Abstract
Introduction
Extracorporeal membrane oxygenation (ECMO) is a mode of extracorporeal life support that has been used to support cardiopulmonary disease refractory to conventional therapy. The experience with the use of ECMO in acute hypoxemic respiratory failure is still limited. The aim of this study was to report clinical outcomes in adult patients with acute hypoxemic respiratory failure refractory to mechanical ventilation treated with ECMO.
Methods
Between July 2011 and October 2017, 18 adult patients with hypoxemic respiratory failure refractory to mechanical ventilation were admitted to the Intensive Care Unit of an acute care tertiary hospital in Barcelona, Spain. These patients were treated with ECMO as salvage respiratory therapy. Outcomes included clinical data, ventilatory and blood gas characteristics, survival, and complications.
Results
Fifteen patients (83.3%) were previously treated in prone position. The indication of VV-ECMO was established at an early stage after a mean (SD) of 3.8 (2.5) days on mechanical ventilation. The mean duration of ECMO was 10.4 days, and 16 patients (88.9%) required venous cannulation, mostly femoral-internal jugular. The mean length of ICU stay was 27 days and the mean hospital stay was 42.1 days. The ICU survival rate was 55.5% (n = 10) and the hospital survival rate was 50% (n = 9).
Conclusions
This clinical study in a small series of ICU patients treated with ECMO confirms the usefulness of this technique as a ventilatory support in patients with refractory hypoxemic respiratory failure. However, the indication of this procedure is also committed to an ethical reflection considering the possible futility of the measure on a case-by-case basis and associated complications.
Keywords: Hypoxemia, Respiratory failure, Extracorporeal membrane oxygenation, salvage respiratory therapy
1. Introduction
The severe forms of acute respiratory distress syndrome (ARDS) have a very high mortality over 45% as defined at the Berlin consensus Conference [1] and remain a challenge for the clinician. The occurrence of severe rapid-onset ARDS causing refractory hypoxemia during the 2009 influenza A (H1N1) pandemic, prompted resurgence of extracorporeal life support techniques, including extracorporeal membrane oxygenation (ECMO). It has been shown that early use of ECMO in combination with protective ventilation gives favorable results in severe hypoxemia [[2], [3], [4], [5]] and should be included in the treatment algorithm of ARDS [2]. The use of veno-venous ECMO (vv-ECMO) in patients with ARDS and refractory hypoxemia and/or hypercapnia promotes lung recovery by allowing ultraprotective ventilation strategies and resting the lungs [3], which may be associated with an increase in survival. The installation of ECMO reduces the risk of lung injury caused by mechanical ventilation and minimizes intraalveolar pressure in positive pressure mechanical ventilation, promoting ultraprotective ventilation [6,7] using plateau airway pressure < 25 cm H2O, tidal volume < 3 mL/kg, high positive-end expiratory pressure (PEEP) > 8–10 cm H2O, with respiratory frequency of ≤10 per minute, and a fraction of inspired oxygen (FiO2) of <0.6 [2,3].
The predictable reversibility of lung lesions and the absence of any other therapeutic limitation are indispensable prerequisites to the use of ECMO [2]. vv-ECMO has evolved substantially and has become a widespread technique with progressively improving outcomes in recent years [8]. Appropriate patient selection, timing and use of validated treatment options, including prone positioning before initiation of extracorporeal support have been reported to be key factors for treatment success [9]. Systematic reviews and meta-analysis have provided encouraging results in patients with refractory ARDS who receive veno-venous ECMO with survival rates around 60% at hospital discharge despite initial high illness severity [9,10]. However, real-life studies are needed to further assess the optimal use, outcomes, and different aspects of ECMO care. The aim of this study was to report clinical outcomes in adult patients with acute hypoxemic respiratory failure refractory to mechanical ventilation treated with ECMO.
2. Materials and methods
2.1. Study design
Between July 2011 and October 2017, a case series study was performed at the ICU of a single acute-care tertiary hospital (Hospital Universitario de Bellvitge) in Barcelona, Spain, in which 34 acute-care beds and 4 intermediate-care beds are available. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, and the protocol was approved by the Institutional Review Board. Since the patients in this study were all sedated and ventilated, written informed consent was obtained from the patient's next of kin.
2.2. Patients
Critically ill adult patients with ARDS and hypoxemia refractory to lung-recruitment maneuvers and prono positioning were eligible for veno-venous ECMO. Extracorporeal life support was considered if despite a protective ventilation strategy (involving the use of prone positioning) the PaO2/FiO2 ratio was below 50 mm Hg when FiO2 = 1 for at least 3 hours, if the PaO2/FiO2 ratio was below 80 mm Hg when FiO2 = 1 for more than 6 hours, and/or if there was respiratory acidosis with pH < 7.20 for over 6 hours. Patients with massive pulmonary embolism and acute right ventricular dysfunction (acute cor pulmonale) were also candidates for ECMO. Veno arterial ECMO must be considered in cardiogenic shock, and vv-ECMO in ARDS with isolated respiratory failure. But there is a special situation in acute cor pulmonale which could have indication of vv-ECMO. In severe forms of ARDS, patients often have evidence of acute right heart failure and pulmonary arterial hypertension because hypoxemia, hypercarbia and acidosis are very potent pulmonary vasoconstrictors. In vv-ECMO therapy the pulmonary perfusión with oxygenated blood leads to a fall in the right heart afterload improving right ventricular cardiac output.
Exclusion criteria were as follows [11]: contraindications to anticoagulation, including active bleeding or high risk of bleeding; intracranial bleeding or potentially hemorrhagic intracranial lesions; duration of mechanical ventilation ≥ 7 days; severe immunosuppression; multiorgan failure syndrome (Sequential Organ Failure Assessment [SOFA] score > 15); coma following cardiac arrest; unpredictable reversibility of lung lesions; age > 70 years; body mass index (BMI) > 35 kg/m2; and moribund patients with a very low chance of meaningful survival with ECMO treatment.
2.3. Study procedures and data collection
Patients underwent ultrasound-guided vascular cannulation following expert panel recommendations [2], with femoral-jugular venous access using a 23–29 F cannula for drainage and 19–21 F for return, and confirmation of correct position of the cannulas. A non-occlusive centrifugal pump system (CARDIOHELP HLS set Advanced, Maquet/Getinge Group Spain, S.L., Madrid, Spain) and the Rotaflow RF-32 Centrifugal Pump, with cannulas of sufficient diameter to allow flow rates of 4–7 L/min were used. The oxygenator membrane is made from polymethylpentene. Percutaneous femoral and internal vein cannulation was performed in the ICU by the cardiac surgery team with the medical support of intensivists. The extracorporeal system and the venous cannulas were also removed in the ICU.
Data recorded in all patients included demographics (age, sex), anthropometric parameters, SOFA score, comorbid diseases, respiratory and ventilation characteristics, hemodynamic and vasoactive features, complete biochemical profile with lactate, complete hemogram and coagulation tests, transfusion requirements, complications related and unrelated to ECMO, and status at ICU discharge.
Descriptive statistics are presented. Categorical variables are expressed as frequencies and percentages, and continuous variables as mean and standard deviation (SD).
3. Results
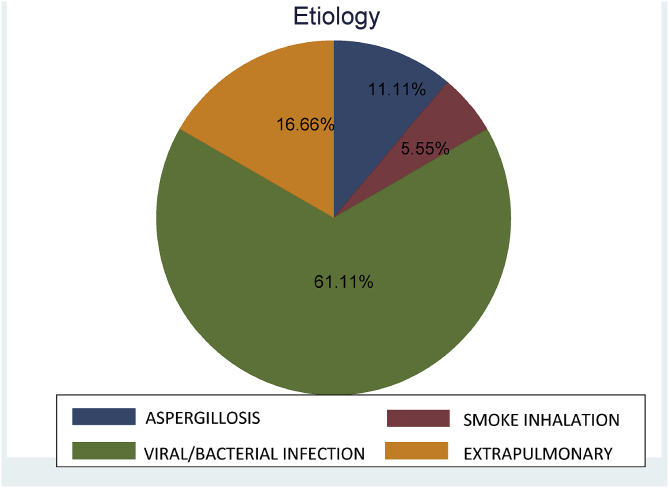
During the study period, a total of 18 patients, 11 men and 7 women, with a mean (SD) age of 44.0 (12.6) years and a mean BMI of 29.1 (3.5) kg/m2 underwent ECMO. The mean SOFA score was 10 (4.0). Eleven patients (61.1%) had a potentially reversible pulmonary cause (bacterial/viral/fungal infections) bacterial or viral (H1N1 virus) in septic patients secondary to pneumonia in 7, invasive pulmonary aspergillosis in 2, Pneumocystis jirovecii pneumonia in 1, smoke inhalation injury in 1. Causes of ARDS in the remaining 7 (38.9%) patients included respiratory distress in the immediate postoperative period after cardiac surgery in 3, immunosuppression in 2 septic patients diagnosed with B-cell and T-cell lymphoma, respectively, acute pancreatitis with septic shock in 1, and thrombotic thrombocytopenic purpura in 1 (Fig. 1). All patients had received corticosteroids and 15 (83.3%) patients required vasopressor support. Severity-related data and respiratory parameters before the introduction of ECMO are shown in Table 1. Prone positioning before ECMO was used in 15 patients (83.3%) and nitric oxide to improve acute pulmonary hypertension in 7.
Fig. 1.
ARDS etiology in VV ECMO patients.
Table 1.
Severity-related characteristics and respiratory parameters before ECMO.
| Variables | Number of patients (%) |
|---|---|
| Total patients | 18 (100) |
| Non-invasive mechanical ventilation (NIMV) before intubation | 7 (38.9) |
| Mechanical ventilation | |
| Pressure-control ventilation (PCV) | 13 (72.2) |
| Volume-controlled ventilation (VCV) | 5 (27.8) |
| Duration of mechanical ventilation, days, mean (SD) | 3.8 (2.4) |
| FiO2 = 1 | 18 (100) |
| PEEP, cm H2O, mean (SD) | 9.8 (2.7) |
| Plateau airway pressure, cm H2O, mean (SD) | 30.0 (2.8) |
| PaO2/FiO2 | 85.6 (42.2) |
| PaCO2, mm Hg, mean (SD) | 72.7 (30.3) |
| pH, mean (SD) | 7.2 (0.1) |
FiO2: fraction of inspired oxygen; PEEP: positive-end expiratory pressure; PaO2: arterial partial oxygen pressure; PaCO2: arterial carbon dioxide partial pressure.
Data expressed as frequencies and percentages in parenthesis unless otherwise stated.
Veno-venous ECMO was performed in 16 (88.9%) patients and veno-arterial ECMO in the remaining 2. The mean duration of ECMO was 10.4 days. The mean flow at 24 hours after ECMO initiation was 4.4 (0.8) L/min and at terminating ECMO 4.1 (0.7) L/min.
Pneumothorax occurred in 6 patients and acute renal failure in 7 patients, 5 of which required real replacement procedures. Transfusion of blood derivatives was necessary in 14 patients and 15 (83.3%) patients required vasopressor support. Complications related to the ECMO procedure are shown in Table 2. Bleeding was the most frequent complication (72.2%) followed by hypovolemia (66.7%), and thrombocytopenia (50%).
Table 2.
ECMO-related complications.
| Variables | Number of patients (%) |
|---|---|
| Total patients | 18 (100) |
| Bleeding complications | 13 (72.2) |
| At the cannulation site | 11 |
| Upper gastrointestinal hemorrhagea | 1 |
| Intracranial hemorrhageb | 1 |
| Hypovolemia | 12 (66.7) |
| Thrombocytopenia | 9 (50) |
| Thrombosis | 3 (16.7) |
| Catheter-related sepsis | 4 (22.2) |
| Embolism | 1 (5.5) |
Anticoagulation was discontinued and ECMO was stopped.
Intracranial hemorrhage was the cause of death.
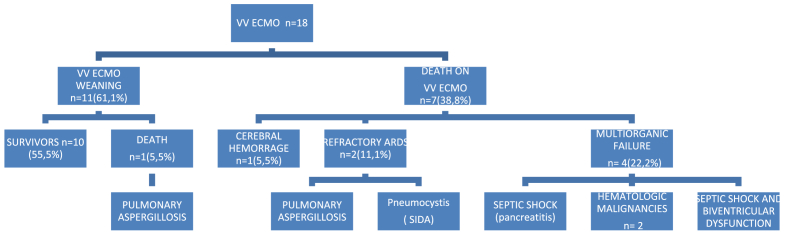
The mean duration of mechanical ventilation was 25.5 (16.7) days. Percutaneous tracheostomy for minimizing airway lesions due to prolonged orotracheal intubation and to facilitate weaning was performed in 14 patients. The mean length of ICU stay was 27.0 (18.2) days and the mean length of hospitalization 42.1 (26.6) days. Weaning from ECMO was performed in 10 (55.5%) patients. Eight patients died, with a mortality rate of 44.4%. Details of respiratory parameters in survivors and non-survivors are shown in Table 3. Four (50%) of the eight patients who died did not fulfilled current indications for veno-venous ECMO because of multiorgan failure due to hematological disease with severe immunosuppression in 2 patients, septic shock and biventricular dysfunction in 1, and septic shock secondary to acute pancreatitis in 1 (Fig. 2).
Table 3.
Characteristics of ventilatory parameters in ECMO survivors and non-survivors.
| Variables | Survivors (n = 10) | Non-survivors (n = 8) |
|---|---|---|
| Age, years | 43.0 (12.3) | 45.4 (13.6) |
| Body mass index (BMI), kg/m2 | 29.4 (3.9) | 28.7 (3.2) |
| SOFA score | 9.8 (3.6) | 10.2 (4.8) |
| Duration of mechanical ventilation before ECMO, days | 4.1 (2.3) | 3.5 (2.7) |
| Total duration of mechanical ventilation, days | 32.3 (17.8) | 17.0 (11.2) |
| Before ECMO | ||
| PEEP, cm H2O | 10.3 (2.2) | 9.2 (3.3) |
| Plateau airway pressure, cm H2O | 29.3 (2.4) | 31.4 (3.1) |
| FiO2 | 1 | 0.9 (9.1) |
| PaO2/FiO2 | 87.9 (45.5) | 82.7 (35.8) |
| PaCO2, mm Hg | 68.8 (36.6) | 78.2 (20.8) |
| pH | 7.2 (0.1) | 7.2 (0.1) |
| After 24 h of ECMO initiation | ||
| PEEP, cm H2O | 9.3 (2.0) | 9.0 (2.9) |
| Plateau airway pressure, cm H2O | 23.3 (2.6) | 28.0 (0) |
| FiO2 | 0.5 (0.3) | 0.6 (0.3) |
| PaO2/FiO2 | 110.5 (71.8) | 67.2 (26.1) |
| PaCO2, mm Hg | 43.1 (8.8) | 49.6 (14.1) |
| pH | 7.4 (0.1) | 7.3 (0.1) |
| At ECMO removal | ||
| PEEP, cm H2O | 11.1 (2.3) | 7.1 (3.5) |
| Plateau airway pressure, cm H2O | 22.4 (4.8) | 23.3 (5.0) |
| FiO2 | 0.4 (0.1) | 0.8 (0.3) |
| PaO2/FiO2 | 150.9 (47.5) | 59.9 (25.5) |
| PaCO2, mm Hg | 43.7 (7.8) | 43.9 (9.2) |
| pH | 7.4 (0.1) | 7.3 (0.1) |
| ECMO sweep gas flow on first day, L/min | 4.2 (1.8) | 5.1 (2.4) |
| ECMO sweep gas flow on last day, L/min | 2.7 (2.2) | 5.6 (2.9) |
FiO2: fraction of inspired oxygen; PEEP: positive-end expiratory pressure; PaO2: arterial partial oxygen pressure; PaCO2: arterial carbon dioxide partial pressure.
Data expressed as mean (SD).
Fig. 2.
VV ECMO patients flow chart.
4. Discussion
This study reports a single-center experience with the use of ECMO in critically ill adult patients treated for refractory hypoxemic ARDS with ECMO. This procedure was successful in 10 out of 18 patients. The mortality rate of our series of 44% is higher than that found in other studies. In a retrospective observational study of three international high-volume ECMO centers with 168 patients, the mortality rate was 29% [12]. In the study carried out in Australia and New Zealand in which 68 patients with severe influenza-associated ARDS were treated with ECMO, the mortality rate was 21% [13]. In the Italian ECMOnet study with 60 patients with influenza A (H1N1)-associated respiratory distress syndrome, the mortality rate was 32% [14]. The development of clinical protocols with a more adequate selection of patients and more specific indications of the technique is essential to improve the survival of patients undergoing ECMO. In our center, a specific ECMO protocol was established in 2016 and some of the patients were reported were selected before implementation of guidelines, which may account in part for the ICU mortality of 44%. In a recent international randomized trial of ECMO in ARDS, EOLIA trial [15], 124 patients were assigned to the ECMO group and 125 to conventional treatment (control group). After 60 days, significant differences in mortality were not found, although the mortality rate was lower in the ECMO group (35% vs 46%). Also, in control patients requiring crossover to ECMO, a high mortality of 57% was reported. As secondary objectives, patients in the ECMO group showed more free days of prone positioning and continuous renal replacement therapy. In this respect, in our study all hematological patients with severe immunosuppression treated with ECMO before 2016 died, with a 100% mortality rate. Our series included 3 immunosuppressed patients, two hematological patients with B- and T-cell lymphomas, respectively, and 1 patient with acquired immunodeficiency syndrome (AIDS). Severe immunosuppression may be a contraindication for ECMO and in these cases, the treatment is probably futile. If an ongoing ECMO support is futile or no longer meets its intended goals, the process of ECMO separation should be thoughtfully coordinated by a multidisciplinary team [16]. In a recent retrospective multicenter study in 10 international ICUs with high ECMO-case volumes, 203 immunocompromised patients with severe ARDS were treated with ECMO [17]. In 203 patients followed for 6 months, survival was only 30%. Patients with hematological malignancies had significantly poorer outcomes than others. This study adds evidence of restricting ECMO to patients with realistic oncological/therapeutic prognoses, acceptable functional status and few pre-ECMO mortality-risk factors.
The SOFA score can be used to evaluate accurately the risk of death in ARSD patients [18] and was calculated before initiation of ECMO, with a mean score of 10. The risk-benefit ratio of ECMO in ARDS should be considered unfavorable in case of multiorgan failure syndrome (SOFA > 15) [2] and has been an exclusion criterion of the study. Also, prone positioning was used before ECMO in the majority of patients (15 out of 18) and muscle relaxation during the first 48 h of severe ADRS. Prone positioning improves oxygenation and respiratory system compliance [19] and has been shown to be associated with a decrease in mortality in patients with hypoxemic severe respiratory failure [[20], [21], [22]]. However, in a systematic review and meta-analysis of 10 trials with 1867 patients, prone positioning showed a statistically significant improvement of survival especially in the more hypoxemic patients, but was also associated with an increased risk of pressure ulcers, endotracheal tube obstruction, and chest tube dislodgements [21].
There is evidence of the role of ECMO referral centers with properly trained staff teams in ECMO management as crucial determinants of survival for patients with severe ARDS [23]. On the other hand, a successful ECMO program requires a significant multidisciplinary and organizational commitment to ensure necessary resources and personnel [4]. In our hospital the intensivists are in close collaboration with the cardiac surgery service (both cardiac surgeons and perfusionists) during cannulation and withdrawal of ECMO, as well as in all possible incidents and mechanical complications related to the technique [2,24]. The timing of ECMO is usually based on the severity of ARDS but there is evidence that early initiation of ECMO improves survival [2,12,25]. In all patients in this series, ECMO was started within 7 days of mechanical ventilation. In relation to the timing of ECMO initiation, different studies have shown the negative impact of duration of mechanical ventilation prior to ECMO, especially after 7 days, with inverse relationship between duration of ventilation mechanical and survival. It's important to highlight the necessity of an early start with the goal of minimizing the pulmonary lesion associated with the mechanical ventilation (VILI).
Usually both ultrasound-guided cannulation and weaning of ECMO are performed in the ICU. In our patients the cannulation technique involved cannulating the femoral vein for drainage with 23–29 F (55 cm length) using higher diameters 29–31 F in cases of extreme hypoxemia or sepsis with hyperdynamia to ensure high flows that exceed 60% of the patient's cardiac output [26,27]. Monitoring of the ECMO system, including the extracorporeal circuit, membrane oxygenation, system pressures, activated clotting time (ACT) is carried out by intensive care nurses. Patients receiving ECMO often require continuous renal replacement therapy (CRRT), and a common method to provide CRRT is via the use of an in-line hemofilter [28]. Pressure-control ventilation (PCV) is the standard mode of ventilation in our ICU and was used in most of the patients. The mean PEEP at 24 h of starting ECMO was 9.1 cm H2O (range 8–10). Of note, that the inclusion of patients treated with ECMO before 2016 and prior to the implementation of the ECMO protocol in our unit may account for the low PEEP in the study group. In the 2016 protocol it is stated the use of an initial PEEP >10 cm H2O as alveolar recruitment maneuver always under ultraprotective ventilation with plateau pressure <25 cm H2O and driving pressure <12 cm H2O. The Extracorporeal Life Support Organization (ELSO) [29] recommends a PEEP of 10 cm H2O, but it has been shown that high PEEP (12 ± 3 cm H2O) at least during the first 3 days of ECMO are associated with a survival benefit [12,26]. We followed the ultraprotective mechanical ventilation strategy targeting the concept of “lung rest”. At initiation of ECMO in severe hypoxemic clinical conditions, we used high flow rates (>4 L/min) with oxygen fraction delivered by the extracorporeal circuit (FECO2) of 100% and >6 L/min seep gas flow to minimize the aggressiveness of the ventilatory settings. Weaning from ECMO has been protocolized with progressive decrease of ECMO parameters, including FECO2 and L/min of gas flow [3,30,31] avoiding reduction of ECMO pump flow and subsequent risk of bleeding events associated with anticoagulation increases.
In our protocol, we prioritized on global accumulated fluid balances for patients with ARDS. Thus, the therapeutic management was stricter for negative balances, especially during the first days when hypoxemia is more critical. There is a bio-trauma component that produces an inflammatory reaction on the lung lesion. That reaction is mediated by macrophages activation and vascular leak with alveolar edema. As a consequence a worsening of respiratory function is produced [3].
Bleeding complications are reported in 30–40% of patients on ECMO [[2], [3], [4],16,24,32]. Hemorrhage is a frequent adverse event in these patients who are critically ill, exposed to anticoagulation and susceptible to coagulopathy and platelet dysfunction, consumption coagulopathies, or circuit induced fibrinolysis [32]. In the present study, bleeding complications occurred in 13 patients, with intracerebral hemorrhage being the cause of death in one of them. Bleeding complications affect the prognosis and requirement of transfusions is a common feature in ECMO patients [3,32]. Bleeding on ECMO is usually managed conservatively and in one of our patients with upper gastrointestinal bleeding, anticoagulation was interrupted and ECMO needed to be stopped.
There has been an increasing interest in identifying prognostic factors for ECMO, and risk scores designed for pre-ECMO mortality prediction in patients with acute ARDS have been developed. Despite the introduction of the ECMOnet score [14] and the PRESERVE score [33], prediction of mortality in EMCO patients remains a challenge due to large patient heterogeneity. Also, these existing validated mortality prediction tools for patients undergoing veno-venous ECMO for refractory lung failure have shown suboptimal performance [34]. However, risk scores may function as a useful supplementary tool for clinicians but we believe that thorough clinical evaluation on a case-to-case basis still remains the cornerstone of ECMO handling.
Our study has several limitations. First, this is a single-center case series report of ECMO data and patient's characteristics in a small study population. Second, although this represents the totality of our experience over more than 5 years, the number of ECMO episodes was not high precluding any subgroup analysis. Also, because of the reduced number of patients included in the study, a multivariate analysis to identify risk factors for ECMO failure was not performed.
5. Conclusions
In our experience veno-venous ECMO is a feasible option for patients with severe ARDS and refractory hypoxemia, but the execution of ECMO is not free of complications which account for a not negligible rate of morbidity. Complications are not infrequent and need to be balanced against the potential benefits of this mode of therapy. Appropriate patient selection is critical for successful ECMO outcomes. Lung injury irreversibility and futility in ECMO are ethical issues inherent in the decisions when the prognosis remains uncertain.
Declarations of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors thank Marta Pulido, MD, for editing the manuscript and editorial assistance.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.rmcr.2018.09.007.
Contributor Information
Elisabet Periche Pedra, Email: lisperiche@hotmail.com, eperiche@bellvitgehospital.cat.
Melinda Rita Koborzan, Email: koborzanmelinda@gmail.com.
Fabrizio Sbraga, Email: sbraga@bellvitgehospital.cat.
Arnau Blasco Lucas, Email: arnaulasco@hotmail.com.
David Toral Sepúlveda, Email: dtoral@bellvitgehospital.cat.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Ferguson N.D., Fan E., Camporota L., Antonelli M., Anzueto A., Beale R. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 2.Richard C., Argaud L., Blet A., Boulain T., Contentin L., Dechartres A. Extracorporeal life support for patients with acute respiratory distress syndrome: report of a Consensus Conference. Ann. Intensive Care. 2014;4:15. doi: 10.1186/2110-5820-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Sorbo L., Cypel M., Fan E. Extracorporeal life support for adults with severe acute respiratory failure. Lancet Respir. Med. 2014;2:154–164. doi: 10.1016/S2213-2600(13)70197-8. [DOI] [PubMed] [Google Scholar]
- 4.Mosier J.M., Kelsey M., Raz Y., Gunnerson K.J., Meyer R., Hypes C.D. Extracorporeal membrane oxygenation (ECMO) for critically ill adults in the emergency department: history, current applications, and future directions. Crit. Care. 2015;19:431. doi: 10.1186/s13054-015-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonastre J., Suberviola B., Pozo J.C., Guerrero J.E., Torres A., Rodríguez A. Extracorporeal lung support in patients with severe respiratory failure secondary to the 2010-2011 winter seasonal outbreak of influenza A (H1N1) in Spain. Med. Intensiva. 2012;36:193–199. doi: 10.1016/j.medin.2011.12.004. [Article in Spanish] [DOI] [PubMed] [Google Scholar]
- 6.Schmidt M., Pellegrino V., Combes A., Scheinkestel C., Cooper D.J., Hodgson C. Mechanical ventilation during extracorporeal membrane oxygenation. Crit. Care. 2014;18:203. doi: 10.1186/cc13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodie D., Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N. Engl. J. Med. 2011;365:1905–1914. doi: 10.1056/NEJMct1103720. [DOI] [PubMed] [Google Scholar]
- 8.Peek G.J., Mugford M., Tiruvoipati R., Wilson A., Allen E., Thalanany M.M. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 9.Vaquer S., de Haro C., Peruga P., Oliva J.C., Artigas A. Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann. Intensive Care. 2017;7:51. doi: 10.1186/s13613-017-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tillmann B.W., Klingel M.L., Iansavichene A.E., Ball I.M., Nagpal A.D. Extracorporeal membrane oxygenation (ECMO) as a treatment strategy for severe acute respiratory distress syndrome (ARDS) in the low tidal volume era: a systematic review. J. Crit. Care. 2017;41:64–71. doi: 10.1016/j.jcrc.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt M., Bréchot N., Combes A. Ten situations in which ECMO is unlikely to be successful. Intensive Care Med. 2016;42:750–752. doi: 10.1007/s00134-015-4013-9. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M., Stewart C., Bailey M., Nieszkowska A., Kelly J., Murphy L. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a retrospective international multicenter study. Crit. Care Med. 2015;43:654–664. doi: 10.1097/CCM.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 13.Davies A., Jones D., Bailey M., Beca J., Bellomo R., Blackwell N. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 14.Pappalardo F., Pieri M., Greco T., Patroniti N., Pesenti A., Arcadipane A. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: the ECMOnet score. Intensive Care Med. 2013;39:275–281. doi: 10.1007/s00134-012-2747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Combes A., Hajage D., Capellier G., Demoule A., Lavoué S., Guervilly C. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N. Engl. J. Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 16.Makdisi T., Makdisi G. Extra corporeal membrane oxygenation support: ethical dilemmas. Ann. Transl. Med. 2017;5:112. doi: 10.21037/atm.2017.01.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt M., Schellongowski P., Patroniti N., Taccone F.S., Reis Miranda D., Reuter J. Six-month outcome of immunocompromised severe ARDS patients rescued by ECMO. An international multicenter retrospective study. Am. J. Respir. Crit. Care Med. 2018 Jan 3 doi: 10.1164/rccm.201708-1761OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roch A., Hraiech S., Masson E., Grisoli D., Forel J.M., Boucekine M. Outcome of acute respiratory distress syndrome patients treated with extracorporeal membrane oxygenation and brought to a referral center. Intensive Care Med. 2014;40:74–83. doi: 10.1007/s00134-013-3135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimmoun A., Roche S., Bridey C., Vanhuyse F., Fay R., Girerd N. Prolonged prone positioning under VV-ECMO is safe and improves oxygenation and respiratory compliance. Ann. Intensive Care. 2015;5:35. doi: 10.1186/s13613-015-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bein T., Grasso S., Moerer O., Quintel M., Guerin C., Deja M. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med. 2016;42:699–711. doi: 10.1007/s00134-016-4325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sud S., Friedrich J.O., Taccone P., Polli F., Adhikari N.K., Latini R. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med. 2010;36:585–599. doi: 10.1007/s00134-009-1748-1. [DOI] [PubMed] [Google Scholar]
- 22.Guérin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 23.Noah M.A., Peek G.J., Finney S.J., Griffiths M.J., Harrison D.A., Grieve R. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306:1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 24.Tulman D.B., Stawicki S.P., Whitson B.A., Gupta S.C., Tripathi R.S., Firstenberg M.S. Veno-venous ECMO: a synopsis of nine key potential challenges, considerations, and controversies. BMC Anesthesiol. 2014;14:65. doi: 10.1186/1471-2253-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z., Gu W.-J., Chen K., Ni H. Mechanical ventilation during extracorporeal membrane oxygenation in patients with acute severe respiratory failure. Can. Respir. J. 2017;2017 doi: 10.1155/2017/1783857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan E., Gattinoni L., Combes A., Schmidt M., Peek G., Brodie D. Venovenous extracorporeal membrane oxygenation for acute respiratory failure: a clinical review from an international group of experts. Intensive Care Med. 2016;42:712–724. doi: 10.1007/s00134-016-4314-7. [DOI] [PubMed] [Google Scholar]
- 27.Levy B., Taccone F.S., Guarracino F. Recent developments in the management of persistent hypoxemia under veno-venous ECMO. Intensive Care Med. 2015;41:508–510. doi: 10.1007/s00134-014-3579-y. [DOI] [PubMed] [Google Scholar]
- 28.Chen H., Yu R.G., Yin N.N., Zhou J.X. Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic review. Crit. Care. 2014;18:675. doi: 10.1186/s13054-014-0675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Extracorporeal Life Support Organization (ELSO) 2017. Guidelines for Adult Respiratory Failure August.https://www.elso.org/Resources/Guidelines.aspx Available from: [Google Scholar]
- 30.Peek G.J., Moore H.M., Moore N., Sosnowski A.W., Firmin R.K. Extracorporeal membrane oxygenation for adult respiratory failure. Chest. 1997;112:759–764. doi: 10.1378/chest.112.3.759. [DOI] [PubMed] [Google Scholar]
- 31.Bréchot N., Luyt C.E., Schmidt M., Leprince P., Trouillet J.L., Léger P. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit. Care Med. 2013;41:1616–1626. doi: 10.1097/CCM.0b013e31828a2370. [DOI] [PubMed] [Google Scholar]
- 32.Aubron C., Cheng A.C., Pilcher D., Leong T., Magrin G., Cooper D.J. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit. Care. 2013;17:R73. doi: 10.1186/cc12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt M., Zogheib E., Rozé H., Repesse X., Lebreton G., Luyt C.E. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39:1704–1713. doi: 10.1007/s00134-013-3037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enger T., Philipp A., Videm V., Lubnow M., Wahba A., Fischer M. Prediction of mortality in adult patients with severe acute lung failure receiving veno-venous extracorporeal membrane oxygenation: a prospective observational study. Crit. Care. 2014;18:R67. doi: 10.1186/cc13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.