Abstract
Recent studies indicate a stage-specific, differential role for the oncogene Akt on various cancers. In prostate cancer (PCa), suppression of Akt activity in the advanced stages promoted transforming growth factor-β (TGFβ) pathway-mediated epithelial-to-mesenchymal transition (EMT) and metastasis to the lungs. In the current study, we performed Affymetrix analysis to compare the expression profile of microRNAs in the mouse prostate tissues collected at the prostatic inter-epithelial neoplasia (PIN) stage from Transgenic adenocarcinoma of the mouse (TRAMP)/Akt1+/+ versus TRAMP/Akt1–/– mice, and at the advanced stage from TRAMP/Akt1+/+ mice treated with triciribine (Akt inhibitor) versus DMSO-treated control. Our analysis demonstrates that in the early stage, Akt1 in the TRAMP prostate tumors express a set of miRNAs responsible for regulating cancer cell survival, proliferation, and tumor growth, whereas, in the advanced stages, a different set of miRNAs that promote EMT and cancer metastasis is expressed. Our study has identified novel Akt-regulated signature microRNAs in the early and advanced PCa and demonstrates their differential effects on PCa growth and metastasis.
Keywords: Biochemistry, Bioinformatics, Cancer research
1. Introduction
Metastatic prostate cancer (PCa) is the leading cause of cancer-related deaths in men in the US and the Europe [1]. Although slow-growing cancer, PCa that has metastasized to the bone, lungs, and brain are difficult to treat [2]. Uncertainties in the molecular mechanisms leading to the switch from early to advanced PCa is the underlying reason for the unreliable screening measures and ineffective treatments that are currently used in the management of PCa [3]. Recent studies from our laboratory have indicated that transforming growth factor-β (TGFβ)-induced epithelial-to-mesenchymal transition (EMT) plays an important role in this process [4]. TGFβ, that plays a tumor suppressor role in the early stages switches to a metastasis promoter in the advanced stages [4, 5, 6]. However, the mechanisms that regulate this switch are not clearly understood.
Recently we showed that Akt1, the predominant Akt isoform in the PCa cells [7] and vascular cells [8, 9, 10] plays a dual, reciprocal role in tumor growth and metastasis [11]. Similar results have also been reported in four other types of cancer such as the breast [12, 13], liver [14], non-small cell lung [15] and head and neck [16]. Furthermore, a very recent study from our lab has indicated that the specific loss of Akt1 in endothelial cells promotes prostate cancer metastasis [17]. These studies have identified Akt1 to promote tumor growth but suppress cancer metastasis. The above studies also have identified a reciprocal link between Akt1 and TGFβ pathways in promoting cancer cell EMT and metastasis. Until today, the molecular mechanisms connecting these two pathways in the regulation of EMT and metastasis have not been identified.
Micro-RNAs are novel players in the modulation of cellular signaling in various physiological and pathological processes [18]. There are several microRNAs that have been identified to regulate the tumor progression, EMT, and metastasis in PCa [19]. Interestingly, one of the studies linking Akt1 suppression to EMT in breast cancer demonstrated the involvement of microRNAs, mir200 cluster in particular in the process [12]. However, such a link between Akt1 activity, microRNAs expression regulation, tumor growth, EMT, and metastasis has not been shown in other cancer types.
In the current study, we performed microRNA array on an Affymetrix platform to identify the signature microRNAs followed by bioinformatics analysis to identify the potential microRNA regulated pathways in the early prostatic inter-epithelial neoplasia (PIN) [20] stage and the advanced stage (31 week old mice) TRansgenic Adenocarcinoma of the Mouse Prostate (TRAMP) PCa tissues in the presence and absence of Akt1 gene in the early stage (12-week old mice; PIN stage) and between DMSO and triciribine (Akt inhibitor) treatment in the advanced stage. Our results indicate different signatures of the microRNA by Akt1 in the PIN and advanced PCa, with a clear role of Akt1-regulated microRNAs in the regulation of cell survival and proliferation in the early stages and EMT and metastasis in the advanced stages.
2. Materials and methods
2.1. Generation and genotyping of TRAMP/Akt1+/– and TRAMP/Akt1–/– mice
Akt1–/–mice (C57BL/6 background) were generated and maintained as reported previously [8]. In order to generate TRAMP/Akt1–/– transgenic mice, C57BL/6 Akt1+/– male was crossed with TRAMP (C57BL/6 background) female mice (Jackson, Bar Harbor, ME). All experiments were carried out in accordance with guidelines set by Augusta VA Medical Center. DNA was extracted from the tails of 10- to 21-day old litters (Qiagen, Valencia, CA). TRAMP transgene (600 bp) was detected by PCR (forward: 5′-GCGCTGCTGACTTTCTAAACATAAG-3′ and reverse: 5′-GAGCTCACGTTAAGTTTTGATGTGT-3′) with an annealing temperature of 55 °C. The internal positive control (forward: 5′-CTAGGCCACAGAATTGAAAGATCT-3′ and reverse: 5′-GTAGGTGGAAATTCAGCATCATCC-3′) produced a 324 bp fragment. Primers to confirm Akt1 gene knockout (forward: 5′-TCCAGGACCAGGGGAGGATGTTTCTACTG-3′ and reverse: 5′-ACGACATGGTGCAGCAATGGCCAGCG-3′) yielded a 600 bp band. Primers for Neo gene (forward: 5′-TGAGACGTGCTACTTCCATTTGTCACGTCC-3′ and reverse: 5′-ACAGGCCGCTACTATGCCATGAAGATCCTC-3′) generated a 1200 bp fragment [11]. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals. All tests were performed with the approval of the Charlie Norwood VAMC Institutional Animal Care and Use Committee (approval reference #15-08-083).
2.2. TRAMP prostate miRNA isolation and microarray profiling
We subjected the prostate tissues collected from TRAMP/Akt1+/+ and TRAMP/Akt1–/– mice at 12 weeks (PIN stage) age for Affymetrix® technology-based microRNA array analysis. To determine the specific effect of pharmacological suppression of Akt in advanced PCa, we subjected the prostate tissues collected from TRAMP/Akt1+/+ mice treated with DMSO (control) or triciribine (Selleckchem, Houston, TX) for 5 weeks starting from week 26 and collecting at 31 weeks for the microRNA array analysis. miRNAs were isolated from mouse prostates using Qiagen miRNeasy Kit according to manufacturer's protocol. The concentration of miRNA was determined using a NanoDrop spectrophotometer (Thermo Scientific) and the quality of miRNA was analyzed using an Agilent 2100 Bioanalyzer. Microarrays were performed on miRNA using an Affymetrix GeneChip® miRNA 4.0 Array at the Integrated Genomics Core, Augusta University, GA. The miRNA profiles for the early stage prostate tumors with or without the Akt1 gene and the advanced prostate tumors with DMSO (control) or triciribine treatment were determined and analyzed.
2.3. Normalization and pathway analysis of microRNA array
The miRNA expression was normalized to the average of the house keeping genes (snoRNA251, snoRNA202, snoRNA142, and U6) provided in the miRNA PCR arrays. The miRNA profile of TRAMP/Akt1–/– was normalized to TRAMP/Akt1+/+ (early stage), while the miRNA profile of triciribine treated advanced tumor-bearing TRAMP/Akt1+/+ was normalized with the respective DMSO treated controls (late stage). T-tests were used to calculate the p-value to determine the significant difference in miRNA expression between the groups. The p-value cutoff of 0.05 and the miRNAs with a fold change above 1.5 were considered differentially expressed for further analyses. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathway analyses were performed using DIANA-miRPath version 3.0 (http://diana.imis.athena-innovation.gr/DianaTools/index.php) on differentially expressed microRNAs target genes [21]. Analysis of EMT genes regulated by microRNAs was determined using the epithelial-to-mesenchymal transition gene database (dbEMT; http://dbemt.bioinfo-minzhao.org/). Principal component analysis (PCA) was performed between control and test TRAMP tumors both in the early and advanced stages.
2.4. Ingenuity pathway analysis
Ingenuity Pathway Analysis (IPA, Qiagen Bioinformatics) is a software that transforms a list of molecules into a set of relevant networks associated with pathology based on extensive records maintained in the Ingenuity Pathways Knowledge Base [22]. Highly interconnected networks are predicted to represent a significant biological function [23]. IPA was used to connect 132 genome-wide association study (GWAS)- implicated cancer genes along with microRNA and various cancer pathways [24, 25]. Significantly changed miRNAs associated with Akt1 inhibition from the two experimental sets were uploaded in IPA and core analyzed. Genes that are differentially regulated by miRNAs, as well as miRNAs, were mapped to molecular pathways, canonical pathways, and biological functions that are predominantly associated with cancer. All genes that were directly affected by the pathway in cancer are shown.
2.5. Data and statistical analysis
All the studies performed using the KEGG, Ingenuity, and miR-Path databases were performed in an unbiased manner without focusing on any specific targets or signaling pathways. dbEMT database analysis was performed specifically to look into the known and potential genes/targets regulated by each or combination of the most up- or down-regulated miroRNAs as obtained from the KEGG and miR-Path analysis on EMT and cancer metastasis. All the data are presented as mean ± SD and were calculated from multiple independent experiments performed in quadruplicates. For normalized data analysis, data was confirmed that normality assumption was satisfied and analyzed using paired sample t-test (dependent t-test) and/or further confirmed with non-parametric test Wilcoxon signed rank test. For all other analyses, Student's two-tailed t-test or ANOVA test were used to determine significant differences between treatment and control values using the GraphPad Prism 4.03 software and SPSS 17.0 software. Data with P < 0.05 were considered significant.
3. Results
3.1. Akt1 gene deletion in the early (PIN) and pharmacological suppression in the advanced (metastasis) PCa in TRAMP prostate reveal expression changes in microRNAs involved in different signaling pathways
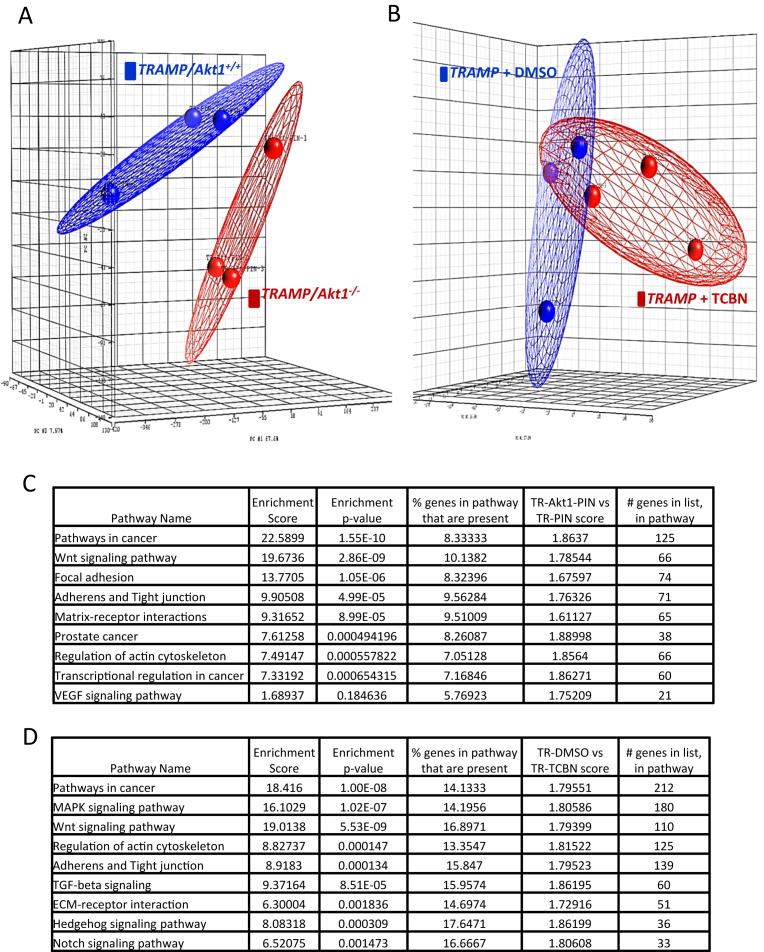
Principle component analysis (PCA) mapping of TRAMP/Akt1+/+ and TRAMP/Akt1–/– showed that TRAMP/Akt1+/+ group was clustered distinctly from TRAMP/Akt1–/– group (Fig. 1A). KEGG pathway based all microRNA target prediction analysis indicated changes in the expression of several genes involved in the regulation of cancer growth, Wnt signaling pathway, focal adhesion, extracellular matrix interactions and cell-cell junctions etc. (Fig. 1C). As supported by the literature, these results indicated that Akt1 predominantly regulates cancer pathways, Wnt Signaling pathways, Focal adhesions, junctional proteins, extracellular matrix interactions, actin cytoskeleton and VEGF signaling pathway in the promotion of tumor growth in the early stages and that the absence of Akt1 gene suppresses these effects.
Fig. 1.
Akt-regulated microRNAs differentially regulate PCa pathways in the early and advanced stages. (A) Principle component analysis (PCA) mapping of TRAMP/Akt1+/+ and TRAMP/Akt1–/– profiling. TRAMP/Akt1+/+ group (indicated by red color) was clustered distinctly from TRAMP/Akt1–/– group (indicated by blue color). (B) Principle component analysis (PCA) mapping of 31 weeks old, 5 weeks treated TRAMP + DMSO and TRAMP+ Triciribine prostate tissue profiling. TRAMP + DMSO group (indicated by blue color) was clustered distinctly from TRAMP+ Triciribine group (indicated by red color). (C) Table showing pathways affected by the microRNA expression in TRAMP/Akt1–/– compared to TRAMP/Akt1+/+ mouse prostates as determined by the KEGG pathway analysis. (D) Table showing pathways affected by the microRNA expression in TRAMP + Triciribine compared to TRAMP + DMSO mouse prostates as determined by the KEGG pathway analysis.
Principle component analysis (PCA) mapping of TRAMP/Akt1+/+ + DMSO and TRAMP/Akt1+/+ + triciribine in the advanced stages showed that TRAMP/Akt1+/+ + DMSO group was clustered distinctly from TRAMP/Akt1+/+ + triciribine group (Fig. 1B). KEGG pathway based all microRNA target prediction analysis of the TRAMP/Akt1+/+ + DMSO and TRAMP/Akt1+/+ + triciribine treated advanced stage prostate cancer tissues indicated changes in the expression of several genes predominantly involved in the regulation of the Cancer pathways, Wnt signaling pathway and cytoskeletal remodeling, similar to what was observed in the early stages. Interestingly, Akt suppression by triciribine in the late stages also promoted EMT-regulating pathways such as the MAP kinase signaling, TGFβ pathway, Notch and Hedgehog signaling etc. (Fig. 1D). A highly diverse group of microRNA repertoire was observed in these mouse prostate samples (TRAMP/Akt1–/– compared to TRAMP/Akt1+/+ prostates versus TRAMP + DMSO compared to TRAMP + triciribine) at two different stages of the disease (Figs. 2 and 3, respectively) suggesting an important role of microRNAs in stage-specific effects of Akt suppression on PCa.
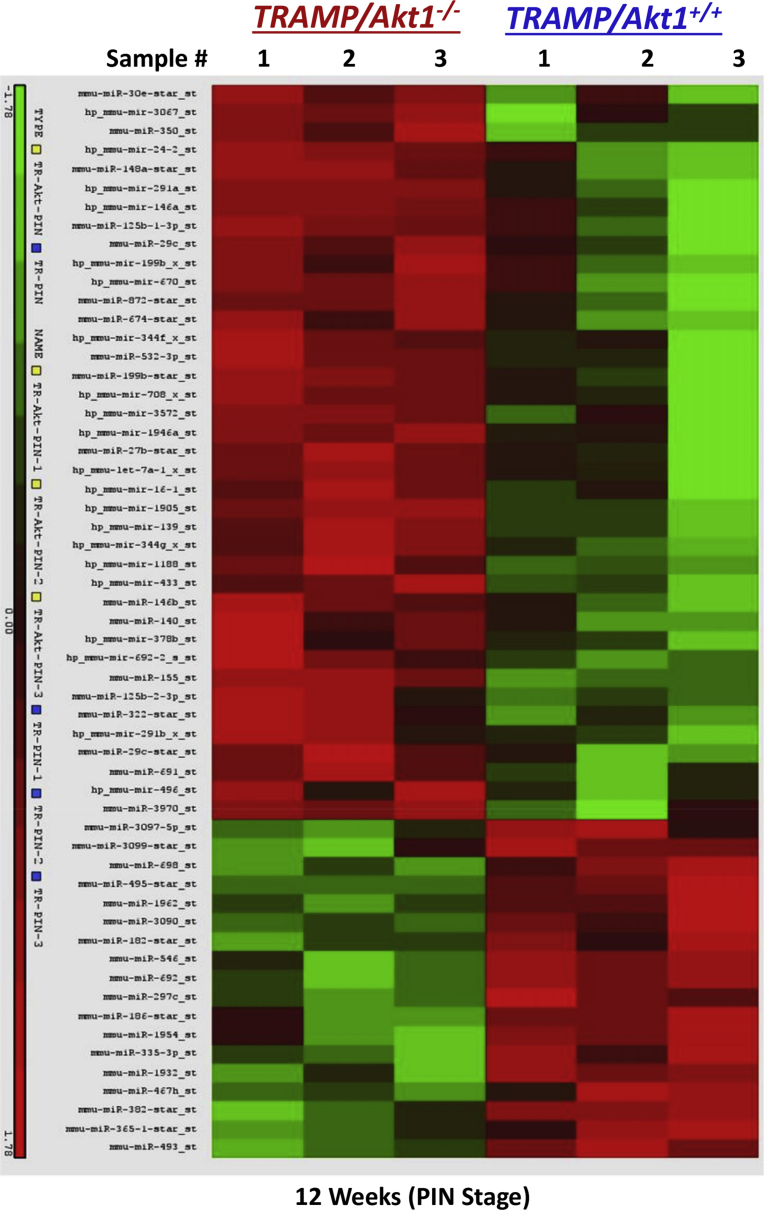
Fig. 2.
Akt suppression in the early and advanced stages of PCa modulates a different set of microRNAs. Alteration in the miRNAs in TRAMP/Akt1–/– mouse prostates compared to TRAMP/Akt1+/+ shown in a Heat-map (n = 3).
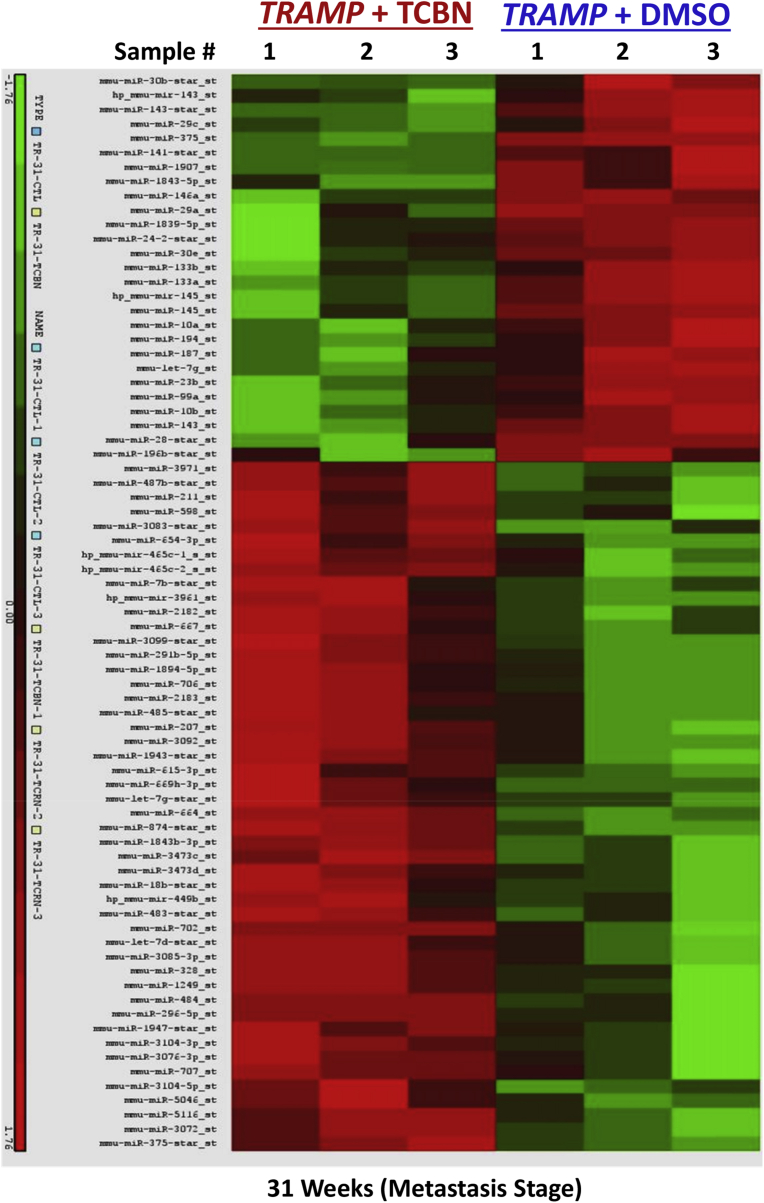
Fig. 3.
Akt suppression in the early and advanced stages of PCa modulates a different set of microRNAs. Alteration in the miRNAs in TRAMP + Triciribine mouse prostates compared to TRAMP + DMSO shown in a Heat-map (n = 3).
3.2. Akt1 deletion in TRAMP mice alters expression changes in selective microRNAs that regulate cell survival and proliferation in early PCa
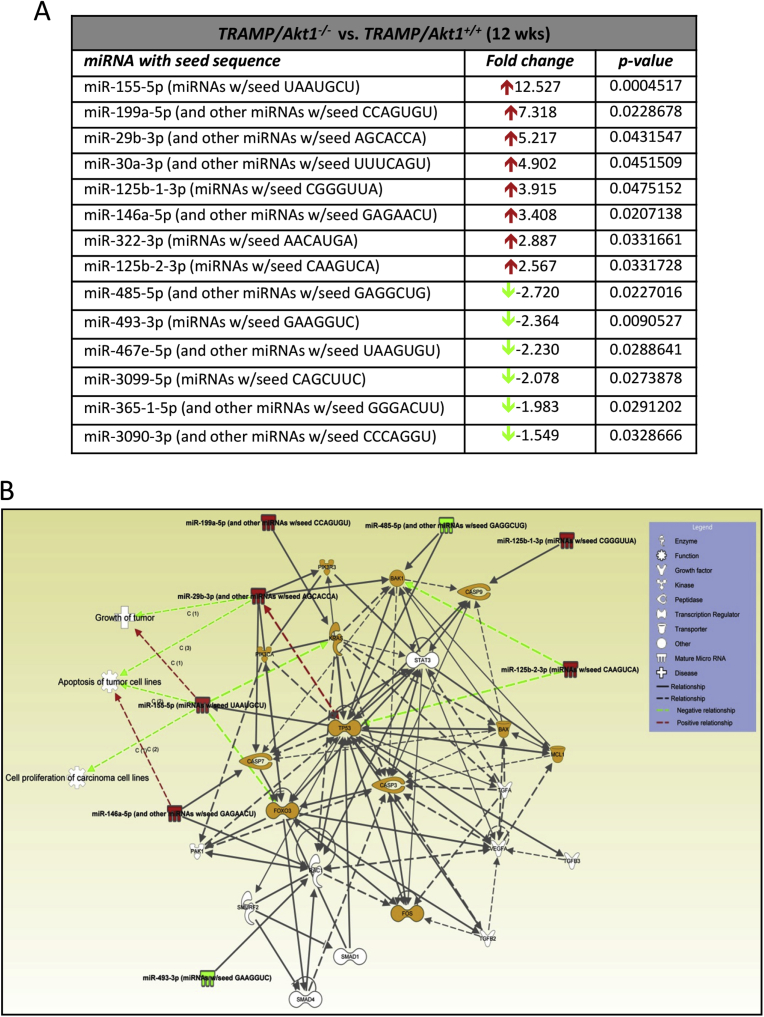
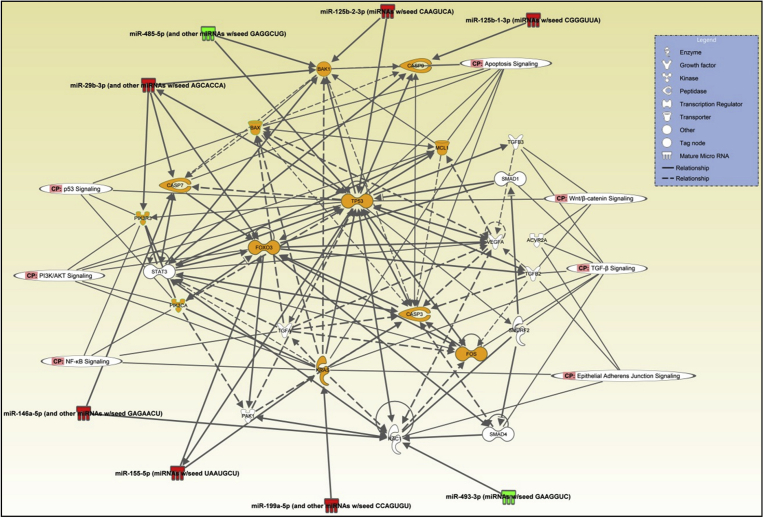
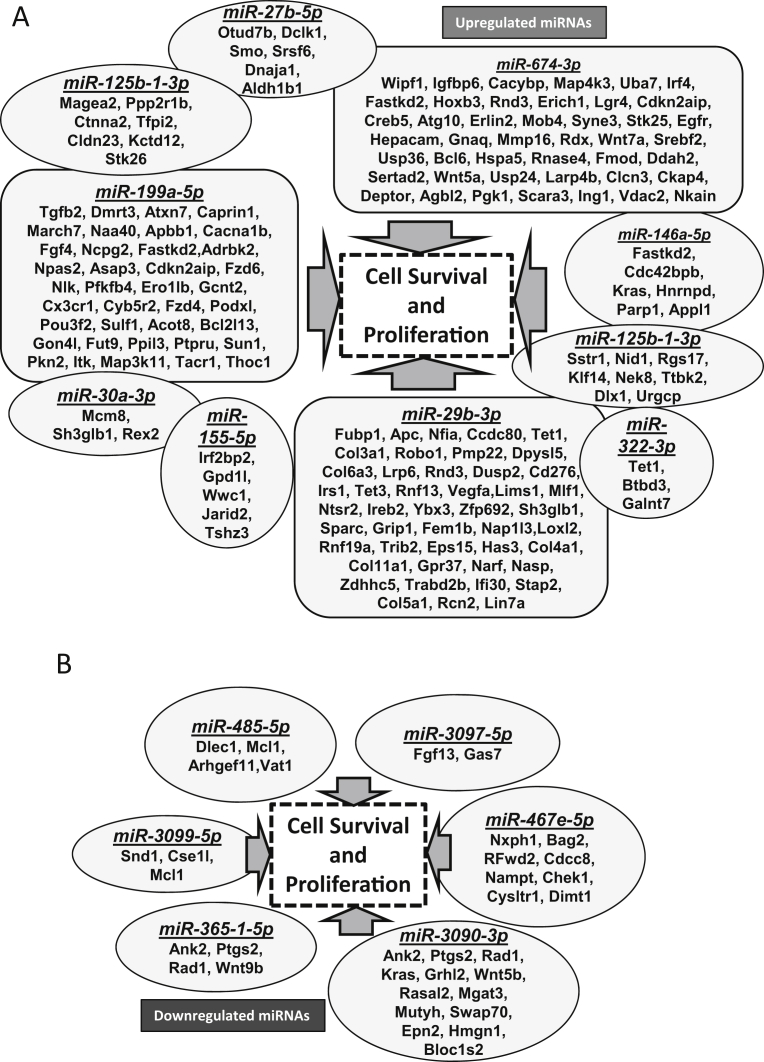
There were significant changes in the repertoire of microRNA expression in TRAMP/Akt1–/– compared to TRAMP/Akt1+/+ prostates (Figs. 2 and 4A). While ∼5–13-fold increase in miR-155-5p, miR199a-5p, and miR-29b-3p was observed in TRAMP/Akt1–/– compared to TRAMP/Akt1+/+ prostates, this was also associated with a 2–3-fold decrease in miR-485-5p and miR-493-3p (Fig. 4A). Based on the Ingenuity Pathway Analysis® system that converts a list of microRNAs and/or genes of interest in particular disease pathology into a set of functional networks based on the reported biological interactions, we identified that the net effect of Akt1 gene deletion in TRAMP prostate at early cancer stage such as PIN stage will be suppression of proliferation and promotion of apoptosis (Figs. 4B and 5), thus inhibiting oncogenic transformation and tumor growth. All the microRNAs that were modulated by Akt1 gene deletion in the PIN stage TRAMP prostate were previously characterized for their target genes and cellular function in various cancers. The gene targets of the upregulated microRNAs in the PIN-stage TRAMP prostates, such as the mir155-5p, mir29b-3p, mir199a-5p, mir125b-1-3p, mir674-3p and mir29b-3p because of Akt1 gene knockdown, as identified by the Gene ontology and KEGG pathway (DIANA-miRPath database) analyses has informed about the integral role of these microRNAs and their target genes in the promotion of cell survival and/or proliferation (Fig. 6A; Supplemental Table 1). Similarly, GO and KEGG analysis on the target genes of downregulated miRNAs such as mir485-5p, mir3097-5p, mir460e-5p, mir3090-3p, mir365-1-5p and mir3099-5p identified their role in promoting cellular arrest and apoptosis (Fig. 6B; Supplemental Table 2), suggesting that Akt inhibition in the early stages of PCa has a tumor suppressive effect.
Fig. 4.
MicroRNA expression changes in TRAMP/Akt1–/– mouse prostates compared to TRAMP/Akt1+/+ show the integral role of Akt1 in cell survival and proliferation. (A) Selected miRNAs differentially regulated in TRAMP/Akt1–/– mouse prostates compared to TRAMP/Akt1+/+. (B) Signaling network analysis using Ingenuity Pathway Analysis software involving microRNAs identified from the study indicating the integral role of Akt1-regulated microRNAs in cell survival, proliferation and growth in the early stage PCa.
Fig. 5.
Signaling network analysis using Ingenuity Pathway Analysis software involving microRNAs identified from the study indicating the integral role of Akt1-regulated microRNAs in cell survival, proliferation and growth in the early stage PCa.
Fig. 6.
KEGG and Gene Ontology (mirPath) analysis indicate modulation cell survival and proliferation by Akt-regulated microRNAs in the early PCa. (A) Diagram showing highly upregulated miRNAs in TRAMP/Akt1–/– mouse prostates compared to TRAMP/Akt1+/+, and their predicted and known targets indicating their predominant involvement in the cell survival and proliferation in the early PCa. (B) Diagram showing highly down-regulated miRNAs in TRAMP/Akt1–/– mouse prostates compared to TRAMP/Akt1+/+, and their predicted and known targets indicating their predominant involvement in the cell survival and proliferation in the early PCa.
3.3. Pharmacological inhibition of Akt in the advanced PCa-bearing TRAMP mice alters expression changes in selective microRNAs that regulate EMT and metastasis
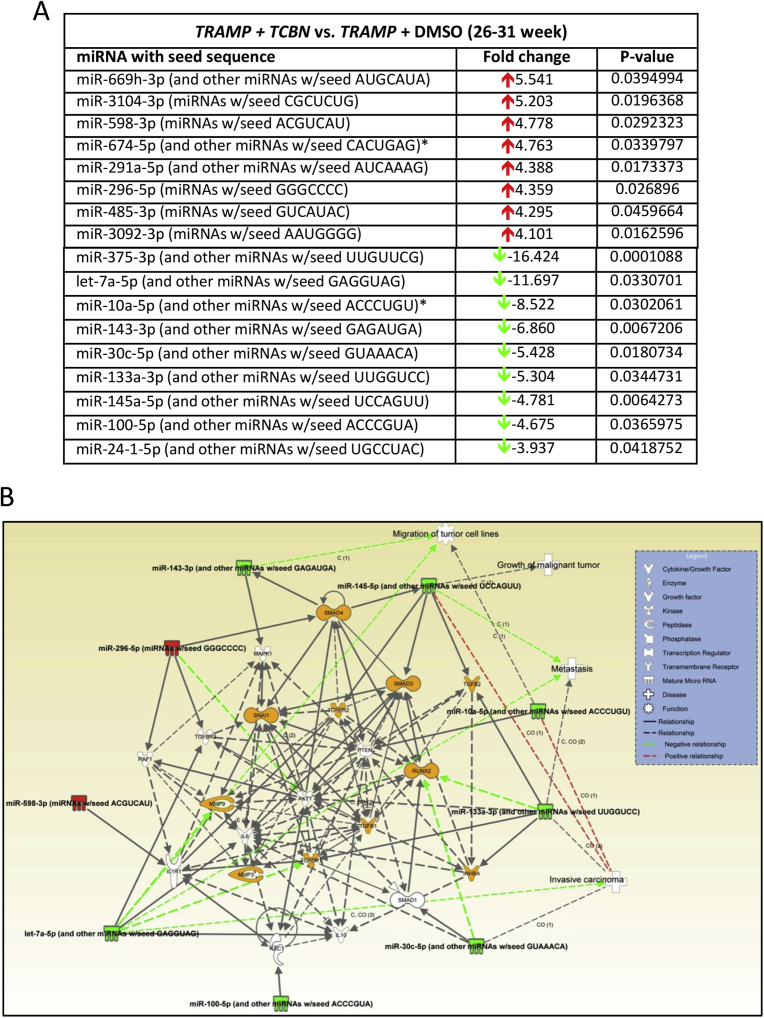
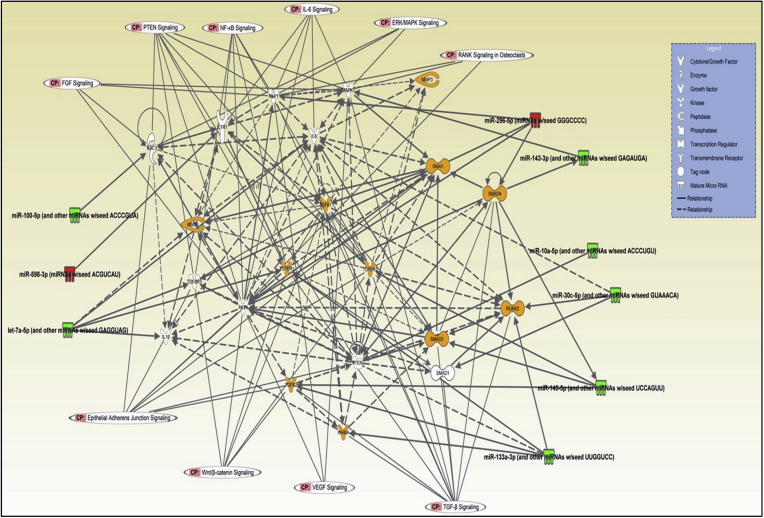
We observed significant changes in the repertoire of microRNA expression in triciribine treated compared to DMSO treated control prostates, which are entirely different from the early stage tumors (Figs. 3 and 7A). While ∼5-fold increase in miR-669h-3p, miR3104-3p and miR-598-3p were observed in triciribine treated compared to DMSO treated control prostates, more changes were observed in the downregulated microRNAs resulted in ∼7–17-fold decrease in miR-375-3p, let-7a-5p, miR-10a-5p and miR-143-3p (Fig. 7A). Based on the Ingenuity Pathway Analysis®, we identified that the net effect of Akt activity suppression using triciribine in TRAMP prostate in the advanced stages will be the promotion of cellular migration, invasion, malignancy and differentiation to mesenchymal type as demonstrated by changes in the expression of smooth muscle cell actin-α and TGFβ signaling (Figs. 7B and 8), thus promoting metastatic ability. Analysis based on KEGG pathway analysis and dbEMT database analysis also indicated that the changes in these microRNAs with Akt suppression in advanced PCa will promote EMT and metastasis.
Fig. 7.
MicroRNA expression changes in TRAMP/Triciribine mouse prostates compared to TRAMP/DMSO show promotion of EMT with Akt suppression. (A) Selected miRNAs differentially regulated in Triciribine treated TRAMP+ mouse prostates compared to DMSO treated control TRAMP+. (B) Signaling network analysis using Ingenuity Pathway Analysis software involving microRNAs identified from the study indicating the integral role of Akt-regulated microRNAs in EMT and PCa metastasis in the advanced stages.
Fig. 8.
Signaling network analysis using Ingenuity Pathway Analysis software involving microRNAs identified from the study indicating the integral role of Akt-regulated microRNAs in EMT and PCa metastasis in the advanced stages.
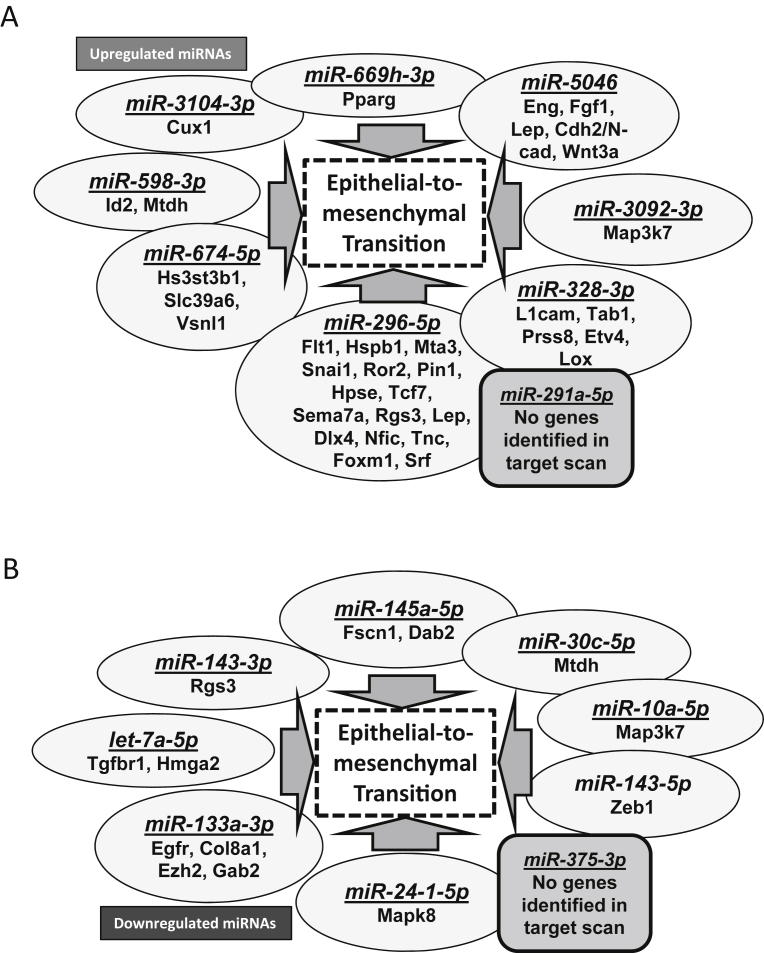
Although several microRNAs that were modulated by Akt suppression in the advanced stage TRAMP prostate were previously characterized for their target genes and cellular function in various cancers, information regarding some of the highly downregulated microRNAs such as mir375-3p was not available in these databases or in the literature. The gene targets of the up-regulated microRNAs such as the mir669h-3p, mir5046, mir3092-3p, mir328-3p, mir296-5p and mir674-5p because of Akt suppression by triciribine treatment in the advanced PCa tissues as identified by the dbEMT database analyses has informed about the integral role of these microRNAs and their target genes in the promotion of EMT and metastasis (Fig. 9A and Supplemental Table 3). Similarly, the gene targets of the down-regulated microRNAs such as the mir145a-5p, mir30c-5p, mir10a-5p, mir143-5p, let7a-5p and mir133a-5p because of Akt activity suppression by triciribine treatment in the advanced PCa tissues as identified by the dbEMT database has informed about the integral role of these microRNAs in the suppression of EMT and metastasis (Fig. 9B and Supplemental Table 4). Overall, the results suggest that Akt inhibition in the advanced stages of PCa would promote metastasis. Complete lists of microRNAs identified in the Affymetrix microarrays comparing TRAMP/Akt1–/–to TRAMP/Akt1+/+ prostates versus TRAMP + DMSO to TRAMP + Triciribine treated prostates are provided in Supplemental Tables 5 and 6, respectively.
Fig. 9.
KEGG and Gene Ontology (mirPath) analysis indicate modulation epithelial-to-mesenchymal transition and metastasis by Akt-regulated microRNAs in the advanced PCa. (A) Diagram showing highly upregulated miRNAs in Triciribine-treated TRAMP+ mouse prostates compared to DMSO treated control TRAMP+ prostates, and their predicted and known targets indicating their predominant involvement in the regulation of EMT in the advanced PCa. (B) Diagram showing highly downregulated miRNAs in Triciribine-treated TRAMP+ mouse prostates compared to DMSO treated control TRAMP+ prostates, and their predicted and known targets indicating their predominant involvement in the regulation of EMT in the advanced PCa.
4. Discussion & conclusion
Our study has demonstrated for the first time that Akt(1) suppression during the early and advanced stages of PCa induces stage-specific changes in the repertoire of microRNAs involved in the differential regulation of oncogenic transformation, tumor growth, and metastasis. Mechanistically this involves microRNA-mediated regulation of genes involved in cell survival and proliferation in the early stages and deregulation of TGFβ, MAP kinase, Notch and Hedgehog signaling in the later stages.
Akt has been indisputably regarded as a pro-tumorigenic kinase in various cancers [26, 27]. Several studies from our laboratory have indicated that Akt is indispensable for the survival, motility, and proliferation of PCa cells in vitro and tumor growth in vivo [7, 28, 29, 30, 31, 32]. Intriguingly, recent studies from various laboratories in different cancer types such as the breast [12, 13], liver [14], non-small cell lung [15] head and neck [16], have reported a different, paradoxical effect of Akt suppression on cancer metastasis. Our most recent study in PCa has clearly demonstrated that although Akt1 gene deletion in TRAMP mice prevents oncogenic transformation and tumor growth in the prostate, the pharmacological suppression of Akt kinase activity in TRAMP mice bearing advanced PCa using triciribine-augmented metastasis to distant tissues such as the lungs, liver, and kidney [11]. One of the key mechanisms by which Akt suppression leading to increased metastasis in PCa [11] and breast cancer [12, 13] has been identified to be the deregulation of various genes involved in the TGFβ-mediated EMT of cancer cells.
How Akt(1) suppression leads to deregulation of the TGFβ pathway to promote EMT and cancer metastasis is not clearly understood and literature in this area is scarce. One of the first studies reporting a connection between Akt1 suppression, TGFβ expression and EMT in breast cancer indicated the down-regulation of mir200 clusters such as mir200a, mir200b, and mir200c, which subsequently led to reduced expression of E-Cadherin and increased expression of vimentin and EMT transcription factor Zeb1 [12]. Although the involvement of microRNAs was not investigated, a causal relationship between Akt1 suppression and promotion of EMT via increased transcription factor Twist1 expression was also reported by another group in breast cancer cells [13]. Although increased invasion as a result of Akt suppression has also been reported by other laboratories in NSCLC [15], liver [14] and head and neck [16] cancer, the involvement of microRNAs and TGFβ pathway in the process have not been investigated. Similarly, our recent study in PCa demonstrated changes in the expression of a plethora of genes involved in the TGFβ and EMT pathways. Results reported in the current study is the second in any cancers, after breast cancer [13] and is the first report in PCa that demonstrate the involvement of stage-specific expression of various microRNAs linking Akt1 activity suppression, activation of TGFβ pathway and EMT.
Unlike breast cancer cells, analysis of TRAMP PCa tissues did not reveal a difference in the expression of mir200 family with Akt1 activity suppression in either of the early or advanced stages, indicating that different sets of microRNAs are involved in various cancers. In the early (PIN) stages, Akt1 gene deficiency in the TRAMP prostate resulted in significant increase in mir155-5p, mir199a-5p, mir29b-3p and mir30a-3p as well as a decrease in the expression of mir485-5p, mir493-3p and mir467e-5p, all of which that have been demonstrated to regulate the cell survival and proliferation in the early stages of cancer as analyzed by the KEGG, GO and IPA databases. Among these, mir155-5p has been shown to induce gastric cancer cell apoptosis [33] and promote autophagy in cervical cancer cells [34]. On the other end, in hepatocellular carcinoma [35] and colorectal cancer [36] mir155-5p has demonstrated its ability to resist apoptosis and promotes cellular proliferation, respectively. Intriguingly, although mir199a-5p was found to suppress tumor growth from colorectal cancer cells [37], papillary thyroid carcinoma [38], triple-negative breast cancer [39] and proliferation of esophageal cancer cells [40], its down-regulation was shown to promote prostate adenocarcinoma progression [41]. Furthermore, mir29b-3p has been shown to act as a tumor suppressor in glioblastoma where it can inhibit cell growth and induce apoptosis in vitro [42]. In addition, a reciprocal correlation was found between miR-30a-3p expression and esophageal cancer cells proliferation [43]. This clearly underlines the cell type-specific effect of miRNAs despite the nature of the disease. Although miR-485-5p has been shown to suppress breast cancer and hepatocellular carcinoma progression [44, 45], the proliferation of NSCLC [46], its reduced expression was associated with poor gastric cancer prognosis [47]. Such a complexity indicate that the stage-specific effects of Akt on PCa growth and metastasis is orchestrated by several but not a single miRNA. In general, the microRNAs detected in the early PCa stage with Akt1 gene deletion were not involved in the regulation of TGFβ pathway, MAP Kinase pathway or EMT indicating that the effect of Akt1 suppression on these events is limited to the advanced stages.
In the advanced stages, Akt1 inhibition by triciribine treatment for 6 weeks resulted in the increased expression of mir669h-3p and mir3104-3p as well as decreased expression of mir375-3p, le7a-5p, mir10a-5p and mir143-3p all of which are the signature microRNAs in the modulation of TGFβ and EMT pathway as analyzed using the KEGG, GO, IPA and dbEMT databases. In spite of the significant upregulation of miR-375 in the serum of castration-resistant PCa patients [48], we observed a significant reduction of miR-375-3p with tricirbine treatment in the advanced tumor-bearing TRAMP mice. Interestingly, during their investigation for the miRNA-Runx1/2 signaling network in the regulation of PCa progression in TRAMP mice and by looking at the temporal miRNAs expression in TRMAP's tumors, Farina et al have also noticed a significant reduction in miR-375-3p expression as the tumor develops in these mice compared to wild-type controls [49]. Although its expression was measured up to 21-week-old mice, the expression of Runx1/2, which are targets for miR-375-3p, was elevated in 33week-old TRAMPs indicating the potential reduction of miR-375-3p during that stage. However, since we had TRAMP + DMSO as our control, treatment with TCBN was the only reason responsible for the further reduction in this miRNA, assuming its low level in the control animals. Another study reported that loss of let-7a expression in human PCa specimens was correlated to higher Gleason score and more importantly to higher EZH2 expression [50], which is known to regulate molecular features of cancer stem cells (CSC), thus EMT [51]. The suppressive activity of miR-143-3p on ovarian cancer progression was reported through downregulation of TGFβ activated kinase-1 (TAK1) [52]. Interestingly, we observed a significant reduction in miR-143-3p with TCBN treatment, which is potentially involved in augmenting TGFβ-induced PCa metastasis upon Akt inhibition in the advanced stage PCa. Currently, there is no information related to the role of miR-669h-3p and miR-3104-3p in cancer, which represents novel topics for further investigation. Our analysis thus demonstrates a significant role of Akt-regulated microRNAs in the stage-specific regulation of PCa.
In conclusion, our study provides the necessary clues that the expression of different sets of microRNAs during the early and the advanced stages of PCa plays a major role in the differential regulation of many signaling pathways such as the Akt and TGFβ pathways and that the microRNAs are also responsible for linking these pathways together. Our results will lay the foundation for many future discoveries that may lead to the development of various tools in the management of PCa by identifying the key microRNAs involved in the regulation of different signaling pathways, determining changes in the microRNA expression in cancer biopsies and/or body fluids as a biomarker for staging and for future therapies. A major limitation of our study is that the data is specific to a murine model of PCa and hence have limited clinical relevance. Nevertheless, cellular studies involving human PCa cell lines in our laboratory have yielded similar effects of Akt suppression on EMT and metastasis [11]. However, because of the significant differences between the murine and the human microRNAs involved in various pathologies, more studies on the specific microRNAs involved in human PCa and their specific effects on cell signaling pathways, EMT and metastasis are warranted. This will be the focus of future research in our laboratory.
Declarations
Author contribution statement
Abdulrahman Alwhaibi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Fei Gao, Sandeep Artham: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Bernard M. Hsia: Performed the experiments; Analyzed and interpreted the data.
Ashish Mondal: Performed the experiments.
Ravindra Kolhe: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Payaningal R. Somanath: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the National Institutes of Health grants (R01HL103952 and UL1TR002378). Payaningal R. Somanath was supported by the Wilson Pharmacy Foundation and Translational Research Initiative Grant. Abdulrahman Alwhaibi was supported by a fellowship provided by the King Saud University.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This work has been accomplished using the resources and facilities at the VA Medical Center in Augusta, GA. The funders had no role in the study design, data collection, analysis, and decision to publish the data. The contents of the manuscript do not represent the views of the Department of Veteran Affairs or the United States Government.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [published Online First: 2017/01/06] [DOI] [PubMed] [Google Scholar]
- 2.Hussain M., DiPaola R.S. Clinical research in metastatic prostate cancer: a focus on impact and value. Am. Soc. Clin. Oncol. Educ. Book. 2015:17–21. doi: 10.14694/EdBook_AM.2015.35.17. [published Online First: 2015/05/21] [DOI] [PubMed] [Google Scholar]
- 3.Pezaro C., Woo H.H., Davis I.D. Prostate cancer: measuring PSA. Intern. Med. J. 2014;44(5):433–440. doi: 10.1111/imj.12407. [published Online First: 2014/05/13] [DOI] [PubMed] [Google Scholar]
- 4.Al-Azayzih A., Gao F., Somanath P.R. P21 activated kinase-1 mediates transforming growth factor beta1-induced prostate cancer cell epithelial to mesenchymal transition. Biochim. Biophys. Acta. 2015;1853(5):1229–1239. doi: 10.1016/j.bbamcr.2015.02.023. [published Online First: 2015/03/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [published Online First: 2003/06/18] [DOI] [PubMed] [Google Scholar]
- 6.Al-Azayzih A., Gao F., Goc A. TGFbeta1 induces apoptosis in invasive prostate cancer and bladder cancer cells via Akt-independent, p38 MAPK and JNK/SAPK-mediated activation of caspases. Biochem. Biophys. Res. Commun. 2012;427(1):165–170. doi: 10.1016/j.bbrc.2012.09.035. [published Online First: 2012/09/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goc A., Al-Husein B., Kochuparambil S.T. PI3 kinase integrates Akt and MAP kinase signaling pathways in the regulation of prostate cancer. Int. J. Oncol. 2011;38(1):267–277. [PubMed] [Google Scholar]
- 8.Chen J., Somanath P.R., Razorenova O. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat. Med. 2005;11(11):1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somanath P.R., Kandel E.S., Hay N. Akt1 signaling regulates integrin activation, matrix recognition, and fibronectin assembly. J. Biol. Chem. 2007;282(31):22964–22976. doi: 10.1074/jbc.M700241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somanath P.R., Razorenova O.V., Chen J. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5(5):512–518. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao F., Alwhaibi A., Sabbineni H. Suppression of Akt1-beta-catenin pathway in advanced prostate cancer promotes TGFbeta1-mediated epithelial to mesenchymal transition and metastasis. Cancer Lett. 2017;402:177–189. doi: 10.1016/j.canlet.2017.05.028. [published Online First: 2017/06/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iliopoulos D., Polytarchou C., Hatziapostolou M. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci. Signal. 2009;2(92):ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C.W., Xia W., Lim S.O. AKT1 inhibits epithelial-to-mesenchymal transition in breast cancer through phosphorylation-dependent Twist1 degradation. Canc. Res. 2016;76(6):1451–1462. doi: 10.1158/0008-5472.CAN-15-1941. [published Online First: 2016/01/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q., Yu W.N., Chen X. Spontaneous hepatocellular carcinoma after the combined deletion of Akt isoforms. Canc. Cell. 2016;29(4):523–535. doi: 10.1016/j.ccell.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao G., Pierobon M., Kim I.K. Inhibition of AKT1 signaling promotes invasion and metastasis of non-small cell lung cancer cells with K-RAS or EGFR mutations. Sci. Rep. 2017;7(1):7066. doi: 10.1038/s41598-017-06128-9. [published Online First: 2017/08/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brolih S., Parks S.K., Vial V. AKT1 restricts the invasive capacity of head and neck carcinoma cells harboring a constitutively active PI3 kinase activity. BMC Canc. 2018;18(1):249. doi: 10.1186/s12885-018-4169-0. [published Online First: 2018/03/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao F., Alwhaibi A., Artham S. Endothelial Akt1 loss promotes prostate cancer metastasis via beta-catenin-regulated tight-junction protein turnover. Br. J. Cancer. 2018 doi: 10.1038/s41416-018-0110-1. [published Online First: 2018/05/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo L., Zhang Y., Zhang L. MicroRNAs, TGF-beta signaling, and the inflammatory microenvironment in cancer. Tumour Biol. 2016;37(1):115–125. doi: 10.1007/s13277-015-4374-2. [published Online First: 2015/11/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oom A.L., Humphries B.A., Yang C. MicroRNAs: novel players in cancer diagnosis and therapies. BioMed Res. Int. 2014;2014:959461. doi: 10.1155/2014/959461. [published Online First: 2014/08/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson D., Van Allen E.M., Wu Y.M. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.05.001. [published Online First: 2015/05/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolhe R., Hunter M., Liu S. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep. 2017;7(1):2029. doi: 10.1038/s41598-017-01905-y. [published Online First: 2017/05/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvano S.E., Xiao W., Richards D.R. A network-based analysis of systemic inflammation in humans. Nature. 2005;437(7061):1032–1037. doi: 10.1038/nature03985. [published Online First: 2005/09/02] [DOI] [PubMed] [Google Scholar]
- 23.Ravasz E., Somera A.L., Mongru D.A. Hierarchical organization of modularity in metabolic networks. Science. 2002;297(5586):1551–1555. doi: 10.1126/science.1073374. [published Online First: 2002/08/31] [DOI] [PubMed] [Google Scholar]
- 24.Gao L., Barnes K.C. Recent advances in genetic predisposition to clinical acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;296(5):L713–L725. doi: 10.1152/ajplung.90269.2008. [published Online First: 2009/02/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang R.T., Wu D., Meliton A. Experimental lung injury reduces Kruppel-like factor 2 to increase endothelial permeability via regulation of RAPGEF3-rac1 signaling. Am. J. Respir. Crit. Care Med. 2017;195(5):639–651. doi: 10.1164/rccm.201604-0668OC. [published Online First: 2016/11/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LoPiccolo J., Blumenthal G.M., Bernstein W.B. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist. Updat. 2008;11(1-2):32–50. doi: 10.1016/j.drup.2007.11.003. [published Online First: 2008/01/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martini M., De Santis M.C., Braccini L. PI3K/AKT signaling pathway and cancer: an updated review. Ann. Med. 2014;46(6):372–383. doi: 10.3109/07853890.2014.912836. [published Online First: 2014/06/06] [DOI] [PubMed] [Google Scholar]
- 28.Goc A., Liu J., Byzova T.V. Akt1 mediates prostate cancer cell microinvasion and chemotaxis to metastatic stimuli via integrin beta(3) affinity modulation. Br. J. Cancer. 2012;107(4):713–723. doi: 10.1038/bjc.2012.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goc A., Kochuparambil S.T., Al-Husein B. Simultaneous modulation of the intrinsic and extrinsic pathways by simvastatin in mediating prostate cancer cell apoptosis. BMC Canc. 2012;12:409. doi: 10.1186/1471-2407-12-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kochuparambil S.T., Al-Husein B., Goc A. Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J. Pharmacol. Exp. Therapeut. 2011;336(2):496–505. doi: 10.1124/jpet.110.174870. [DOI] [PubMed] [Google Scholar]
- 31.Gao F., Al-Azayzih A., Somanath P.R. Discrete functions of GSK3alpha and GSK3beta isoforms in prostate tumor growth and micrometastasis. Oncotarget. 2015;6(8):5947–5962. doi: 10.18632/oncotarget.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goc A., Al-Husein B., Katsanevas K. Targeting Src-mediated Tyr216 phosphorylation and activation of GSK-3 in prostate cancer cells inhibit prostate cancer progression in vitro and in vivo. Oncotarget. 2014;5(3):775–787. doi: 10.18632/oncotarget.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S., Zhang T., Zhou X. The tumor suppressor role of miR-155-5p in gastric cancer. Oncol. Lett. 2018;16(2):2709–2714. doi: 10.3892/ol.2018.8932. [published Online First: 2018/07/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F., Shan S., Huo Y. MiR-155-5p inhibits PDK1 and promotes autophagy via the mTOR pathway in cervical cancer. Int. J. Biochem. Cell Biol. 2018;99:91–99. doi: 10.1016/j.biocel.2018.04.005. [published Online First: 2018/04/09] [DOI] [PubMed] [Google Scholar]
- 35.Fu X., Wen H., Jing L. MicroRNA-155-5p promotes hepatocellular carcinoma progression by suppressing PTEN through the PI3K/Akt pathway. Cancer Sci. 2017;108(4):620–631. doi: 10.1111/cas.13177. [published Online First: 2017/01/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu Y.L., Wang H.F., Sun Z.Q. Up-regulated miR-155-5p promotes cell proliferation, invasion and metastasis in colorectal carcinoma. Int. J. Clin. Exp. Pathol. 2015;8(6):6988–6994. [published Online First: 2015/08/12] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Q.D., Zhou Q.Q., Dong L. MiR-199a-5p inhibits the growth and metastasis of colorectal cancer cells by targeting ROCK1. Technol. Cancer Res. Treat. 2018;17 doi: 10.1177/1533034618775509. 1533034618775509. [published Online First: 2018/05/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma S., Jia W., Ni S. miR-199a-5p inhibits the progression of papillary thyroid carcinoma by targeting SNAI1. Biochem. Biophys. Res. Commun. 2018;497(1):181–186. doi: 10.1016/j.bbrc.2018.02.051. [published Online First: 2018/02/11] [DOI] [PubMed] [Google Scholar]
- 39.Chen J., Shin V.Y., Siu M.T. miR-199a-5p confers tumor-suppressive role in triple-negative breast cancer. BMC Canc. 2016;16(1):887. doi: 10.1186/s12885-016-2916-7. [published Online First: 2016/11/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrnes K.A., Phatak P., Mansour D. Overexpression of miR-199a-5p decreases esophageal cancer cell proliferation through repression of mitogen-activated protein kinase kinase kinase-11 (MAP3K11) Oncotarget. 2016;7(8):8756–8770. doi: 10.18632/oncotarget.6752. [published Online First: 2015/12/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong J., Huang R., Su Z. Downregulation of miR-199a-5p promotes prostate adeno-carcinoma progression through loss of its inhibition of HIF-1alpha. Oncotarget. 2017;8(48):83523–83538. doi: 10.18632/oncotarget.18315. [published Online First: 2017/11/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin J., Shim H.G., Hwang T. Restoration of miR-29b exerts anti-cancer effects on glioblastoma. Cancer Cell Int. 2017;17:104. doi: 10.1186/s12935-017-0476-9. [published Online First: 2017/11/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi B., Wang Y., Chen Z.J. Down-regulation of miR-30a-3p/5p promotes esophageal squamous cell carcinoma cell proliferation by activating the Wnt signaling pathway. World J. Gastroenterol. 2017;23(45):7965–7977. doi: 10.3748/wjg.v23.i45.7965. [published Online First: 2017/12/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M., Cai W.R., Meng R. miR-485-5p suppresses breast cancer progression and chemosensitivity by targeting survivin. Biochem. Biophys. Res. Commun. 2018;501(1):48–54. doi: 10.1016/j.bbrc.2018.04.129. [published Online First: 2018/04/22] [DOI] [PubMed] [Google Scholar]
- 45.Sun X., Liu Y., Li M. Involvement of miR-485-5p in hepatocellular carcinoma progression targeting EMMPRIN. Biomed. Pharmacother. 2015;72:58–65. doi: 10.1016/j.biopha.2015.04.008. [published Online First: 2015/06/10] [DOI] [PubMed] [Google Scholar]
- 46.Huang R.S., Zheng Y.L., Li C. MicroRNA-485-5p suppresses growth and metastasis in non-small cell lung cancer cells by targeting IGF2BP2. Life Sci. 2018;199:104–111. doi: 10.1016/j.lfs.2018.03.005. [published Online First: 2018/03/07] [DOI] [PubMed] [Google Scholar]
- 47.Jing L.L., Mo X.M. Reduced miR-485-5p expression predicts poor prognosis in patients with gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2016;20(8):1516–1520. [published Online First: 2016/05/11] [PubMed] [Google Scholar]
- 48.Nguyen H.C., Xie W., Yang M. Expression differences of circulating microRNAs in metastatic castration resistant prostate cancer and low-risk, localized prostate cancer. Prostate. 2013;73(4):346–354. doi: 10.1002/pros.22572. [published Online First: 2012/08/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farina N.H., Zingiryan A., Akech J.A. A microRNA/Runx1/Runx2 network regulates prostate tumor progression from onset to adenocarcinoma in TRAMP mice. Oncotarget. 2016;7(43):70462–70474. doi: 10.18632/oncotarget.11992. [published Online First: 2016/09/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong D., Heath E., Chen W. Loss of let-7 up-regulates EZH2 in prostate cancer consistent with the acquisition of cancer stem cell signatures that are attenuated by BR-DIM. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033729. [published Online First: 2012/03/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanacore D., Boccellino M., Rossetti S. Micrornas in prostate cancer: an overview. Oncotarget. 2017;8(30):50240–50251. doi: 10.18632/oncotarget.16933. [published Online First: 2017/04/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi H., Shen H., Xu J. MiR-143-3p suppresses the progression of ovarian cancer. Am. J. Transl. Res. 2018;10(3):866–874. [published Online First: 2018/04/11] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.