Abstract
Photodynamic therapy uses photosensitizers (PS) to kill cancer cells by generating reactive oxygen species – like singlet oxygen (SO) - upon illumination with visible light. PS membrane anchoring augments local SO concentration, which in turn increases photodynamic efficiency. The latter may suffer from SO’s escape into the aqueous solution or premature quenching. Here we determined the time constants of SO escape and quenching by target molecules to be in the nanosecond range, the former being threefold longer. We confined PS and dipolar target molecules either to different membrane monolayers or to the same leaflet and assessed their abundance by fluorescence correlation spectroscopy or membrane surface potential measurements. The rate at which the contribution of the dipolar target molecules to membrane dipole potential vanished, served as a measure of the photo-oxidation rate. The solution of the reaction–diffusion equations did not indicate diffusional rate limitations. Nevertheless, reducing the PS-target distance increased photodynamic efficiency by preventing other SO susceptible moieties from protecting the target. Importantly, our analytical model revealed a fourfold difference between SO generation rates per molecule of the two used PSs. Such analysis of PS quantum yield in a membrane environment may help in designing better PSs.
Introduction
Photosensitizers (PS) play a key role in cancer photodynamic therapy1–3. They adhere to cancer cells and kill them when excited by light due to the generation of reactive oxygen species (ROS)4. PS that respond to visible light may be tuned to mainly produce singlet oxygen (SO), which, in turn, preferentially targets membrane proteins. The efficacy of this approach crucially depends on (i) PS’ membrane affinity5, (ii) the quantum yield of SO generation, (iii) SO lifetime, τl, and (iv) SO dwell time τdw in the membrane.
SO residence time τr may be determined by either τl or τdw. Taking into account 1O2 decay6, SO may travel δ = √Dτl ≈ 120 nm in first case assuming a diffusion constant of D = 5 × 10−5 cm2 s−1 7. τl ≈ 3 μs has been reported for a liposomal environment8. Thus, once born within the membrane, the likelihood of SO hitting the desired target would appear to be rather high, even if the cell membrane is sparsely decorated by PS molecules. In the second case τr is limited by SO’s escape into the aqueous environment. The oxygen water/membrane distribution coefficient is equal to Kp ≈ 4.49. Assuming the same Kp for SO suggests that SO may hit the membrane-water interface no more than 4.4 times before escaping into the cytoplasm or the extracellular solution. If we take membrane thickness d = 4 nm as the characteristic diffusion span between the hits, we find τdw = d2/D = 3 ns. This estimate is close to τdw = 12 ns as predicted by molecular dynamics simulations10. Accordingly, τdw, would be much smaller than τl, indicating that the actual distance between PS and target molecules determines photodynamic efficiency. If so, an increase in diffusion span due to PS burial into the hydrophobic membrane interior should augment τdw (and thus τr), which in turn, could increase photodynamic efficiency. A correlation between PS penetration depth and photo effects has indeed been observed11, however, the molecular mechanism has not yet been identified.
To distinguish between the possible scenarios: τr ≈ τl, or τr ≈ τdw, we adsorbed PS and dipolar target (DT) molecules at different densities either to the same or to opposing leaflets of a lipid membrane and analyzed effective encounters between SO and DT in terms of the rate at which DT’s contribution to membrane dipole potential vanished. We observed τl to be in the nanosecond range indicating that SO rarely escapes from the membrane and that augmenting photodynamic efficiency requires shortening of the DT to PS distances.
Materials and Methods
Black lipid membranes (BLMs) were formed by the Mueller Rudin technique12 from a solution containing 15 mg/ml L-α- diphytanoylphosphocholine (Avanti Polar Lipids, USA) per ml of n-decane in an aperture (diameter 0.8–1.2 mm) of a Teflon diaphragm that separated two aqueous compartments of equal volumes. Both compartments were continuously stirred by magnetic stirrers. Buffer solutions were prepared in twice distilled water with 100 mM KCl (chemically pure, Reachim, Russia) and 10 mM HEPES (Calbiochem, USA) at pH 7.5, adjusted by KOH (chemically pure, Reachim, Russia). The styryl dye 4-(2-(6-(Dibutylamino)-2-naphthalenyl)ethenyl)-1-(3-sulfopropyl)pyridinium hydroxide (di-4-ANEPPS; Sigma, USA) and aluminum phthalocyanines, AlPcSn, with various numbers n (1 ≤ n ≤ 4) of peripheral sulfo-groups from (Porphyrin Products, USA) served as DT and PS, respectively.
The electrical measurements were performed with the aid of silver-chloride electrodes that were connected to the aqueous compartments via agar bridges. Total electrode resistance did not exceed 30 kOhm. Membrane capacitance and conductance were continuously measured as previously described13. The difference of BLM boundary potentials, Δφb, was monitored by using the intramembrane field compensation (IFC) method13,14 (see also reviews15,16). IFC uses a variable dc offset to a sine wave input (300–700 Hz) to minimize membrane capacitance. We measured Δφb and determined the photo–oxidation rate of DT as previously described17. In brief, we placed the planar lipid bilayer into the focused beam of a monochromatic light source (semiconductor diode laser with a wavelength of 670 nm, optical power 1 mW). Subsequently, we added the PS into the distant aqueous compartment (also called cis compartment) with respect to the light source. DT was added either to the cis compartment or to the opposite (trans) compartment. Since both substances are membrane impermeable, their location remained well defined throughout the experiment”.
The membrane surface densities, T and P, as well as the lateral diffusion coefficients, DDT and DPS of both DT and PS were assessed by placing the horizontal BLM formed by Montal-Mueller technique18 into the focus of a laser scanning microscope (LSM 510 META/ConfoCor 3, Carl Zeiss, Jena, Germany) and exploiting fluorescence correlation spectroscopy, FCS19,20:
| 1 |
where r and τd are the lateral focus radius of the confocal volume and the characteristic residence time of DT or PS in the confocal volume, respectively. τd was obtained by fitting a one-component model for 2D translational diffusion21 to the autocorrelation function G of the time, τ, dependent fluorescence intensity:
| 2 |
where N is the average number of fluorescent molecules in the confocal volume.
Results
PS adsorption to the lipid bilayer
PS membrane adsorption alters bilayer boundary potential φb. The introduced change Δφb depends upon PS’ aqueous concentration, Pa5,13 (Fig. 1A). Generally, Δφb is a superposition of changes in membrane surface potential Δφs and membrane dipole potential Δφd15,16:
| 3 |
φs is accessible via measurements of the electrophoretic mobility of lipid vesicles. The mobility reflects the so-called ζ – potential that, in 0.1 M KCl, describes the electrostatic potential at 0.2 nm distance from the vesicle surface. Using the Gouy Chapman theory, φs can be calculated from ζ. Upon AlPcS3 and AlPcS4 membrane adsorption, we measured Δφb values that can entirely be attributed to changes in ζ5, i.e. Δφb and Δφs are roughly identical.
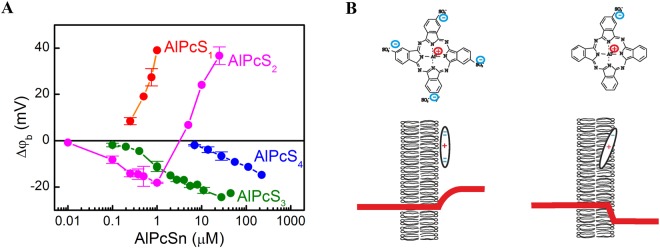
Figure 1.
Adsorption of phthalocyanines on the bilayer lipid membrane. (A) Dependence of the boundary potential change Δφb upon concentration of aluminum phthalocyanines with various numbers of sulfo groups in the solution. (B) The various positions of the phthalocyanines in the membrane with four and one sulfo groups resulting in the generation of the boundary potential of opposite signs on the surface of the lipid membrane. Red lines – the profiles of the potential change across the membrane due to adsorption of these phthalocyanines on the right side of the membrane.
In contrast, for AlPcS1 and AlPcS2 negative ζ – values5 (and thus negative Δφs values) have been observed for all aqueous PS concentrations Pa, while Δφb was positive for some AlPcS2 concentrations and all AlPcS1 concentrations. The observation suggests that both AlPcS2 and AlPcS1 significantly alter Δφd. The underlying dipole moment of the phthalocyanine molecule is generated by the separation of the positive charge at the aluminum cation in the center and the negative charges of the sulfo-groups on its periphery. Thus, in contrast to AlPcS4, the charges in AlPcS1 are distributed asymmetrically (Fig. 1B). The positive sign of Δφd indicates that AlPcS1 inserts the positive Al-moiety into the lipid bilayer, whereas the negatively charged S-group faces the aqueous solution (Fig. 1B). AlPcS2 is likely to adopt a similar orientation (Fig. 1B). Our result is in line with AlPcSn accessibility by fluorides5 and molecular dynamic simulations with porphyrin22.
Determination of membrane surface PS and DT densities
We performed FCS experiments to find T and P (Fig. 2). DT elicits Δφb changes that are proportional to its aqueous concentrations17. That is, DT’s limited aqueous solubility prevents the linear relation between Ta and T from breaking down as might be expected in case of a Langmuir adsorption isotherm. Since Δφb and the aqueous DT concentration Ta are the proportional to each other17, there must be a linear relationship between Ta, and T. It allowed calculating that T amounts to 30 molecules per μm2 and per nM of Ta. Thus, for Ta = 60 nM we find T = 1800 molecules μm−2 (Fig. 2A). Accordingly, DT’s contribution to Δφb amounts to 0.003 molecules/nm2/mV.
Figure 2.
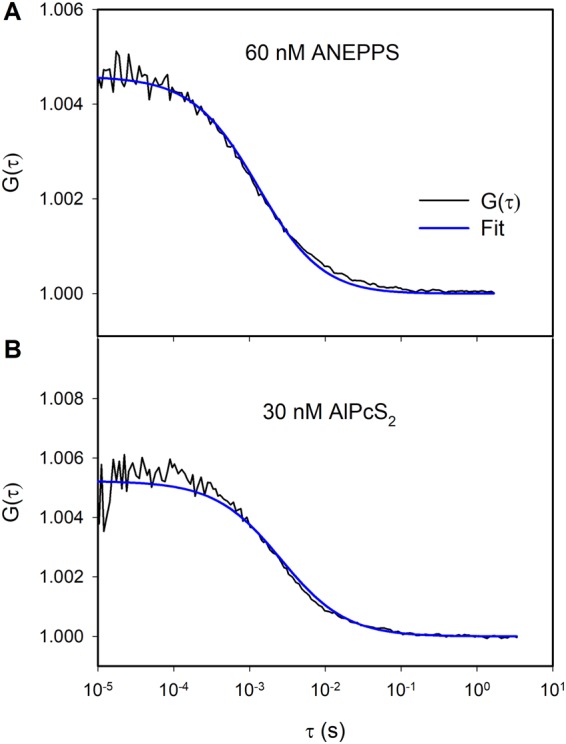
(A) Autocorrelation function of fluorescence of di-4-ANEPPS (concentration in solution is 60 nM) and its approximation by the equation (2). (B) Autocorrelation function of fluorescence of AlPcS2 (concentration in solution is 30 nM) and its approximation by the equation (2). BLM formed by the Montal-Mueller technique by adding the solution of diphytanoylphosphatidylcholine in hexane (15 mg/ml) to the water-air interface.
From τDT = 1.31 ms, we found DDT = 7.6 µm2/s (Eq. 2). We used FITC (fluorescein isothiocyanate) for calibration experiments. Its diffusion coefficient DFITC is equal to 565 µm2/s23,24. From the equation:
| 4 |
we determined the confocal volume V = 0.22 fl. The structural parameter S (defined as the ratio of the long (ωz) to the short radii (ωx = ωy = ωr) of the ellipsoidal confocal volume and τFITC were equal to 5 and 18 µs, respectively.
Similarly, we obtained DPS = 3.4 µm2/s from PS residence time in the focal plane τPS = 2.6 ms. At an aqueous AlPcS2 concentration Pa = 30 nM (Fig. 2B), we observed P = 1700 molecules/μm2. The corresponding adsorption coefficient amounts to 57 molecules/μm2 per nM Pa. The focal volume of the red laser was calibrated with Cy5. We took Cy5’s diffusion coefficient and S to be equal to 280 µm2/s25,26 and 8, respectively. Cy5’s residence time τCy5 amounted to 31.6 µs.
The photodynamic activity of phthalocyanines with various numbers of sulfo-groups
When both DT and PS were adsorbed to the BLM surface, membrane illumination at 670 nm led to a drop in Δφb due to DT’s oxidation by SO17. Δφb recovered in the dark due to the adsorption of intact DT molecules from the aqueous bulk solution (Fig. 3). The kinetics of photo damage depended on whether DT and PS were added into the solution to the same (cis configuration) side or to the opposite (trans configuration) side of the membrane. Dividing Δφb(t) by its value φads at time t = 0 we define the rate R of DT oxidation as17:
| 5 |
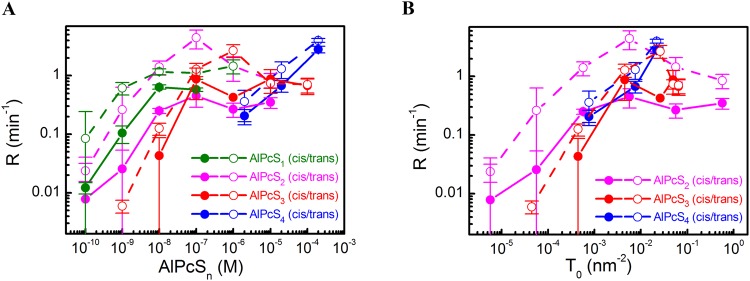
enabling a quantitative analysis of the photodynamic effects. We found that R depended (i) on the number of sulfo-groups per PS molecule (Fig. 4A), (ii) the geometrical arrangement (cis or trans configuration), and (iii) the aqueous PS concentration. For PS molecules with two sulfo-groups, R depended non-monotonically on Pa in trans configuration. Similar bell-shaped concentration dependencies were reported for SO-mediated gramicidin inactivation5. In contrast, R increased monotonically with Pa in cis configuration, suggesting that SO quenching by PS may be involved. This would be in line with chemoluminescence-based observations of SO quenching by PS27.
Figure 3.
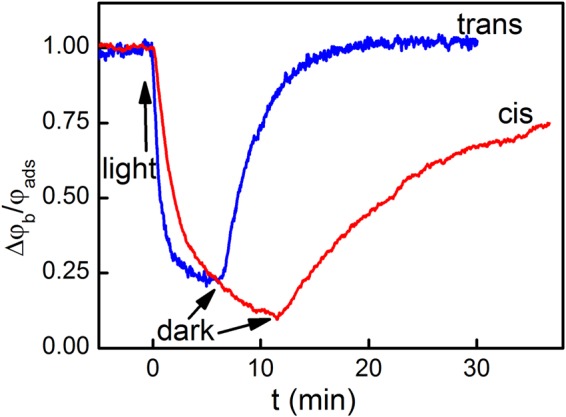
Comparison of the kinetics of relative potential decay during illumination and its recovery in the dark in case of cis and trans photo effects. Either the cis or the trans solutions contained 2 μM di-4-ANEPPS (“cis” photo effect or “trans” photo effect, respectively). 0.2 μM AlPcS2 were added to the cis solution.
Figure 4.
Dependence of the rate R of oxidation of di-4-ANEPPS adsorbed on the cis (open symbols) or trans (closed symbols) side of the BLM on the aqueous AlPcSn concentration (A) or as a function of AlPcS2 and AlPcS4 membrane surface densities (B).
In order to account for differences in AlPcSn membrane affinities, we replotted R as a function of P (Fig. 4B). For AlPcS3 and AlPcS4, we calculated P from the increment in surface charge5, whereas we used FCS to determine P of the weakly charged AlPcS2 and AlPcS1. R did not significantly vary with the number of sulfogroups in cis configuration: R was roughly proportional to P for P < 0.01 molecules/nm2, and it approached saturation at higher P. However, in trans configuration, we observed a 10-fold augmented R for AlPcS2 and the absence of saturation for AlPcS4. The latter may be due to AlPcS4’s low binding constant.
Mathematical model
Our counterintuitive observation that R is smaller if PS and DT are adsorbed to the same leaflet than to opposite ones has three possible explanations: (1) PS and DT molecules interact with each other thereby decreasing the quantum yield of SO, (2) PS molecules quench SO or (3) DT molecules possess two different moieties that may serve as a SO target: damage of only one of these moieties - of the aniline group - changes membrane dipole potential, whereas targeting the second moiety - the unsaturated hydrocarbon chain in the middle of the molecule - remains electrically silent.
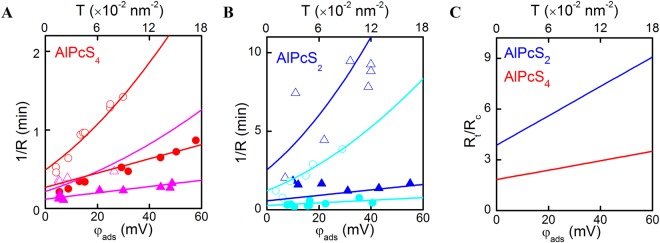
First, we ruled out the potential interaction by recording the effect of DT on the fluorescence spectra of liposomal PS (see Supplementary Fig. S1). Since there was none, a DT-PS interaction is unlikely. Second, we estimated the impact of PS mediated SO quenching. Since it should depend on the concentration of both molecules, we would expect the ratio Rc/Rt to vary with PS concentration. The invariance of that ratio at low PS concentrations (Fig. 4) excludes SO quenching by PS from being responsible for the difference between Rc and Rt. Third, to estimate the impact of SO quenching by DT molecules, we plotted 1/R against φads (Fig. 5). The slopes of the curves in “cis” configuration exceeded the slopes in “trans” configuration for all φads, thereby confirming DT’s quenching effect. The difference between the two configurations was more pronounced when AlPcS4 was substituted for AlPcS2, suggesting a deeper bilayer penetration depth of AlPcS2, and thus, a smaller PS to DT distance.
Figure 5.
Dependence of the inverse rate of cis and trans photo effects on the potential caused by adsorption of di-4-ANEPPS at (A) 20 μM (squares) or 200 μM of AlPcS4 (down triangles) and (B) 4 nM (up triangles) or 200 nM (circles) of AlPcS2 in water solution. The filled symbols represent the experiments where di-4-ANEPPS was at trans side of the BLM, the open ones – at the cis side. The spline lines are best fits of the theoretical model (equations T4 and T5) to the data. (C) Rt/Rc = f(T0) plot from the results of the fit.
Differences in the oxidation rates of styryl dyes analogues17 indicate that DT possess two different moieties that may serve as a SO target: damage of only one of these moieties - of the aniline group - changes membrane dipole potential, whereas targeting the second moiety - the unsaturated hydrocarbon chain in the middle of the molecule - remains electrically silent. This peculiarity generates the different R in cis and trans configurations: In cis configuration, DT’s naphthalene ring is only ~ 1 nm farther away from the PS molecule than DT’s double bond (Fig. 6). Consequently, the double bond may provide protection for the naphthalene ring. In trans configuration, the naphthalene ring is closer to the PS molecule, and thus, it may represent the primary SO target. This hypothesis only makes sense if τr is very short – small enough to prevent SO from reaching distant targets, and definitely much shorter than suggested by the previously estimated τl = 3 µs in a liposomal suspension.
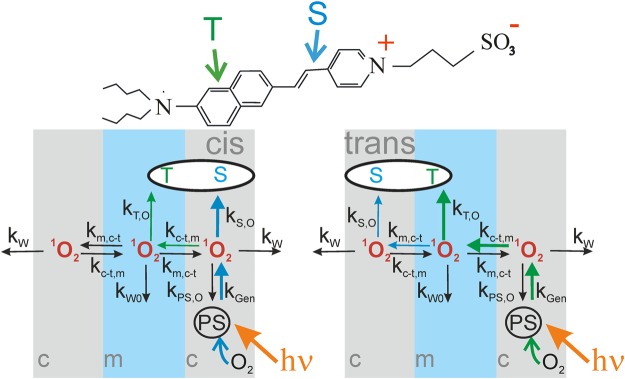
Figure 6.
Model of SO generation, quenching and transport. (A) ANEPPS’ structure reveals two different moieties that can be targeted by SO: the aniline ring (T) and the unsaturated hydrocarbon chain (S). (B) SO generation, membrane transport, and quenching. After oxidation of the aniline ring, the target molecule loses its dipole moment (or its orientation), but the hydrocarbon double bond may still quench SO.
We supported the hypothesis about 2 SO susceptible DT moieties with a mathematical model that takes into account all chemical reactions and membrane diffusion processes (see Theory). The numerical solution of the differential equations satisfactorily fits the experimental data (Fig. 5) for the parameter set displayed in Table 1. The model divides the lipid membrane into three layers and assumes that SO is generated by PS molecules in one of the outer layers. In contrast, DT molecules extend from the outer to the middle layer. The Δφd affecting moiety (designated by letter T in Fig. 6) - the naphthalene group at the end of the chromophore molecule - is immersed into the middle layer17. The other moiety that is susceptible to SO – the unsaturated hydrocarbon chain in the middle of the molecule (designated by letter S in the Fig. 6) – localizes to one of the outer membrane layers. SO may quench the S-group even when the T-moiety is oxidized and DT’s contribution to Δφd has vanished.
Table 1.
Model parameters.
| Phthalocyanine | AlPcS4 | AlPcS2 |
|---|---|---|
| kGen (m2s−1) | 0.6 ± 0.1 | 2.4 ± 0.3 |
| kW (s−1) | (5.0 ± 0.9) · 107 | |
| kW0 (s−1) | (2.9 ± 0.3) · 107 | |
| kT,O (m2s−1) | >10 −10 | |
| kS,O (m2s−1) | (1.5 ± 0.2) · 10−10 | (6.0 ± 0.7) · 10−10 |
| kc−t,m (s−1)fixed, from Pm | 2.4 · 108 | |
| km,c−t (s−1)fixed, from Pm | 5.5 · 107 | |
They represent the best fit of the system of differential equations (T4) to the experimental data (Fig. 5A,B). The global parameters (independent on the choice of the phtalocyanine) are highlighted in bold. The local parameters, i.e. the rate, kGen, of SO generation and the rate, kS,O, of SO quenching by S, are valid for all concentrations of the specific phtalocyanine.
We noticed that - contrary to the expectations - the limit of the oxidation rate at zero DT density depends on which side of the membrane contains DT (Fig. 5). This observation suggests that the DT preparation already contained oxidized molecules, which were capable of capturing SO, while being unable to contribute to Δφb.
Theory
Mathematical model of SO generation, membrane permeation, and quenching
The model describes the permeation of singlet oxygen through the membrane taking into account the non-uniform distribution of SO across the membrane. SO mirrors the bell–shaped distribution of ground-state oxygen with the concentration maximum in the middle of the membrane10. The steady-state concentration of SO not only depends on this distribution, but also on its lifetime. To simplify this distribution, we will consider it to be discrete. Let the membrane consist of three layers, two of them are the fields of the lipid bilayer lying near its interface with water, and the third one – the internal hydrophobic region of the membrane, is located in the layers of hydrocarbon chains of the lipids. We will refer to these layers as cis (the layer with the PS molecules), medium (the middle of the membrane) and trans (the layer opposite to cis). The concentrations of singlet oxygen in these layers will be designated as Oc, Om and Ot, correspondingly. The singlet oxygen is generated from the excited molecules of PS adsorbed on the cis-side of membrane. No matter which of the locations of the PS molecules is considered, ground state oxygen is present at saturating concentrations with respect to SO generation. Consequently, both AlPcS2 and AlPcS4 operate at a rate that is solely governed by light intensity (see for example Fig. 5 in13 or Fig. 8 in17).
The target of SO can be located either in the cis or trans layer. The model assumes that two parts of the target molecule react with singlet oxygen (Fig. 6). The first one, the aniline group is designated by “T”. The oxidation of this group by singlet oxygen in the middle layer (Om in Fig. 6) nullifies their contribution to membrane dipole potential. The concentrations of this group in cis and trans layers are correspondingly designated, as Tc and Tt. The other part – the unsaturated hydrocarbon chain in the middle of the molecule (designated by “S” in Fig. 6) can react with singlet oxygen in the surface layers (Oc, or Ot) without changing the dipole potential. The approach neglects the incremental diffusion span of no more than 0.5 nm that a SO molecule has to cross if born on the “wrong” (opposite to the direction of movement) side of a PS molecule. It corresponds to travel time of only ~ 1 ns, which is at least 10 times smaller than the most conservative estimate of τdw = 12 ns from molecular dynamics simulations10.
The reactions and transfer of singlet oxygen in cis and trans photoeffects are shown in Fig. 6. The singlet oxygen can react with target molecules, be quenched by the medium, the molecules or photosensitizer and transfer to the adjacent layer. Once SO has left the membrane, the probability of its retrieval is negligible since (i) the volume of the aqueous bulk so much larger than that of the membrane, (ii) and the lifetime in aqueous solutions is very limited. The equations describe the damage of the target and the processes with singlet oxygen in the experiment, where the target molecules located at the cis side of the membrane are
| T1 |
where
ka — rate constant of adsorption of target molecules from solution to membrane;
kd — rate constant of desorption of target molecules from membrane to the solution;
kT,O — rate constant of damaging of target molecules by singlet oxygen;
kGen — rate constant of generation of singlet oxygen;
kS,O — rate constant of quenching of singlet oxygen by the target molecules;
kPS,O — rate constant of quenching of singlet oxygen by the photosensitizer molecules;
kc-t,m — rate constant of transfer of singlet oxygen from cis or trans layers to the middle one;
km,c-t — rate constant of transfer of singlet oxygen from the middle layer to cis or trans ones;
kW — rate constant of quenching of singlet oxygen in cis and trans layers;
kW0 — rate constant of quenching of singlet oxygen in the middle layer.
Similarly, the equations describing the damage of the target at the trans side are
| T2 |
To simplify the solution of these equations, we will consider that the processes described here are fast when compared with the processes of oxidation and diffusion of the targets described in17. If the concentration of the singlet oxygen changes much faster than that of the targets, it reaches the steady-state value before the concentration of the target significantly begins to change. Therefore, we can solve the equations assuming the steady-state distribution of singlet oxygen and equilibrium distribution of the targets.
| T3 |
In this case, the equations for the singlet oxygen concentration transform to the algebraic ones. By solving these equations and introducing the steady-state concentrations of the singlet oxygen into the first equations in (T1) and (T2) we only have single differential equations describing the kinetics of oxidation of the target either in cis or in trans positions. Thus we will instead solve these equations by determining the rate of oxidation of the target Rc and Rt at cis and trans positions, respectively, as a slope (time derivative) of the relative change of the potential at the beginning of illumination according to Eq. 5. The relation between the potential measured in the experiment and the surface density of the target molecules can be established experimentally by comparing the dipole potentials measured by IFC method at given concentration of the ANEPPS in the solution (the data in17) and the surface density of these molecules measured by the FCS technique (Fig. 2). These values are proportional to each other:
Relative potential can be derived through the ratio of current surface density of the target to the initial one corresponding to equilibrium of targets between the membrane and solution before the illumination
where
In these designations, the Rc can be derived through the steady-state concentrations of singlet oxygen Om determined by equations (T1)
and similarly Rt – through Om determined by equations (T2)
For further simplification of equations (T1 and T2), we will neglect SO quenching by PS, putting the corresponding constant, kPS,O, equal to 0. It may be valid if we use very low concentrations of photosensitizers in the linear region of the dependence of the rate R on the concentrations of photosensitizers in the solution or on their surface densities (Fig. 4). It seems natural to assume that the surface densities of two parts of the same targets molecule, T and S, are equal. However, one cannot exclude the existence of a fraction of the target molecules, which are partially damaged and do not contribute to the dipole potential, but still can react with singlet oxygen and quench it. To take into account these “invisible” target molecules we will assume that surface density at each side of the membrane consists of two members: “visible” T and “invisible” S0
Solving the equations (T1 and T2) with the above-mentioned simplifications gives the rates Rc and Rt as functions of the ANEPPS adsorption potential
| T4 |
with
We used equation T4 to fit the data in Fig. 5. kc-t,m was not used as a fitting parameter. but was obtained from the known membrane permeability PM = 80 cm/s of O228,29 that we assume to be equal to PM of SO: , where δ is the membrane thickness. Substituting SO’s membrane diffusion coefficient DM by the Einstein relation, we find . Since we are not interested in the time τ that SO takes to cross the bilayer, but in the time that SO requires to diffuse from the middle of one of the outer layers to the middle of the membrane, we substitute δ and 1/τ for δ/3 and kc-t,m, respectively. This yields kc−t,m = 2.4 × 108 s−1.
S0 was treated as a constant parameter for each of PS and was found from the global fit as αS0 = 30 mV, equal both for AlPcS2 and AlPcS4.
One of the simplest consequences of the model is that the ratio of Rc and Rt is always less than 1. This ratio is equal to
| T5 |
Discussion
Our experiments revealed that SO in membranes is extremely short-lived: instead of spending microseconds in the membrane interior, SO does not survive tens of nanoseconds. In other words, τr ≈ τl. τl is mainly governed by the time it takes SO to reach its nearest target. The conclusion is based on experiments, in which a SO molecule that was born in the membrane headgroup region was free to target molecules in (i) the same headgroup region, (ii) the inner hydrophobic layer, and (iii) the opposing headgroup region. Although the increment in distance between these targets was smaller than 2 nm, the probability of SO reacting with the targets sharply decreased with distance.
A lower limit of τl maybe obtained by assuming a diffusion limited reaction. In our experiments, the DT molecules (for φads = 20 mV) were only 4 nm apart as indicated by FCS measurements. Thus, in cis configuration, the distance r between a PS and the nearest DT neighbor molecule did not exceed 2 nm. Taking into account SO’s diffusion coefficient D = 5 × 10−5 cm2 s−1 7, we find the SO travel time
| 6 |
τt is probably smaller in biological membranes since they are densely packed with SO scavengers that are represented by unsaturated lipids and membrane proteins containing aromatic residues30–33.
In order to obtain a more accurate estimate of τl, we performed a quantitative analysis of our experiments by solving the differential equations for the combined system of chemical reactions and diffusion processes (see Theory). We found the simplified solution represented by Eq. (7):
| 7 |
where T0, S0, Rc, and Rt denote T prior to illumination, the initial surface density of DT’s oxidizable moiety that does not contribute to Δφb (Fig. 6), R in cis and trans configurations, respectively. The Rt/Rc ratio depends on the combined rate kW of SO membrane exit and quenching by the aqueous medium, the transfer rate kc-t,m from the outer membrane layer to the medium layer, and the rate kS,O of SO quenching by the S moiety of DT (Fig. 6). Eq. 7 assumes a non-negligible value S0, i.e. it does not work for T0 ≫ S0. Equation (7) predicts that Rc is always smaller than Rt, which is in perfect agreement with the experiment.
We first globally fitted the complete set of differential equations (see Theory) to individual sets of Rc = f(T0) and Rt = f(T0) that have been experimentally obtained for both AlPcS4 and AlPcS2 (Table 1 and Fig. 5A,B) and then constructed the Rt/Rc = f(T0) plot from the results of the fit (Fig. 5C). Solving the complete set of equations also allowed us to obtain the rate kGen of SO generation. kGen is fourfold higher for AlPcS2 than for AlPcS4, indicating either an environmental effect on SO yield or immediate SO escape into the aqueous solution without ever entering the lipid membrane. Indeed, in contrast to AlPcS4’s four negative charges, which ensure that it lies flat on the membrane surface, AlPcS2 penetrates into the bilayer. Its chromophore ring inclines towards the hydrocarbon core as indicated by a substantial contribution of Δφd to Δφb. Thus, AlPcS2’s ring is exposed to a four-fold higher oxygen concentration than that belonging to AlPcS4. The observed increment in kGen agrees well with the previously reported increase of photosensitizer efficiency with increasing hydrophobicity11,34,35.
Our mathematical model revealed the reaction rates of SO with targets: The rate kT,O of SO quenching by the T moiety of DT is roughly equal to kS,O ≥ 6.0·10−10 m2s−1. Taking into account the target density of about 0.1 molecule nm−2, this value translates into a reaction time τT,O ≈ 16 ns of the T moiety with SO. τT,O is three orders of magnitude smaller than the estimate for τl from experiments36 with non-oxidizable lipids, underpinning the conclusion that the main determinant of SO lifetime is target density. Moreover, τl is not diffusion limited, i.e. it is not governed by τt: τl ≈ τT,O ≫ τt.
The mathematical model also allows assessing τdw. From km,c−t = 5.5 × 107 s−1 and kw = (5.0 ± 0.9) × 107 s−1 (Table 1) we find τdw ~ (1/km,c−t) + (1/kw) ≈ (40 ± 8) ns. This estimate agrees reasonably very well with the one derived from the literature values of SO’s distribution coefficient and diffusion constant (see Introduction). τl < τdw indicates that most of the reactive oxygen species stay in the membrane long enough to react with the target, i.e. that τr is determined by τl. However, the values of τl and τdw are too close to each other to ensure a photodynamic efficiency of 100%. An increase in targeting efficiency can be realized by two different approaches: (i) by reducing the separation between PS and target so that other molecules susceptible to SO, like unsaturated lipids, cannot protect the proteinaceous target from encounters with SO and (ii) by increasing the quantum yield of PS molecules. Our model allows determining kGen in a membrane environment, thereby offering an important tool for PS optimization.
Electronic supplementary material
Acknowledgements
The work was supported in part by the Russian Science Foundation (grant #14-13-01373P) and by the Ministry of Education and Science of the Russian Federation in the framework of Increase of Competitiveness Program of “MISiS”. The authors are grateful to Asya Vargaftik for the help with measurements and Quentina Beatty for editorial help.
Author Contributions
V.S.S. designed the experiments and wrote the manuscript, P.P. wrote the manuscript, O.V.B., S.A.A. and T.R.G. developed the theoretical model, A.N.K. and E.M. performed electrical measurements on planar bilayers, Y.G.G. provided compounds and advised on the chemistry of phtalocyanines, D.G.K. performed fluorescence measurements. All authors reviewed the manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31901-9.
References
- 1.O’Connor AE, Gallagher WM, Byrne AT. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol. 2009;85:1053–1074. doi: 10.1111/j.1751-1097.2009.00585.x. [DOI] [PubMed] [Google Scholar]
- 2.DeRosa MC, Crutchley RJ. Photosensitized singlet oxygen and its applications. Coordin Chem Rev. 2002;233:351–371. doi: 10.1016/S0010-8545(02)00034-6. [DOI] [Google Scholar]
- 3.Photosensitizers in Medicine, Environment, and Security. (Springer, 2012).
- 4.Baptista MS, et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017;93:912–919. doi: 10.1111/php.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rokitskaya TI, Block M, Antonenko YN, Kotova EA, Pohl P. Photosensitizer binding to lipid bilayers as a precondition for the photoinactivation of membrane channels. Biophys J. 2000;78:2572–2580. doi: 10.1016/S0006-3495(00)76801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackbarth, S., Bornhütter, T. & Röder, B. In Singlet Oxygen: Applications in Biosciences and Nanosciences Vol. 2, Ch. 26, 27–42 (The Royal Society of Chemistry, 2016).
- 7.Fischkoff S, Vanderkooi JM. Oxygen diffusion in biological and artificial membranes determined by the fluorochrome pyrene. J. Gen. Physiol. 1975;65:663–676. doi: 10.1085/jgp.65.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hackbarth S, Roder B. Singlet oxygen luminescence kinetics in a heterogeneous environment - identification of the photosensitizer localization in small unilamellar vesicles. Photochem Photobiol Sci. 2015;14:329–334. doi: 10.1039/C4PP00229F. [DOI] [PubMed] [Google Scholar]
- 9.Battino R, Evans FD, Danforth WF. The solubilities of seven gases in olive oil with reference to theories of transport through the cell membrane. J. Am. Oil Chem. Soc. 1968;45:830–833. doi: 10.1007/BF02540163. [DOI] [PubMed] [Google Scholar]
- 10.Cordeiro RM. Reactive oxygen species at phospholipid bilayers: Distribution, mobility and permeation. Bba-Biomembranes. 2014;1838:438–444. doi: 10.1016/j.bbamem.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Lavi A, Weitman H, Holmes RT, Smith KM, Ehrenberg B. The depth of porphyrin in a membrane and the membrane’s physical properties affect the photosensitizing efficiency. Biophys J. 2002;82:2101–2110. doi: 10.1016/S0006-3495(02)75557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller P, Rudin DO, Tien HT, Wescott WC. Metgods for the Formation of Single Bimolecular Lipid Membranes in Aqueous Solution. J Phys Chem. 1963;67:534–535. doi: 10.1021/j100796a529. [DOI] [Google Scholar]
- 13.Sokolov VS, Pohl P. Membrane transport of singlet oxygen monitored by dipole potential measurements. Biophys. J. 2009;96:77–85. doi: 10.1529/biophysj.108.135145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokolov VS, Kuz’min VG. [Measurement of differences in the surface potentials of bilayer membranes according to the second harmonic of a capacitance current] Biofizika. 1980;25:170–172. [PubMed] [Google Scholar]
- 15.Ermakov, Y. A. & Sokolov, V. S. In Planar Lipid Bilayers (BLMs) and their applications (eds Tien, H. T. & Ottova-Leitmannova, A.) 109–141 (Elsevier, 2003).
- 16.Sokolov, V. S. & Mirsky, V. M. In Ultrathin Electrochemical Chemo- and Biosensors: Technology and Performance (ed. Mirsky, V. M.) 255–291 (Springer, 2004).
- 17.Sokolov VS, et al. Voltage-sensitive styryl dyes as singlet oxygen targets on the surface of bilayer lipid membrane. J Photochem Photobiol B, Biology. 2016;161:162–169. doi: 10.1016/j.jphotobiol.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Montal M, Mueller P. Formation of Bimolecular Membranes from Lipid Monolayers and a Study of Their Electrical Properties. Proc Natl Acad Sci USA. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horner A, Antonenko YN, Pohl P. Coupled diffusion of peripherally bound peptides along the outer and inner membrane leaflets. Biophys. J. 2009;96:2689–2695. doi: 10.1016/j.bpj.2008.12.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horner A, Akimov SA, Pohl P. Long and short lipid molecules experience the same interleaflet drag in lipid bilayers. Phys. Rev. Lett. 2013;110:268101. doi: 10.1103/PhysRevLett.110.268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magde D, Elson EL, Webb WW. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974;13:29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- 22.Cordeiro RM, Miotto R, Baptista MS. Photodynamic Efficiency of Cationic meso-Porphyrins at Lipid Bilayers: Insights from Molecular Dynamics Simulations. J Phys Chem B. 2012;116:14618–14627. doi: 10.1021/jp308179h. [DOI] [PubMed] [Google Scholar]
- 23.Galambos, P. & Forster, F. K. In Proceedings from the µTAS ’98 Workshop. (eds Harrison, D. J. & Van Den Berg, A.) 189–191 (Kluwer Academic Publishers: Dordrechht, Boston, London), (1998).
- 24.Rani SA, Pitts B, Stewart PS. Rapid diffusion of fluorescent tracers into Staphylococcus epidermidis biofilms visualized by time lapse microscopy. Antimicrobial agents and chemotherapy. 2005;49:728–732. doi: 10.1128/AAC.49.2.728-732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bark N, Földes-Papp Z, Rigler R. The incipient stage in thrombin-induced fibrin polymerization detected by FCS at the single molecule level. Biochem Biophys Res Commun. 1999;260:35–41. doi: 10.1006/bbrc.1999.0850. [DOI] [PubMed] [Google Scholar]
- 26.Mozziconacci J, Sandblad L, Wachsmuth M, Brunner D, Karsenti E. Tubulin dimers oligomerize before their incorporation into microtubules. Plos One. 2008;3:e3821. doi: 10.1371/journal.pone.0003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krasnovsky AA, et al. Quenching of singlet molecular oxygen by phthalocyanines and naphthalocyanines. Photochem Photobiol. 1992;55:691–696. doi: 10.1111/j.1751-1097.1992.tb08512.x. [DOI] [PubMed] [Google Scholar]
- 28.Subczynski WK, Hyde JS, Kusumi A. Oxygen permeability of phosphatidylcholine–cholesterol membranes. Proc Natl Acad Sci USA. 1989;86:4474–4478. doi: 10.1073/pnas.86.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Widomska J, Raguz M, Subczynski WK. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim Biophys Acta Biomembr. 2007;1768:2635–2645. doi: 10.1016/j.bbamem.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rokitskaya TI, Kotova EA, Agapov II, Moisenovich MM, Antonenko YN. Unsaturated lipids protect the integral membrane peptide gramicidin A from singlet oxygen. FEBS Lett. 2014;588:1590–1595. doi: 10.1016/j.febslet.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 31.Antonenko YN, et al. Protective effects of mitochondria-targeted antioxidant SkQ in aqueous and lipid membrane environments. J Membr Biol. 2008;222:141–149. doi: 10.1007/s00232-008-9108-6. [DOI] [PubMed] [Google Scholar]
- 32.Egorov S, Kurella EG, Boldyrev AA, Krasnovsky AA., Jr. Quenching of singlet molecular oxygen by carnosine and related antioxidants. Monitoring 1270-nm phosphorescence in aqueous media. Biochem Mol Biol Int. 1997;41:687–694. doi: 10.1080/15216549700201731. [DOI] [PubMed] [Google Scholar]
- 33.Krasnovsky AA, Jr., Kagan VE. Photosensitization and quenching of singlet oxygen by pigments and lipids of photoreceptor cells of the retina. FEBS Lett. 1979;108:152–154. doi: 10.1016/0014-5793(79)81198-9. [DOI] [PubMed] [Google Scholar]
- 34.Stozhkova IN, Cherny VV, Sokolov VS, Ermakov Yu A. Adsorption of haematoporphyrins on the planar bilayer membrane. Membr Cell Biol. 1997;11:381–399. [PubMed] [Google Scholar]
- 35.Engelmann FM, et al. Interaction of cationic meso-porphyrins with liposomes, mitochondria and erythrocytes. J Bioenerg Biomembr. 2007;39:175–185. doi: 10.1007/s10863-007-9075-0. [DOI] [PubMed] [Google Scholar]
- 36.Ehrenberg B, Anderson JL, Foote CS. Kinetics and yield of singlet oxygen photosensitized by hypericin in organic and biological media. Photochem Photobiol. 1998;68:135–140. doi: 10.1111/j.1751-1097.1998.tb02479.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.