Abstract
This research studied various types of packaging to prolong the shelf life of non-preservative white bread. Three types of blown film packages were used, i.e. a single LDPE layer incorporated with an oxygen scavenger, a single LDPE layer containing an oxygen absorber sachet, and three layers of LDPE laminated with O-nylon. The effects of modified packaging atmosphere, i.e. 5, 10, and 21 vol. % of oxygen in nitrogen balance, on the shelf life was also included. Characterization of the packaging films was carried out using several techniques, such as Oxygen Transmission Rate (OTR) and an optical microscopy. Headspace gases, microbial count, as well as physical appearance were used to evaluate the shelf life. The optical microscopic images showed that incorporating the oxygen scavenger into the plastic film produced small pores, contributing to a passive function of the films as their OTRs were significantly enhanced. However, the microbial growth on bread stored in those packages was suppressed, implying that the intermediate generated from scavenging reaction might act as a fungistatic. Even though the scavenging capability of the oxygen absorber sachet lasted only for 4 days, the fungi and mould development thereafter was still lower compared to the package without the sachet. The prolonging white bread shelf life was found to be primarily dependent on two factors. The package with a high oxygen barrier such three-layer films defeated microorganisms. With a low initial oxygen level of around 5% by volume, the bread shelf life could be prolonged up to 5–7 days.
Keywords: Food science, Chemical engineering
1. Introduction
Bakery products, bread, in particular, are widely consumed in Western societies and becoming more common for Asians with a modern and busy lifestyle. Bread can be consumed directly or used as a part of other food such as pizza and pudding. It is easy to prepare and hence, a popular choice for breakfast to suit a city lifestyle. Lately, there are a large variety of types of bread to fulfil consumer need for beneficial nutrients such as dietary fibre, minerals and vitamins. However, bakery products usually have a short shelf life of only a few days at room temperature due to their high water activity. Refrigerating storage of these types of products is not applicable to their texture and taste change with temperature and therefore, they are best consumed at room temperature. Even though some viable fungi are destroyed during baking, some spores can survive or fungi contamination from other processes such as cooling and packaging can occur and are the most spoilage reason in bakery products. The use of proper packaging has been the main choice for satisfying the “green” consumerism market demands to extend the shelf life of bakery products without using any preservatives [1, 2].
Advanced packaging technologies have been playing an important role in food industries for more than a decade and becoming more and more common in recent years especially in a health-concerned society. As these types of packaging successfully extend the shelf life for several foodstuffs including bakery products, uses of chemical preservatives which cause health hazard problems can be reduced significantly. According to Smith [3], food packages are required to have multi-functions in terms of chemical, physical, and biological alterations of the food to prolong its shelf life. Chemically, the packages should be able to control and/or prevent oxidation and some other chemical reactions, such as hydrolytic rancidity and Maillard reaction, in food products. The packages should maintain the moisture level in the products which is a critical parameter controlling bread staling and changes in texture and physical appearance. Most importantly, microbial growth in food during storage is a serious problem and a major cause to shorten food shelf life. Packages that can inhibit microbial proliferation are currently in demand.
Modified atmosphere packaging (MAP) is one of the methods used to extend product shelf life. This can be done by the use of gases to replace air around non-respiring foods regardless of whether or not the atmosphere changes over time of storage or packing. Most MAP for bakery products is mainly high CO2 contents with the elimination of O2 due to the fact that CO2 is considered as an antimicrobial agent. In high water content food like bakery products, it is found that CO2 can dissolve in water to form carbonic acid, lowering the pH. This acidification of the cell contents causes the death of bacteria. Smith et al [4] concluded that mould growth could not be prevented, but could be delayed by N2 and/or CO2 up to 5–10 days. The only possibility to prevent mould growth was to maintain the level of O2 to be below 0.4%. Results of the dependency of O2 content on fungi growth on other types of products under MAP were confirmed [5, 6, 7, 8]. However, the high concentration of CO2 may lead to increased perceived acidity in the taste [5, 9, 10]. Furthermore, the conclusive effect of CO2 in the MAP on the bread quality currently cannot be made due to conflicting results from various research. While Hasan [11] found no trend of effects of CO2 on pita bread texture, Sourki et al [12] reported that Iranian flatbread hardness increased significantly as CO2 concentration increased. On the other hand, Rasmussen and Hansen [13] and Khoshakhlagh et al [14] concluded that CO2 altered neither a staling rate nor texture of white and part-baked Sangak bread, respectively. The ability of CO2 on delaying the firming of bread and biscuits was also testified [15].
Another packaging technique that has been focused is active packaging (AP), the packages that not only act as a protective barrier but interact with the product in order to protect food from adulteration by, for example, moisture, oxygen, ethylene, and microorganisms. Two types of AP, i.e. sachet-based and plastic film-based packaging, have been developed. Sachets, containing active ingredients such as oxygen absorber, ethylene scavenger, and moisture absorber, were developed in the late 1970s in Japan. Although this technique is considerably practical for packaging industries, some disadvantages are inevitable. They cannot be used in liquid products or in the tight fitting film as their functionalities would be stifled. Furthermore, risks of accidental ingestion of these sachets are a serious concern. These drawbacks can be alleviated if those functionality components are incorporated to the plastic film itself. In addition to that, recent studies have revealed that combinations of various packaging techniques to prolong shelf life are more effective. The study of Berenzon and Saguy [16] suggested that even though using oxygen absorber sachets was very effective in terms of controlling the headspace oxygen, they could not slow down lipid oxidation of crackers when stored at high temperatures, attaining comparable sensory evaluation of crackers kept in absence of oxygen absorber sachets. An oxygen absorber sachet was found to extend the shelf life of pita bread and bakery products by impeding mould and yeast growth even in CO2/N2 MAP due to the fact that it absorbed oxygen trapped in food and in the air that permeated through the package [4]. As ethanol was well known as a disinfectant in the medical field, the ability to delay the microbial growth on rye bread depended on the size of ethanol emitter sachets [17]. While Latou et al [18] could not see any advantages of using a combination of an oxygen absorber sachet and an ethanol emitter sachet over to the use of an ethanol emitter sachet alone, Janjarasskul et al [19] reported a synergistic action of the two. Several research disclosed more effectiveness of sachet based AP or the combination of AP with a MAP over MAP alone [4, 10, 20]. Saladino et al [21] added paper filters and a small plastic bag containing paper filters soaked with allyl, benzyl and phenyl isothiocyanates oil into bread plastic trays and found that these essential oils could reduce aflatoxins (AFs) and prevent microflora growth on the bread. Similarly, Balaguer et al [22] adopted the use of essential oil, namely cinnamaldehyde, as an antibacterial and antifungal substance to bioplastic wheat gliadin films and found the dependency of fungicidal and fungistatic activities against Penicillium expansum and Aspergillus niger on the cinnamaldehyde loading. Drastic reduction in fungi and hence the extension of shelf life of bread and cheese were clearly observed. Busolo and Lagaron [23] prepared active polyolefin composites by melt extrusion of iron particles, kaolinite clay and high and linear low-density polyethylene (HDPE and LLDPE) and the nanocomposites films were subsequently prepared by compression moulding. The agglomeration of nanoclay was more pronounced in HDPE, resulting in less oxygen scavenger activity. Besides an active role, by incorporating the nanoclay into the films could reduce the oxygen and water permeability in passive action by 22–28 and 33%, respectively [23, 24]. Particle sizes of iron powder were one of the factors controlling the oxygen scavenging activity [25]. As one expected, the nanoscale iron powder had a higher oxygen absorption rate and capacity compared to a commercial iron-based oxygen scavenger masterbatch (Shelfplus® O2). While these advanced packaging technologies using nanocomposites continue to remain competitive in a global market, an emerging issue of food safety due to direct contact with those nanocomposites should be widely explored and concerned [26].
Considering that a high number of studies have been carried out on advanced packaging to prolong the shelf life of bread, there is a limitation of the MAP that has been focused on which was the usage of CO2 to replace O2. However, in terms of economic point of view, air and N2 are cheap and easy to use. This study investigated and compared the effects of O2/N2 MAP incorporating with plastic-based AP on the shelf life of white bread by considering headspace oxygen contents, microbial counts and appearance. In addition, the effects of film thickness were also examined using multilayer film packages. Besides the scavenging activity, barrier properties of the films were also determined.
2. Experimental
2.1. Materials and bread packaging and storage
Chiba® Shelfplus O2-2400 (currently owned by Albis Plastic GmbH, Germany) masterbatch was purchased from a local supplier. Low-density polyethylene (LDPE) resins were obtained from Thai Polyethylene Co., Ltd. The packaging film was manufactured using a film blowing technique at Seesan 56 Co., Ltd., Thailand, for a single layer film and at Prepack Co., Ltd., Thailand, for a three layer film laminated with O-nylon. The blown film packages were classified as three types, i.e. (1) single layer of LDPE incorporated with different oxygen scavenger concentrations, (2) single layer of LDPE containing an oxygen absorber sachet, and (3) three layers of LDPE incorporated with different oxygen scavenger concentrations in the middle layer and laminated with O-nylon. Details of the three types of packages are illustrated in Table 1. The packages of the size of 200 × 150 mm2 were heat sealed at the bottom. The 2.5 g KEEPIT oxygen absorber sachets purchased from a local supplier were 100% iron oxide powder. White bread prepared without preservatives was purchased from Big C supermarket in Chonburi, Thailand, on the day it was baked. According to the bakery advice, the bread shelf life was only 2 days. The bread was packed in the packages which were subsequently vacuum sealed and then filled with the 300 cm3 O2/N2 gas mixture. For the O2/N2 gas mixture, O2 was obtained from the air in the atmosphere with approximately 65% relative humidity (RH) and N2 used as a balanced gas was from a Linde gas cylinder with a purity of 99%. Each package containing one slide of the bread under three different packaging atmosphere of 5, 10, and 21% by volume of O2 was stored in a room temperature of around 30 °C during the period of the study of 7 days. As one package of the samples was used to perform the tests each day, hence 7 packages of each sample were prepared to complete the test.
Table 1.
Details and packaging codes of packages used in this research.
| Packaging code | Details | Oxygen scavenger content (% w/w) | Thickness (μm) |
|---|---|---|---|
| 1L0 | 1 layer LDPE film | 0 | 37 ± 5 |
| 1L5 | 1 layer LDPE film | 5 | 46 ± 6 |
| 1L10 | 1 layer LDPE film | 10 | 47 ± 7 |
| 1L0S | 1 layer LDPE film with a 2.5 g oxygen absorber sachet | 0 | 37 ± 5 |
| 3L0 | 3 layer LDPE films with O-nylon lamination | 0 | 87 ± 2 |
| 3L10 | 3 layer LDPE films (the middle layer contain oxygen scavenger) with O-nylon lamination | 10 | 87 ± 5 |
2.2. Oxygen scavenger characterization
Scanning Electron Microscopy equipped with Energy Dispersive X-Ray Analysis (SEM-EDX) technique was used to provide detailed high-resolution images of the Ciba® Shelfplus O2-2400 oxygen scavenger masterbatch along with the elemental identification. The analysis using an LEO 1450 VP operated at 20 kV. A Polaron Range Model SC 7620 Ion Sputter Coater was used for gold sputtering. In addition, the quantitative compositional information of the oxygen scavenger masterbatch was carried out using a Thermogravimetric Analysis (TGA). Mettler TGA850 was heated from 30–1000 °C at a heating rate of 10 °C/min under a nitrogen blanket.
2.3. Packaging film characterization
The film thickness was measured using an ID-C112B Digimatic Indicator, Mitutoyo, Japan, having a resolution of 1 μm. Five measurements were done for each film and four films for each packaging sample were carried out to obtain an average and standard deviation values as shown in Table 1. The opacity of the film was verified using percent haze measured by an UltraScan XE, HunterLab, Virginia, USA. It is a dual beam xenon flash spectrophotometer with a wavelength range from 360 to 750 nm. The test was performed in a transmission mode with an area viewport size of 1 inch. An investigation on the microstructure of the films was carried out using a Carton Micro System CM 400 series optical microscope (Japan) equipped with a digital DinoLite eyepiece camera (Anmo Electronics Corporation, Taiwan). Image processing and analysis were performed using the DinoCapture 2.0 software. Oxygen transmission rate (OTR) of the films was tested using N500 Gas Permeability Analyzer (GBPI Packaging Instrument Co., Ltd) according to standard method ASTM D1434. The films of the size of 110 cm in diameter were tested at 23 °C, 0% RH, and 0.1 MPa partial pressure difference. The flow oxygen was an ultrahigh purity grade. Film compositions were analyzed using the TGA technique in a similar way to the analysis of the oxygen scavenger masterbatch.
2.4. Oxygen permeability of the films and headspace gases analysis
The oxygen permeability of the films and oxygen and carbon dioxide contents in the headspace of the packages were investigated using a CP 3800 Variant Gas Chromatography Analyzer (GC). The analysis was performed using two columns, i.e. HP Plot Q and MolSieve, connecting in series in an oven which was set at 60 °C. A Thermal Conductivity Detector (TCD) was equipped with the GC. For oxygen permeability, the packages only contained gas mixture without a slide of bread and the gas measurements were performed every 2 hours for 24 hours and then every 12 hours after that until reached 72 hours. The headspace gas analysis of the packages containing a slice of bread was, on the other hand, carried out daily for a storage period of 7 days.
2.5. Physical appearance
Inspection for visible microbial growth on white bread was performed prior to the microbial count analysis. Photos of the fungi and moulds, if detectable, were taken and the sizes of the visible colonies were measured. The period from packaging to when visible microorganism was detected was considered as “bread mould-free shelf life”.
2.6. Microbial analysis
Spread plate technique was used to analyze yeast and mould counts. 39 g of potato dextrose agar (PDA) was dissolved in 1 L of distilled water and the solution was autoclaved at 121 °C under 15 psi for 15 min. 15–20 ml of the solution was poured into each petri dish and kept in a laminar flow clean bench. 22 g of crumb bread were added to 198 ml of 0.85% sodium chloride (NaCl) solution previously autoclaved and stirred until becoming homogeneous. Serial decimal dilutions were made using the 0.85% NaCl solution. Once the desired concentration was reached, 0.1 ml of the solution was spread on the surface of the prepared culture medium and incubated at 35 ± 2 °C for 48 hours prior to colony counts. According to food safety guidance of the Department of Medical Sciences, Ministry of Public Health, Thailand, microbial counts for bread products must not exceed 104 CFU/g while yeast and mould counts must not exceed 102 CFU/g.
3. Results and discussion
3.1. Characterization of oxygen scavenger masterbatch and packaging films
3.1.1. SEM-EDX
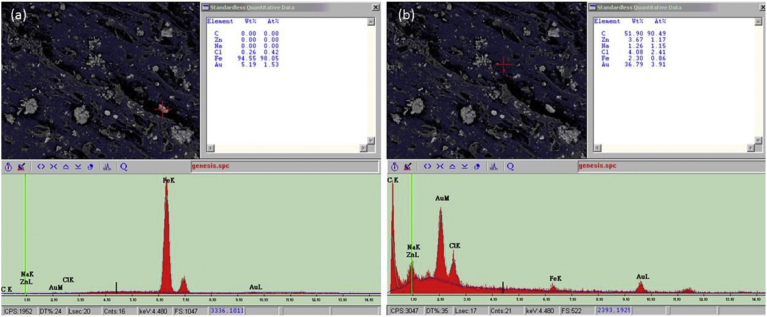
Fig. 1 illustrates SEM images of the Ciba® Shelfplus O2-2400 oxygen scavenger masterbatch. It can be seen that there are a number of particles of the sizes of few microns with few agglomerates of the size up to 30–50 microns dispersed on the surface of the material. Once the EDX technique was utilized, Fig. 2(a) and (b) showed that those particles were pure iron whereas the main component of the material (base polymer in the masterbatch) was carbon, respectively. It can be concluded that the oxygen scavenger masterbatch composes of iron powder in a low-density polyethylene matrix. Similarly to the oxygen absorber sachets, the mechanism of this type of oxygen scavenger follows the following reactions.
| Fe → Fe2+ + 2e− | (1) |
| ½ O2 + H2O + 2e− → OH− | (2) |
| Fe2+ + OH− → Fe(OH)2 | (3) |
| Fe(OH)2 + ¼O2 + ½ H2O → Fe(OH)3 | (4) |
Fig. 1.
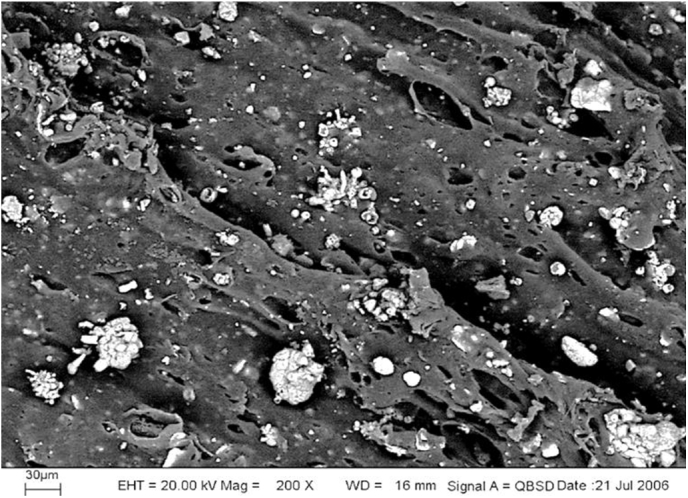
SEM image of Shelf-plus O2-2400 oxygen scavenger master-batch.
Fig. 2.
SEM-EDX of dispersion phase (a), and matrix phase (b) of Shelf-plus O2-2400 oxygen scavenger master-batch.
3.1.2. TGA
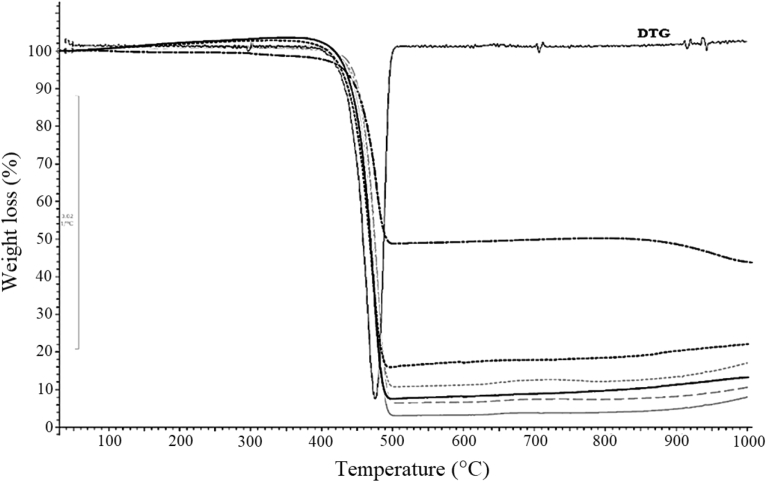
The TGA analysis in Fig. 3 shows that the oxygen scavenger masterbatch composed of around 50% of a base polymer which was LDPE and 50% of iron powder which still remained at the temperature of 1000 °C. The decomposition temperature of the base polymer in the masterbatch at 475 °C (see DTG curve) was similar to that of LDPE, confirming that it was LDPE. The unleveled baseline of the TGA could be seen from the results especially with the tests of three layer films and hence, % remained residues in the curves could not be read directly. Percent residues were calculated from % weight loss at the decomposition temperature of 475 °C and found that for the single layer films containing oxygen scavenger at 0, 5, and 10% w/w were 1, 6, and 10.5%, respectively. The slightly higher amount of residues found from TGA might be due to some other impurities in the LDPE such as metal catalysts and/or reaction terminators. For the three layer films, the remainings were also in the same range of % oxygen scavenger contents in the packages.
Fig. 3.
TGA and DTG chromatograms of Shelfplus O2-2400 oxygen scavenger masterbatch and packaging films.
3.1.3. Film appearance and microstructure
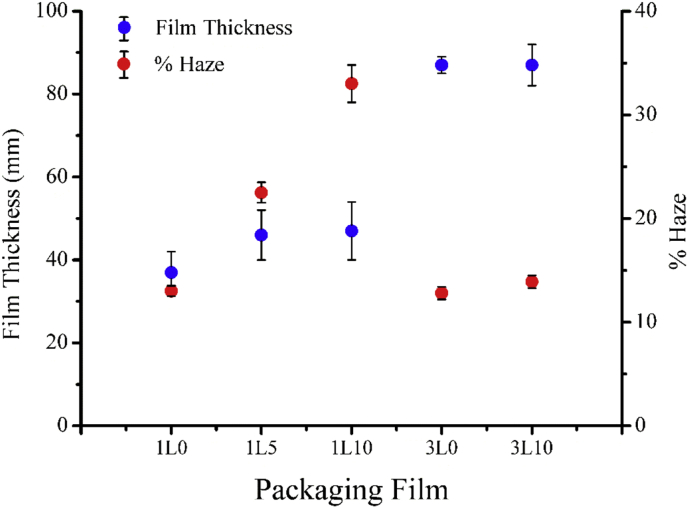
Film thickness and % haze are illustrated in Fig. 4. When incorporating the oxygen scavenger into the LDPE film, the thickness of the films increased from 37 μm to around 46 μm, equivalent to 24% increase. However, there was no difference between the thickness of the films with 5 and 10% w/w oxygen scavenger. In opposite, %haze increased significantly and monotonically with the oxygen scavenger concentration. With 5 and 10% w/w oxygen scavenger, the % haze increased 73 and 154%, respectively. On the other hand, with three layer films, there was no dissimilarity between film thickness as well as % haze of the films with (3L10) and without (3L0) incorporating oxygen scavenger. In addition, % haze of the films was similar to that of the single layer neat film (1L0). This might be because the iron powder was incorporated only with the middle layer. As one would expect, the three-layer films had thickness almost 3 times of the thickness of the single layer neat film.
Fig. 4.
Thickness and opacity of the packaging films.
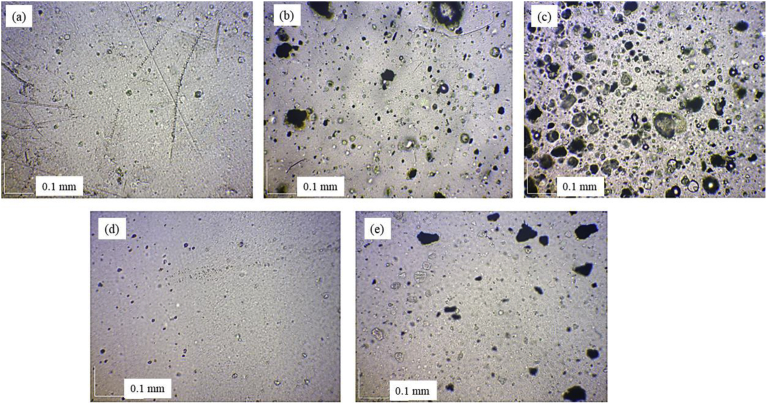
Fig. 5(a–e) show optical microscopy images of different packaging films. It can be seen that the single layer neat film (1L0) had few small pores of the size of few microns. These small holes might occur during the film processing by blown film extrusion. However, once the oxygen scavenger was incorporated into the film, those pores became larger in size. The pore size and amount of the pores increased as the oxygen scavenger concentration increased. In addition, the images show iron powder heterogeneously distributed in the film with some agglomeration. Busolo and Lagaron [23] suggested that the non-homogeneity of these particles may be due to insufficient shear during the film processing and/or affinity with the matrix. This is reasonable since blown film technique does not achieve high shear. The affinity of iron particles and the base polymer was a crucial issue in terms of aggregation of the particles in polymer matrix since it was found that with a higher viscosity matrix, a less homogeneity the film. This iron powder agglomeration was the cause of an increase in film thickness as well as opacity as previously presented in Fig. 4. In addition, a large number of small holes in the film incorporating with oxygen scavenger was due to the fact that those particles could not be bonded with the polymer matrix. Those small pores had an effect on the gas transmission rate of the films as will be discussed in the next section. For the three layer films, those small pores could barely be seen. Even though holes were generated during the film processing for the three layer films, the O-nylon lamination could obscure the gas permeability as observed by the OTR presented in Table 2. Similar to the single layer films, iron particle agglomerations were also detected in the three layer films (3L10).
Fig. 5.
Optical microscopy images of packing films: (a) 1L0, (b) 1L5, (c) 1L10, (d) 3L0, and (e) 3L10.
Table 2.
Oxygen transmission rate of the packaging films.
| Packaging films | Thickness (μm) | OTR (cc/m2·24 h·0.1 MPa) | Testing conditions | Storage product | Reference |
|---|---|---|---|---|---|
| 1L0 | 37 | 154.44 | 23 °C, 0% RH, 0.1 MPa | White bread | |
| 1L5 | 46 | 219.65 | 23 °C, 0% RH, 0.1 MPa | White bread | |
| 1L10 | 47 | 287.89 | 23 °C, 0% RH, 0.1 MPa | White bread | |
| 3L0 | 87 | 7.94 | 23 °C, 0% RH, 0.1 MPa | White bread | |
| 3L10 | 87 | 8.09 | 23 °C, 0% RH, 0.1 MPa | White bread | |
| PVDC/nylon/CPP | 2/14/44 | 6.73 | 23 °C, 0% RH, 0.1 MPa | Sponge cake | Janjarasskul et al [19] |
| nylon/LLDPE | 15/65 | 66.03 | 23 °C, 0% RH, 0.1 MPa | Sponge cake | Janjarasskul et al [19] |
| PET-SiOx/LDPE | 62 | 4 | 25 °C, 75% RH, 0.1 MPa | Wheat bread | Latou et al [18] |
| OPP | 33 | 186.19 | N/A | Iranian flatbread | Sourki et al [12] |
| PET-Al-LLDPE | 12/7/65 | 0.04 | N/A | Iranian flatbread | Sourki et al [12] |
| PET/PET/LLDPE | 12/12/65 | 0 | N/A | Iranian flatbread | Sourki et al [12] |
| Laminated EVA | 95 | <2 | 25 °C, 75% RH | Wheat bread | Rasmussen and Hansen [13] |
| Laminated PA | 90 | 90 | 20 °C, 50% RH | Mackerel | Stamatis and Arkoudelos [5] |
| PE/EVOH/PE | N/A | 7 | 23 °C, 0% RH, 0.1 MPa | Beef steak | Limbo et al [20] |
| PE/TIE/PVDC/TIE/E | 100 | 6.89 | 23 °C, 75% RH | Buckwheat noodles | Bai et al [27] |
| PET | 30 | 56 | 23 °C | Pasta | Sanguinetti et al [7] |
| EVOH/OPET/PE | 54 | 4 | 23 °C | Pasta | Sanguinetti et al [7] |
3.1.4. OTR for passive function
Table 2 summarizes the OTR of the packaging films used in this research as well as in other works. The OTR was measured under 0% HR in which the scavenging activity was expected to be inefficient according to the results of Foltynowicz [25] and Busolo and Lagaron [23]. For single layer films, even though the neat film (1L0) was thinnest, the OTR was the lowest. The OTR of the LDPE film of 37 μm in this study (154.44 cc/m2⋅24 h⋅0.1 MPa) were found to be closed to that of the OPP of the similar thickness of 33 μm studied by Sourki et al [12] (186.19 cc/m2⋅24 h⋅0.1 MPa). Incorporating the films with the oxygen scavenger (1L5 and 1L10) generated small holes as seen in Fig. 5, consequently the OTRs were increased. As the amount of incorporating oxygen scavenger increased, the number of small pores increased and so did the OTR. However, the result showed here conflicted with the findings from previous studies where the OTR reduction was achieved with the iron-based oxygen scavenger active films [23, 24]. This is because those research works incorporated iron powder into nanoclay and the nanoclay acted as a media to bond with polyolefin matrix. As a result, there were no holes appearing in the films.
For the three layer films (3L0 and 3L10), OTRs of the film were much lower than those of the single layer films and comparable to those of multi-layer and/or laminated films in other studies. Even though small pores appeared in the oxygen scavenger incorporating film in the middle layer, the OTRs of the three layer films were identical.
3.1.5. Oxygen permeability in active mode
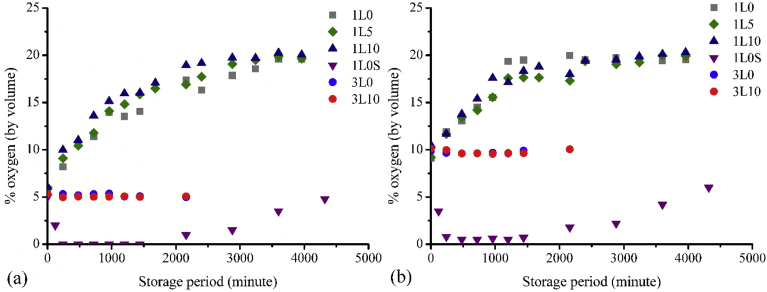
The O2 contents in the packages under the initial O2 atmosphere of 5 and 10% by volume during the storage period are shown in Fig. 6(a) and (b), respectively. The experiments of the packages containing air (21% O2 atmosphere) were not performed as there was no driving force for mass transfer between inside and outside the packages. It was obvious that three-layer films were a very good barrier for oxygen transfer, and probably for other gases as well. This can be confirmed from the OTR shown in Table 2. As the oxygen scavenger was incorporated in the middle layer of the film, its scavenging activity was impeded and hence there was no difference in O2 content for the films without (3L0) and with (3L10) incorporating oxygen scavenger. For the single layer film, the initial oxygen permeable rate was higher in the packages with 5% O2 atmosphere compared to that with 10% O2 atmosphere due to the fact that the pressure gradients between inside and outside the packages which were a driving force for oxygen transfer were higher. However, once the pressure differences were smaller as O2 permeated into the packages the permeable rates of the two packages decreased and were closed to each other. As the initial O2 concentration in the packages with 10% O2 atmosphere was higher, the environment inside the packages reached the normal air atmosphere (21% O2) quicker. It took a couple days for oxygen in the air to permeate into both packages completely. It seemed that the passive function of the films as a barrier medium was more pronounced than the active function as an oxygen scavenger. In accordance with the film morphology, the films with higher amounts of incorporating oxygen scavenger resulted in a slightly higher O2 content and a faster permeable rate due to a larger number of holes in the films. This was again agreed with OTR results shown in Table 2.
Fig. 6.
Oxygen permeability test of packaging films under an initial 5% O2 atmosphere (a) and an initial 10% O2 atmosphere (b).
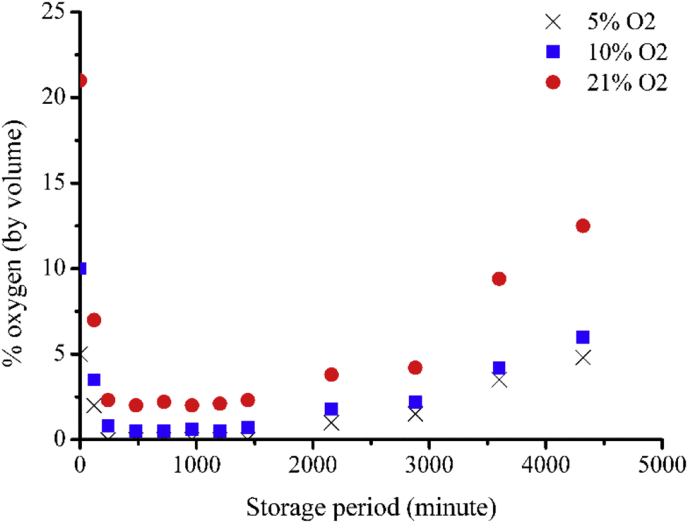
Oppositely, the single layer neat film containing an oxygen absorber sachet (1L0S) acted differently. The O2 rapidly diminished within a couple hours. For the packages with an initial 5% O2 atmosphere, O2 was completely disappeared; however, there was a very low level of O2 (<1% v/v) left in the packages with an initial 10% O2 atmosphere and a low level of around 2.2% v/v in the packages with air (21% v/v O2) as illustrated in Fig. 7. This was sensible since the initial amount of O2 was the highest in the package filled with air and lowest in the package with the initial 5% O2 atmosphere. The very high O2 absorbing performance of sachet based AP in comparison to the scavenging ability of the film based AP was due to the fact that the iron powder exposed to the O2 easily without any barrier. In addition, the amount of the iron powder in the sachet was considerably higher than that incorporating into the LDPE films. In all three packages, the low O2 levels were remained constant for about a day before they started to gradually emerge. The increased level of O2 was because the ambient air continued to permeate through the films while the sachets were already filled up with O2 completely and became inactive.
Fig. 7.
Oxygen permeability test of sachet based packages under different oxygen content atmosphere.
3.2. Characterization of white bread after storage
3.2.1. Headspace gases
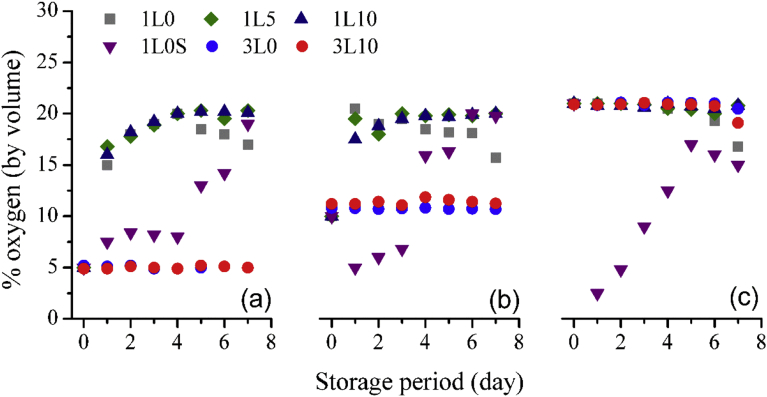
One slide of white bread was packed in each package under different packaging atmosphere. The headspace oxygen was monitored daily and shown in Fig. 8(a–c). In the gas permeability experiments, O2 content in the packages monotonically increased due to air outside the packages permeated into the packages; however, for these experiments, as microbial contaminated in the bread used O2 to cultivate, O2 consumption occurred at the same time as the permeation of the outside air. Consequently, the headspace O2 was a net result of these two phenomena. For three layer packages, it was obvious that there was no O2 permeated from outside air so the reduction of O2 in the packages due to fungi and mould cultivation was expected. However, the O2 levels were barely changed except for the last couple days of the packages filled with air, implying there were particularly low levels of microorganism proliferation. Oppositely, the headspace O2 content of the single layer film packages under 5 and 10% O2 atmosphere increased sharply and within the first couple days, the levels were considered similar to those empty packages. This was because, for the first couple days, the number of microbial counts was still low and hence minimal amounts of O2 were consumed. But on the 4th and 5th day, the headspace O2 levels started to decline for the packages with the initial 10 and 5% O2 atmosphere, respectively, because of the microbial growth. The results were in accordance with the results of headspace CO2 and microbial counts which would be discussed later on. Among the packages with different initial O2 levels, it was revealed that the packages with higher O2 concentrations, the higher O2 consumption due to the microbial growth was more accelerated. Interestingly, the reduction in the O2 level of the air packages was not clear and indistinguishable with the packages with 10% O2 atmosphere. The microbial count results confirmed this finding. This suggested that to minimize the spoilage of the bread from microorganisms, the O2 level must be lower than 10% v/v. For the single layer films incorporating without and with different oxygen scavenger contents, the slightly lower O2 levels were found in the packages without the oxygen scavenger and no difference in O2 levels was found in the packages with 5 and 10% w/w oxygen scavenger, conflicting with the results of OTR and oxygen permeability in active mode. The hypothesis for this phenomenon was that the oxygen scavenger inhibited the microbial growth not only physically by scavenging headspace O2 as mentioned in previous publications [4, 10, 20, 28], but also biochemically as confirmed by the results from the microbial count. The intermediate agent from the scavenging reaction might act as a fungistatic.
Fig. 8.
Headspace oxygen during the storage period of different packages under initial 5% O2 atmosphere (a), 10% O2 atmosphere (b), and 21% O2 atmosphere (c).
For the sachet based packaging, the headspace O2 was absorbed immediately but soon started to gradually increase after 1 day of storage due to the air permeation. While the sachets in the 5% O2 atmosphere packages absorbed less O2, the headspace O2 levels were lowest. Whereas the sachets in the air packages absorbed more O2, the headspace O2 levels were highest. The decrease of headspace O2 at the final days of storage (6th and 7th days) was observed only in the packages filled with air. This was due to the fact that a large number of fungi and mould had visibly grown on the bread as would be mentioned in appearance and mould-free shelf life section.
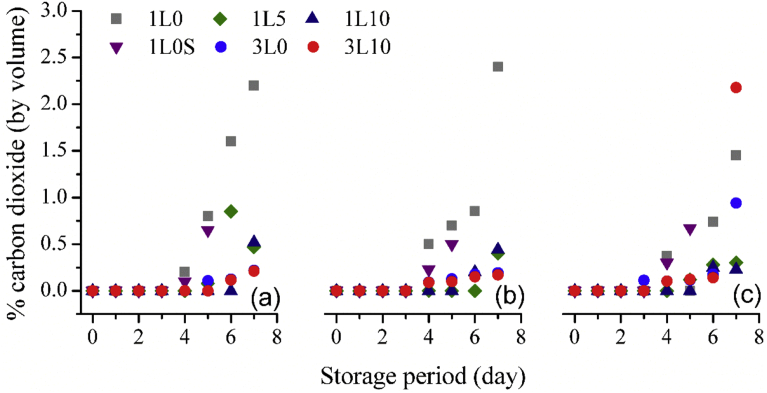
The results of headspace CO2 presented in Fig. 9(a–c) were corresponding to the results of headspace O2 previously discussed. Even though the headspace O2 levels of the three layer film packages was unchanged, very low levels of around 0.2% v/v of headspace CO2 were detected except for the air packages on the last day of storage that approximately 1–2% v/v CO2 were identified. For the single layer film packages, the headspace CO2 levels started to progressively increase from 4th day of storage for the packages of neat LDPE with and without an oxygen absorber sachet whereas the headspace CO2 was noticeable only in the last couple days of storage for the 5 and 10% w/w oxygen scavenger incorporating film packages. This could be due to two reasons. Firstly, as the oxygen scavenger incorporating films contained relatively more pores than the neat films, CO2 could easily diffuse through the films to outside environment. Secondly, the low amounts of CO2 were simply due to the low numbers of microorganisms and this hypothesis was proved by the results from the microbial investigation, revealing lower amounts of fungi and mould in the oxygen scavenger incorporating film packages compared to those in the neat film packages. Since the low levels of less than 3% v/v of CO2 were detected, the dependency of initial O2 concentration in packaging atmosphere on the headspace CO2 was not cleared.
Fig. 9.
Headspace carbon dioxide during the storage period of different packages under initial 5% O2 atmosphere (a), 10% O2 atmosphere (b), and 21% O2 atmosphere (c).
3.2.2. Microbial counts
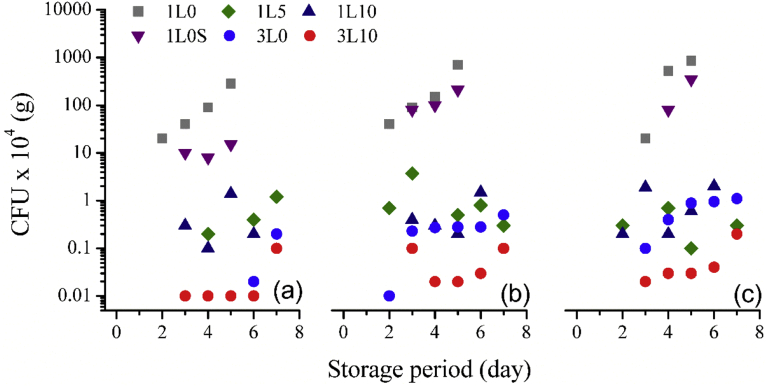
As mentioned earlier that low levels of headspace CO2 were identified in the three-layer film packages, the microbial investigation disclosed that the found headspace CO2 was a result of microorganisms as seen in Fig. 10(a–c). Even though the results of headspace gases could not differentiate the differences between the three layer films incorporating with (3L10) and without (3L0) oxygen scavenger, higher microbial counts in the neat films were noticeable. A similar trend was observed for the single layer film packages especially in those packages with the low initial O2 headspace (5% O2 atmosphere). By incorporating an oxygen scavenger into the LDPE films, microbial growth could be delayed. It was likely that the higher oxygen scavenger concentration, the lower microbial counts although the headspace gases of the 5 and 10% w/w oxygen scavenger film packages were not distinguishable. The scavenging capacity of the oxygen absorber sachet started to deplete after 4 days of storage; however, during those periods, the results showed that the microbial growth rate was suppressed. As a result, one might conclude that there was no significant advantage of using an oxygen absorber sachet in the single layer neat film. Nevertheless, the number of fungi and mould appeared after 4 days of storage period were slightly lower in the packages contained the oxygen absorber sachet, proving its beneficial role in active packaging. Another factor influencing a delay in the microbial development was the amount of O2 content in the package. With a low O2 atmosphere of 5% v/v, the number of microorganisms was about a magnitude lower than those in the 10 and 21% O2 atmosphere. The findings were agreed with the work of Knorr and Tomlins [15] who reported that 100% N2 in packaging atmosphere was as effective as 100% CO2 in terms of extending the white bread shelf life from 5 days in air packaging to 13 days. However, this was contradicted to the conclusion made by Bai et al [27] who did not discover any significant difference in the number of total plate counts in buckwheat noodles packed in normal air and in 100% N2. Comparable microbial counts in the packages with 10 and 21% O2 atmosphere implied that 10% v/v O2 content in the headspace of the packages were sufficient for the microbial growth. Interestingly, the food safety guidance of the Department of Medical Sciences, Ministry of Public Health, Thailand, sets the limitation of yeast and mould counts as 102 CFU/g where those bread with invisible fungi and mould colony possessed microbial counts over the limit and might be mistakenly consumed by customers.
Fig. 10.
Microbial counts during the storage period of different packages under initial 5% O2 atmosphere (a), 10% O2 atmosphere (b), and 21% O2 atmosphere (c).
3.2.3. Appearance and mold-free shelf life
During the storage period of 7 days, fungi and mould colonies on slides of bread in the three-layer film packages both with (3L10) and without (3L0) incorporating with oxygen scavenger under all three O2 concentration atmosphere (5, 10, and 21% v/v) were not visible. The appearance of fungi and mould on the bread in the single layer films packages depended mainly on the initial O2 contents in the packaging headspace as well as the oxygen scavenger concentrations. With the packages containing air inside, the microbial development appeared earlier than those with lower O2 concentrations (5 and 10% O2 atmosphere). Similar findings were reported by Smith et al [4] that 100% N2 atmosphere could prolong the crusty rolls' shelf life compared to a normal air package. By incorporating an oxygen scavenger into film packages, the visible microorganisms growth could be delayed for air headspace packages and prevented for low O2 headspace packages (5 and 10% O2 atmosphere). Unlike the report on the substantial extension of shelf life of bakery products using an oxygen absorber sachet by Salminen et al [17] and Smith et al [4] the use of the oxygen absorber sachet in this research did not impede the appearance of fungi and mould; however, the colony sizes were much smaller as seen in Fig. 11(a) and (b). Except for an extreme condition of a single layer neat film (1L0) packed under air headspace (21% O2), the detectable microbial colony sizes were considerably small as shown in Fig. 11(a). Only with the bread kept under the extreme condition showed extensive fungi and mould colony sizes illustrated in Fig. 11(b). Table 3 summarized the storage day that visible microbial on bread was spotted, or in other words, the mould-free shelf life of the bread.
Fig. 11.
Bread appearance after storage in most packages (a) and in 1L0 package under 21% O2 atmosphere (b).
Table 3.
Bread mould-free shelf life.
| Packaging code | Headspace oxygen content (with N2 balance) |
||
|---|---|---|---|
| 5% | 10% | 21% | |
| 1L0 | 5 days | 5 days | 4a days |
| 1L5 | - | - | 6 days |
| 1L10 | - | - | 6 days |
| 1L0S | - | 5 days | 4 days |
| 3L0 | - | - | - |
| 3L10 | - | - | - |
- Means no visible microbial on bread and hence the shelf life >7 days.
Indicates large colonies as shown in Fig. 11(b).
4. Conclusions
Prolonging of white bread shelf life was accomplished with the use of novel advanced packaging technologies. The combination of AP and MAP demonstrated a high potential for bakery product industries. It was illustrated that CO2 was not a necessity in the MAP as long as the amount of O2 was minimized by using N2 as a balance gas. By incorporating an iron-based oxygen scavenger into the petroleum plastic films, the intermediate from the scavenging reaction acted as an antimicrobial agent in a similar way to CO2. If the packaging film possesses a high barrier for O2 transmission property, the usage of incorporating oxygen scavenger as well as the MAP is almost unnecessary. These technologies could be extended to other bakery products.
Declarations
Author contribution statement
Settakorn Upasen: Performed the experiments.
Piyachat Wattanachai: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Faculty of Engineering, Burapha University, Thailand under the grant no. WJP. 13/2558.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank undergrad students, Ms. S. Hawlee, Mr. T. Chancheu, Ms. M. Katesopa, Mr. T. Sukwattana, Ms. P. Suyanang, and Ms. S. Prampan, who carry out experiments as their senior projects.
References
- 1.Kotsianis I.S., Giannou V., Tzia C. Production and packaging of bakery products using MAP technology. Trends Food Sci. Technol. 2002;13(9–10):319–324. [Google Scholar]
- 2.Gutiérrez L., Sánchez C., Batlle R., Nerín C. New antimicrobial active package for bakery products. Trends Food Sci. Technol. 2009;20(2):92–99. [Google Scholar]
- 3.Smith J.P. Bakery products. In: Parry R.T., editor. Principles and Applications of Modified Atmosphere Packaging of Foods. Springer; Boston, MA: 1993. [Google Scholar]
- 4.Smith J.P., Ooraikul B., Koersen W.J., Jackson E.D., Lawrence R.A. Novel approach to oxygen control in modified atmosphere packaging of bakery products. Food Microbiol. 1986;3(4):315–320. [Google Scholar]
- 5.Stamatis N., Arkoudelos J. Quality assessment of Scomber colias japonicus under modified atmosphere and vacuum packaging. Food Contr. 2007;18(4):292–300. [Google Scholar]
- 6.Halouat A.E., Debevere J.M. Effect of water activity, modified atmosphere packaging and storage temperature on spore germination of moulds isolated from prunes. Int. J. Food Microbiol. 1997;35(1):41–48. doi: 10.1016/s0168-1605(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 7.Sanguinetti A.M., Del Caro A., Scanu A., Fadda C., Milella G., Catzeddu P., Piga A. Extending the shelf life of gluten-free fresh filled pasta by modified atmosphere packaging. LWT Food Sci. Technol. 2016;71:96–101. [Google Scholar]
- 8.Taniwaki M.H., Hocking A.D., Pitt J.I., Fleet G.H. Growth of fungi and mycotoxin production on cheese under modified atmospheres. Int. J. Food Microbiol. 2001;68(1–2):125–133. doi: 10.1016/s0168-1605(01)00487-1. [DOI] [PubMed] [Google Scholar]
- 9.Fik M., Surówka K., Maciejaszek I., Macura M., Michalczyk M. Quality and shelf life of calcium-enriched wholemeal bread stored in a modified atmosphere. J. Cereal Sci. 2012;56(2):418–424. [Google Scholar]
- 10.Suppakul P., Thanathammathorn T., Samerasut O., Khankaew S. “Shelf life extension of “fios de ovos”, an intermediate-moisture egg-based dessert, by active and modified atmosphere packaging”. Food Contr. 2016;70:58–63. [Google Scholar]
- 11.Hasan S.M., Naje S.A., Abosalloum S. Shelf life extrusion of pita bread by modified atmosphere packaging. J. Food Dairy Sci. Mansoura Univ. 2014;5(2):55–62. [Google Scholar]
- 12.Hematian Sourki A., Yazdi F.T., Ghiafeh Davoodi M., Mortazavi S.A., Karimi M., Jahromi S.H.R., Pourfarzad A. Staling and quality of Iranian flat bread stored at modified atmosphere in different packaging. Int. J. Soc. Behav. Educ. Econ. Bus. Ind. Eng. 2010;4(5):567–572. [Google Scholar]
- 13.Rasmussen P.H., Hansen A. Staling of wheat bread stored in modified atmosphere. LWT Food Sci. Technol. 2001;34(7):487–491. [Google Scholar]
- 14.Khoshakhlagh K., Hamdami N., Shahedi M., Le-Bail A. Quality and microbial characteristics of part-baked Sangak bread packaged in modified atmosphere during storage. J. Cereal Sci. 2014;60(1):42–47. [Google Scholar]
- 15.Knorr D., Tomlins R.I. Effect of carbon dioxide modified atmosphere on the compressibility of stored baked goods. J. Food Sci. 1985;50(4):1172–1173. [Google Scholar]
- 16.Berenzon S., Saguy I.S. Oxygen absorbers for extension of crackers shelf-life. LWT Food Sci. Technol. 1998;31(1):1–5. [Google Scholar]
- 17.Salminen A., Latva-Kala K., Randell K., Hurme E., Linko P., Ahvenainen R. The effect of ethanol and oxygen absorption on the shelf-life of packed sliced rye bread. Packaging Technology and Science. LWT Food Sci. Technol. 1996;9(1):29–42. [Google Scholar]
- 18.Latou E., Mexis S.F., Badeka A.V., Kontominas M.G. Shelf life extension of sliced wheat bread using either an ethanol emitter or an ethanol emitter combined with an oxygen absorber as alternatives to chemical preservatives. J. Cereal Sci. 2010;52(3):457–465. [Google Scholar]
- 19.Janjarasskul T., Tananuwong K., Kongpensook V., Tantratian S., Kokpol S. Shelf life extension of sponge cake by active packaging as an alternative to direct addition of chemical preservatives. LWT Food Sci. Technol. 2016;72:166–174. [Google Scholar]
- 20.Limbo S., Uboldi E., Adobati A., Iametti S., Bonomi F., Mascheroni E., Santagostino S., Powers T.H., Franzetti L., Piergiovanni L. Shelf life of case-ready beef steaks (Semitendinosus muscle) stored in oxygen-depleted master bag system with oxygen scavengers and CO2/N2 modified atmosphere packaging. Meat Sci. 2013;93(3):477–484. doi: 10.1016/j.meatsci.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Saladino F., Quiles J.M., Luciano F.B., Mañes J., Fernández-Franzón M., Meca G. Shelf life improvement of the loaf bread using allyl, phenyl and benzyl isothiocyanates against Aspergillus parasiticus. LWT Food Sci. Technol. 2017;78:208–214. [Google Scholar]
- 22.Balaguer M.P., Lopez-Carballo G., Catala R., Gavara R., Hernandez-Munoz P. Antifungal properties of gliadin films incorporating cinnamaldehyde and application in active food packaging of bread and cheese spread foodstuffs. Int. J. Food Microbiol. 2013;166(3):369–377. doi: 10.1016/j.ijfoodmicro.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Busolo M.A., Lagaron J.M. Oxygen scavenging polyolefin nanocomposite films containing an iron modified kaolinite of interest in active food packaging applications. Innovat. Food Sci. Emerg. Technol. 2012;16:211–217. [Google Scholar]
- 24.Khalaj M.-J., Ahmadi H., Lesankhosh R., Khalaj G. Study of physical and mechanical properties of polypropylene nanocomposites for food packaging application: nano-clay modified with iron nanoparticles. Trends Food Sci. Technol. 2016;51:41–48. [Google Scholar]
- 25.Foltynowicz Z., Bardenshtein A., Sängerlaub S., Antvorskov H., Kozak W. Nanoscale, zero valent iron particles for application as oxygen scavenger in food packaging. Food Packag. Shelf Life. 2017;11:74–83. [Google Scholar]
- 26.Huang J.-Y., Li X., Zhou W. Safety assessment of nanocomposite for food packaging application. Trends Food Sci. Technol. 2015;45(2):187–199. [Google Scholar]
- 27.Bai Y.-P., Guo X.-N., Zhu K.-X., Zhou H.-M. Shelf-life extension of semi-dried buckwheat noodles by the combination of aqueous ozone treatment and modified atmosphere packaging. Food Chem. 2017;237:553–560. doi: 10.1016/j.foodchem.2017.05.156. [DOI] [PubMed] [Google Scholar]
- 28.Hutter S., Rüegg N., Yildirim S. Use of palladium based oxygen scavenger to prevent discoloration of ham. Food Packag. Shelf Life. 2016;8:56–62. [Google Scholar]