resources. Finally, HCRU studies should ideally assess the impact of HCRU on HRQoL. Unfortunately, economic data, costs estimation, and QoL data were not available for this population.
Conclusion
Although targeted therapy-treated patients for mRCC may exhibit lower monthly use of healthcare, our study shows these patients ultimately will need more healthcare resources than those without any targeted therapy, due primarily to an increased survival length.
Footnotes
Competing interests: Dr. Richard has attended advisory boards for Sanofi and has received payment from AbbVie, Astellas, and Janssen. The remaining authors report no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.Canadian Cancer Society. Toronto (ON): Canadian Cancer Society’s Advisory Committee on Cancer Statistics; 2018. [Accessed Aug. 28. 2018]. [updated 2017]. Available at http://www.cancer.ca/en/cancer-information/cancertype/kidney/statistics/?region=qc/ [Google Scholar]
- 2.Cancer.net. American Society of Clinical Oncology; c2005–16. [Accessed Aug. 28, 2018]. [updated August 2017]. Available at http://www.cancer.net/cancer-types/kidney-cancer/statistics/ [Google Scholar]
- 3.Beaumont JL, Butt Z, Baladi J, et al. Patient-reported outcomes in a phase 3 study of everolimus vs. placebo in patients with metastatic carcinoma of the kidney that has progressed on vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy. Oncologist. 2011;16:632–40. doi: 10.1634/theoncologist.2010-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukowski R, Cella D, Gondek K, et al. Effects of sorafenib on symptoms and quality of life: Results from a large randomized placebo-controlled study in renal cancer. Am J Clin Oncol. 2007;30:220–7. doi: 10.1097/01.coc.0000258732.80710.05. [DOI] [PubMed] [Google Scholar]
- 5.Cella D, Davis MP, Negrier S, et al. Characterizing fatigue associated with sunitinib and its impact on health-related quality of life in patients with metastatic renal cell carcinoma. Cancer. 2014;120:1871–80. doi: 10.1002/cncr.28660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cella D, Michaelson MD, Bushmakin AG, et al. Health-related quality of life in patients with metastatic renal cell carcinoma treated with sunitinib vs. interferon-alpha in a phase 3 trial: Final results and geographical analysis. Br J Cancer. 2010;102:658–64. doi: 10.1038/sj.bjc.6605552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cella D, Pickard AS, Duh MS, et al. Health-related quality of life in patients with advanced renal cell carcinoma receiving pazopanib or placebo in a randomized phase 3 trial. Eur J Cancer. 2012;48:311–23. doi: 10.1016/j.ejca.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Hansen RN, Hackshaw MD, Nagar SP, et al. Healthcare costs among renal cancer patients using pazopanib and sunitinib. J Manag Care Spec Pharm. 2015;21:37–44. doi: 10.18553/jmcp.2015.21.1.37. https://doi.org/10.18553/jmcp.2015.21.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih YC, Chien CR, Xu Y, Pan IW, et al. Economic burden of renal cell carcinoma: Part I - an updated review. Pharmacoeconomics. 2011;29:315–29. doi: 10.2165/11586100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Shih YC, Chien CR, Xu Y, et al. Economic burden of renal cell carcinoma in the US: Part II - an updated analysis. Pharmacoeconomics. 2011;29:331–41. doi: 10.2165/11586110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib vs. sunitinib in metastatic renal cell carcinoma. N Engl J Med. 2013;369:722–31. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North SA, Basappa N, Basiuk J, et al. Management of advanced kidney cancer: Canadian Kidney Cancer Forum consensus update. Can Urol Assoc J. 2015;9:164–70. doi: 10.5489/cuaj.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer RJ, Bacik J, Mazumdar M. Prognostic factors for survival of patients with stage IV renal cell carcinoma: Memorial Sloan-Kettering Cancer Center experience. Clin Cancer Res. 2004;10:6302s–3s. doi: 10.1158/1078-0432.CCR-040031. [DOI] [PubMed] [Google Scholar]
- 15.Schmidinger M, Larkin J, Ravaud A. Experience with sunitinib in the treatment of metastatic renal cell carcinoma. Ther Adv Urol. 2012;4:253–65. doi: 10.1177/1756287212454933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Giorgi U, Scarpi E, Sacco C, et al. Standard vs. adapted sunitinib regimen in elderly patients with metastatic renal cell cancer: Results from a large retrospective analysis. Clin Genitourin Cancer. 2014;12:182–9. doi: 10.1016/j.clgc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Burke JP, Liu Z, Zheng J, et al. Incidence, annualized cost, and utilisation burden in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009 abstr e17529. [Google Scholar]
- 18.Oh WK, McDermott F, Duh MS, et al. Healthcare costs in patients (pts) with metastatic renal cell carcinoma (mRCC) treated with angiogenesis inhibitors (AI), ASCO 2010. unpublished. [Google Scholar]