Abstract
BACKGROUND: Presurgical carbohydrate antigen 19-9 (CA19-9) level predicts overall survival (OS) in resected pancreatic adenocarcinoma (PaC). The aim of this pooled analysis was to evaluate if presurgical CA19-9 level can also predict local control (LC) and distant metastasis-free survival (DMFS). METHODS: Seven hundred patients with PaC from eight institutions who underwent surgical resection ± adjuvant treatment between 2000 and 2014 were analyzed. Patients were divided based on four presurgical CA19-9 level cutoffs (5, 37, 100, 353 U/ml). Weibull regression model to identify independent predictors of OS on 404 patients with complete information was fitted. RESULTS: Median follow-up was 17 months (range: 2-225 months). Univariate analysis showed a better prognosis in pT1-2, pN0, diameter <30 mm, or grade 1 tumors and in patients undergoing R0 resection, distal pancreatectomy, or adjuvant chemotherapy and with lower CA19-9 levels. Five-year OS, LC, and DMFS were as follows: CA19-9 <5.0: 5.7%, 47.2%, 17.0%; CA19-9 5.1-37.0: 37.9%, 63.3%, 46.0%; CA19-9 37.1-100.0: 27.1%, 59.4%, 39.0%; CA19-9 100.1-353.0: 17.4%, 43.4%, 26.7%; CA19-9 >353.1: 10.9%, 50.2%, and 23.4%, respectively. At multivariate analysis, CA19-9 >100 and <353 level (P=.002), CA19-9 ≥353.1 (P<.001) level, G3 tumor (P=.002), and tumor diameter >30 mm (P<.001) correlated with worse OS. Patients treated with postoperative chemoradiation doses >50.0 Gy showed improved OS (P<.001). CONCLUSION: Presurgical CA19-9 predicts both OS and pattern of failure. Therefore, CA19-9 should be included in predictive models in order to customize treatments based on prognostic factors. Moreover, future studies should stratify patients according to presurgical CA19-9 level.
Introduction
According to Cancer Stat Facts (National Cancer Institute, Surveillance, Epidemiology, and End Results program), 55,440 news cases of pancreatic carcinoma (PaC) are estimated in 2018, and 5-year survival was only 8.5% between 2008 and 2014 in the United States [1]. In Europe, PaC mortality has recently increased [2], and in the United States, PaC represents the fourth cause of cancer mortality, while in 2030, it will become the second leading cause of cancer mortality [3]. Prognosis is unfavorable even in the minority (20%) of patients with resectable disease despite the contribution of adjuvant therapies which have produced some marginal improvement in survival [4].
Based on these disappointing results, more effective and tailored adjuvant treatments are needed to improve patients’ outcome. Furthermore, identification of cancer biology surrogate biomarkers able to define patient groups with higher risk of local recurrence and distant metastasis would be useful [5], [6].
In 1979, Koprowski and colleagues first discovered carbohydrate antigen 19-9 (CA19-9) originally isolated from a human colorectal cancer cell line [7]. The prognostic role of presurgical CA19-9 has been suggested in patients with resected PaC [8], [9]. Moreover, CA19-9 determination is rapid and cost-effective. Therefore, presurgical assessment of CA19.9 may improve patients’ selection and stratification in clinical trials evaluating innovative PaC treatments different from the traditional approach of upfront surgery followed by adjuvant chemotherapy (CT) with or without postoperative chemoradiation (CRT).
However, evidence on the predictive role of CA19-9 is limited. In particular, studies evaluating the possibility to predict both local and distant failures are lacking. Based on this background, we analyzed a multicenter patient population treated with radical surgery for PaC. The aim of the analysis was to evaluate whether CA19-9 could predict local control (LC) and distant metastasis-free survival (DMFS) and to confirm in a large series its correlation with survival.
Material and Methods
A multicenter retrospective review of 1300 patients who underwent surgical resection of PaC with or without adjuvant treatment [CT, CRT, postoperative and/or intraoperative radiotherapy (RT)] from eight institutions [Roma, Madrid, Verona, Milano, Campobasso, Baltimore (two institutions), Rochester] was performed. A total of 700 patients with available presurgical CA19-9 value were identified and included in the analysis.
Data collection and design of this pooled analysis were presented in a previous publication [10]. Briefly, participating centers selected data of patients with PaC who underwent radical surgery ± adjuvant therapies. Patients with metastatic disease, histology other than adenocarcinoma, ampullary and periampullary tumors, neoadjuvant treatment, intraoperative RT, and lack of presurgical CA19-9 value were not included in the analysis. Also, postoperative CRT treatment modalities were previously described [11]. Briefly, RT was administered with linear accelerators using multiple-field technique. Median prescribed total dose was 50.4 Gy in 1.8 Gy daily fractionation. Concomitant CT was based on 5-fluorouracil or gemcitabine, while adjuvant CT was based on gemcitabine in most patients. Patients were treated between 2000 and 2014. After the treatment, most patients underwent physical examination, complete blood count, and computed tomography scan of chest and abdomen every 3 months in the first 2 years, every 6 months from the third to fifth year, and annually thereafter. In some patients treated before 2002, computed tomography scans were alternated with abdominal ultrasound and chest X-rays. Follow-up visits and imaging studies were anticipated in case of symptoms.
In this analysis, patients were divided according to presurgical CA19-9 levels based on four different cutoffs: ≤5 U/ml, 37 U/ml, 100 U/ml, and 353 U/ml and five relative CA19-9 classes: 0.0-5.0, 5.1-37.0, 37.1-100, 100.1-353.0, >353.1 U/ml. The first cutoff (≤5 U/ml) was applied to discriminate patients who are Lewis antigen negative and thus unable to express CA19-9 but similar to what was done in previous studies [6], [12], [13]. The second cutoff (37 U/ml) represented the upper limit of the normal reference CA19-9 value among normal subjects. The third cutoff (100 U/ml) was selected in analogy to several previous publications [14], [15], [16]. The fourth cutoff (353 U/ml) was the median value of patients with CA19-9 values >100 U/ml.
Continuous variables were expressed as median and range, while categorical variables were presented as number and percentages. Trend for trend across ordered variables was studied by means of score test for linear trend of the log odds. Survival functions were plotted using the Kaplan-Meier method and compared through the log-rank test or the modified log-rank test for trend (ordered variables) [17].
The correlation between presurgical CA19-9 values and outcomes was studied both on the general population of 700 patients and on a subgroup of 404 patients with complete data also in terms of grading, margins status, and stage.
The analysis presented in Figure 1, Figure 2, Figure 3 and in the supplementary material was adjusted by participating center to account for baseline survival differences among hospitals. We adapted the Weibull regression models to study predictors of OS assuming shared frailty (gamma distributed) by participating center to account for latent center-level effects [18]. The Weibull distribution was chosen based on preliminary analysis of the baseline hazard function. At first, we fitted spline Weibull survival regression models to explore the shape of the association between CA19-9 levels and OS using data of all patients [19]. Covariates included in multivariable models were selected with a stepwise backward elimination (P removal ≥.10; P addition <.10) based on likelihood ratio test. Median OS expressed in months was estimated as average marginal effects.
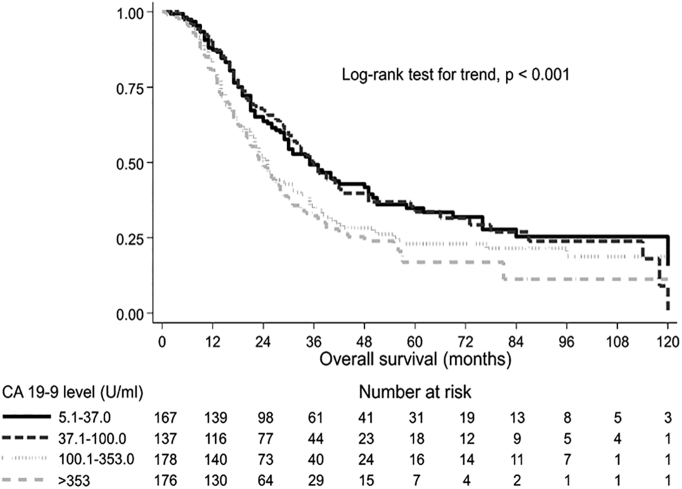
Figure 1.
Overall survival by presurgical CA19-9 level. Kaplan-Meier survival curves and log-rank test adjusted by center.
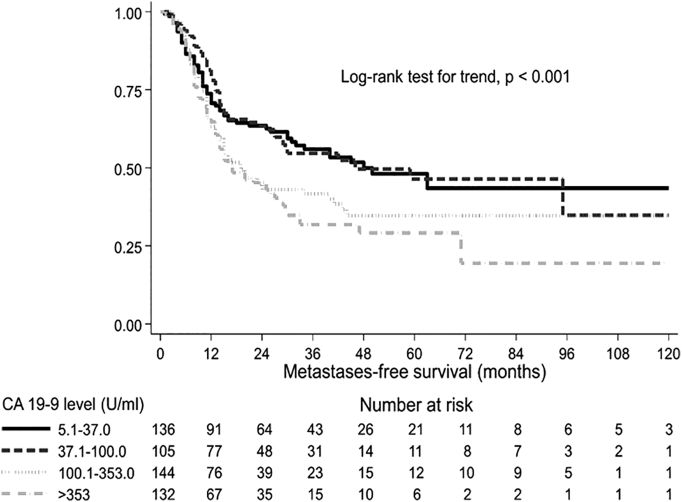
Figure 2.
Distant metastases-free survival by presurgical CA19-9 level. Kaplan-Meier survival curves and log-rank test adjusted by center.
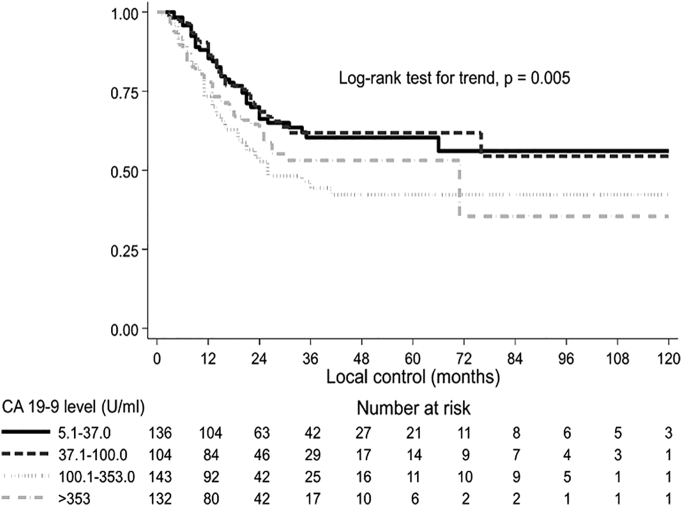
Figure 3.
Local control by presurgical CA19-9 level. Kaplan-Meier survival curves and log-rank test adjusted by center.
Statistical analysis was carried out using Stata 12.1 SE (Stata Corp, College Station, TX). All tests were two-sided, and a P value <.05 was considered statistically significant.
Results
Based on the inclusion criteria, 700 patients with available presurgical CA19-9 value were included in this pooled analysis. The median age was 63 years (range: 17-86 years), and the median follow-up was 17 months (range: 2-225 months). Four hundred ninety-four patients (70.6%) underwent pancreaticoduodenectomy, 99 patients (14.1%) distal pancreatectomy, and 34 patients (4.9%) total pancreatectomy.
Table 1 shows patients characteristics and survival results in details. Univariate analysis showed a better prognosis in pT1-2, pN0, diameter <30 mm, or grade 1 tumors, and in patients undergoing R0 resection, distal pancreatectomy, or adjuvant chemotherapy and with lower CA19-9 levels.
Table 1.
Patient Characteristics of the Analyzed Cohort with the Number of Patients and Percentage of the Total Number of Patients [%] and Survival Differences by Clinicopathologic and Treatment Details
| Variable | Value | No. of Patients [%] | 5-year OS [%] | Median OS [months] | P |
|---|---|---|---|---|---|
| Age | Median (range) | 63 (17-86) | |||
| Gender | Male | 360 [51.4] | 18.8 | 25 | .099 |
| Female | 340 [48.6] | 25.8 | 26 | ||
| CA 19-9 level (U/ml) | 0.0-5.0 | 39 [5.6] | 5.7 | 25 | <.001 |
| 5.1-37.0 | 167 [23.9] | 37.9 | 38 | ||
| 37.1-100.0 | 139 [19.9] | 27.1 | 32 | ||
| 100.1-353.0 | 178 [25.4] | 17.4 | 22 | ||
| >353.1 | 177 [25.3] | 10.9 | 20 | ||
| Tumor site | Head | 583 [83.3] | 20.8 | 25 | .102 |
| Body | 74 [10.6] | 31.8 | 37 | ||
| Tail | 31 [4.4] | 27.5 | 22 | ||
| Unknown | 12 [1.7] | ||||
| Type of pancreatectomy | PD | 494 [70.6] | 21.4 | 25 | .032 |
| DP | 99 [14.1] | 31.7 | 35 | ||
| TP | 34 [4.9] | 9.9 | 21 | ||
| Unknown | 73 [10.4] | ||||
| Grade | 1 | 68 [9.7] | 33.2 | 29 | .001 |
| 2 | 276 [39.4] | 21.0 | 29 | ||
| 3 | 272 [38.9] | 19.3 | 21 | ||
| 4 | 14 [2.0] | 22.0 | 28 | ||
| Unknown | 70 [10.0] | ||||
| Margin status | R0 | 350 [50.0] | 27.7 | 29 | <.001 |
| R1 | 203 [29.0] | 12.5 | 21 | ||
| Unknown | 147 [21.0] | ||||
| Tumor diameter (mm) | < 30 | 269 [38.4] | 35.3 | 31 | <.001 |
| ≥ 30 | 223 [31.9] | 17.4 | 22 | ||
| Unknown | 208 [29.7] | ||||
| pT stage | 1 | 21 [3.0] | 61.5 | 84 | <.001 |
| 2 | 78 [11.1] | 27.5 | 36 | ||
| 3 | 549 [78.4] | 19.5 | 24 | ||
| 4 | 44 [6.3] | 23.6 | 22 | ||
| Unknown | 8 [1.2] | ||||
| pN stage | N0 | 208 [29.7] | 36.5 | 34 | <.001 |
| N+ | 486 [69.4] | 16.1 | 23 | ||
| Unknown | 6 [0.9] | ||||
| Adjuvant CT | No | 302 [43.1] | 21.8 | 22 | .009 |
| Yes | 398 [56.9] | 22.3 | 28 | ||
| Postoperative RT ± CCT | No | 311 [44.4] | 25.9 | 25 | .271 |
| Yes | 389 [55.6] | 19.4 | 25 | ||
| RT dose (Gy) | <50.4 | 85 [12.2] | 24.4 | 25 | .660 |
| >50.4 | 222 [31.7] | 20.1 | 26 | ||
| Unknown | 393 [56.1] |
CCT: concurrent CT; DP, distal pancreatectomy; PD, pancreaticoduodenectomy; TP, total pancreatectomy.
Analyzing the association between presurgical CA19-9 level, excluding patients considered to be unable to express the marker (≤5 U/ml), those with a higher CA19-9 level had more advanced disease in terms of pathological tumor (P<.001) and nodal stage (P<.001), while no significant correlation with the margin status was recorded (P=.256) (Table 2).
Table 2.
Association between Presurgical CA19-9 Level and Tumor Stage, Nodal Stage, and Margins Status
| Ca 19-9 Level (U/ml) | Tumor Stage |
Nodal Stage |
Margin Status |
|||
|---|---|---|---|---|---|---|
| T1-2 (%) | T3-4 (%) | N0 (%) | N1 (%) | R0 (%) | R1 (%) | |
| 0.0-5.0 | 2.8 | 97.2 | 27.8 | 72.2 | 57.1 | 42.9 |
| 5.1-37.0 | 21.6 | 78.4 | 39.4 | 60.6 | 60.7 | 39.3 |
| 37.1-100.0 | 17.3 | 82.7 | 36.0 | 64.0 | 60.7 | 39.3 |
| 100.1-353.0 | 12.4 | 87.6 | 22.6 | 77.4 | 66.7 | 33.3 |
| >353.1 | 9.6 | 90.4 | 24.3 | 75.7 | 65.9 | 34.7 |
| P trend* | <.001 | <.001 | .256 | |||
Score test for linear trend of the log odds across CA 19-9 level categories 5.1-37.0, 37.1-100.0, 100.1-353.0, and >353.1.
Analyzing the impact of presurgical CA19-9 on outcome, increasing values of the marker were significantly correlated with worse OS (P<.001), LC (P=.008), and DMFS (P<.001), with the exception of patients with CA19-9 ≤5 U/ml (Table 3). Supplementary Material Figure shows the median OS curve fitted by presurgical CA19-9 levels. For patients with CA19-9 >200 U/ml, median OS did not further worsen and was less than 2 years. Figure 1, Figure 2, Figure 3 represent OS, DMFS, and LC stratified by presurgical CA19-9 classes and adjusted by participating center.
Table 3.
Impact of Presurgical CA19-9 on LC and DMFS
| Variable | Value (U/ml) | 5-year LC (%) | Median LC (Months) | P | 5-year DMFS (%) | Median DMFS (Months) | P |
|---|---|---|---|---|---|---|---|
| CA19-9 | 0.0-5.0 | 47.2 | 24 | .008 | 17.0 | 20 | <.001 |
| 5.1-37.0 | 63.3 | NR | 46.0 | 45 | |||
| 37.1-100.0 | 59.4 | NR | 39.0 | 29 | |||
| 100.1-353.0 | 43.4 | 26 | 26.7 | 15 | |||
| >353.1 | 50.2 | 71 | 23.4 | 14 |
NR, not reached.
Supplementary Material Figure.
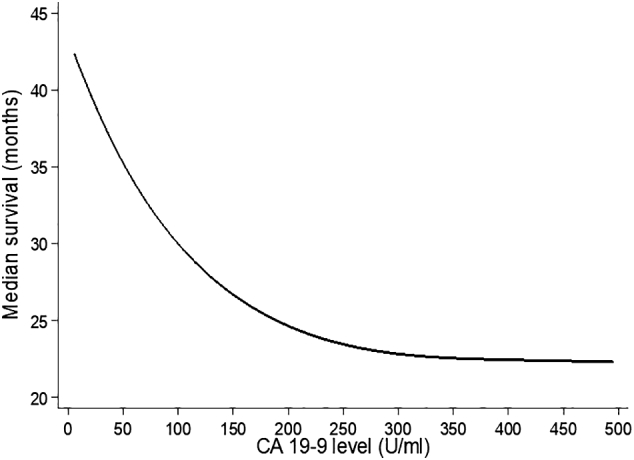
Median overall survival by CA 19-9 levels. Estimates from a spline Weibull proportional-hazards regression model assuming shared frailty among centers. Analysis restricted to subjects with CA 19-9 level above 5 U/ml.
A further subgroup analysis of 404 patients with complete information was performed (Supplementary Material Table). Univariate analysis showed a better prognosis in females, in younger (<59 years) patients, and in subjects undergoing postoperative CRT, R0 resection, or pancreaticoduodenectomy. Higher survival rates were recorded even in grade 1, pT1-2, and pN0 stage tumors and in PaCs with diameter <30 mm. Furthermore, patients with a normal CA19-9 (5.1-37.0 U/ml) value showed better OS (median: 36 months) compared to the rest of the cohort.
At multivariable analysis, grade 3 (HR: 1.85, 95% CI 1.26-2.70, P=.001) and lymph node involvement (HR: 1.85, 95% CI: 1.35-2.53, P<.001) were associated with worse OS. Patients treated with a CRT dose >50 Gy (HR: 0.38, 95% CI: 0.23-0.64, P<.001) showed a reduced risk of mortality. Median OS progressively worsened in patients with CA19-9 levels between 100.1 and 353.0 U/ml (HR: 1.79, 95% CI: 1.24-2.58, P=.002) and CA19-9 levels ≥353.1 (HR: 1.91, 95% CI: 1.33-2.74, P<.001).
Discussion
To the best of our knowledge, this study is one of the largest analyses on the prognostic role of presurgical CA19-9 value in resected PaC [10], [20], [21], [22]. The results of this analysis clearly confirmed the role of CA19-9 as a predictor of OS. More interestingly, they demonstrated the role of this marker in predicting both LC and DMFS. Finally, they identified on an experimental basis a cutoff level (200 U/ml) able to define the patients’ population with worse prognosis.
This analysis presents evident limits including the retrospective design, heterogeneity in the methods and timing of follow-up, and lack of correction of CA19-9 levels based on bilirubin values to prevent the confounding effect on the marker level due to the unavailability of this information. However, correcting CA19-9 values based on bilirubin levels does not seem to be mandatory as shown by the study of Hartwig and colleagues [7].
Our results confirm several previous reports [15], [20], [23], [24], [25], [26] showing a relationship between higher presurgical CA19-9 level (>100 U/ml or >1000 U/ml) and poor prognosis. Only Kondo and colleagues reported lack of correlation between presurgical CA19-9 and OS, probably due to the relatively small number of patients included in their series [16].
Furthermore, in our analysis, a CA19-9 value >200 U/ml was particularly indicative of poor OS and could be used as cutoff level for patients’ stratification in future studies (Supplementary Material Figure).
Previous studies on carcinogenesis showed that CA19-9 is related to the Lewis blood group antigens by a mechanism of gene silencing of a sialyltransferase, which implies early abnormal synthesis and accumulation of the sialyl Lewis-a [27], [28]. Approximately 7%-10% of Caucasian individuals have Lewis α−β− phenotype [14], [28] and therefore are unable to express this antigen.
In this analysis, contrary to the results reported by Berger and colleagues [9] and Hartwig and coworkers [6], nonsecretor patients showed a worse 5-year OS compared to those with CA 19-9 level between 5 and 37 U/ml (5.7% vs. 37.9%, respectively). Therefore, further analysis on the outcome of these patients’ subgroups is justified to avoid undertreatment of this group of subjects.
Some previous studies reported a correlation between CA 19-9 and resectability [29], [30]. These findings are confirmed by the relationship we observed between the five CA19-9 classes and tumor (P<.001) and nodal stage (P<.001). Our analysis also showed a significant impact of CA19-9 level on patients’ outcome, independently of other well-known prognostic factors such as tumor grade and stage. These findings suggest a relationship between CA19-9 values with biological tumor aggressiveness and therefore the opportunity to include this marker in predictive models [30].
This study demonstrated a significant correlation between presurgical CA19-9 levels and LC and DMFS. Kim and colleagues [13] reported a worse DMFS in patients with higher CA19-9 values, while a correlation with LC was never reported before. It has been hypothesized that through a different ligand, CA19-9 enhances metastasizing via a mechanism leading to extravascular invasion [31].
From a clinical point of view, this information may be of little practical use due to the dismal prognosis of PaC patients. In fact, while LC and DMFS rates were significantly different based on the CA19-9 classes defined in this study, their values were low in all cases. For example, in the class (5.1-37.0 U/ml) with lower incidence of disease recurrences, 5-year LC and DMFS were 63.3% and 46.0%, respectively. Therefore, these values cannot justify a deescalation of adjuvant therapies. However, this information suggests that it would be appropriate to stratify patients by presurgical CA19-9 values in future trials on PaC having LC and DMFS as end points. Finally, considering the significant impact of the presurgical value of this marker on both LC and metastases, new studies could be specifically designed for patients with presurgical CA19-9 >200 U/ml. These trials should provide an intensification of both systemic (for example, by combining CT with immunotherapy) and local treatments (for example, with a dose escalation of postoperative RT achievable using the most modern RT techniques).
Conclusion
Our pooled analysis showed that presurgical CA19-9 levels significantly predict OS, LC, and DMFS. Future researches in this field seem justified in order to test the possibility of increasing the predictive power of CA19-9 through a dynamic evaluation of its level and/or in association with other tumor biomarkers and/or together with radiomic analysis. Furthermore, our report suggests the inclusion of CA19-9 in predictive models and in patients’ stratification in future trials on resectable PaC.
The following are the supplementary data related to this article.
Overall Survival: Estimates from Weibull Proportional-Hazards Regression Models Assuming Shared Frailty Among Participating Centers. Analysis of 404 Patients with Complete Information
Disclosure
The authors have no actual or potential conflicts of interest existing regarding this paper. The abstract has previously been presented as a poster at the 35th Annual meeting of the ESTRO (European Society for Radiotherapy & Oncology), Torino, Italy, April 29-May 2, 2016.
References
- 1.https://seer.cancer.gov/statfacts/html/pancreas.html
- 2.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2014. Ann Oncol. 2014;25:1650–1656. doi: 10.1093/annonc/mdu138. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 5.Arcelli A, Cammelli S, Frakulli R, Farina E, Guido A, Cellini F, Macchia G, Mattiucci GC, Buwenge M, Morganti AG. Translational research in radiotherapy of pancreatic adenocarcinoma: a review. Transl Biomed. 2015;5:1–5. [Google Scholar]
- 6.Hartwig W, Strobel O, Hinz U, Fritz S, Hackert T, Roth C. CA19-9 in potentially resectable pancreatic cancer: perspective to adjust surgical and perioperative therapy. Ann Surg Oncol. 2013;20:2188–2196. doi: 10.1245/s10434-012-2809-1. [DOI] [PubMed] [Google Scholar]
- 7.Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 8.Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21:441–447. doi: 10.1093/annonc/mdp332. [DOI] [PubMed] [Google Scholar]
- 9.Berger AC, Meszoely IM, Ross EA, Watson JC, Hoffman JP. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2004;11:644–649. doi: 10.1245/ASO.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Morganti AG, Falconi M, van Stiphout RG, Mattiucci GC, Alfieri S, Calvo FA, Dubois JB, Fastner G, Herman JM, Maidment BW., 3rd. Multi-institutional pooled analysis on adjuvant chemoradiation in pancreatic cancer. Int J Radiat Oncol Biol Phys. 2014;90:911–917. doi: 10.1016/j.ijrobp.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Morganti AG, Valentini V, Macchia G, Alfieri S, Trodella L, Brizi MG. Adjuvant radiotherapy in resectable pancreatic carcinoma. Eur J Surg Oncol. 2002;28:523–530. doi: 10.1053/ejso.2002.1289. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Xu H, Wang W, Wu C, Chen Y, Yang J, Cen P, Xu J, Liu C, Long J. A preoperative serum signature of CEA+/CA125+/CA19-9 >/= 1000 U/mL indicates poor outcome to pancreatectomy for pancreatic cancer. Int J Cancer. 2015;136:2216–2227. doi: 10.1002/ijc.29242. [DOI] [PubMed] [Google Scholar]
- 13.Kim TH, Han SS, Park SJ, Lee WJ, Woo SM, Yoo T, Moon SH, Kim SH, Hong EK, Kim DY. CA 19-9 level as indicator of early distant metastasis and therapeutic selection in resected pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:743–748. doi: 10.1016/j.ijrobp.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol. 2012;3:105–119. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiura T, Uesaka K, Kanemoto H, Mizuno T, Sasaki K, Furukawa H. Serum CA19-9 is a significant predictor among preoperative parameters for early recurrence after resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:977–985. doi: 10.1007/s11605-012-1859-9. [DOI] [PubMed] [Google Scholar]
- 16.Kondo N, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Nakashima A, Sakabe R, Shigemoto N, Kato Y. Prognostic impact of perioperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2010;17:2321–2329. doi: 10.1245/s10434-010-1033-0. [DOI] [PubMed] [Google Scholar]
- 17.Harrington DP, Fleming TR. A class of rank test procedures for censored survival data. Biometrika. 1982;69:553–566. [Google Scholar]
- 18.Gutierrez RG. Parametric frailty and shared frailty survival models. Stata J. 2002;2:22–44. [Google Scholar]
- 19.Royston P, Sauerbrei W. Multivariable modeling with cubic regression splines: a principled approach. Stata J. 2007;7:45. [Google Scholar]
- 20.Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A. Fernandez-delCastillo C, Warshaw AL, Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897–2902. doi: 10.1200/JCO.2005.05.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waraya M, Yamashita K, Katagiri H, Ishii K, Takahashi Y, Furuta K. Preoperative serum CA19-9 and dissected peripancreatic tissue margin as determiners of long-term survival in pancreatic cancer. Ann Surg Oncol. 2009;16:1231–1240. doi: 10.1245/s10434-009-0415-7. [DOI] [PubMed] [Google Scholar]
- 22.Barugola G, Partelli S, Marcucci S, Sartori N, Capelli P, Bassi C. Resectable pancreatic cancer: who really benefits from resection? Ann Surg Oncol. 2009;16:3316–3322. doi: 10.1245/s10434-009-0670-7. [DOI] [PubMed] [Google Scholar]
- 23.Brown EG, Canter RJ, Bold RJ, Preoperative CA. 19-9 kinetics as a prognostic variable in radiographically resectable pancreatic adenocarcinoma. J Surg Oncol. 2015;111:293–298. doi: 10.1002/jso.23812. [DOI] [PubMed] [Google Scholar]
- 24.Hallemeier CL, Botros M, Corsini MM, Haddock MG, Gunderson LL, Miller RC, Preoperative CA. 19-9 level is an important prognostic factor in patients with pancreatic adenocarcinoma treated with surgical resection and adjuvant concurrent chemoradiotherapy. Am J Clin Oncol. 2011;34:567–572. doi: 10.1097/COC.0b013e3181f946fc. [DOI] [PubMed] [Google Scholar]
- 25.Tzeng CW, Balachandran A, Ahmad M, Lee JE, Krishnan S, Wang H, Crane CH, Wolff RA, Varadhachary GR, Pisters PW. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2014;16:430–438. doi: 10.1111/hpb.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Q, Yang XH, Zhang Y, Jing W, Zheng LQ, Liu YP. Elevated serum CA19-9 level is a promising predictor for poor prognosis in patients with resectable pancreatic ductal adenocarcinoma: a pilot study. World J Surg Oncol. 2014;12:171. doi: 10.1186/1477-7819-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duraker N, Hot S, Polat Y, Hobek A, Gencler N, Urhan N. CEA, CA 19-9, and CA 125 in the differential diagnosis of benign and malignant pancreatic diseases with or without jaundice. J Surg Oncol. 2007;95:142–147. doi: 10.1002/jso.20604. [DOI] [PubMed] [Google Scholar]
- 28.Vestergaard EM, Hein HO, Meyer H, Grunnet N, Jorgensen J, Wolf H. Reference values and biological variation for tumor marker CA 19-9 in serum for different Lewis and secretor genotypes and evaluation of secretor and Lewis genotyping in a Caucasian population. Clin Chem. 1999;45:54–61. [PubMed] [Google Scholar]
- 29.Zhang S, Wang YM, Sun CD, Lu Y, Wu LQ. Clinical value of serum CA19-9 levels in evaluating resectability of pancreatic carcinoma. World J Gastroenterol. 2008;14:3750–3753. doi: 10.3748/wjg.14.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamburrino D, Partelli S, Crippa S, Manzoni A, Maurizi A, Falconi M. Selection criteria in resectable pancreatic cancer: a biological and morphological approach. World J Gastroenterol. 2014;20:11210–11215. doi: 10.3748/wjg.v20.i32.11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnani JL. The discovery, biology, and drug development of sialyl Lea and sialyl Lex. Arch Biochem Biophys. 2004;426:122–131. doi: 10.1016/j.abb.2004.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overall Survival: Estimates from Weibull Proportional-Hazards Regression Models Assuming Shared Frailty Among Participating Centers. Analysis of 404 Patients with Complete Information