Summary
Background
Intracerebral haemorrhage growth is associated with poor clinical outcome and is a therapeutic target for improving outcome. We aimed to determine the absolute risk and predictors of intracerebral haemorrhage growth, develop and validate prediction models, and evaluate the added value of CT angiography.
Methods
In a systematic review of OVID MEDLINE—with additional hand-searching of relevant studies' bibliographies— from Jan 1, 1970, to Dec 31, 2015, we identified observational cohorts and randomised trials with repeat scanning protocols that included at least ten patients with acute intracerebral haemorrhage. We sought individual patient-level data from corresponding authors for patients aged 18 years or older with data available from brain imaging initially done 0·5–24 h and repeated fewer than 6 days after symptom onset, who had baseline intracerebral haemorrhage volume of less than 150 mL, and did not undergo acute treatment that might reduce intracerebral haemorrhage volume. We estimated the absolute risk and predictors of the primary outcome of intracerebral haemorrhage growth (defined as >6 mL increase in intracerebral haemorrhage volume on repeat imaging) using multivariable logistic regression models in development and validation cohorts in four subgroups of patients, using a hierarchical approach: patients not taking anticoagulant therapy at intracerebral haemorrhage onset (who constituted the largest subgroup), patients taking anticoagulant therapy at intracerebral haemorrhage onset, patients from cohorts that included at least some patients taking anticoagulant therapy at intracerebral haemorrhage onset, and patients for whom both information about anticoagulant therapy at intracerebral haemorrhage onset and spot sign on acute CT angiography were known.
Findings
Of 4191 studies identified, 77 were eligible for inclusion. Overall, 36 (47%) cohorts provided data on 5435 eligible patients. 5076 of these patients were not taking anticoagulant therapy at symptom onset (median age 67 years, IQR 56–76), of whom 1009 (20%) had intracerebral haemorrhage growth. Multivariable models of patients with data on antiplatelet therapy use, data on anticoagulant therapy use, and assessment of CT angiography spot sign at symptom onset showed that time from symptom onset to baseline imaging (odds ratio 0·50, 95% CI 0·36–0·70; p<0·0001), intracerebral haemorrhage volume on baseline imaging (7·18, 4·46–11·60; p<0·0001), antiplatelet use (1·68, 1·06–2·66; p=0·026), and anticoagulant use (3·48, 1·96–6·16; p<0·0001) were independent predictors of intracerebral haemorrhage growth (C-index 0·78, 95% CI 0·75–0·82). Addition of CT angiography spot sign (odds ratio 4·46, 95% CI 2·95–6·75; p<0·0001) to the model increased the C-index by 0·05 (95% CI 0·03–0·07).
Interpretation
In this large patient-level meta-analysis, models using four or five predictors had acceptable to good discrimination. These models could inform the location and frequency of observations on patients in clinical practice, explain treatment effects in prior randomised trials, and guide the design of future trials.
Funding
UK Medical Research Council and British Heart Foundation.
Research in context.
Evidence before this study
We did a systematic review of studies of the risk of intracerebral haemorrhage growth, and associations with it, published in OVID MEDLINE (from Jan 1, 1970, to Dec 31, 2015) using a comprehensive search strategy, limited to humans, combining terms for intracerebral haemorrhage (“exp basal ganglia hemorrhage/”, “intracranial hemorrhages/”, “cerebral hemorrhage/”, “intracranial hemorrhage, hypertensive/”, and other text words) with text words suggesting growth (“expansion”, “growth”, or “enlargement”), with no language restrictions. When we updated the search to March 1, 2018, we identified reports of five new cohorts, representing a maximum of a 10% increase in the number of eligible patients compared with those from the 36 cohorts that provided individual patient data in this meta-analysis. We did not include these five new cohorts in our analyses. Intracerebral haemorrhage growth risk is known to be highest soon after intracerebral haemorrhage symptom onset, but its absolute risks over time and by baseline volume are unclear. Studies have identified several risk factors associated with intracerebral haemorrhage growth, but many associations are not consistent across studies, and the predictive values of these risk factors remain to be determined.
Added value of this study
Our systematic review led to the pooling of 5435 eligible patients from 36 cohorts, which is, to the best of our knowledge, the largest patient-level meta-analysis to explore the absolute risk and predictors of intracerebral haemorrhage growth. We found that the risks of growth over time and by baseline intracerebral haemorrhage volume were not linear. The sample size enabled us to model these associations with good precision and construct and validate multivariable models adjusted for 13 categorical or continuous covariates. Four predictors (time from symptom onset to baseline imaging, intracerebral haemorrhage volume on baseline imaging, antiplatelet use, and anticoagulant use) were independent predictors of intracerebral haemorrhage growth (C-index 0·78, 95% CI 0·75–0·82). Addition of information about the presence of spot sign on CT angiography to the model increased the C-index by just 0·05 (95% CI 0·03–0·07).
Implications of all the available evidence
Models using four or five predictors that are simple to collect had acceptable to good discrimination for predicting intracerebral haemorrhage growth, which was slightly improved by the addition of information on spot sign from CT angiography. These models could guide the monitoring of patients at risk of clinical deterioration as well as the interpretation and investigation of treatment effects in randomised trials.
Introduction
Haemorrhagic stroke is responsible for around 11% of strokes in high-income countries but 22% of strokes in low-income and middle-income countries,1 where 75% of deaths due to haemorrhagic stroke occur.2 Spontaneous (non-traumatic) intracerebral haemorrhage is the most frequent type of haemorrhagic stroke and has the worst outcome: almost half of patients die within the first month and 80% of survivors are dependent on a caregiver.3
Intracerebral haemorrhage volume increases after vessel rupture and growth can continue after intracerebral haemorrhage is first diagnosed on brain imaging. Intracerebral haemorrhage growth is associated with poor clinical outcome.4 Therefore, immediately after confirmation of intracerebral haemorrhage diagnosis on brain imaging, accurate prediction of the risk of later intracerebral haemorrhage growth could help to target patients' monitoring, treatment and transfer to specialist care, and the design and interpretation of randomised trials of treatments to limit intracerebral haemorrhage growth.5
The timing of the first brain imaging done after intracerebral haemorrhage onset and the intracerebral haemorrhage volume found on imaging are two consistently identified risk factors for intracerebral haemorrhage growth, although the association of other potential risk factors has been inconsistent in many small observational studies. Interest has grown in whether a so-called spot sign due to contrast extravasation on additional angiography at the time of diagnostic imaging is a predictor of intracerebral haemorrhage growth.6 There are several multivariable prediction models for intracerebral haemorrhage growth,7, 8, 9, 10, 11 but the identified predictors have varied across models, and several have relied on CT angiography,12 which is not readily available in low-income and middle-income countries. Identifying more accurate predictors of intracerebral haemorrhage growth is recognised to be a research priority.13
Therefore, we aimed to identify the risk and predictors of acute intracerebral haemorrhage growth, develop and validate prediction models that could be used worldwide, and evaluate the added value of CT angiography.
Methods
Search strategy and selection criteria
We conducted a systematic review to identify studies of intracerebral haemorrhage growth that would share individual patient data for a patient-level meta-analysis of the absolute risks and predictors of intracerebral haemorrhage growth.14 A prespecified protocol (finalised on June 20, 2013, and not registered; appendix) guided our data collection and analyses.
One author (JF) identified potentially eligible cohorts by searching OVID MEDLINE from Jan 1, 1970, to Dec 31, 2015, using a comprehensive search strategy (appendix); hand-searching relevant studies' bibliographies; contacting authors of collaborating studies; and accessing patient-level data from eligible cohorts in the Virtual International Stroke Trials Archive. We included the largest single report of any observational or randomised cohort—regardless of language of publication—if it included at least ten eligible patients with acute intracerebral haemorrhage who had brain imaging (by CT with or without angiography or by MRI) to diagnose intracerebral haemorrhage and used a predefined protocol for repeat imaging (done regardless of clinical need), which would minimise the risks of selection and information biases about intracerebral haemorrhage growth.
We included patients from these cohorts if they were aged 18 years or older; had non-traumatic intracerebral haemorrhage that was probably due to cerebral small vessel disease and not secondary to an underlying structural cause identified by brain imaging; had data available from brain imaging initially done 0·5–24 h and repeated fewer than 6 days after symptom onset; had baseline intracerebral haemorrhage volume of less than 150 mL; and did not undergo acute treatment that might reduce intracerebral haemorrhage volume (ie, surgical evacuation,15 haemostatic therapy,5 or blood pressure lowering16). We excluded patients if the time from symptom onset to baseline imaging was not known in hours or if they had not been included in the published report of their cohort.
We emailed our protocol and an invitation to collaborate to the corresponding authors of cohorts that were eligible for inclusion, followed by one reminder. We included cohorts if corresponding authors of studies reporting them confirmed their eligibility and provided patient-level data on eligibility criteria and other variables at baseline, information on type and timing of baseline and repeat brain imaging, intracerebral haemorrhage characteristics (location, volume on baseline and repeat imaging, presence of intraventricular haemorrhage), and the presence of the spot sign on CT angiography if done (appendix).
Research ethics committees or other entities overseeing the use of patients' data had approved the collaborating cohorts. Cohorts shared only anonymised data, so neither individual consent nor specific approval for this individual patient data meta-analysis were required.
Data analysis
We used reports of the included cohorts to categorise their method of intracerebral haemorrhage volume measurement as a cohort-level characteristic into either the manual ABC/2 method17 or an automated or semi-automated planimetric method.18 We assessed risk of bias across cohorts by identifying the studies that did not meet our eligibility criteria, did not share data, or did not provide data on a sufficient number of the variables of interest (appendix). We checked data completeness and consistency within each cohort and resolved any queries directly with the relevant collaborators. We standardised the format, coding, and units of measurement of variables to maximise the number available for analysis in all cohorts. We did not use or request aggregate data from cohorts that did not share patient-level data.
We prespecified that the primary outcome measure of intracerebral haemorrhage growth would be an increase in intracerebral haemorrhage volume between baseline and repeat imaging of more than 6 mL; we chose an absolute measure of intracerebral haemorrhage growth in volume because such measures seem to have higher positive predictive values for more severe clinical outcomes than does the combination of absolute or relative increases in intracerebral haemorrhage volume (eg, >33%).19
We prespecified the variables that might be predictors of intracerebral haemorrhage growth in our protocol (appendix) on the basis of their clinical relevance, likelihood of being associated with outcome, and reliability and accuracy of measurement (appendix). To these variables, we added history of liver disease and history of stroke; we also added CT angiography spot sign in view of the increasing interest in its role as a predictor since the protocol had originally been written (appendix).6 Of these prespecified variables, we selected potential predictors on the basis of their completeness and availability at the time of diagnosis in the available cohorts and the extent to which their selection maximised the total sample size available for multivariable analyses. Many cohorts excluded patients taking anticoagulant therapy at onset and only a few cohorts conducted CT angiography, so we took a hierarchical approach to investigating univariable and multivariable associations and predictors of intracerebral haemorrhage growth.
First, we analysed patients not taking anticoagulant therapy at intracerebral haemorrhage symptom onset because they constituted the vast majority of the included cohorts. In this dataset, we examined the associations between intracerebral haemorrhage growth and a subset of the variables, which were chosen on the basis of their completeness and availability at the time of intracerebral haemorrhage diagnosis in the participating cohorts. We visually inspected plots of cohort-specific estimates of association for each variable to exclude major heterogeneity. We then used a one-stage approach to meta-analysis to obtain unadjusted and adjusted estimates pooled across the cohorts using logistic regression models with random intercepts and random coefficients. For all continuous predictors, we used either a linear term or, where there was strong evidence (p<0·01) of non-linearity on the log-odds scale, a fractional polynomial. We described the univariable associations between intracerebral haemorrhage growth and two of the continuous variables (time to baseline imaging and intracerebral haemorrhage volume at baseline) by plotting the predicted probability of intracerebral haemorrhage growth derived from the model against the predictor. For the remaining continuous variables, we quantified the unadjusted and adjusted associations using the odds ratio for the upper quartile compared with the lower quartile based on the fitted linear or fractional polynomial terms in the logistic regression model. We had a sufficient sample size to split those patients who were not taking anticoagulant therapy by contributing cohort into two datasets: one to develop a prediction model and another to validate its performance. We did this temporal validation with patients from earlier cohorts (1994–2007) allocated to the development dataset and patients from more recent cohorts (2008–15) allocated to the validation dataset. We chose a subset of potential predictors for entry into a multivariable model on the basis of their combined availability in the development dataset and the number of patients with intracerebral haemorrhage growth (to avoid overfitting), without considering the results of the unadjusted and adjusted associations between each predictor and intracerebral haemorrhage growth. We did not examine interactions between other covariates and these associations. We derived a prediction index for intracerebral haemorrhage growth with the predictors that remained in a multivariable logistic regression model after backwards elimination. We assessed the performance of the prediction model using calibration plots of predicted versus observed probabilities, receiver operating characteristic curves, and the C-index to assess discrimination in both the development and validation datasets and in patients from cohorts that included patients taking anticoagulant therapy at intracerebral haemorrhage onset.
Second, we assessed the performance of the prediction model in patients taking anticoagulant therapy at intracerebral haemorrhage onset.
Third, we split by contributing cohort those patients from cohorts that included at least some patients taking anticoagulant therapy at intracerebral haemorrhage onset into one dataset to develop a prediction model and another to validate its performance (using temporal validation, as described above). We considered the same subset of potential predictors as for the first prediction model, with the addition of anticoagulant therapy use at intracerebral haemorrhage onset. We derived a prediction index for intracerebral haemorrhage growth and assessed its performance using the same approaches as for the first prediction model.
Fourth, in cohorts that included at least some patients with data available on the spot sign identified by CT angiography and that also included and distinguished patients taking anticoagulant therapy at onset, we assessed whether spot sign presence was independently associated with intracerebral haemorrhage growth and the predictive performance when it was added to the predictors in the second prediction model.
We did a prespecified sensitivity analysis to compare our findings using a definition of intracerebral haemorrhage growth as an absolute increase of more than 6 mL versus an absolute increase of more than 6 mL or a relative increase of more than 33% in intracerebral haemorrhage volume. We did post-hoc sensitivity analyses to compare associations between time from intracerebral haemorrhage symptom onset to baseline brain imaging and intracerebral haemorrhage volume on baseline imaging with intracerebral haemorrhage growth in cohorts using ABC/2 versus planimetric methods of measuring intracerebral haemorrhage volume and in cohorts from earlier versus later time periods.
Analyses were done using SAS software version 9.4 (SAS Institute) and Stata version 12.1 (StataCorp).
Role of the funding source
The study sponsors had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The data were available to all authors on request. The corresponding author had final responsibility for the decision to submit for publication.
Results
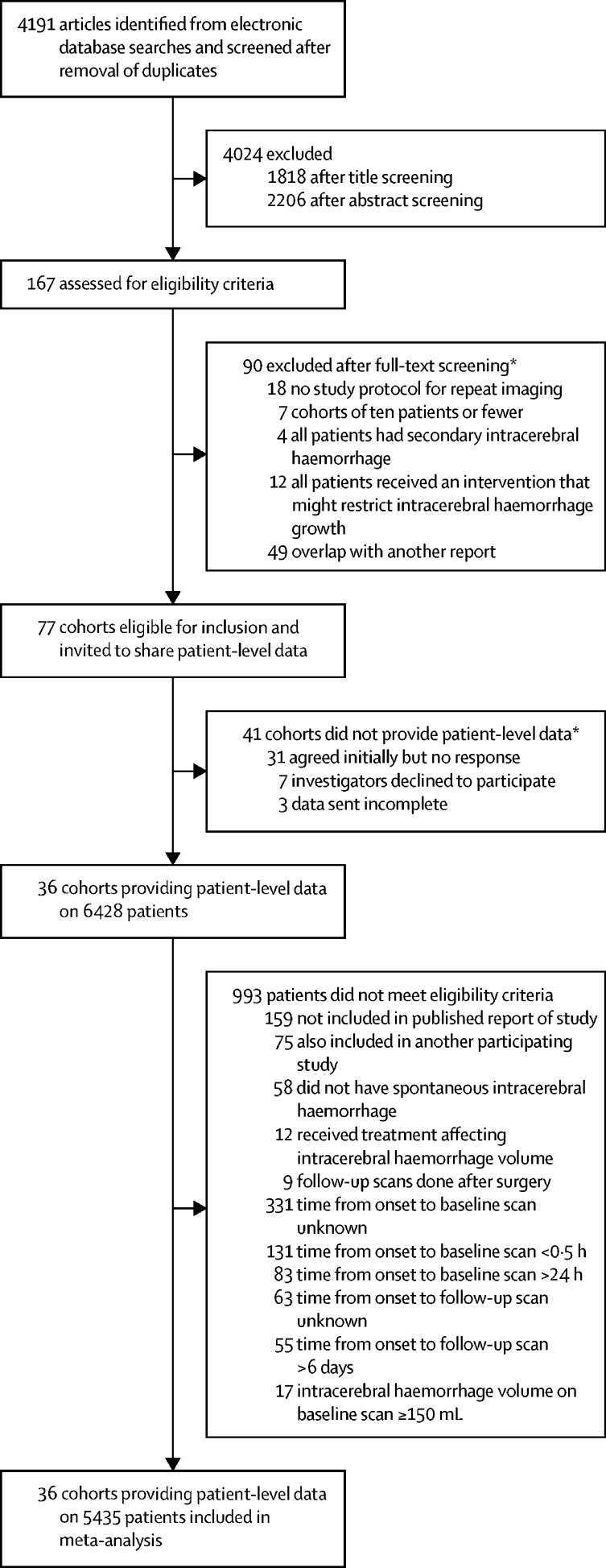
We screened 4191 studies identified by our searches, assessed 167 for eligibility, invited 77 eligible cohorts to share data, and obtained patient-level data from 36 (47%) cohorts18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 involving 6428 patients with repeat brain imaging after intracerebral haemorrhage between 1985 and 2015 (no data up to 1984 were obtained; figure 1; appendix).20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 Countries classified as high income by the World Bank contributed to 26 (72%) of 36 collaborating cohorts versus 30 (73%) of 41 eligible cohorts that did not collaborate. Planimetric methods of measuring intracerebral haemorrhage volume were used by 19 (53%) of 36 collaborating cohorts versus six (15%) of 41 eligible cohorts that did not collaborate.
Figure 1.
Study selection
*Excluded studies and cohorts are listed in the appendix.
After confirming the integrity of the data from eligible cohorts and excluding patients who were ineligible, we created a dataset of 5435 patients (appendix), from which we identified four groups of patients for further analysis: 5076 patients not taking anticoagulant therapy at intracerebral haemorrhage onset, 351 patients taking anticoagulant therapy at intracerebral haemorrhage onset, 3550 patients from cohorts that included at least some patients taking anticoagulant therapy at intracerebral haemorrhage onset, and 868 patients for whom both information about anticoagulant therapy at intracerebral haemorrhage onset and spot sign on acute CT angiography were known (table 1; appendix). The availability of potential predictors varied between the collaborating cohorts such that their overall completeness was 86% in the patients not taking anticoagulant therapy at intracerebral haemorrhage onset, 88% in the patients taking anticoagulant therapy at intracerebral haemorrhage onset, 89% in patients from cohorts that included at least some patients taking anticoagulant therapy at intracerebral haemorrhage onset, and 91% in the patients with information about anticoagulant therapy at intracerebral haemorrhage onset and spot sign on acute CT angiography. More than 80% of patients in all groups had repeat imaging done within 48 h of intracerebral haemorrhage onset and less than 2% of patients had repeat imaging done more than 4 days after intracerebral haemorrhage onset (appendix).
Table 1.
Characteristics of patients included in the four datasets for meta-analysis
| Not taking anticoagulant therapy (n=5076) | Taking anticoagulant therapy (n=351) | From cohorts with some patients taking anticoagulant therapy (n=3550) | CT angiography (n=868) | ||
|---|---|---|---|---|---|
| Sex | |||||
| Female | 1971/4884 (40%) | 135 (38%) | 1449 (41%) | 379 (44%) | |
| Male | 2913/4884 (60%) | 216 (62%) | 2101 (59%) | 489 (56%) | |
| Age, years | 67 (56–76) | 76 (69–82) | 69 (58–78) | 70 (57–79) | |
| Previous stroke | 607/4560 (13%) | 77/317 (24%) | 481/3308 (15%) | 97/829 (12%) | |
| Previous intracerebral haemorrhage* | 179/2753 (7%) | 9/246 (4%) | 113/2051 (6%) | 29/805 (4%) | |
| Previous ischaemic stroke* | 246/2755 (9%) | 53/246 (22%) | 213/2051 (10%) | 70/805 (9%) | |
| History of hypertension | 3787/5050 (75%) | 291 (83%) | 2739/3547 (77%) | 616/866 (71%) | |
| History of diabetes mellitus | 727/4197 (17%) | 82/343 (24%) | 626/3475 (18%) | 137/807 (17%) | |
| History of liver disease | 256/3360 (8%) | 14/220 (6%) | 96/1946 (5%) | 15/360 (4%) | |
| History of excessive alcohol consumption† | 568/3091 (18%) | 20/177 (11%) | 221/1455 (15%) | 73/554 (13%) | |
| Antiplatelet therapy at symptom onset | 913/5030 (18%) | 102 (29%) | 855/3543 (24%) | 225/837 (27%) | |
| Anticoagulant therapy at symptom onset | 0 | 351 (100%) | 349/3547 (10%) | 87/841 (10%) | |
| Systolic blood pressure at presentation, mm Hg | 177 (158–198); n=4882 | 170 (147–190); n=320 | 177 (157–197); n=3333 | 175 (150–200); n=860 | |
| Blood glucose at presentation, mmol/L | 7·0 (5·9–8·7); n=4265 | 7·4 (6·0–9·3); n=340 | 7·0 (5·9–8·7); n=3417 | 7·3 (6·1–8·9); n=864 | |
| Platelet count (×109/L) at presentation | 221 (181–266); n=3857 | 209 (174–260); n=289 | 222 (185–267); n=2284 | 227 (181–273); n=862 | |
| Glasgow Coma Scale score at presentation | |||||
| 3–6 | 285/4564 (6%) | 42/342 (12%) | 248/3502 (7%) | 73/831 (9%) | |
| 7–12 | 1033/4564 (23%) | 81/342 (24%) | 824/3502 (24%) | 193/831 (23%) | |
| 13–14 | 1157/4564 (25%) | 70/342 (20%) | 830/3502 (24%) | 151/831 (18%) | |
| 15 | 2089/4564 (46%) | 149/342 (44%) | 1600/3502 (46%) | 414/831 (50%) | |
| NIHSS score at presentation | 12 (7–18); n=2661 | 13 (7–17); n=126 | 12 (7–17); n=2014 | 14 (6–18); n=325 | |
| Time from symptom onset to baseline imaging, h | 2·4 (1·3–4·7) | 3·3 (1·7–6·4) | 2·2 (1·3–4·2) | 2·9 (1·5–5·1) | |
| Intracerebral haemorrhage volume on baseline imaging, mL | 13·2 (6·3–30·0) | 16·0 (6·4–39·0) | 13·4 (6·6–30·3) | 15·0 (6·6–34·1) | |
| Lobar location of intracerebral haemorrhage on baseline imaging | 1080/4920 (22%) | 129/344 (38%) | 907/3439 (26%) | 267/866 (31%) | |
| Intraventricular haemorrhage present on baseline imaging | 1834/4980 (37%) | 157/348 (45%) | 1265/3452 (37%) | 344 (40%) | |
| CT angiogram spot sign present | .. | .. | .. | 204 (24%) | |
| >6 mL intracerebral haemorrhage growth | 1009 (20%) | 110 (31%) | 771 (22%) | 177 (20%) | |
| >6 mL or >33% intracerebral haemorrhage growth | 1301 (26%) | 139 (40%) | 986 (28%) | 219 (25%) | |
Data are n (%), n/N (%), or median (IQR). NIHSS=National Institutes of Health Stroke Scale.
Available in a subgroup of cohorts that quantified the subtype of previous stroke. Not all cohorts that quantified the subtype included both intracerebral haemorrhage and ischaemic stroke.
Definition of excessive consumption varied across cohorts.
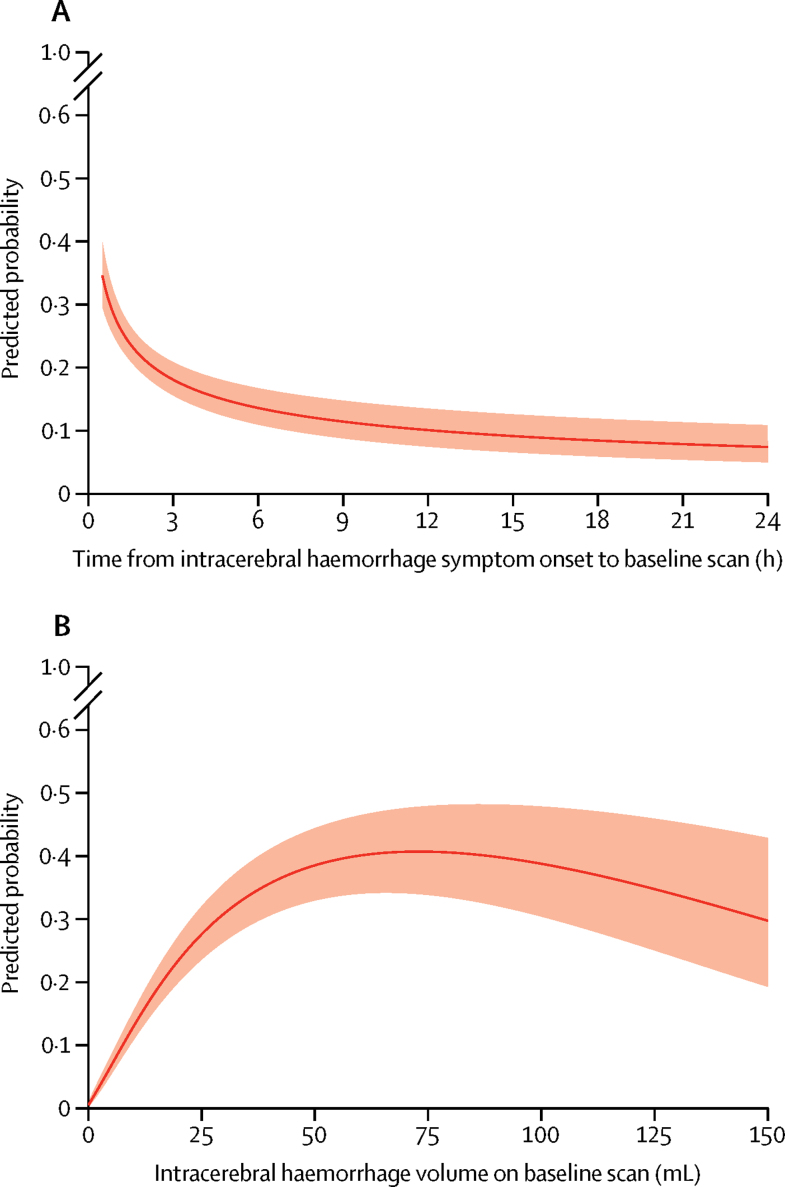
When assessing the two variables with non-linear associations, we found that in patients not taking anticoagulant therapy at intracerebral haemorrhage onset, the predicted probability of intracerebral haemorrhage growth declined with increasing time from intracerebral haemorrhage symptom onset to baseline imaging: the rate of decline was steepest 0·5–3 h after intracerebral haemorrhage symptom onset (figure 2A). The predicted probability of intracerebral haemorrhage growth increased with increasing intracerebral haemorrhage volume on baseline brain imaging and peaked at about 75 mL, above which it declined (figure 2B). We aimed to quantify the associations between 17 additional variables and the occurrence of intracerebral haemorrhage growth (appendix). There were too few patients with data for six variables (previous intracerebral haemorrhage, previous ischaemic stroke, history of liver disease, history of excessive alcohol consumption, platelet count at presentation, and National Institutes of Health Stroke Scale [NIHSS] score at presentation). Therefore, we selected 13 of the 19 variables as potential predictors for a multivariable model in patients not taking anticoagulant therapy, on the basis of maximising the number of predictors being considered while also maximising the number of patients with complete data for all the predictors chosen for the subset: time from symptom onset to baseline imaging, intracerebral haemorrhage volume on baseline imaging, sex, age, previous stroke, history of hypertension, history of diabetes, antiplatelet therapy at symptom onset, systolic blood pressure at presentation, blood glucose at presentation, Glasgow Coma Scale score at presentation, intracerebral haemorrhage location on baseline scan, and intraventricular haemorrhage on baseline scan. We restricted all further analyses to datasets of patients with complete data on these 13 potential predictors.
Figure 2.
Predicted probability of intracerebral haemorrhage growth >6 mL
Data calculated on 5076 patients who were not taking anticoagulant therapy at symptom onset. (A) Predicted probability by time from intracerebral haemorrhage symptom onset to baseline imaging, and (B) according to intracerebral haemorrhage volume on baseline imaging. The solid line indicates predicted probability and the shaded region indicates the 95% CIs.
3479 patients who were not taking anticoagulant therapy at intracerebral haemorrhage onset had data available for the 13 predictors. We developed a prediction model for intracerebral haemorrhage growth using a dataset of 2534 (73%) of these patients from 18 earlier cohorts (ie, 1994–2007; appendix). From the 13 potential predictors considered, three significant predictors constituted the final model (table 2):
where the predictive index (PI) is given by
Table 2.
Multivariable models of predictors of intracerebral haemorrhage growth >6 mL
| Comparison | Odds ratio (95% CI) | p value | |
|---|---|---|---|
| Patients not taking anticoagulant therapy at symptom onset* | |||
| Time from symptom onset to baseline imaging, h† | 3·4 vs 1·2 | 0·65 (0·51–0·82) | 0·0003 |
| Intracerebral haemorrhage volume on baseline imaging, mL† | 28 vs 7 | 4·73 (3·81–5·87) | <0·0001 |
| Antiplatelet therapy at symptom onset | Yes vs no | 1·38 (1·06–1·79) | 0·016 |
| Patients from cohorts including at least some patients taking anticoagulant therapy at symptom onset‡ | |||
| Time from symptom onset to baseline imaging, h† | 3·5 vs 1·2 | 0·59 (0·42–0·82) | 0·0021 |
| Intracerebral haemorrhage volume on baseline imaging, mL† | 29 vs 7 | 4·81 (3·82–6·05) | <0·0001 |
| Antiplatelet therapy at symptom onset | Yes vs no | 1·36 (1·04–1·78) | 0·023 |
| Anticoagulant therapy at symptom onset | Yes vs no | 2·91 (1·97–4·26) | <0·0001 |
Data were calculated on 2534 patients from 18 cohorts (appendix).
The odds ratios for time from symptom onset to baseline imaging and intracerebral haemorrhage volume on baseline imaging are for upper quartile compared with lower quartile.
Data were calculated on 2381 patients from ten cohorts (appendix).
with time measured in hours, volume measured in mL, and antiplatelet an indicator variable for antiplatelet therapy at intracerebral haemorrhage onset taking values 1 for yes and 0 for no.
This first prediction model had good calibration (appendix) and its discrimination was good in both the development dataset (C-index 0·75, 95% CI 0·72–0·77) and the temporal validation dataset of 945 (27%) patients from six later cohorts (0·76, 0·73–0·79). This prediction model, derived in patients who were not taking anticoagulant therapy at symptom onset, underestimated the probability of intracerebral haemorrhage growth in the 351 patients in 21 cohorts who were taking anticoagulant therapy at symptom onset (appendix), but its discrimination remained good (0·73, 0·68–0·79).
We also developed a prediction model for intracerebral haemorrhage growth using a dataset of 2381 patients from ten cohorts that included at least some patients taking anticoagulant therapy at intracerebral haemorrhage onset (appendix). From the 13 potential predictors plus anticoagulant therapy at intracerebral haemorrhage symptom onset, four predictors constituted the final model (table 2), where PI is given by
where anticoagulant is an indicator variable for anticoagulant therapy at intracerebral haemorrhage onset taking values 1 for yes and 0 for no.
This second prediction model was well calibrated (appendix) and its discrimination was good in both the development dataset (C-index 0·75, 95% CI 0·73–0·78) and the validation dataset of 895 patients from five cohorts (0·74, 0·71–0·78).
Finally, to assess the additional predictive value of spot sign on CT angiography, we assessed the performance of a third prediction model in the 837 patients from six cohorts with available data on all covariates (appendix), where PI is given by
where spot is an indicator variable for presence of CT angiography spot sign taking values 1 for present and 0 for absent.
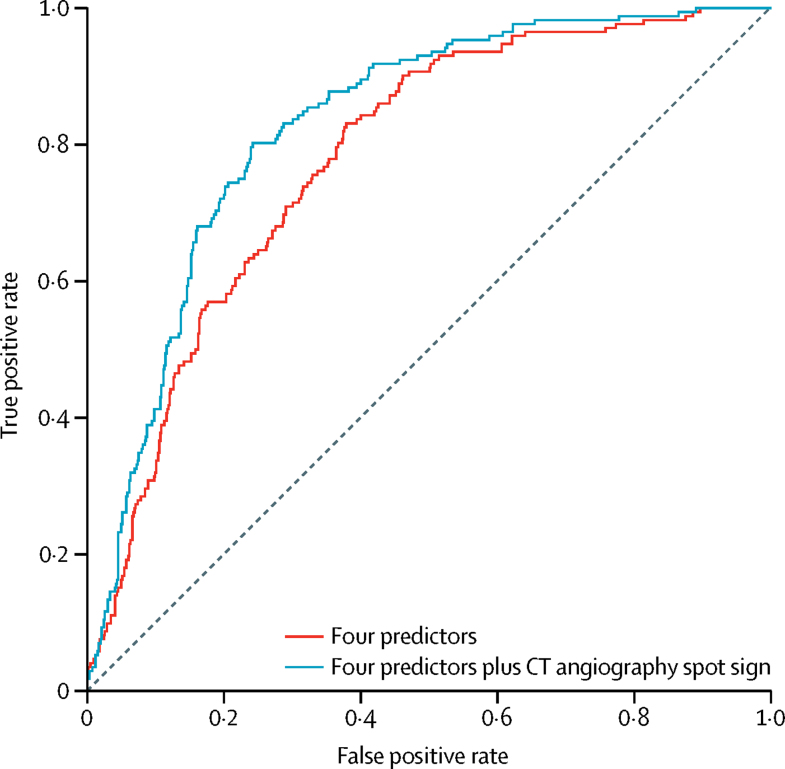
The presence of the spot sign was strongly and independently associated with the occurrence of intracerebral haemorrhage growth (table 3) and improved the C-index of the prediction model by 0·05 (95% CI 0·03–0·07) from 0·78 (0·75–0·82) to 0·83 (0·80–0·86; figure 3).
Table 3.
Multivariable models of predictors of intracerebral haemorrhage growth >6 mL in patients with assessment of CT angiography spot sign, data on antiplatelet therapy, and data on anticoagulant therapy use at symptom onset
| Comparison |
Four predictors |
Four predictors with the addition of CT angiography spot sign |
|||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | ||
| Time from symptom onset to baseline imaging, h* | 5·1 vs 1·5 | 0·50 (0·36–0·70) | <0·0001 | 0·61 (0·44–0·84) | 0·0030 |
| Intracranial haemorrhage volume on baseline imaging, mL* | 33 vs 6 | 7·18 (4·46–11·56) | <0·0001 | 5·35 (3·25–8·81) | <0·0001 |
| Antiplatelet therapy at symptom onset | Yes vs no | 1·68 (1·06–2·66) | 0·026 | 1·45 (0·89–2·35) | 0·13 |
| Anticoagulant therapy at symptom onset | Yes vs no | 3·48 (1·96–6·16) | <0·0001 | 2·80 (1·53–5·10) | 0·0008 |
| CT angiography spot sign | Present vs absent | .. | .. | 4·46 (2·95–6·75) | <0·0001 |
Data were calculated on 837 patients from six cohorts (appendix).
Odds ratios for time from symptom onset to baseline imaging and intracranial haemorrhage volume on baseline imaging are for upper quartile vs lower quartile.
Figure 3.
Receiver operating characteristic curves for the predicted probability of intracerebral haemorrhage growth >6 mL
Data calculated on 837 patients with assessment of CT angiography spot sign, data on antiplatelet therapy, and data on anticoagulant therapy use at symptom onset. Receiver operating characteristic curves used four predictors (time from symptom onset to baseline imaging [h], intracerebral haemorrhage volume on baseline imaging [mL], antiplatelet therapy at symptom onset, and anticoagulant therapy at symptom onset) and four predictors plus CT angiography spot sign.
We assessed the performance of the second and third prediction models at different thresholds of predicted probability of intracerebral haemorrhage growth and found very few significant differences in sensitivity, specificity, positive predictive value, and negative predictive value (appendix).
In a prespecified sensitivity analysis, when we defined intracerebral haemorrhage growth as an absolute increase of more than 6 mL or a relative increase of more than 33% in intracerebral haemorrhage volume between baseline and follow-up imaging, the direction, strength, and significance of the adjusted associations between almost all predictors and intracerebral haemorrhage growth remained the same (appendix), and the C-index of our second prediction model improved from 0·71 (95% CI 0·67–0·75) to 0·76 (0·72–0·80) with the addition of information from CT angiography (appendix). In a post-hoc sensitivity analysis, we found no evidence that the risk of intracerebral haemorrhage growth according to time from symptom onset to baseline imaging or according to intracerebral haemorrhage volume on baseline imaging differed by cohort epoch or volumetric method used (appendix).
Discussion
This collaborative meta-analysis evaluated 19 covariates in one or more analyses of predictors of intracerebral haemorrhage growth from 5435 eligible patients in 36 cohorts. We identified novel non-linear associations between the probability of intracerebral haemorrhage growth and both the time from symptom onset to baseline imaging and baseline intracerebral haemorrhage volume. We showed that only four predictors that are simple to collect (time from symptom onset to baseline imaging, intracerebral haemorrhage volume on baseline imaging, antiplatelet use, and anticoagulant use) were independently associated with intracerebral haemorrhage growth in multivariable models, and a prediction model that we developed using these predictors not only had good calibration and discrimination but also done well in an external validation dataset. The addition of information about the presence of spot sign on CT angiography to this prediction model gave a small increase in discrimination.
Although many studies have investigated unadjusted and adjusted associations between a wide variety of clinical, blood, genetic, imaging, and pharmacological factors and the occurrence of intracerebral haemorrhage growth, only a few prediction models have been developed and the predictors used have varied considerably.7, 8, 9, 10, 11, 51 Since 2011, there has been growing interest in use of the spot sign on CT angiography for predicting intracerebral haemorrhage growth,10, 30 but the clinical utility of the small increase in discrimination that resource-intensive advanced vascular imaging adds to simple clinical and imaging predictors that are available worldwide is unclear.
The strengths of this study include its large sample size and availability of many predictors from geographically diverse cohorts to develop and externally validate prediction models involving simple predictors that could be used in any health-care setting, as well as the added value of CT angiography in high-income countries. We minimised the risk of selection and information biases by restricting eligibility to cohorts that had defined when they would repeat brain imaging soon after intracerebral haemorrhage onset in all survivors and not according to clinical need alone.
Although our study was large, only half of the investigators of the available cohorts shared patient-level data. Most cohorts were assembled in high-income countries. A shortage of data on the following variables precluded their inclusion in our prediction models: previous intracerebral haemorrhage, previous ischaemic stroke, history of liver disease, history of excessive alcohol consumption, platelet count at presentation, and NIHSS score at presentation. Since the end of the literature search that defined inclusion in our analyses, our update of the search to March 1, 2018, identified reports of five new cohorts involving 669 patients, representing a maximum of a 10% increase over the 6428 patients from 36 cohorts that provided individual patient data. Nonetheless, the sample size we achieved allowed us to develop and validate prediction models using a large number of widely available predictors, without omitting any predictors that had been identified by previous prediction models. Included cohorts with data collected in the 1990s might not have used multiple-row detector array technology and digitisation, which might have affected their accuracy of intracerebral haemorrhage volume measurement, although there was no evidence that our findings differed by cohort epoch in sensitivity analyses. 19 (53%) of 36 cohorts used planimetric methods to estimate intracerebral haemorrhage volume but 17 (47%) of 36 cohorts used the ABC/2 method (which can marginally overestimate intracerebral haemorrhage volume18), although we found no evidence that our findings differed by volumetric method in sensitivity analyses. Since these cohorts were studied, a variety of new imaging signs (eg, density, irregularity, fluid level, hypodensity, island, satellite, swirl,56 blend,37 and black hole57) have been described, but we were unable to evaluate them because they were not collected by the collaborating cohorts and we could not re-evaluate patients' imaging. However, our simple prediction models provide the basis upon which the added value of these new signs can be assessed, as we have done for the CT angiography spot sign.
We found that the rate of decline in the probability of intracerebral haemorrhage growth was steepest during the 0·5–3 h after intracerebral haemorrhage symptom onset and that the predicted probability of intracerebral haemorrhage growth peaked at an intracerebral haemorrhage volume of about 75 mL. These findings could in part explain the neutral results of recent randomised trials of acute interventions designed to limit intracerebral haemorrhage growth, which enrolled many patients towards or beyond the time of greatest risk of intracerebral haemorrhage growth and most patients had small intracerebral haemorrhages at low probability of growth. For example, the average time to randomisation after intracerebral haemorrhage symptom onset and average intracerebral haemorrhage volume were 3·7 h and 13 mL in TICH2,58 3·7 h and 11 mL in INTERACT2,22 3·1 h and 10 mL in ATACH2,59 and 2·7 h and 22–24 mL in FAST.40 In particular, our findings about the association between time after intracerebral haemorrhage symptom onset and the probability of intracerebral haemorrhage growth emphasise the importance of extremely rapid assessment, investigation, and randomisation in future trials of therapies to improve outcome by limiting intracerebral haemorrhage growth.
The prediction models that we have developed could be useful in clinical practice for predicting the risk of intracerebral haemorrhage growth, which is recommended in the emergency assessment of acute intracerebral haemorrhage. The clinically useful threshold for the predicted probability of intracerebral haemorrhage growth will vary according to its desired accuracy (appendix), the clinical setting, and future therapeutic advances, such that our models might help in determining patients' place of care and frequency of observation.60
Acknowledgments
Acknowledgments
This study was funded by the UK Medical Research Council (senior clinical fellowship G1002605 and the Edinburgh Hub for Trials Methodology Research G0800803) and the British Heart Foundation (travel fellowship FS/13/72/30531).
Contributors
RA-SS conceived and designed the project. JF designed the literature search strategies and searched the literature. RA-SS, JF, and RJL co-wrote the protocol and arbitrated cohort eligibility. JF described included studies and communicated with coauthors. RJL processed data and did the data analyses, with oversight from RA-SS. RA-SS, JF, and RJL wrote the first draft of the manuscript. PDL, TWKB, AMA, JNG, SAM, TS, XW, HA, HH, MO, DAG, LM, DD, DR-L, CAM, D-KJ, AD, JCa, XY, JCl, BV, SK, YO, SF, KT, QL, JK, PD, JÁS, MH-G, LP-S, CC, MPK, RM, CV, MND, YI, HW, WCZ, CDd'E, RIA, PR, YM, ARZ, KSB, SMS, JCG, JM-F, JM, JB, HY, DS, ESC, MS, RL, BHM, AMD, MDN, YF, CSA, and JR acquired data, revised the work critically for important intellectual content where required, approved the final version to be published, and agreed to be accountable for all aspects of the work.
Declaration of interests
RJL reports grants from the UK Medical Research Council, during the conduct of the study. JNG reports personal fees from CSL Behring and Octapharma; and grants from Pfizer, Boehringer Ingelheim, and Portola, outside of the submitted work. HA reports personal fees from Asuka, Bayer, Daiichi-Sankyo, and Takeda, outside of the submitted work. JCl reports grants from the DANA Foundation and personal fees from SAGE Therapeutics, outside of the submitted work. BV reports personal fees from Pfizer/Bristol-Myers Squibb and Bayer, outside of the submitted work. JK reports grants from the National Institutes of Health/National Institute of Neurological Disorders and Stroke, during the conduct of the study. CV reports grants from the Neurocritical Care Society, during the conduct of the study. MS reports grants from National Institutes of Health/National Institute of Neurological Disorders and Stroke and the American Heart Association, outside of the submitted work. CSA reports grants from the National Health and Medical Research Council of Australia, during the conduct of the study; and personal fees from Takeda and Amgen, outside of the submitted work. JR reports grants from the National Institutes of Health and personal fees from Boehringer Ingelheim and Pfizer, outside of the submitted work. All remaining authors declare no competing interests.
Contributor Information
Rustam Al-Shahi Salman, Email: rustam.al-shahi@ed.ac.uk.
VISTA-ICH Collaboration:
Daniel F Hanley, Jr, Kenneth S Butcher, Stephen Davis, Barbara Gregson, Kennedy R Lees, Patrick D Lyden, Stephan A Mayer, Keith W Muir, and Thorsten Steiner
ICH Growth Individual Patient Data Meta-analysis Collaborators:
Peng Xie, Babak Bakhshayesh, Mark McDonald, Thomas Brott, Paolo Pennati, Adrian R Parry-Jones, Craig J Smith, Stephen J Hopkins, Mark Slevin, Veronica Campi, Puneetpal Singh, Francesca Papa, Aurel Popa-Wagner, Valeria Tudorica, Ryo Takagi, Akira Teramoto, Karin Weissenborn, and Heinrich Lanfermann
Supplementary Material
References
- 1.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Krishnamurthi RV, Parmar P. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013: the GBD 2013 Study. Neuroepidemiology. 2015;45:161–176. doi: 10.1159/000441085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 4.Davis SM, Broderick J, Hennerici M. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 5.Al-Shahi Salman R, Law ZK, Bath PMW, Steiner T, Sprigg N. Haemostatic therapies for acute spontaneous intracerebral haemorrhage. Cochrane Database Syst Rev. 2018;4 doi: 10.1002/14651858.CD005951.pub4. CD005951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du FZ, Jiang R, Gu M, He C, Guan J. The accuracy of spot sign in predicting hematoma expansion after intracerebral hemorrhage: a systematic review and meta-analysis. PLoS One. 2014;9:e115777. doi: 10.1371/journal.pone.0115777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda R, Ogura T, Ooigawa H. A practical prediction model for early hematoma expansion in spontaneous deep ganglionic intracerebral hemorrhage. Clin Neurol Neurosurg. 2013;115:1028–1031. doi: 10.1016/j.clineuro.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Brouwers HB, Chang Y, Falcone GJ. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71:158–164. doi: 10.1001/jamaneurol.2013.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan S, Conell C, Veerina KT, Rao VA, Flint AC. Prediction of intracerebral haemorrhage expansion with clinical, laboratory, pharmacologic, and noncontrast radiographic variables. Int J Stroke. 2015;10:1057–1061. doi: 10.1111/ijs.12507. [DOI] [PubMed] [Google Scholar]
- 10.Huynh TJ, Aviv RI, Dowlatshahi D. Validation of the 9-Point and 24-Point Hematoma Expansion Prediction Scores and Derivation of the PREDICT A/B Scores. Stroke. 2015;46:3105–3110. doi: 10.1161/STROKEAHA.115.009893. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Arima H, Al-Shahi Salman R. Clinical prediction algorithm (BRAIN) to determine risk of hematoma growth in acute intracerebral hemorrhage. Stroke. 2015;46:376–381. doi: 10.1161/STROKEAHA.114.006910. [DOI] [PubMed] [Google Scholar]
- 12.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 13.Brouwers HB, Greenberg SM. Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc Dis. 2013;35:195–201. doi: 10.1159/000346599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 15.Hanley DF, Thompson RE, Muschelli J. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. 2016;15:1228–1237. doi: 10.1016/S1474-4422(16)30234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. How should we lower blood pressure after cerebral hemorrhage? A systematic review and meta-analysis. Cerebrovasc Dis. 2017;43:207–213. doi: 10.1159/000462986. [DOI] [PubMed] [Google Scholar]
- 17.Kothari RU, Brott T, Broderick JP. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan K, Mukhtar SF, Lingard J. Performance characteristics of methods for quantifying spontaneous intracerebral haemorrhage: data from the Efficacy of Nitric Oxide in Stroke (ENOS) trial. J Neurol Neurosurg Psychiatry. 2015;86:1258–1266. doi: 10.1136/jnnp-2014-309845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowlatshahi D, Demchuk AM, Flaherty ML. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–1244. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abilleira S, Montaner J, Molina CA, Monasterio J, Castillo J, Alvarez-Sabin J. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J Neurosurg. 2003;99:65–70. doi: 10.3171/jns.2003.99.1.0065. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Sabin J, Delgado P, Abilleira S. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke. 2004;35:1316–1322. doi: 10.1161/01.STR.0000126827.69286.90. [DOI] [PubMed] [Google Scholar]
- 22.Anderson CS, Heeley E, Huang Y. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 23.Anderson CS, Huang Y, Wang JG. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7:391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 24.Bakhshayesh B, Hosseininezhad M, Saadat SN, Ansar MM, Ramezani H, Saadat SM. Iron overload is associated with perihematoma edema growth following intracerebral hemorrhage that may contribute to in-hospital mortality and long-term functional outcome. Curr Neurovasc Res. 2014;11:248–253. doi: 10.2174/1567202611666140530124855. [DOI] [PubMed] [Google Scholar]
- 25.Biffi A, Battey TW, Ayres AM. Warfarin-related intraventricular hemorrhage: imaging and outcome. Neurology. 2011;77:1840–1846. doi: 10.1212/WNL.0b013e3182377e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brott T, Broderick J, Kothari R. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Brouwers HB, Battey TW, Musial HH. Rate of contrast extravasation on computed tomographic angiography predicts hematoma expansion and mortality in primary intracerebral hemorrhage. Stroke. 2015;46:2498–2503. doi: 10.1161/STROKEAHA.115.009659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butcher KS, Jeerakathil T, Hill M. The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke. 2013;44:620–626. doi: 10.1161/STROKEAHA.111.000188. [DOI] [PubMed] [Google Scholar]
- 29.Delgado P, Alvarez-Sabin J, Abilleira S. Plasma d-dimer predicts poor outcome after acute intracerebral hemorrhage. Neurology. 2006;67:94–98. doi: 10.1212/01.wnl.0000223349.97278.e0. [DOI] [PubMed] [Google Scholar]
- 30.d'Esterre CD, Chia TL, Jairath A, Lee TY, Symons SP, Aviv RI. Early rate of contrast extravasation in patients with intracerebral hemorrhage. AJNR Am J Neuroradiol. 2011;32:1879–1884. doi: 10.3174/ajnr.A2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Napoli M, Parry-Jones AR, Smith CJ. C-reactive protein predicts hematoma growth in intracerebral hemorrhage. Stroke. 2014;45:59–65. doi: 10.1161/STROKEAHA.113.001721. [DOI] [PubMed] [Google Scholar]
- 32.Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998;29:1160–1166. doi: 10.1161/01.str.29.6.1160. [DOI] [PubMed] [Google Scholar]
- 33.Kawano-Castillo J, Ward E, Elliott A. Thrombelastography detects possible coagulation disturbance in patients with intracerebral hemorrhage with hematoma enlargement. Stroke. 2014;45:683–688. doi: 10.1161/STROKEAHA.113.003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28:2370–2375. doi: 10.1161/01.str.28.12.2370. [DOI] [PubMed] [Google Scholar]
- 35.Leira R, Davalos A, Silva Y. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461–467. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 36.Li N, Worthmann H, Heeren M. Temporal pattern of cytotoxic edema in the perihematomal region after intracerebral hemorrhage: a serial magnetic resonance imaging study. Stroke. 2013;44:1144–1146. doi: 10.1161/STROKEAHA.111.000056. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Zhang G, Huang YJ. Blend sign on computed tomography: novel and reliable predictor for early hematoma growth in patients with intracerebral hemorrhage. Stroke. 2015;46:2119–2123. doi: 10.1161/STROKEAHA.115.009185. [DOI] [PubMed] [Google Scholar]
- 38.Lyden PD, Shuaib A, Lees KR. Safety and tolerability of NXY-059 for acute intracerebral hemorrhage: the CHANT Trial. Stroke. 2007;38:2262–2269. doi: 10.1161/STROKEAHA.106.472746. [DOI] [PubMed] [Google Scholar]
- 39.Marti-Fabregas J, Borrell M, Silva Y. Hemostatic proteins and their association with hematoma growth in patients with acute intracerebral hemorrhage. Stroke. 2010;41:2976–2978. doi: 10.1161/STROKEAHA.110.595868. [DOI] [PubMed] [Google Scholar]
- 40.Mayer SA, Brun NC, Begtrup K. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 41.Mayer SA, Brun NC, Begtrup K. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 42.Mayer SA, Brun NC, Broderick J. Safety and feasibility of recombinant factor VIIa for acute intracerebral hemorrhage. Stroke. 2005;36:74–79. doi: 10.1161/01.STR.0000149628.80251.b8. [DOI] [PubMed] [Google Scholar]
- 43.Mayer SA, Brun NC, Broderick J. Recombinant activated factor VII for acute intracerebral hemorrhage: US phase IIA trial. Neurocrit Care. 2006;4:206–214. doi: 10.1385/NCC:4:3:206. [DOI] [PubMed] [Google Scholar]
- 44.Moon BH, Jang DK, Han YM, Jang KS, Huh R, Park YS. Association factors for CT angiography spot sign and hematoma growth in Korean patients with acute spontaneous intracerebral hemorrhage: a single-center cohort study. J Korean Neurosurg Soc. 2014;56:295–302. doi: 10.3340/jkns.2014.56.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murai Y, Takagi R, Ikeda Y, Yamamoto Y, Teramoto A. Three-dimensional computerized tomography angiography in patients with hyperacute intracerebral hemorrhage. J Neurosurg. 1999;91:424–431. doi: 10.3171/jns.1999.91.3.0424. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Luna D, Rubiera M, Ribo M. Serum low-density lipoprotein cholesterol level predicts hematoma growth and clinical outcome after acute intracerebral hemorrhage. Stroke. 2011;42:2447–2452. doi: 10.1161/STROKEAHA.110.609461. [DOI] [PubMed] [Google Scholar]
- 47.Toyoda K, Okada Y, Minematsu K. Antiplatelet therapy contributes to acute deterioration of intracerebral hemorrhage. Neurology. 2005;65:1000–1004. doi: 10.1212/01.wnl.0000179178.37713.69. [DOI] [PubMed] [Google Scholar]
- 48.Venkatasubramanian C, Mlynash M, Finley-Caulfield A. Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke. 2011;42:73–80. doi: 10.1161/STROKEAHA.110.590646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volbers B, Willfarth W, Kuramatsu JB. Impact of perihemorrhagic edema on short-term outcome after intracerebral hemorrhage. Neurocrit Care. 2016;24:404–412. doi: 10.1007/s12028-015-0185-y. [DOI] [PubMed] [Google Scholar]
- 50.Witsch J, Bruce E, Meyers E. Intraventricular hemorrhage expansion in patients with spontaneous intracerebral hemorrhage. Neurology. 2015;84:989–994. doi: 10.1212/WNL.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao X, Xu Y, Siwila-Sackman E, Wu B, Selim M. The HEP Score: a nomogram-derived hematoma expansion prediction scale. Neurocrit Care. 2015;23:179–187. doi: 10.1007/s12028-015-0147-4. [DOI] [PubMed] [Google Scholar]
- 52.Zazulia AR, Videen TO, Diringer MN, Powers WJ. Poor correlation between perihematomal MRI hyperintensity and brain swelling after intracerebral hemorrhage. Neurocrit Care. 2011;15:436–441. doi: 10.1007/s12028-011-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zazulia AR, Videen TO, Powers WJ. Transient focal increase in perihematomal glucose metabolism after acute human intracerebral hemorrhage. Stroke. 2009;40:1638–1643. doi: 10.1161/STROKEAHA.108.536037. [DOI] [PubMed] [Google Scholar]
- 54.Ziai WC, Torbey MT, Kickler TS, Oh S, Bhardwaj A, Wityk RJ. Platelet count and function in spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2003;12:201–206. doi: 10.1016/S1052-3057(03)00075-2. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez-Luna D, Dowlatshahi D, Aviv RI. Venous phase of computed tomography angiography increases spot sign detection, but intracerebral hemorrhage expansion is greater in spot signs detected in arterial phase. Stroke. 2014;45:734–739. doi: 10.1161/STROKEAHA.113.003007. [DOI] [PubMed] [Google Scholar]
- 56.Selariu E, Zia E, Brizzi M, Abul-Kasim K. Swirl sign in intracerebral haemorrhage: definition, prevalence, reliability and prognostic value. BMC Neurol. 2012;12:109. doi: 10.1186/1471-2377-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q, Zhang G, Xiong X. Black hole sign: novel imaging marker that predicts hematoma growth in patients with intracerebral hemorrhage. Stroke. 2016;47:1777–1781. doi: 10.1161/STROKEAHA.116.013186. [DOI] [PubMed] [Google Scholar]
- 58.Sprigg N, Flaherty K, Appleton JP. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391:2107–2115. doi: 10.1016/S0140-6736(18)31033-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qureshi AI, Palesch YY, Barsan WG. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375:1033–1043. doi: 10.1056/NEJMoa1603460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hemphill JC, 3rd, Greenberg SM, Anderson CS. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.