Abstract
The price of whole-genome sequencing is now within the budget of the average American consumer. This has resulted in the commercialization of genome sequencing for a variety of applications, including health-related risk assessment. Direct-to-consumer marketing of personal DNA sequence information uncouples the generation of personal health-related data from the physician-patient relationship. Here, I discuss the status of consumer genomics and the current and potential concerns about bypassing physicians in the analysis and interpretation of personal genomic information and subsequent health care decision-making.
Introduction
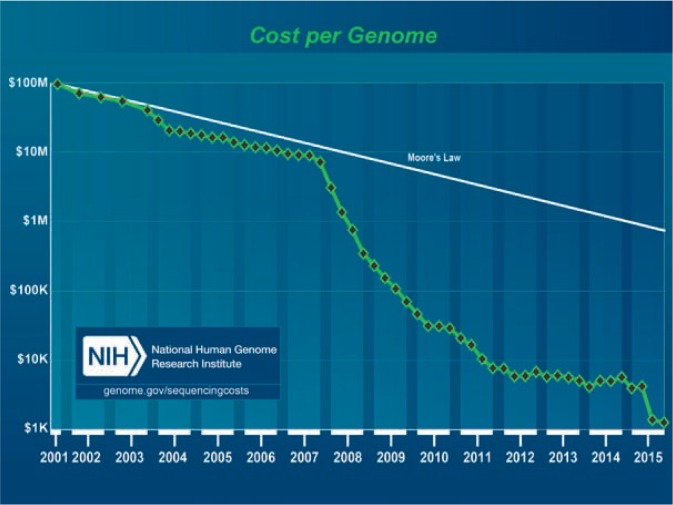
The sequencing of the human genome is transforming medicine. In a few years, the underlying molecular basis for thousands of genetic disorders will be known. A key dividend of the Human Genome Project was to drive down the cost of DNA sequencing by five orders of magnitude (100,000-fold) in 15 years. To put this transformation in perspective, Moore’s law (as modified by David House) describes the progressive increase in computer speed over the last several decades as doubling in performance every 18 months. From 2001 to 2007, the decrease in genome sequencing cost followed Moore’s Law, but after 2008, the drop in DNA sequencing cost sank far faster (See Figure 1).
Figure 1.
Decline of genome sequencing cost (green diamonds) compared to Moore’s Law.
Source: https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/
Recent revolutions in DNA sequencing technology and computational speed and storage have reduced the cost of genome sequencing and analysis to a level that makes it practical for middle-class Americans to obtain the sequence of their genome in few days. For a few hundred dollars, it is now possible for anyone to get their DNA analyzed for a variety of personal traits and disease risk factors. What effect will this information have on patient care? How much do we currently know about the predictive power and clinical validity of DNA sequence-disease associations? How should the clinical care community respond to this information revolution?
Genetic disease and genetic risk
The language used in the popular press to describe inherited disease is confusing. People often speak of “gene for sickle cell disease” or the “gene for hemophilia,” for example. There is no gene for sickle cell disease. The gene altered in sickle cell anemia is the adult beta globin gene, and everyone has two copies of it. What distinguishes patients with sickle cell disease is that they have a particular variant—a so-called “allele”— of the adult beta globin gene that makes their red blood cells clump abnormally in their capillaries under low oxygen tension, resulting in transient painful crises and long-term organ damage. It is also approximately 20% of individuals who carry two copies of important to understand that all mutations are not alike. Sickle cell disease occurs in people with two copies of the same adult beta globin gene allele, both copies of which program an abnormal protein. Other alleles of the adult beta globin gene result in little or none of the beta globin protein, a condition called beta thalassemia. The severity of beta thalassemia can vary, depending on the nature of the allele, and whether one or both copies of the adult beta globin gene are altered, but the clinical presentation is distinct from sickle cell disease. Personal genomics testing can certainly determine whether a patient carries a mutant adult beta globin gene, and the clinical data concerning this gene are sufficient to provide useful interpretations of such genomics data.
If the public discourse surrounding simple Mendelian diseases is sometimes misleading or confusing, the idea of disease risk for complex disease is infinitely more challenging. For common medical conditions like age-related macular degeneration, asthma, depression, heart disease, hypertension, obesity and type 2 diabetes, there are multiple genetic factors with various contributions, as well as environmental inputs. In some cases, gene sequence variants are statistically more often encountered in affected individuals, yet others may have the same variants without symptoms. An illustration of this is the association of the APOL1 allele with chronic kidney disease (CKD). While CKD was thought to be a complex disease, the presence of the APOL1 allele can account for much of the risk. But only the APOL1 allele actually get CKD unless they also suffer from another condition such as HIV infection. For most people with APOL1 CKD, the influence of such secondary factors is unknown, making it hard to predict who will get CKD. Thus, it is important to stress that genetic risk is not the same as genetic destiny. Critical to the utility of genomics data is the predictive power associated with specific alleles at specific genes, and appreciating the clinical validity behind a given prediction. In most cases of sequence variants uncovered by personal genomics, the clinical validity of most allele associations with complex diseases is weak or nonexistent, making the predictive power of genomics testing problematic at best.
The Direct-To-Consumer Genomics Business Model
A variety of genomics testing services are presently being marketed directly to consumers without a doctor’s order. Some companies test for a handful of specific genes, looking for alleles for which there are clinically informative data or that have significance for ancestry. Other companies sequence the “exome,” that fraction of the genome that contains genes encoding proteins. This represents only about 1% of the human genome, making the amount of data and the task of assembling and validating it much more manageable than sequencing the entire genome. The remainder of the genome encodes (1) sequences that control protein coding genes, (2) non-coding RNAs that regulate protein coding genes, (3) transposable elements, (4) sequences that control chromosome behavior and (5) sequences with no known function. However, with the rapidly declining cost of DNA sequencing and rapidly growing computational speed and data storage capacity, many companies offer whole genome sequencing. Table 1 tabulates several privately held companies offering direct-to-consumer personalized genomic analysis and their services
Table 1.
List of personal genomics companies providing direct-to-consumer DNA testing in the US as of June, 2016
| Company | Services |
|---|---|
| 23andME | ancestry and ethnicity, traits, genetic disease carrier status, wellness |
| AncestryDNA | ancestry and ethnicity |
| Counsyl | inherited cancer gene screening, pre-natal and pre-conceptional screening |
| DNA4Life | drug sensitivity report, wellness review, skin care report |
| DNA-CardioCheck, Inc | clotting and cardiovascular disorders |
| FullGenomes | deep ancestry |
| Gene by Gene | research, exome sequencing, whole genome sequencing |
| Genographic Project | deep ancestry |
| Genomic Express | ancestry, nutrition, pharmacogenetics, sports, traits |
| *Genos | exome sequencing for disease risk, athletic or nutritional predispositions. |
| Healthspek PGT | pharmacogenetics |
| Interleukin Genetics | periodontal disease, weight management, heart health, bone health and nutritional needs |
| InVitae | cardiology, hematology, hereditary cancer, metabolic diseases and newborn screening, neurology, ophthalmology, pediatric genetics |
| Kailos Genetics | cancer screening, pharmacogenetics, ADHD |
| Sure Genomics | health, whole genome sequencing |
| Ubiome | gut microbiome sequencing |
| **Veritas Genetics | whole genome sequencing, BRCA, prenatal testing |
does not accept orders from NY or FL
according to their web site, requires a doctor’s order
Obtaining a highly accurate entire genome sequence of a single person is relatively easy compared to making sense of that information. To begin with, the raw sequence information is fragmentary; hundreds of millions of fragments must be assembled by computer into the linear sequences comprising the three billion nucleotide sequence units in a human genome. Then, there is the reality that the chemistry of DNA sequencing isn’t flawless, so sequencing errors have to be distinguished from actual variants. Finally, there is the question of whether the validated variants are simply random and benign or have meaning for the person’s ancestry or health.
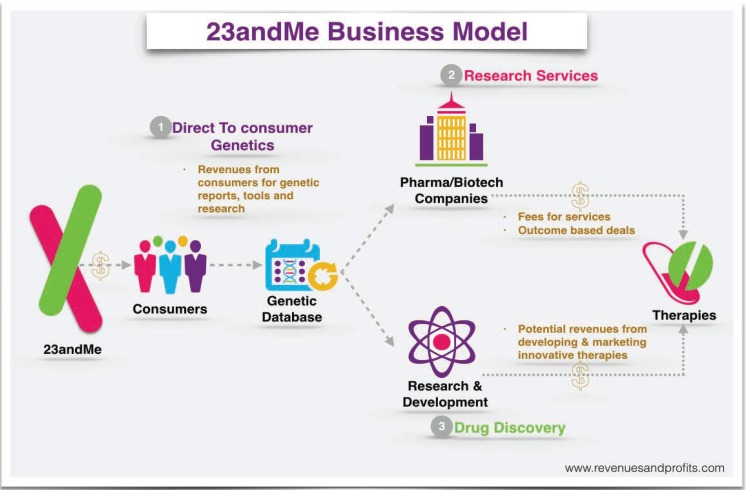
Probably the most widely advertised consumer genomics provider is 23andMe, a privately held biotechnology company based in Mountain View, California. 23andMe provides the customer with a container in which they deposit a saliva sample. When the sample is returned, the company extracts and genotypes the DNA. 23andME then sends the customer a research report showing whether they are a carrier for any of over 35 diseases and provides information about benign traits and likely ancestry. The 23andMe business model is to collect genetic data in order to identify patterns and aggregate information to sell to researchers, insurers and pharmaceutical companies. (See Figure 2.) Anticipating the future market value of this database to commercial partners, 23andMe steeply subsidizes the cost of its services to individual consumers. In all but one case, the companies listed in Table 1 work directly with consumers without a doctor’s order.
Figure 2.
Source:http://revenuesandprofits.com/how-23andme-makes-money-understanding-23andme-business-model/
Direct-To-Consumer Genomics: Disintermediating Access to Medical Data
Disintermediation is the process of bringing customers closer to products or services by cutting out intermediaries. A familiar and long-established example of disintermediation is the farmer’s market, where farmers sell produce directly to consumers without the intermediation of a grocery store. More recent examples include the Uber app, which connects people directly with a car and driver without the intermediation of a taxi company, and airbnb, which gives travelers access to overnight accommodations without the intermediation of hotel companies.
Consumer genomics disintermediates personal genetic information by removing the physician and genetic counselor. The expertise required of physicians is orders of magnitude more complex than that required for farming, driving a car or renting a room, so the idea of removing the doctor from the collection and interpretation of personal medical data would seem to threaten the traditional medical information hierarchy.
In reality, disintermediation has been occurring in medicine for decades. Digital thermometers, blood pressure cuffs, blood sugar monitors, and pregnancy testing kits are examples of only somewhat more sophisticated commercialized technologies that permit the lay public direct access to actionable health-related parameters without overt symptoms or physician intermediation
The public is increasingly turning to online applications called “symptom checkers” to seek diagnoses and advice on appropriate care. In a recent study, 23 such online applications were evaluated for accuracy in a total of 770 standardized patient evaluations for diagnosis and 532 standardized patient evaluations for triage.1 Surprisingly, the correct diagnosis was listed first in only 43% of standardized patient evaluations overall, and was listed in the top 20 diagnoses only 58% of the time. Appropriate triage advice was given in 57% of the cases. This compares with in-person physician diagnostic accuracy rates of 85–90%. With regard to online diagnostics, caveat emptor.
Direct-to-consumer marketing of genomics data may be compared to the widely accepted direct marketing of MRI and CT imaging of the coronary arteries for calcification and of the breasts, lungs, abdominal organs and pelvis for cancer. In contrast to sampling DNA from cheek swabs or sputum samples, CT imaging involves ionizing radiation exposure, a known cancer risk; radiation exposure during a full-body CT scan is approximately 500 times that of a chest X-ray. While a small number of individuals could discover tumors this way, only a fraction of these people will have an altered outcome. On the other hand, benign lesions uncovered incidental to imaging—so-called “incidentalomas”—can lead to needless invasive testing and anxiety. For screening the “worried well,” the risks of asymptomatic CT imaging clearly outweigh the benefits. While MRIs lack the risks associated with ionizing radiation exposure, the possibilities of actionable false positives remain.
The critical health care issues that emerge from direct-to-consumer marketing of genomic data are (1) should consumer access to personal genomics data be denied or controlled, and (2) what can and cannot be claimed concerning the medical implications of the data.
On the first issue, I will argue that people should have unfettered, disintermediated access to their personal genome data. On the second, there are legitimate controls that should be imposed on the marketing of claims about personal genomic data.
Access
In an open society, maximizing autonomy is a virtue. Knowledge is power. The concerns expressed around disintermediated access to personal genomics data are focused on whether personal genomics data could cause significant harm to the subject. There is certainly anecdotal evidence for severe emotional distress associated with disintermediated genetic testing that resulted in unexpected risk findings.2 Fortunately, several recent studies have been published that address these concerns.
Does genetic risk information concerning the ApoE4 allele increase distress and anxiety? The presence of one copy of the ApoE4 allele increases the risk of Alzheimers by approximately three-fold, while two copies increases risk approximately fifteen-fold. In a study of 162 asymptomatic adults who had a parent with Alzheimer’s disease, each subject’s symptoms of anxiety, depression, and stress were measured after test results were revealed.3 Genetic counselors monitored all subjects for adverse psychological effects and made referrals when appropriate. The study found evidence that disclosure of genotyping information provides a benefit to those who are ApoE4-negative and results in only transient, modest distress to ApoE4-positive individuals. A caveat here is that there is no proven medical intervention for Alzheimers. In the case of bilateral prophylactic mastectomy in response to increased genetic risk for breast cancer, it is possible that anxiety and distress may be quite different when contemplating invasive therapy.
Do elevated cancer risk results alter risk perception in genomic testing customers? In surveys of over 1,000 people for the effect of risk information for four common cancers, a study found that DTC genomic testing results indicating elevated risk did lead to increased risk perception, while an average risk result left risk perception unchanged or lower.4
Do genetic risk data motivate risk-reducing behavior? Based on a review of 14 papers reporting results of seven clinical studies and six analogue studies, Marteau et al.5 reported that “... communicating DNA-based disease risk estimates has little or no effect on smoking and physical activity.” The same could well be said of the common long-term patient response to standard DNA-free doctor’s admonitions concerning weight loss, smoking cessation, alcohol moderation, sunscreen use, and regular exercise
Taken together, the current published literature suggests no lasting harm comes from giving people access to their genomics data, although little benefit could be quantified, either.6 On the issue of DTC genomics testing, there is no justification for medical paternalism, defined as “ … usurpation of decision-making power, by preventing people from doing what they have decided, interfering in how they arrive at their decisions, or attempting to substitute one’s judgment for theirs, expressly for the purpose of promoting their welfare.”7 Thus, disintermediated access to personal genomics data is not contraindicated.
Data Interpretation
The second major concern for direct-to-consumer genomics testing is that the current state of medical knowledge has not kept up with the detail with which we can examine genomes. Therefore, what should direct-to-consumer genomics testing companies be allowed to tell their clients about the meaning of sequence variation?
Many or most genomic testing clients are interested in whether they carry a mutation that affects their health or the health of their descendants. An obvious place to look for such mutations is in genes that code for proteins, and whether there is a mutation that would inactivate one or both copies of that gene. A 2012 paper reported an exhaustive analysis of 185 human genomes of healthy people to look specifically for changes that would result in complete loss of function in any of their protein-coding genes.8 The analysis found that the typical “healthy” person has about 100 such mutations in their genome, with about 20 of these changing both copies of the gene. Most of the loss-of-function variants found in specific individuals appear to be common variants that occur in non-essential genes, and there is little or no evidence implicating these loss-of-function mutations in common complex diseases like heart disease or type 2 diabetes. Some loss-of-function variants are rare, suggesting that they are deleterious, and some of these could be associated with serious disease conditions.
However, our current ability to predict risk from genomics data is weak or nonexistent for most sequence variants, so simply knowing that there are sequence variants that disrupt a specific gene is not enough reason to seek medical intervention. Indeed, a recent study comparing the ability of three direct-to-consumer genomics companies to assess risk for six common complex diseases (age-related macular degeneration, atrial fibrillation, celiac disease, Crohn’s disease, prostate cancer and type 2 diabetes) using data sets simulating 100,000 individuals found that the company genomic data analysis varied significantly in their predictive ability for each disease and in the risks predicted for individual consumers.9
For now, stringent standards are essential for published disclaimers concerning the limitations of current knowledge of risks attached to most sequence variants in light of current scientific evidence. Of course, the standards must be evolvable as the evidence inevitably improves with time.
Even with better data on gene function, low health literacy (health reporting jargon) and low genetic literacy are barriers to the ability of consumers to use personal genomic data as a guide to health care decision-making. Both barriers necessarily affect perception of risk, which is also influenced by education, ethnicity and culture. Here lies a critical role for intermediation, either by a genetically literate physician or a genetic counselor. Of course, clinically significant responses to genomic data will still require standard medical protocol; patients who seek a mastectomy or ovariectomy based on genomics data will still face physician intermediation in this country.
With the widespread availability of disintermediated genomics data, are we really better informed about our health? What happens when a consumer of genomics data learns he or she carry a mutation associated with an elevated risk for colon or breast cancer? Or more dramatically, consider a 25-year-old who learns that he or she carries a mutation giving the certainty of dying of Huntington disease in their fourth or fifth decade when no mitigating therapies exist. While psychological evaluation and genetic counseling are standards advocated by the medical community prior to specific testing for Huntington disease, disintermediated testing provides no such mechanism to prepare consumers of commercial testing for bad news and the risk of anxiety, emotional distress, depression and suicidal ideation.10 Table 2 summarizes some of the cost/benefit considerations of commercial genomics testing.
Table 2.
Predictive testing cost/benefit comparisons
| Potential advantages | Potential disadvantages |
|---|---|
| More information | Costs to individuals of tests that yield little determinate information |
| Allows early intervention | Social harms when private testing can undermine equal access to healthcare |
| Allows more personal control | Costs of consequences of having information: a) for individual when inaccurate or hard to interpret, b) for individual when nothing can be done, c) for individual if inaccurate risk assessments lead to false reassurance or misplaced anxiety, d) for the individual, if results lead to stigma or other effects that may be regretted, given that information once known cannot be ‘un-known’ (e.g., for insurance declarations), e) for taxpayers when unnecessary follow-up testing and treatment is carried out |
| Possibility of saving public healthcare resources if testing and treatment are conducted privately | Costs and harms to third parties – when children or third parties are tested without consent, or when embryos are tested for conditions whose risks may be hard to determine |
| Can alert relatives to important genetic conditions | Costs and harms to third parties – when children or third parties are tested without consent, or when embryos are tested for conditions |
Adapted from: Nuffield Council on Bioethics (2010) Medical Profiling and Online Medicine: the ethics of ‘personalised healthcare’ in a consumer age, NCB, London, Chapter 3.
Studies conducted in collaboration with 23andMe so far have shown no evidence of adverse effects, but current users of 23andMe skew toward an emotionally, intellectually and educationally prepared demographic with sufficient affluence and access to health care to cope with adverse news. Nevertheless, the terms of service on the 23andMe web site stipulates that “ … information you learn from 23andMe is not designed to independently diagnose, prevent, or treat any condition or disease or to ascertain the state of your health in the absence of medical and clinical information,” and “23andMe makes no warranty that … the results that may be obtained from the use of the services will be accurate or reliable.”
Regardless, it is clear that more and more personal medical data will be finding their way unfiltered directly to consumers. This represents a significant shift in control from doctors to the general public. In the absence of physician oversight, will this new access result in a significant impact on outcomes? In the cases of diabetes and heart disease, most people don’t need test data or a doctor to know that a better diet and regular exercise will improve their health. Will whole genome data change patient behavior for the better? So far, it looks like the risk data currently available from direct-to-consumer personal genomics are neither as empowering as claimed by the companies promoting this product, nor are they as dangerous as critics have feared.11
The Future of Personalized Genomics and the Doctor-Patient Relationship
At its best, direct-to-consumer genomics testing could eventually become like other forms of home medical testing—another way for people to take personal control of their health and wellness. According to a recent study, the reliability of current genomic sequencing technology surpasses the accuracy of Sanger DNA sequencing, long held to be the gold standard.12 Today, however, genomics testing to assess risk for complex diseases rests on a weak foundation of clinical validation. Thus, in most cases genomics data cannot serve as a guide to action. While medical paternalism is not warranted in controlling access to personal genomics data, there may be a benefit for some genomics testing clients to access pre-test intermediation by physicians or genetic counselors to prepare for adverse news, and a stronger post-hoc role for physician intermediation in interpreting risk, prescribing risk response where appropriate and counseling caution in over-interpretation. Indeed, the American College of Genetics and Genomics has published their recommendations for health care professional involvement in DTC genomic testing (see sidebar). For their part, regulation of direct-to consumer companies should be limited to proper lab and confidentiality practices and avoiding over-reach in making interpretive claims concerning medical risk.6
SIDEBAR.
Direct-To-Consumer Genetic Testing: A Revised Position Statement of the Amercian College of Medical Genetics and Genomics
Source: American College of Medical Genetics and Genomics/Genetics in Medicine
With ongoing genetic discoveries and improvements in technology, more genetic tests are available than ever before. Along with greater availability has come increased consumer demand for genetic tests and expansion of direct-to-consumer (DTC) testing. The American college of Medical Genetics believes that it is critical for the public to realize that genetic testing is only one part of a complex process which has the potential for both positive and negative impact on health and well-being. The college believes that the following should be considered minimum requirements for any genetic testing protocol:
A knowledgeable health care professional should be involved in the process of ordering and interpreting a genetic test. Genetic testing is highly technical and complex. A genetics expert such as a certified medical geneticist or genetic counselor can help the consumer determine, for example, whether a genetic test should be performed and how to interpret test results in light of personal and family history. A number of risks can be reduced if a genetics professional is involved in genetic testing. These risks include lack of informed consent, inappropriate testing, misinterpretation of results, testing that is inaccurate or not clinically valid, lack of follow-up care, misinformation, and other adverse consequences.
The consumer should be fully informed regarding what the test can and cannot say about his or her health. Many DTC genetic tests do not give a definitive answer as to whether an individual will develop a given condition, but provide only a risk or probability of developing a disease. The interpretation of such results is often highly nuanced and such information needs to be communicated to the consumer in the appropriate context and in an understandable fashion that is linguistically and culturally appropriate.
The scientific evidence on which a test is based should be clearly stated. DTC genetic test providers should provide easy-to-understand information with primary references documenting the scientific data on which a specific test is based.
The clinical testing laboratory must be accredited by CLIA, the state and/or other applicable accrediting agencies. The accreditation process ensures that laboratories adhere to strict standards and guidelines for clinical testing. Test result reports to consumer should indicate the specifics of the lab’s accreditation.
Biography
Joel C. Eissenberg, PHD, is a Professor and Associate Dean for Research, Edward A. Doisy Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine.
Contact: eissenjc@slu.edu

Footnotes
Disclosure
None reported.
References
- 1.Semigran HL, Linder JA, Gidengil C, Mehrotra A. Evaluation of symptom checkers for self diagnosis and triage: audit study. BMJ. 2015;351:h3480. doi: 10.1136/bmj.h3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahon SM. Impact of direct-to-consumer genetic testing. J Oncol Pract. 2012;9:260. doi: 10.1200/JOP.2012.000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green RC, Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, Brown T, Eckert SL, Butson M, Sadovnick AD, Quaid KA, Chen C, Cook-Deegan R, Farrer LA, for the REVEAL Study Group Disclosure of APOE genotype for risk of Alzheimer’s Disease. N Engl J Med. 2009;361:245–254. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carere DA, VanderWeele T, Moreno TA, Mountain JL, Roberts JS, Kraft P, Green RC, PGen Study Group The impact of direct-to-consumer personal genomic testing on perceived risk of breast, prostate, colorectal, and lung cancer: findings from the PGen study. BMC Med Genomics. 2015 Oct;8:15. 63. doi: 10.1186/s12920-015-0140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marteau TM, French DP, Griffin SJ, Prevost AT, Sutton S, Watkinson C, Attwood S, Hollands GJ. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Chchrane Database Sys Rev. 2010;10:CD007275. doi: 10.1002/14651858.CD007275.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green RC, Farahany NA. The FDA is overcautious on consumer genomics. Nature. 2014;505:286–287. doi: 10.1038/505286a. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan DR. Autonomy, paternalism, and justice: Ethical priorities in public health. Am J Public Health. 2008;98:15–21. doi: 10.2105/AJPH.2007.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacArthur DG, Balasubramanian S, Frankish A, et al. A systematic survey of loss-of-function variants in human protein-coding genes. Science. 2012;333:823–828. doi: 10.1126/science.1215040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalf RRJ, Mihaescu R, Kundu S, de Knijff P, Green RC, Janssens ACJW. Variations in predicted risks in personal genome testing for common complex diseases. Genet Med. 2014;16:85–91. doi: 10.1038/gim.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robins Wahlin TB. To know or not to know: a review of behaviour and suicidal ideation in preclinical Huntington’s disease. Patent Educ Couns. 2007;65:279–287. doi: 10.1016/j.pec.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Nordgren A. Neither as harmful as feared by critics nor as empowering as promised by providers: risk information offered direct to consumer by personal genomics companies. J Community Genet. 2014;5:59–68. doi: 10.1007/s12687-012-0094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck TF, Mullikin JC, NISC Comparative Sequencing Program, Biesecker LG Systematic evaluation of Sanger validation of next-generation sequencing variants. Clin Chem. 2016;62:647–654. doi: 10.1373/clinchem.2015.249623. [DOI] [PMC free article] [PubMed] [Google Scholar]